Zebrafish Paralogs brd2a and brd2b Are Needed for Proper Circulatory, Excretory and Central Nervous System Formation and Act as Genetic Antagonists during Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance and Handling
2.2. RNA Isolation and RT-PCR
2.3. Brd2b Peptide Antibody Production, Western Blot and Peptide Competition
2.4. Full-Length cDNA Cloning
2.5. Antisense Morpholino Treatments
2.6. Crispr-Cas9 Disruption
2.7. In Situ Hybridization
2.8. Immunohistochemistry
2.9. TUNEL Analysis
2.10. Phenotypic Assessment and Population Data
3. Results
3.1. Zebrafish brd2b Exhibits Transcript Variants Differentially Regulated during Development
3.2. brd2b Encodes a Maternal/Zygotic Factor That Is Localized to the Animal Pole in Oocytes and Enriched in the CNS and Ventral Trunk in Embryos
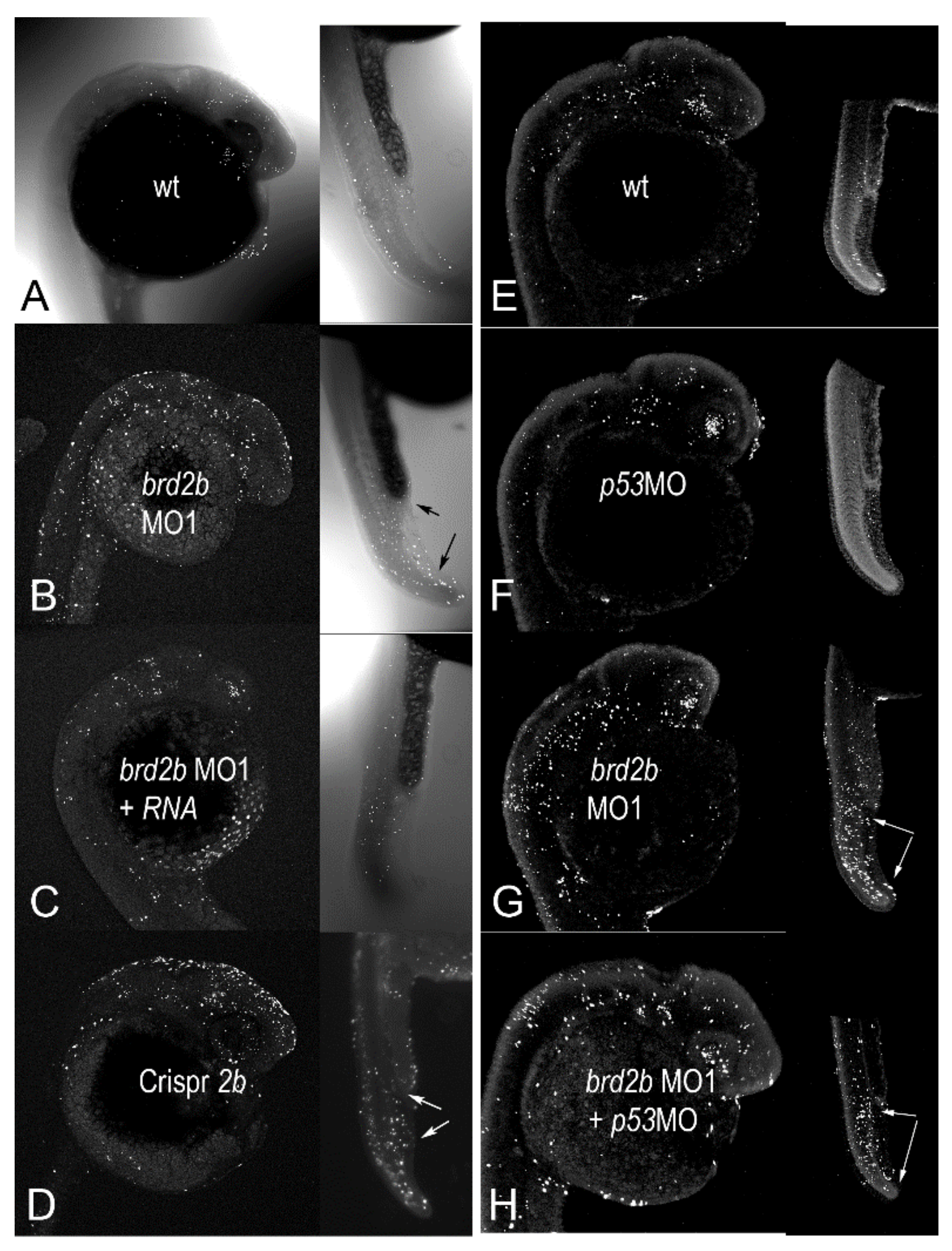
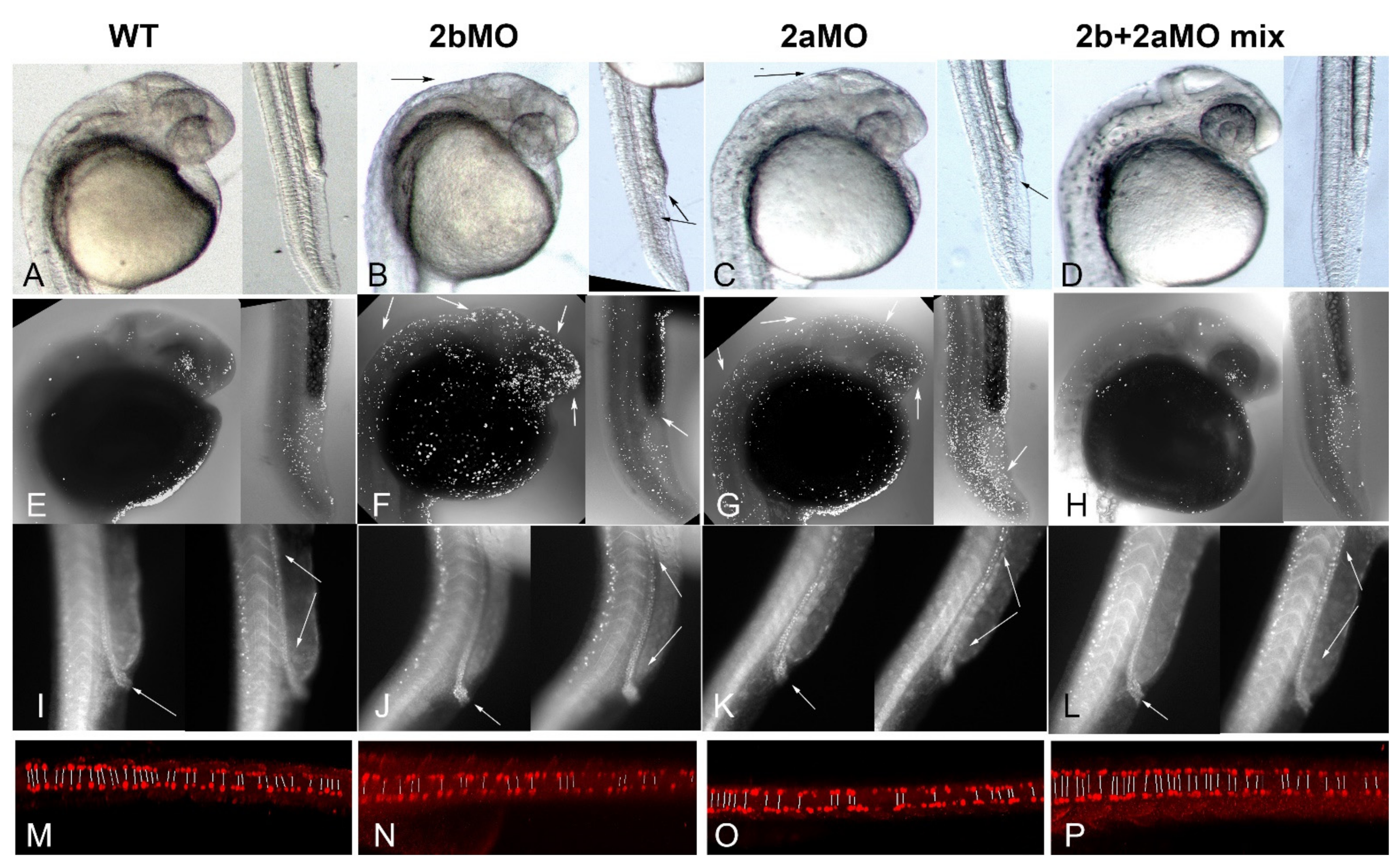
3.3. Brd2b Knockdown Results in Reduced Hindbrain, Ill-Defined MHB, and Trunk Abnormalities Similar to Brd2a Morphants, but Presents Unique Circulatory and Pronephric Defects
3.4. Brd2b Knockdown Increases Cell Death in the CNS of Prim 5 Morphant Embryos but Reduces Cell Death in the Cloaca of the Pronephros
3.5. Patterning of pax2a (+) Spinal Interneurons and Distribution of Pronephric Cells Is Disrupted in Both brd2b and brd2a Morphants
3.6. Co-Knockdown of Brd2a and Brd2b Paralogs Restores Wild Type Phenotype to Both Brain and Pronephros of Morphant Embryos at Prim 5
3.7. Enhancement of Morphant Brain Phenotypes by Injection of Paralogous RNA Corroborates Genetic Antagonism between brd2a and brd2b Loci
4. Discussion
4.1. Shared and Distinct Functions of Brd2b/2a Paralogs
4.1.1. RNA Variants and Protein Isoforms
4.1.2. Maternal-Zygotic Function
4.1.3. Cell Death, Differentiation, and Lineage Specification
4.2. Functional Antagonism between Brd2 Paralogs
4.3. Gene Duplication, Divergence, and Antagonism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taniguchi, Y. The bromodomain and extra-terminal domain (BET) family: Functional anatomy of BET paralogous proteins. Int. J. Mol. Sci. 2016, 17, 1849–1872. [Google Scholar] [CrossRef]
