Abstract
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) and common buckwheat (Fagopyrum esculentum Moench) are important sources of proteins with balanced amino-acid compositions, and thus of high nutritional value. The polyphenols naturally present in Tartary buckwheat and common buckwheat lower the true digestibility of the proteins. Digestion-resistant peptides are a vehicle for fecal excretion of steroids, and in this way, for bile acid elimination and reduction of cholesterol concentrations in serum. Buckwheat proteins are more effective compared to soy proteins for the prevention of gallstone formation. Tartary and common buckwheat grain that contains appropriate amounts of selenium-containing amino acids can be produced as functional food products. The protein-rich by-products of buckwheat are a good source of bioactive substances that can suppress colon carcinogenesis by reducing cell proliferation. The grain embryo is a rich source of proteins, so breeding buckwheat with larger embryos is a possible strategy to increase protein levels in Tartary and common buckwheat grain. However, chemical analysis of the grain is the most relevant criterion for assessing grain protein levels and quality.
1. Introduction
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) and common buckwheat (Fagopyrum esculentum Moench) are traditionally grown in the Himalayas, south-east Asia, and central and eastern Europe. Buckwheat is a low input plant and is suitable for cultivation in mountainous regions. In some parts of the world, buckwheat is an important component of the diet, and thus an important source of carbohydrate, protein, and secondary metabolites in the human diet [1,2,3,4,5]. Buckwheat noodles are a popular dish in Japan, and traditionally in some rural areas of China, Korea, and Bhutan, and in regions on the southern slopes of the European Alps, including Slovenia, Italy, Switzerland, and France.
In Slovenia, bread made with Tartary and common buckwheat is used traditionally, and its consumption is again becoming popular [5]. Buckwheat groats (i.e., husked buckwheat grain) are traditionally produced and consumed in central and eastern Europe (i.e., Slovenia, Croatia, Poland, Ukraine, Russia), and can be used in a similar way to rice [5,6].
While breeding of common buckwheat has been widespread in all buckwheat growing areas, Tartary buckwheat is a specialty crop that is appreciated in less favorable environmental conditions. Until recently, Tartary and common buckwheat breeding were designed to achieve high yields and resistance to less favorable environmental conditions. More recently, special attention has been paid towards the optimization of the nutritional quality parameters of Tartary buckwheat and common buckwheat, which has included high protein content and optimal amino-acid composition of the proteins. Here, we review the possibilities to develop Tartary and common buckwheat for grain with higher protein levels with improved amino-acid composition.
2. Protein Levels in Buckwheat Grain and Milling Fractions
Protein levels in buckwheat grain are relatively low (~12%) compared to leguminous plants (soybean meal, ~51%), although they are higher compared to most cereals [7]. It is possible to concentrate proteins by milling and separation of the fractions of common buckwheat. The milling fractions with the highest protein levels is the bran, with ~30% protein in terms of dry matter [8]. The highest levels of the flavonoid rutin were also detected in the same milling fraction. The lowest protein levels are seen for the flour fraction, with only 4.4% [8].
By milling Tartary buckwheat, bran with 25% protein can be obtained, with the flour with 10% protein [9,10]. Thus, by adapting the milling methods, it is possible to obtain products for diverse uses and nutritional values.
3. Quality of Buckwheat Protein Compared to Soybean and Cereals
According to Eggum [7], common buckwheat contains 5.1% lysine in its protein fraction (i.e., 5.1 g/16 g N). For comparison, lysine levels in the protein fractions measured by the same method were 2.6% for wheat, 2.8% for maize, and 3.7% for barley (Table 1). Lysine levels for the protein in soybean meal are 6.0% and the same in fava bean. Due to the high levels of lysine, and the well-balanced levels of the other amino acids, the biological value of buckwheat proteins is 93%. From the same type of experiments with rats, soybean meal proteins have a biological value of 68%, compared to that of pork protein, at ~84%. Despite the higher lysine levels in soybean and fava bean, the buckwheat protein is superior, with the lower biological value of the soybean and fava bean proteins due to their lower levels of sulfur-containing amino acids (Table 1) [7].

Table 1.
Contents of essential amino acids and biological value of various protein sources (adapted from Eggum [7]).
These low levels of sulfur-containing amino acids in beans means that quality is compensated for by quantity. However, according to Eggum [7], high quantities of protein are not an advisable solution to the problem of this less balanced amino acid composition in beans. These bean proteins that contain amino acids with lower nutritional quality is the superficial amount of nonessential amino acids. This superficial amount is used by an organism only as a source of energy. However, this represents a relative waste of energy, as proteins are not a good energy source [7].
The distribution of sulfur in grain can be used as a marker of protein distribution among different grains and within the different parts of the grain [11,12]. Recently, the use of microparticle-induced X-ray emission (micro-PIXE) has improved the detection of sulfur content and its allocation to the different structures of buckwheat grain, which might serve for estimation of the relative levels of proteins containing sulfur amino acids in the cotyledons and the aleurone [13,14,15].
Seed storage proteins in buckwheat are mainly water and saline soluble, in contrast to gluten-containing cereals [16,17,18]. Buckwheat grain proteins consist of up to 50 to 60% albumins and glutelins [19]. Buckwheat samples also have high concentrations of lysine, although threonine and sulfur-containing amino acids are the first limiting amino acids in regard to the need for amino acids in human nutrition [20,21,22,23,24].
The amino acids serine, proline, glycine, histidine, and arginine, obtained after in-vitro digestion of buckwheat bran appears to have high cytotoxic effects on cultured colon cancer cells. The final cytotoxic effects arise from not only the levels of bioactive substances, but also as a function of the synergistic actions among these. Thus, buckwheat protein-rich by-products are a good source of bioactive substances [25,26,27,28,29]. However, in-vitro antiproliferative effects on cancer cells do not always predict health-promoting effects, as the impacts must also be studied and confirmed in vivo. Some literature data [26] have stressed the need for a complex evaluation of the biological activities of buckwheat metabolites on intestinal health. Consumption of buckwheat protein extracts has been shown to slow mammary carcinogenesis in rats, which was connected with muscle hypertrophy and reduced hepatic triglyceride concentrations [30,31,32,33].
In selenium-enriched buckwheat plants, the resulting proteins are interesting in terms of their selenium content [34]. It is feasible to increase the selenium concentration in Tartary and common buckwheat grain by fertilizing them with selenium compounds so that they become a good source of seleno amino acids [35,36]. In selenium-enriched buckwheat grain, 39% of the selenium is present as water-soluble selenium, such as in peptides, proteins, and other selenium compounds; also, 93% of this water-soluble selenium is selenomethionine [37], which is one of the most bioavailable forms of selenium for animals and humans.