- Mujtaba, S.; Zeng, L.; Zhou, M. Structure and acetyl-lysine recognition of the bromodomain. Oncogene 2007, 26, 5521–5527. [Google Scholar] [CrossRef]
- Denis, G.V. Bromodomain motifs and scaffolding? Front. Biosci. 2001, 6, D1065–D1068. [Google Scholar] [CrossRef]
- Chang, Y.L.; King, B.; Lin, S.C.; Kennison, J.A.; Huang, D.H. A double bromodomain protein, FSF-S, activates the homeotic gene Ultrabithorax through a critical promoter-proximal region. Mol. Cell. Biol. 2007, 27, 5486–5498. [Google Scholar] [CrossRef]
- Denis, G.V. Duality in bromodomain-containing complexes. Front. Biosci. 2001, 6, D849–D852. [Google Scholar] [CrossRef]
- Krishna, V.; Yin, X.; Song, Q.; Walsh, A.; Pocalyko, D.; Bachman, K.; Anderson, I.; Madakamutil, L.; Nagpal, S. Integration of the transcriptome and genome-wide landscape of BRD2 and BRD4 binding motifs identifies key superenhancer genes and reveals the mechanism of Bet inhibitor action in rheumatoid arthritis synovial fibroblasts. J. Immunol. 2021, 206, 422–431. [Google Scholar] [CrossRef]
- Lambert, J.P.; Picaud, S.; Fujisawa, T.; Hou, H.; Savitsky, P.; Uusküla-Reimand, L.; Gupta, G.D.; Abdouni, H.; Lin, Z.Y.; Tucholska, M.; et al. Interactome Rewiring Following Pharmacological Targeting of BET Bromodomains. Mol. Cell. 2019, 73, 621–638.e17. [Google Scholar] [CrossRef]
- Manterola, M.; Brown, T.M.; Oh, M.Y.; Garyn, C.; Gonzalez, B.J.; Wolgemuth, D.J. BRDT is an essential epigenetic regulator for proper chromatin organization, silencing of sex chromosomes and crossover formation in male meiosis. PLoS Genet. 2018, 14, e1007209. [Google Scholar] [CrossRef] [PubMed]
- Berkovits, B.D.; Wolgemuth, D.J. The role of the double bromodomain-containing BET genes during mammalian spermatogenesis. Curr Top. Dev. Biol. 2013, 102, 293–326. [Google Scholar]
- Nerlakanti, N.; Yao, J.; Nguyen, D.T.; Patel, A.K.; Eroshkin, A.M.; Lawrence, H.R.; Ayaz, M.; Kuenzi, B.M.; Agarwal, N.; Chen, Y.; et al. Targeting the BRD4-HOXB13 coregulated transcriptional networks with bromodomain-kinase inhibitors to suppress metastatic castration-resistant prostate cancer. Mol. Cancer Ther. 2018, 12, 2796–2810. [Google Scholar] [CrossRef]
- Borck, P.C.; Guo, L.W.; Plutzky, J. BET epigenetic reader proteins in cardiovascular transcriptional programs. Circ. Res. 2020, 126, 1190–1208. [Google Scholar] [CrossRef]
- Trivedi, A.; Mehrotra, A.; Baum, C.E.; Lewis, B.; Basuroy, T.; Blomquist, T.; Trumbly, R.; Filipp, F.V.; Setaluri, V.; de la Serna, I.L. Bromodomain and extra-terminal domain (BET) proteins regulate melanocyte differentiation. Epigenetics Chromatin. 2020, 13, 14. [Google Scholar] [CrossRef]
- Stonestrom, A.J.; Hsu, S.C.; Werner, M.T.; Blobel, G.A. Erythropoiesis provides a BRD’s eye view of BET protein function. Drug Discov. Today Technol. 2016, 19, 23–28. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Meng, G.; Lin, H.; Wu, S.; Wang, J.; Luo, J.; Xu, X.; Tough, D.; Lindon, M.; et al. BET bromodomain inhibition promotes neurogenesis while inhibiting gliogenesis in neural progenitor cells. Stem Cell Res. 2016, 17, 212–221. [Google Scholar] [CrossRef]
- Sansam, C.G.; Pietrzak, K.; Majchrzycka, B.; Kerlin, M.A.; Chen, J.; Rankin, S.; Sansam, C.L. A mechanism for epigenetic control of DNA replication. Genes Dev. 2018, 32, 224–229. [Google Scholar] [CrossRef]
- Shao, Z.; Yao, C.; Khodadadi-Jamayran, A.; Xu, W.; Townes, T.M.; Crowley, M.R.; Hu, K. Reprogramming by de-bookmarking the somatic transcriptional program through targeting of BET bromodomains. Cell Rep. 2016, 16, 3138–3145. [Google Scholar] [CrossRef]
- Belkina, A.C.; Denis, G.V. BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 2012, 12, 465–477. [Google Scholar] [CrossRef]
- Spriano, F.; Stathis, A.; Bertoni, F. Targeting BET bromodomain proteins in cancer: The example of lymphomas. Pharmacol. Ther. 2020, 215, 107631. [Google Scholar] [CrossRef]