The selenium content in Tartary buckwheat under ambient UV-B radiation is higher compared to common buckwheat. Tartary buckwheat can grow at higher altitudes than common buckwheat. In mountain areas, the UV-B radiation is more intense, and selenium might protect the plants from strong UV-B radiation. Enhanced UV-B radiation increases the selenium content in the vegetative parts of common buckwheat without selenium treatment [37]. In addition, higher selenium levels have been shown for selenium-treated Tartary buckwheat compared to selenium-treated common buckwheat [38]. The grain of selenium-treated hybrid buckwheat also shows increased accumulation of selenium when exposed to reduced UV radiation, compared to plants exposed to ambient UV radiation levels [39].
With regards to selenium and flavonoids, there are similar effects of UV-B radiation on flavonoid content in buckwheat. Tartary buckwheat contains more flavonoids than common buckwheat [40]. Kreft et al. [41] studied the impact of UV-B radiation on rutin content and reported that this was higher in plants exposed to ambient levels of UV-B radiation, compared to plants grown under reduced UV-B radiation.
4. Buckwheat Protein Digestibility
Proteins from Tartary buckwheat and common buckwheat have low digestibility [42,43]. The polyphenols that are naturally present in Tartary buckwheat and common buckwheat, including rutin and quercetin, lower the true digestibility of the proteins [43,44,45,46]. Ikeda et al. [47] reported that phenolic substances have significant inhibitory effects on in-vitro peptic and pancreatic digestion of globulin, and thus Tartary buckwheat and common buckwheat secondary metabolites might have an impact on protein digestibility [48]. Considerable interactions between polyphenols and proteins are observed after hydrothermal treatment [45]. In buckwheat, the situation is similar to millet, where the protein digestibility is slower compared to other cereals, potentially because of the binding of polyphenolics to proteins [49].
The interactions between proteins and phenolic substances slow down the digestion of proteins in the small and large intestine. However, microbial fermentation in the colon can enhance the digestibility of proteins blocked by polyphenols in hydrothermally processed buckwheat [44,45]. Digestion-resistant peptides are important for the fecal excretion of steroids. Buckwheat proteins prevent gallstone formation more strongly in comparison to soy proteins. They might also slow mammary carcinogenesis by lowering serum estradiol and suppress colon carcinogenesis by reducing cell proliferation [50,51,52,53,54,55,56].
It would be expected that breeding Tartary buckwheat and common buckwheat for lower grain polyphenols content will enhance the nutritional value of the proteins; however, this might instead be unfavorable for the beneficial impact of polyphenol-protein complexes for preventing diseases [45,51].
5. Bioactivity of Buckwheat Peptides
Buckwheat peptides can be obtained by the fermentation of buckwheat grain or sprouts.
The buckwheat grain is the source of biofunctional peptides [28,57,58,59,60,61], which are fermented in the process of malting [62] and during the preparation of buckwheat beer or distillation products [63,64].
Sprouts from Tartary and common buckwheat grain are prepared to produce buckwheat vegetable, which is rich in flavonoids and mineral elements [65,66,67]. Sprouts can be further processed by fermentation, and valuable products with reported blood-pressure-lowering effects can be obtained [68,69,70]. Purified buckwheat proteins and peptides can be used due to their beneficial effect in atherosclerosis [71,72] and hypercholesterolemia [73,74,75,76].
There are possibilities to develop novel buckwheat products based on the functional and technological values of buckwheat proteins and peptides [74,75,77,78,79,80]. The properties of buckwheat proteins and isolates can be altered by thermal and ultrasound treatment. Maillard reaction can change the proteins to modify their allergenicity [81,82,83]. Another method to obtain novel buckwheat protein-rich food products is to fractionate buckwheat grain by milling [84]. However, none of these methods appears to be satisfactory for sufficient removal or inactivation of allergenic buckwheat proteins in the products. This is a pity because some buckwheat products are used for gluten-free products, and some customers might have multiple food intolerance syndromes [85,86,87]. Tartary and common buckwheat, alone or in combination with rice or corn, are used in the development of gluten-free food products [88].
Buckwheat leaf flour and grain can be used to produce protein isolates [89,90,91,92,93]. However, the process should include the removal of polyphenolic substances [94]. On the other hand, tannin-protein complexes can be used in buckwheat products due to their radical scavenging effects; these complexes can provide effective radical sinks and modify protein digestibility [17,45,95,96].
6. Buckwheat Breeding for High Protein Levels: Prospects for the Future
Buckwheat grain is rich in high-quality proteins and contains well-balanced amounts of essential amino acids (i.e., lysine, methionine, cysteine, tryptophan). In addition, common buckwheat is important as a nectariferous plant, and both Tartary and common buckwheat are pharmaceutical plants [97,98,99,100]. In recent buckwheat breeding programs, particular properties have been investigated, such as high grain yield, frost resistance, increased protein content, reduced allergenic protein content [97]. People with multiple allergies can also develop an allergy when eating buckwheat [85], which can be caused by low-molecular-weight proteins in the grain embryo (i.e., 18–29 kD) [84,100,101,102,103,104,105]. However, there have not been any reports on the allergenicity of endosperm proteins and of proteins with diverse molecular weights in the aleurone. Besides the embryo, the aleurone is the main grain tissue with a high protein content (Figure 1). In the preparation of buckwheat food products, it is important to understand how endosperm and embryo cells are damaged under the technological processes to make the content of the cells available for intestinal digestion [106,107]. Temperature treatments and intestinal microbial activity are other factors of importance in the production of buckwheat foods with health promoting effects [68,108,109,110,111]. It would be expected that breeding Tartary buckwheat and common buckwheat for larger embryo and thicker aleurone will enhance the amount of the grain proteins. Genetic variability of embryo size has been reported to have an impact on the chemical composition of grains, as reported for barley and genus Avena [112,113]. Microscopic analyses of buckwheat grain might represent a method for screening for buckwheat breeding, in terms of a larger embryo and aleurone, and thus also for higher protein levels in the grain [15] (Figure 1).
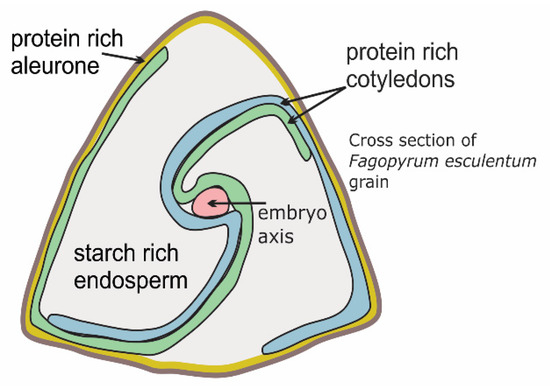
Figure 1.
Cross-section of a common buckwheat grain.
At the same time, there is greater protein polymorphism within varieties of common buckwheat than between varieties [114]. This high polymorphism can be exploited in breeding, although like globulin, most of the proteins show single Mendelian gene inheritance, with a codominant expression of multiple alleles, which complicates plant breeding [115]. Cross-fertilization of common buckwheat has an impact on the breeding approach. The suggested procedure for common buckwheat breeding, according to Kreft [116], is presented in Figure 2. Common buckwheat is a cross-pollinating plant. In the field breeding experiments, diploid common buckwheat must be in physical isolation (in tents or glasshouses), so uncontrolled pollination is prevented in common buckwheat breeding (Figure 2). Such isolation is not needed in the breeding of self-compatible Tartary buckwheat varieties.
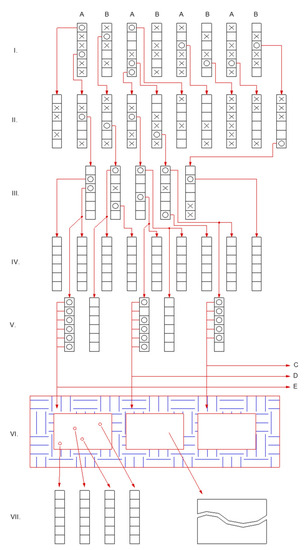
Figure 2.
Stages of buckwheat breeding over time. The Roman numerals (I–VII) indicate the years (generations) of the breeding. The starting material in the first year is two-parent varieties or populations, indicated as A and B. Both parents are sown in parallel rows. For one parent, after opening the first flower on each plant, the plants with thrum flowers are removed. In the other parent, the plants with pin flowers are removed. So in generation (I), only fertilization between A and B flowers is possible, and not fertilization among the plants within each parent. Each square symbolizes a plant, and the plants marked with “x” are removed from breeding, while the plants marked with “o” are selected for further propagation and breeding. Groups of plants with empty squares are those that are used for testing according to the phenotype in the field trial, but not for further propagation or breeding. The best-selected plant material is isolated and taken forward for further propagation or breeding or for submission to official variety testing. In generation (VI), the plants are sown in isolation, for evaluation of yield and for propagation. The plant groups C, D, and E are taken on to the first field tests on larger isolated plots.
Under Slovenian and similar climatic conditions, it is possible to grow two generations of buckwheat in 1 year. Under these Slovenian conditions, the first generation of the year is sown at the beginning of May and harvested in mid-July. The second generation is thus sown about a week after the harvest of the first generation and is harvested in early October. The second-generation (Figure 2, II) can theoretically be uniform if the parent plants are homozygous. However, in common buckwheat populations or varieties the plants are never homozygous, because the populations or varieties are obligatory cross-pollinated. Therefore a certain selection is already feasible and reasonable in the second generation (Figure 2, II). Isolation is possible by means of greenhouse compartments or tents, which prevents insects from visiting from outside, while bumblebees in each compartment can pollinate the plants. Another method of isolation is to use a tetraploid buckwheat area between the diploid breeding material lots. It has been shown [116] that 12 m to 20 m of such isolation is sufficient to prevent undesirable plant crossings [116,117,118]. The pollination of diploid plants with pollen grains from tetraploid plants or vice versa, results in aborted or sterile seeds.
The number of proteins per seed can be estimated by screening the embryo size in cross-sections of grain [112,113]. However, the more accurate methods are by chemical analyses. The problem is that for chemical analysis, some of the seeds are milled, and hence, they are lost from further seed propagation of the respective sample. It is thus necessary to use as small a sample for protein analyses as possible or to cut off the upper third of the seed only, which covers part of the cotyledons and aleurone. The remaining part of the seed can then be sown for the next generation.
Breeding programs include the development of adapted high-yield varieties that have other good agronomic characteristics, such as high levels of rutin, the very high biological value of the proteins, or reduced levels of antinutritional factors and zero allergenic protein [19,119,120]. In the future, it would make sense for buckwheat breeding programs to include genotypes with larger endosperm and smaller embryo as an option to reduce allergenic proteins to produce specific low allergenicity buckwheat varieties.
A recently published draft sequence of genes of the common buckwheat [121,122] has allowed the use of genomic selection for quantitative traits based on DNA markers, as well as other methods related to the quality of proteins [123]. Furthermore, the availability of such information facilitates the identification of markers for the identification of qualitatively useful genes [121,124]. A major advantage of genomic selection is that, in contrast to the conventional marker-assisted selection, it is suitable for improving quantitative properties managed by multiple quantitative trait loci. Genomic selection and use of DNA markers in breeding buckwheat protein quality are feasible only at the initial stages [125,126,127]. Rapid advances in genomic technology will certainly improve genomics-based breeding for buckwheat quality in the near future [128].
7. Conclusions
Buckwheat is a low-input plant that is suitable for cultivation in mountainous regions. Recently, special attention has been paid to optimizing the nutritional quality parameters of Tartary buckwheat and common buckwheat, including its high protein content and optimal amino acid composition. The distribution of sulfur in the grain can be used as a marker of protein distribution among the different grains and within the different parts of the grain. The use of micro-PIXE has improved the detection of sulfur and its distribution in the grain, which can be used to estimate the relative content of proteins with sulfur amino acids in the cotyledons and aleurone.
It is possible to increase the selenium concentration in buckwheat grain by fertilizing plants with selenium compounds, making them a good source of selenoamino acids. In selenium-enriched buckwheat grain, 39% of selenium is present as water-soluble selenium—e.g., in peptides, proteins, and other selenium compounds. In addition, 93% of this water-soluble selenium is selenomethionine, which is one of the most bioavailable forms of selenium for human nutrition.
Rutin, quercetin, and other polyphenols, that are naturally present in buckwheat, reduce the digestibility of the proteins. Phenolic substances have significant inhibitory effects on the in vitro digestion of globulin in the intestine, so secondary metabolites of buckwheat can have an influence on the digestibility of proteins. Significant interactions between polyphenols and proteins are observed after hydrothermal treatment. In buckwheat, protein digestibility is slower compared to other cereals, possibly due to the binding of the polyphenols to the proteins. The interactions between proteins and phenolic substances slow down proteins’ digestion in the small and large intestine. However, microbial fermentation in the large intestine can improve the digestibility of proteins blocked by polyphenols in hydrothermally treated buckwheat. Digestion-resistant peptides are important for the fecal excretion of steroids. The excretion of bile acids reduces the cholesterol concentration in serum. Buckwheat proteins are more effective at preventing the formation of gallstones than soya proteins. There are opportunities to improve protein content and quality through buckwheat breeding and to develop novel buckwheat products based on buckwheat proteins and peptides’ functional and technological value.