- Morgado-Pascual, J.L.; Rayego-Mateos, S.; Tejedor, L.; Suarez-Alvarez, B.; Ruiz-Ortega, M. Bromodomain and extraterminal proteins as novel epigenetic targets for renal diseases. Front. Pharmacol. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Deeney, J.T.; Belkina, A.C.; Shirihai, O.S.; Corkey, B.E.; Denis, G.V. BET bromodomain proteins Brd2, Brd3 and Brd4 selectively regulate metabolic pathways in the pancreatic β-cell. PLoS ONE. 2016, 11, e0151329. [Google Scholar]
- Rudman, M.D.; Choi, J.S.; Lee, H.E.; Tan, S.K.; Ayad, N.G.; Lee, J.K. Bromodomain and extraterminal domain-containing protein inhibition attenuates acute inflammation after spinal cord injury. Exp. Neurol. 2018, 309, 181–192. [Google Scholar] [CrossRef]
- Cochran, A.G.; Conery, A.R.; Sims, R.J., 3rd. Bromodomains: A new target class for drug development. Nat. Rev. Drug Discov. 2019, 18, 609–628. [Google Scholar] [CrossRef]
- Digan, M.E.; Haynes, S.R.; Mozer, B.A.; Dawid, I.B.; Forquignon, F.; Gans, M. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev.Biol. 1986, 114, 161–169. [Google Scholar] [CrossRef]
- Rhee, K.; Brunori, M.; Trousdale, R.; Wolgemuth, D.J. Expression and potential role of Fsrg1, a bromodomain-containing homologue of the Drosophila gene female sterile homeotic. J. Cell Sci. 1998, 111, 3541–3550. [Google Scholar] [CrossRef]
- DiBenedetto, A.J.; Guinto, J.B.; Ebert, T.D.; Bee, K.J.; Schmidt, M.M.; Jackman, T.R. Zebrafish brd2a and brd2b are paralogous members of the bromodomain-ET (BET) family of transcriptional coregulators that show structural and expression divergence. BMC Dev. Biol. 2008, 8, 39. [Google Scholar] [CrossRef]
- Florence, B.L.; Faller, D.V. Drosophila female sterile (1) homeotic is a multifunctional transcriptional regulator that is modulated by Ras signaling. Dev. Dyn. 2008, 237, 554–564. [Google Scholar] [CrossRef]
- Murphy, T.; Melville, H.; Fradkin, E.; Bistany, G.; Branigan, G.; Olsen, K.; Comstock, C.R.; Hanby, H.; Garbade, E.; DiBenedetto, A.J. Knockdown of epigenetic transcriptional co-regulator Brd2a disrupts apoptosis and proper formation of hindbrain and midbrain-hindbrain boundary (MHB) region in zebrafish. Mech. Devel. 2017, 146, 10–30. [Google Scholar] [CrossRef]