Author Contributions
Conceptualization, Z.L. and M.G.; data curation, M.G., M.Z., and A.G.; validation, writing original draft preparation, review and editing, all authors equally responsible; visualization, A.G.; supervision, M.G.; project administration and funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was the result of a study financed by the Slovenian Research Agency, through the programs P1-0212 “Biology of Plants” and P3-0395 “Nutrition and Public Health”, and the applied project L4-9305, co-financed by the Ministry of Agriculture, Forestry and Food, Republic of Slovenia.
Acknowledgments
The authors are grateful for the collaboration with Christian Zewen, Luxemburg, and to Christopher Berrie for revising the English text.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Luthar, Z. Buckwheat genetic resources in Central Europe. In Buckwheat Germplasm in the World; Zhou, M., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Academic Press, An Imprint of Elsevier: London, UK, 2018; pp. 127–143. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Yan, J.; Zhou, M.; Woo, S.-H.; Weng, W.; Cheng, J.; Zhang, K. Tartary Buckwheat: An Under-utilized Edible and Medicinal Herb for Food and Nutritional Security. Food Rev. Int. 2020, 1–15. [Google Scholar] [CrossRef]
- Dziedzic, K.; Kurek, S.; Mildner–Szkudlarz, S.; Kreft, I.; Walkowiak, J. Fatty acids profile, sterols, tocopherol and squalene content in Fagopyrum tataricum seed milling fractions. J. Cereal Sci. 2020, 96, 103118. [Google Scholar] [CrossRef]
- Skrabanja, V.; Kreft, I. Resistant Starch Formation Following Autoclaving of Buckwheat (Fagopyrum esculentum Moench) Groats. An In Vitro Study. J. Agric. Food Chem. 1998, 46, 2020–2023. [Google Scholar] [CrossRef]
- Eggum, B.O. The protein quality of buckwheat in comparison with other protein sources of plant or animal origin. In Buckwheat: Genetics, Plant Breeding, Utilization; Kreft, I., Javornik, B., Dolinšek, B., Eds.; Biotechnical Faculty: Ljubljana, Slovenia, 1980; pp. 115–120. [Google Scholar]
- Skrabanja, V.; Kreft, I.; Golob, T.; Modic, M.; Ikeda, S.; Ikeda, K.; Kreft, S.; Bonafaccia, G.; Knapp, M.; Kosmelj, K. Nutrient Content in Buckwheat Milling Fractions. Cereal Chem. J. 2004, 81, 172–176. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Vombergar, B.; Luthar, Z. The concentration of flavonoids, tannins and crude proteins in grain fractions of common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum Gaertn.). Folia Boil. Geol. 2019, 59, 101–157. [Google Scholar] [CrossRef]
- Kump, P.; Rupnik, P.; Budnar, M.; Kreft, I. Analysis of sulphur in plant material. Nucl. Instrum. Methods Phys. Res. B 1977, 142, 205–208. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Pelicon, P.; Vavpetič, P.; Kreft, I.; Regvar, M. Elemental analysis of edible grains by micro-PIXE: Common buckwheat case study. Nucl. Instrum. Methods Phys. Res. B 2009, 267, 2884–2889. [Google Scholar] [CrossRef]
- Kaulich, B.; Gianoncelli, A.; Beran, A.; Eichert, D.; Kreft, I.; Pongrac, P.; Regvar, M.; Vogel-Mikuš, K.; Kiskinova, M. Low-energy X-ray fluorescence microscopy opening new opportunities for bio-related research. J. R. Soc. Interface 2009, 6, S641–S647. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Regvar, M.; Vavpetič, P.; Pelicon, P.; Kreft, I. Improved Lateral Discrimination in Screening the Elemental Composition of Buckwheat Grain by Micro-PIXE. J. Agric. Food Chem. 2011, 59, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Pongrac, P.; Kelemen, M.; Vavpetič, P.; Vogel-Mikuš, K.; Regvar, M.; Pelicon, P. Application of micro-PIXE (particle induced X-ray emission) to study buckwheat grain structure and composition. Fagopyrum 2020, 37, 5–10. [Google Scholar] [CrossRef]
- Chrungoo, N.; Dohtdong, L.; Chettry, U. Diversity in Seed Storage Proteins and Their Genes in Buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Academic Press is an Imprint of Elsevier: London, UK, 2016; pp. 387–399. [Google Scholar]
- Škrabanja, V.; Kreft, I. Nutritional Value of Buckwheat Proteins and Starch. In Molecular Breeding and Nutritional Aspects of Buckwheat; Academic Press is an Imprint of Elsevier: London, UK, 2016; pp. 169–176. [Google Scholar]
- Radović, S.R.; Maksimović, V.R.; Varkonyi-Gasic, E. Characterization of Buckwheat Seed Storage Proteins. J. Agric. Food Chem. 1996, 44, 972–974. [Google Scholar] [CrossRef]
- Joshi, D.C.; Zhang, K.; Wang, C.; Chandora, R.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.I.; Zhou, M. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnol. Adv. 2020, 39, 107479. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Khatkar, B.S. Pseudocereals nutritional composition functional properties and food applications. In Food Bioactives: Functionality and Applications in Human Health; Deka, S.C., Seth, D., Hulle, N.R.S., Eds.; Apple Academic Press: Cambridge, MA, USA, 2019; pp. 129–148. [Google Scholar]
- Sytar, O.; Brestic, M.; Zivcak, M.; Tran, L.-S.P. The Contribution of Buckwheat Genetic Resources to Health and Dietary Diversity. Curr. Genom. 2016, 17, 193–206. [Google Scholar] [CrossRef]
- Sytar, O.; Chrenková, M.; Ferencová, J.; Polačiková, M.; Rajský, M.; Brestič, M. Nutrient capacity of amino acids from buckwheat seeds and sprouts. J. Food Nutr. Res. 2018, 57, 38–47. [Google Scholar]
- Sytar, O.; Biel, W.; Smetanska, I.; Brestic, M. Bioactive Compounds and Their Biofunctional Properties of Different Buckwheat Germplasms for Food Processing. In Buckwheat Germplasm in the World; Zhou, M.L., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 191–204. [Google Scholar]
- Krumina-Zemture, G.; Beitane, I.; Gramatin, I. Amino acid and dietary fibre content of pea and buckwheat flours. Res. Rural Dev. 2016, 1, 84–90. [Google Scholar]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Olejnik, A.; Rychlik, J.; Kreft, I.; Drożdżyńska, A.; Walkowiak, J. The cytotoxic effect of artificially digested buckwheat products on HT-29 colon cancer cells. J. Cereal Sci. 2018, 83, 68–73. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Amarowicz, R.; Opyd, P.; Bez, J.; Muranyi, I.; Petersen, I.L.; Laparra, J.M. Protein-Rich Flours from Quinoa and Buckwheat Favourably Affect the Growth Parameters, Intestinal Microbial Activity and Plasma Lipid Profile of Rats. Nutrients 2020, 12, 2781. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, S.; Zhang, W.; Ng, T.B.; Ye, X. Buckwheat Antifungal Protein with Biocontrol Potential To Inhibit Fungal (Botrytis cinerea) Infection of Cherry Tomato. J. Agric. Food Chem. 2019, 67, 6748–6756. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Li, J.; Wang, Y.; Yang, X. Enzyme-assisted development of biofunctional polyphenol-enriched buckwheat protein: Physicochemical properties, in vitro digestibility, and antioxidant activity. J. Sci. Food Agric. 2019, 99, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, R.; Li, Y.M.; Liang, N.; Zhao, Y.; Zhu, H.; He, Z.; Liu, J.; Hao, W.; Jiao, R.; et al. Cholesterol-Lowering Activity of Tartary Buckwheat Protein. J. Agric. Food Chem. 2017, 65, 1900–1906. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Yamazaki, M.; Kato, N. Buckwheat protein extract ameliorates atropine-induced constipation in rats. Curr. Adv. Buckwheat Res. 1995, 5, 941–946. [Google Scholar]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kato, N. Feeding of buckwheat protein extract reduces hepatic triglyceride concentration, adipose tissue weight, and hepatic lipogenesis in rats. J. Nutr. Biochem. 1996, 7, 555–559. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kishida, N.; Kato, N. Consumption of a Buckwheat Protein Extract Retards 7,12-Dimethylbenz[α] anthracene-Induced Mammary Carcinogenesis in Rats. Biosci. Biotechnol. Biochem. 1999, 63, 1837–1839. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kondoh, M.; Hayashi, K.; Kato, N. Muscle Hypertrophy in Rats Fed on a Buckwheat Protein Extract. Biosci. Biotechnol. Biochem. 1999, 63, 1242–1245. [Google Scholar] [CrossRef]
- Stibilj, V.; Kreft, I.; Smrkolj, P.; Osvald, J. Enhanced selenium content in buckwheat (Fagopyrum esculentum Moench) and pumpkin (Cucurbita pepo L.) seeds by foliar fertilisation. Eur. Food Res. Technol. 2004, 219, 142–144. [Google Scholar] [CrossRef]
- Breznik, B.; Germ, M.; Gaberscik, A.; Kreft, I. Combined effects of elevated UV-B radiation and the addition of selenium on common (Fagopyrum esculentum Moench) and tartary [Fagopyrum tataricum (L.) Gaertn.] buckwheat. Photosyntetica 2005, 43, 583–589. [Google Scholar] [CrossRef]
- Tadina, N.; Germ, M.; Kreft, I.; Breznik, B.; Gaberscik, A. Effects of water deficit and selenium on common buckwheat (Fagopyrum esculentum Moench.) plants. Photosyntetica 2007, 45, 472–476. [Google Scholar] [CrossRef]
- Smrkolj, P.; Germ, M.; Kreft, I.; Stibilj, V. Respiratory potential and Se compounds in pea (Pisum sativum L.) plants grown from Se-enriched seeds. J. Exp. Bot. 2006, 57, 3595–3600. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Gadžo, D.; Stibilj, V.; Djikić, M.; Gavrić, T.; Kreft, I.; Germ, M. Sulphur interferes with selenium accumulation in Tartary buckwheat plants. Plant Physiol. Biochem. 2016, 108, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Stibilj, V.; Kreft, I.; Vogel-Mikuš, K.; Gaberscik, A.; Germ, M. Selenium treatment alters the effects of UV radiation on chemical and production parameters in hybrid buckwheat. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2017, 68, 1–11. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Kreft, S.; Štrukelj, B.; Gaberščik, A.; Kreft, I. Rutin in buckwheat herbs grown at different UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002, 53, 1801–1804. [Google Scholar] [CrossRef]
- Ikeda, K.; Kishida, M. Digestibility of proteins in buckwheat seed. Fagopyrum 1993, 13, 21–24. [Google Scholar]
- Kreft, I.; Javornik, B. Chemical composition and protein quality of buckwheat (Fagopyrum esculentum Moench). Plant Foods Hum. Nutr. 1981, 30, 175–179. [Google Scholar] [CrossRef]
- Skrabanja, V.; Laerke, H.; Kreft, I. Effects of hydrothermal processing of buckwheat (Fagopyrum esculentum Moench) groats on starch enzymatic availability in vitro and in vivo in rats. J. Cereal Sci. 1998, 28, 209–214. [Google Scholar] [CrossRef]
- Skrabanja, V.; Lærke, H.N.; Kreft, I. Protein-polyphenol interactions and in vivo digestibility of buckwheat groat proteins. Pflügers Archiv. 2000, 440, R129–R131. [Google Scholar] [CrossRef]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J. Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cereal Sci. 2019, 85, 243–248. [Google Scholar] [CrossRef]
- Ikeda, K.; Oku, M.; Kusano, T.; Yasumoto, K. Inhibitory Potency of Plant Antinutrients towards the In Vitro Digestibility of Buckwheat Protein. J. Food Sci. 1986, 51, 1527–1530. [Google Scholar] [CrossRef]
- Oparin, P.B.; Mineev, K.S.; Dunaevsky, Y.E.; Arseniev, A.S.; Belozersky, M.A.; Grishin, E.V.; Egorov, T.A.; Vassilevski, A. Buckwheat trypsin inhibitor with helical hairpin structure belongs to a new family of plant defence peptides. Biochem. J. 2012, 446, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Wieslander, G.; Fabjan, N.; Vogrincic, M.; Kreft, I.; Janson, C.; Spetz-Nyström, U.; Vombergar, B.; Tagesson, C.; Leanderson, P.; Norbäck, D. Eating Buckwheat Cookies Is Associated with the Reduction in Serum Levels of Myeloperoxidase and Cholesterol: A Double Blind Crossover Study in Day-Care Centre Staffs. Tohoku J. Exp. Med. 2011, 225, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, G.; Fabjan, N.; Vogrinčič, M.; Kreft, I.; Vombergar, B.; Norbäck, D. Effects of common and Tartary buckwheat consumption on mucosal symptoms, headache and tiredness: A double-blind crossover intervention study. J. Food Agric. Environ. 2012, 10, 107–110. [Google Scholar]
- Tomotake, H.; Shimaoka, I.; Kayashita, J.; Yokoyama, F.; Nakajoh, M.; Kato, N. A Buckwheat Protein Product Suppresses Gallstone Formation and Plasma Cholesterol More Strongly than Soy Protein Isolate in Hamsters. J. Nutr. 2000, 130, 1670–1674. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Xiong, Y.L. Antioxidant and bile acid binding activity of buckwheat protein in vitro digests. J. Agric. Food Chem. 2009, 57, 4372–4380. [Google Scholar] [CrossRef]
- Guo, X.-N.; Zhu, K.; Zhang, H.; Yao, H. Purification and Characterization of the Antitumor Protein from Chinese Tartary Buckwheat (Fagopyrum tataricum Gaertn.) Water-Soluble Extracts. J. Agric. Food Chem. 2007, 55, 6958–6961. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, K.; Zhang, H.; Yao, H. Anti-Tumor Activity of a Novel Protein Obtained from Tartary Buckwheat. Int. J. Mol. Sci. 2010, 11, 5201–5211. [Google Scholar] [CrossRef]
- Leung, E.H.W.; Ng, T.B. A relatively stable antifungal peptide from buckwheat seeds with antiproliferative activity toward cancer cells. J. Pept. Sci. 2007, 13, 762–767. [Google Scholar] [CrossRef]
- Chen, X.-W.; Luo, D.-Y.; Chen, Y.-J.; Wang, J.-M.; Guo, J.; Yang, X. Dry fractionation of surface abrasion for polyphenol-enriched buckwheat protein combined with hydrothermal treatment. Food Chem. 2019, 285, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-M.; Ma, C.-Y. Extraction, purification and characterization of globulin from common buckwheat (Fagopyrum esculentum Moench) seeds. Food Res. Int. 2006, 39, 974–981. [Google Scholar] [CrossRef]
- Deng, Y.; Padilla-Zakour, O.; Zhao, Y.; Tao, S. Influences of High Hydrostatic Pressure, Microwave Heating, and Boiling on Chemical Compositions, Antinutritional Factors, Fatty Acids, In Vitro Protein Digestibility, and Microstructure of Buckwheat. Food Bioprocess Technol. 2015, 8, 2235–2245. [Google Scholar] [CrossRef]
- Fujimura, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, Characterization, and Sequencing of a Novel Type of Antimicrobial Peptides, Fa-AMP1 andFa-AMP2, from Seeds of Buckwheat (Fagopyrum esculentum Moench.). Biosci. Biotechnol. Biochem. 2003, 67, 1636–1642. [Google Scholar] [CrossRef]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2013, 152, 349–369. [Google Scholar] [CrossRef]
- Nic Phiarais, B.P.; Schehl, B.D.; Arendt, E.K. Protein Changes during Malting of Buckwheat. J. Am. Soc. Brew. Chem. 2008, 66, 127–135. [Google Scholar] [CrossRef]
- Zhou, X. Toward a novel understanding of buckwheat self-defensive strategies during seed germination and preliminary investigation on the potential pharmacological application of its malting products. J. Med. Plants Res. 2011, 5, 6946–6954. [Google Scholar] [CrossRef]
- Wang, X.; Ullah, N.; Sun, X.; Guo, Y.; Chen, L.; Li, Z.; Feng, X. Development and characterization of bacterial cellulose reinforced biocomposite films based on protein from buckwheat distiller’s dried grains. Int. J. Biol. Macromol. 2017, 96, 353–360. [Google Scholar] [CrossRef]
- Pongrac, P.; Kump, P.; Budič, B.; Vogel-Mikuš, K. Magnesium and phosphorus distributions in developing tartary buckwheat cotyledons/Razporeditev magnezija in fosforja v razvijajočih se kličnih listih tatarske ajde. Folia Boil. Geol. 2017, 57, 45. [Google Scholar] [CrossRef]
- Pongrac, P.; Scheers, N.; Sandberg, A.-S.; Potisek, M.; Arčon, I.; Kreft, I.; Kump, P.; Vogel-Mikuš, K. The effects of hydrothermal processing and germination on Fe speciation and Fe bioaccessibility to human intestinal Caco-2 cells in Tartary buckwheat. Food Chem. 2016, 199, 782–790. [Google Scholar] [CrossRef]
- Pongrac, P.; Potisek, M.; Fraś, A.; Likar, M.; Budič, B.; Myszka, K.; Boros, D.; Nečemer, M.; Kelemen, M.; Vavpetič, P.; et al. Composition of mineral elements and bioactive compounds in tartary buckwheat and wheat sprouts as affected by natural mineral-rich water. J. Cereal Sci. 2016, 69, 9–16. [Google Scholar] [CrossRef]
- Ma, Q.; Zhao, Y.; Wang, H.-L.; Li, J.; Yang, Q.-H.; Gao, L.-C.; Murat, T.; Feng, B. Comparative study on the effects of buckwheat by roasting: Antioxidant properties, nutrients, pasting, and thermal properties. J. Cereal Sci. 2020, 95, 103041. [Google Scholar] [CrossRef]
- Koyama, M.; Hattori, S.; Amano, Y.; Watanabe, M.; Nakamura, K. Blood Pressure-Lowering Peptides from Neo-Fermented Buckwheat Sprouts: A New Approach to Estimating ACE-Inhibitory Activity. PLoS ONE 2014, 9, e105802. [Google Scholar] [CrossRef]
- Koyama, M.; Naramoto, K.; Nakajima, T.; Aoyama, T.; Watanabe, M.; Nakamura, K. Purification and Identification of Antihypertensive Peptides from Fermented Buckwheat Sprouts. J. Agric. Food Chem. 2013, 61, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, L.; Tang, W.; Zhou, Y.; Wang, Q.; Li, Z. A novel buckwheat protein with a beneficial effect in atherosclerosis was purified from Fagopyrum tataricum (L.) Gaertn. Arch. Biol. Sci. 2013, 65, 767–772. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, L.; Li, Z.; Zhou, Y.; Chen, Y.; Lu, Y. Advance on the benefits of bioactive peptides from buckwheat. Phytochem. Rev. 2015, 14, 381–388. [Google Scholar] [CrossRef]
- Tomotake, H.; Yamamoto, N.; Yanaka, N.; Ohinata, H.; Yamazaki, R.; Kayashita, J.; Kato, N. High protein buckwheat flour suppresses hypercholesterolemia in rats and gallstone formation in mice by hypercholesterolemic diet and body fat in rats because of its low protein digestibility. Nutrition 2006, 22, 166–173. [Google Scholar] [CrossRef]
- Tomotake, H.; Yamamoto, N.; Kitabayashi, H.; Kawakami, A.; Kayashita, J.; Ohinata, H.; Karasawa, H.; Kato, N. Preparation of Tartary Buckwheat Protein Product and Its Improving Effect on Cholesterol Metabolism in Rats and Mice Fed Cholesterol-Enriched Diet. J. Food Sci. 2007, 72, S528–S533. [Google Scholar] [CrossRef]
- Kayashita, J.; Nagai, H.; Kato, N. Buckwheat Protein Extract Suppression of the Growth Depression in Rats Induced by Feeding Amaranth (Food Red No. 2). Biosci. Biotechnol. Biochem. 1996, 60, 1530–1531. [Google Scholar] [CrossRef][Green Version]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Yamazaki, M.; Kato, N. Consumption of buckwheat protein lowers plasma cholesterol and raises fecal neutral sterols in cholesterol-Fed rats because of its low digestibility. J. Nutr. 1997, 127, 1395–1400. [Google Scholar] [CrossRef]
- Li, C.-H.; Matsui, T.; Matsumoto, K.; Yamasaki, R.; Kawasaki, T. Latent production of angiotensin I-converting enzyme inhibitors from buckwheat protein. J. Pept. Sci. 2002, 8, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.; Deleu, L.J.; Delcour, J.A. Proteins of Amaranth (Amaranthus spp.), Buckwheat (Fagopyrum spp.), and Quinoa (Chenopodium spp.): A Food Science and Technology Perspective. Compr. Rev. Food Sci. Food Saf. 2017, 16, 39–58. [Google Scholar] [CrossRef]
- Jin, J.; Okagu, O.D.; Yagoub, A.E.A.; Udenigwe, C.C. Effects of sonication on the in vitro digestibility and structural properties of buckwheat protein isolates. Ultrason. Sonochem. 2021, 70, 105348. [Google Scholar] [CrossRef] [PubMed]
- Veličković, T.Ć.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2017, 17, 82–103. [Google Scholar] [CrossRef]
- Xue, F.; Wu, Z.; Tong, J.; Zheng, J.; Li, C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci. Biotechnol. Biochem. 2017, 81, 1891–1898. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Li, C.; Li, Y.-Y.; Wang, Z. Effects of Maillard reaction on allergenicity of buckwheat allergen Fag t 3 during thermal processing. J. Sci. Food Agric. 2012, 93, 1510–1515. [Google Scholar] [CrossRef]
- Lee, C.; In, S.; Han, Y.; Oh, S. Reactivity change of IgE to buckwheat protein treated with high-pressure and enzymatic hydrolysis. J. Sci. Food Agric. 2015, 96, 2073–2079. [Google Scholar] [CrossRef]
- Ličen, M.; Kreft, I. Buckwheat (Fagopyrum esculentum Moench) low molecular weight seed proteins are restricted to the embryo and are not detectable in the endosperm. Plant Physiol. Biochem. 2005, 43, 862–865. [Google Scholar] [CrossRef]
- Wieslander, G.; Norbäck, D. Buckwheat allergy. Allergy 2001, 56, 703–704. [Google Scholar] [CrossRef]
- Wang, T.-C.; Shyur, S.-D.; Wen, D.-C.; Kao, Y.-H.; Huang, L.-H. Buckwheat anaphylaxis: An unusual allergen in Taiwan. Asian Pac. J. Allergy Immunol. 2006, 24, 167–170. [Google Scholar]
- Jungewelter, S.; Airaksinen, L.; Pesonen, M. Occupational buckwheat allergy as a cause of allergic rhinitis, asthma, contact urticaria and anaphylaxis—An emerging problem in food-handling occupations? Am. J. Ind. Med. 2020, 63, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- De Arcangelis, E.; Cuomo, F.; Trivisonno, M.C.; Marconi, E.; Messia, M.C. Gelatinization and pasta making conditions for buckwheat gluten-free pasta. J. Cereal Sci. 2020, 95, 103073. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Tang, C.-H.; Wang, X.-Y.; Liu, F.; Wang, C.-S. Physicochemical and Conformational Properties of Buckwheat Protein Isolates: Influence of Polyphenol Removal with Cold Organic Solvents from Buckwheat Seed Flours. J. Agric. Food Chem. 2009, 57, 10740–10748. [Google Scholar] [CrossRef]
- Zhu, L.W.; Zhou, Y.; Cai, F.; Deng, J.; Huang, J.; Zhang, X.N.; Zhang, J.G.; Chen, Q.F. Quantitative analysis of perennial buckwheat leaves proteinand GABA using near infrared spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 2421–2426. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Franczyk, A.; Zimoch-Korzycka, A.; Appah, P.; Utioh, A.; Neufeld, J.; House, J.D. Impact of Processing on the Protein Quality of Pinto Bean (Phaseolus vulgaris) and Buckwheat (Fagopyrum esculentum Moench) Flours and Blends, As Determined by in Vitro and in Vivo Methodologies. J. Agric. Food Chem. 2017, 65, 3919–3925. [Google Scholar] [CrossRef]
- Tang, C.-H.; Peng, J.; Zhen, D.-W.; Chen, Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009, 115, 672–678. [Google Scholar] [CrossRef]
- Riedl, K.M.; Hagerman, A.E. Tannin−Protein Complexes as Radical Scavengers and Radical Sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zielinski, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Woo, S.H.; Roy, S.K.; Kwon, S.J.; Cho, S.W.; Sarker, K.; Lee, M.S.; Chung, K.Y.; Kim, H.H. Concepts, Prospects, and Potentiality in Buckwheat (Fagopyrum eculentum Moench): A research perspective. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.H., Chrungoo, N., Wieslander, G., Eds.; Academic Press, an Imprint of Elsevier: London, UK, 2016; pp. 21–49. [Google Scholar]
- Yabe, S.; Iwata, H. Genomics-assisted breeding in minor and pseudo-cereals. Breed. Sci. 2020, 70, 19–31. [Google Scholar] [CrossRef]
- Vombergar, B.; Škrabanja, V.; Luthar, Z.; Germ, M. Starting points for the study of the effects of flavonoids, tannins and crude proteins in grain fractions of common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum Gaertn.). Folia Biol. Geol 2017, 58, 101–145. [Google Scholar] [CrossRef]
- Jin, J.; Ohanenye, I.C.; Udenigwe, C.C. Buckwheat proteins: Functionality, safety, bioactivity, and prospects as alternative plant-based proteins in the food industry. Crit. Rev. Food Sci. Nut. 2020, 1–13. [Google Scholar] [CrossRef]
- Yanagihara, Y. Buckwheat hypersensitivity. Kansensho 1980, 10, 184–188. [Google Scholar]
- Yano, M.; Nakamura, R.; Hayakawa, S.; Torii, S. Purification and Properties of Allergenic Proteins in Buckwheat Seeds. Agric. Biol. Chem. 1989, 53, 2387–2392. [Google Scholar] [CrossRef]
- Urisu, A.; Kondo, Y.; Morita, Y.; Yagi, E.; Tsuruta, M.; Yasaki, T.; Yamada, K.; Kuzuya, H.; Suzuki, M.; Titani, K. Isolation and characterization of major allergen in buckwheat seed. In Current Advances in Buckwheat Research; Shinshu University Press: Matsumoto, Japan, 1995; pp. 965–974. [Google Scholar]
- Wang, Z.; Zhang, Z.; Zhao, Z.; Wieslander, G.; Norbäck, D.; Kreft, I. Purification and Characterization of a 24 kDa Protein from Tartary Buckwheat Seeds. Biosci. Biotechnol. Biochem. 2004, 68, 1409–1413. [Google Scholar] [CrossRef]
- Matsumoto, R.; Fujino, K.; Nagata, Y.; Hashiguchi, S.; Ito, Y.; Aihara, Y.; Takahashi, Y.; Maeda, K.; Sugimura, K. Molecular characterization of a 10-kDa buckwheat molecule reactive to allergic patients’ IgE. Allergy 2004, 59, 533–538. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Zannini, E.; Bez, J.; Arendt, E.K.; O’Mahony, J.A. Physical and flow properties of pseudocereal-based protein-rich ingredient powders. J. Food Eng. 2020, 281, 109973. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Q.; Xia, Q.; Zha, B.; Sun, J.; Xu, B.; Shi, Y.-C. Intact endosperm cells in buckwheat flour limit starch gelatinization and digestibility in vitro. Food Chem. 2020, 330, 127318. [Google Scholar] [CrossRef]
- Zhou, X.L.; Yan, B.B.; Xiao, Y.; Zhou, Y.M.; Liu, T.Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef]
- Ge, R.H.; Wang, H. Nutrient components and bioactive compounds in tartary buckwheat bran and flour as affected by thermal processing. Int. J. Food Prop. 2020, 23, 127–137. [Google Scholar] [CrossRef]
- Ji, X.; Han, L.; Liu, F.; Yin, S.; Peng, Q.; Wang, M. A mini-review of isolation, chemical properties and bioactivities of polysaccharides from buckwheat (Fagopyrum Mill). Int. J. Biol. Macromol. 2019, 127, 204–209. [Google Scholar] [CrossRef]
- Guo, X.; Xiong, Y.L. Characteristics and functional properties of buckwheat protein–sugar Schiff base complexes. LWT 2013, 51, 397–404. [Google Scholar] [CrossRef]
- Kreft, I.; Rutar, V.; Luthar, Z.; Javornik, B. Ugotavljanje velikosti kalčka in vsebnost olja z NMR pri žlahtnjenju visokolizinskega ječmena. Zb. BF UEK Ljubl. Slov. Agric. Issue 1986, 47, 25–31. [Google Scholar]
- Rolletschek, H.; Fuchs, J.; Friedel, S.; Börner, A.; Todt, H.; Jakob, P.M.; Borisjuk, L. A novel noninvasive procedure for high-throughput screening of major seed traits. Plant Biotechnol. J. 2014, 13, 188–199. [Google Scholar] [CrossRef]
- Ličen, M.; Dvořáček, V.; Čepková, P.; Michalová, A. Endosperm protein polymorphism of common and tartary buckwheat. Acta Agric. Slov. 2004, 83, 171–179. [Google Scholar]
- Luthar, Z.; Rogl, S.; Kump, B.; Javornik, B. 38–48 kDa subunits of buckwheat 13S globulins are controlled by a single locus. Plant Breed. 2008, 127, 322–324. [Google Scholar] [CrossRef]
- Kreft, I. Ajda; Kmečki Glas: Ljubljana, Slovenia, 1995; 112p. [Google Scholar]
- Adhikari, K.N.; Campbell, C. Natural outcrossing in common buckwheat. Euphytica 1998, 102, 233–237. [Google Scholar] [CrossRef]
- Luthar, Z. Genska banka ajde: Buckwheat genebank—A source of Slovenian genetic variability. Acta Agric. Slov. 2012, 99, 307–316. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Zhang, Y.; Huang, K.; Yang, W.; Li, X.; Zhang, Z.; Wu, K.; Xu, X.; Ruan, R.; et al. De novo transcriptome assembly and identification of genes related to seed size in common buckwheat (Fagopyrum esculentum M.). Breed. Sci. 2019, 69, 487–497. [Google Scholar] [CrossRef]
- Chen, P.; Guo, Y.F.; Yan, Q.; Li, Y.H. Molecular cloning and characterization of Fag t 2: A 16-kDa major allergen from Tartary buckwheat seeds. Allergy 2011, 66, 1393–1395. [Google Scholar] [CrossRef]
- Yasui, Y.; Hirakawa, H.; Ueno, M.; Matsui, K.; Katsube-Tanaka, T.; Yang, S.J.; Aii, J.; Sato, S.; Mori, M. Assembly of the draft genome of buckwheat and its applications in identifying agronomically useful genes. DNA Res. 2016, 23, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.; Hara, T.; Ueno, M.; Enoki, H.; Kimura, T.; Nishimura, S.; Yasui, Y.; Ohsawa, R.; Iwata, H. Potential of Genomic Selection in Mass Selection Breeding of an Allogamous Crop: An Empirical Study to Increase Yield of Common Buckwheat. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, R. Current status and prospects of common buckwheat breeding in Japan. Breed. Sci. 2020, 70, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Tomatsu, T.; Kinouchi, S.; Suzuki, T.; Sato, T. Identification of a gene encoding glutathione S-transferase that is related to anthocyanin accumulation in buckwheat (Fagopyrum esculentum). J. Plant Physiol. 2018, 231, 291–296. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Liu, X.; Geng, F.; Huang, Q.; Zhao, J.; Xiang, D.; Zhao, G. Analysis of tartary buckwheat (Fagopyrum tataricum) seed proteome using offline two-dimensional liquid chromatography and tandem mass spectrometry. J. Food Biochem. 2019, 43, e12863. [Google Scholar] [CrossRef]
- Yasui, Y. History of the progressive development of genetic marker systems for common buckwheat. Breed. Sci. 2020, 70, 13–18. [Google Scholar] [CrossRef]
- Suzuki, T.; Noda, T.; Morishita, T.; Ishiguro, K.; Otsuka, S.; Brunori, A. Present status and future perspectives of breeding for buckwheat quality. Breed. Sci. 2020, 70, 48–66. [Google Scholar] [CrossRef]
- Zhang, K.; He, M.; Fan, Y.; Zhao, H.; Gao, B.; Yang, K.; Li, F.; Tang, Y.; Gao, Q.; Lin, T.; et al. Resequencing of global Tartary buckwheat accessions reveals multiple domestication events and key loci associated with agronomic traits. Genome Biol. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).