- Deschamps, J.; van Nes, J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development 2005, 132, 2931–2942. [Google Scholar] [CrossRef]
- Bielczyk-Maczyńska, E.; Serbanovic-Canic, J.; Ferreira, L.; Soranzo, N.; Stemple, D.L.; Ouwehand, W.H.; Cvejic, A. A loss of function screen of identified genome-wide association study loci reveals new genes controlling hematopoiesis. PLOS Genet. 2014, 10, e1004450. [Google Scholar] [CrossRef]
- Belkina, A.C.; Blanton, W.P.; Nikolajczyk, B.S.; Denis, G.V. The double bromodomain protein Brd2 promotes B cell expansion and mitogenesis. J. Leukoc. Biol. 2014, 95, 451–460. [Google Scholar] [CrossRef]
- Shang, E.; Wang, X.; Wen, D.; Greenberg, D.A.; Wolgemuth, D.J. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev. Dyn. 2009, 238, 908–917. [Google Scholar] [CrossRef]
- Gyuris, A.; Donovan, D.J.; Seymour, K.A.; Lovasco, L.A.; Smilowitz, N.R.; Halperin, A.I.P.; Klysik, J.E.; Freiman, R.N. The chromatin-targeting protein Brd2 is required for neural tube closure and embryogenesis. Biochim. Biophys. Acta 2009, 1789, 413–421. [Google Scholar] [CrossRef]
- Denis, G.V.; Vaziri, C.; Guo, N.; Faller, D.V. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ. 2000, 11, 417–424. [Google Scholar] [PubMed]
- Greenwald, R.J.; Tumang, J.R.; Sinha, A.; Currier, N.; Cardiff, R.D.; Rothstein, T.L.; Faller, D.V.; Denis, G.V. Em-Brd2 transgenic mice develop B cell lymphoma and leukemia. Blood 2004, 103, 1475–1484. [Google Scholar] [CrossRef][Green Version]
- Crowley, T.E.; Brunori, M.; Rhee, K.; Wang, X.; Wolgemuth, D.J. Change in nuclear cytoplasmic localization of a double-bromodomain protein during proliferation and differentiation of mouse spinal cord and dorsal root ganglia. Dev. Brain Res. 2004, 149, 93–101. [Google Scholar] [CrossRef]
- Tsume, M.; Kimura-Yoshida, C.; Mochida, K.; Shibukawa, Y.; Amazaki, S.; Wada, Y.; Hiramatsu, R.; Shimokawa, K.; Matsuo, I. Brd2 is required for cell cycle exit and neuronal differentiation through the E2F1 pathway in mouse neuroepithelial cells. Biochem. Biophys. Res. Commun. 2012, 425, 762–768. [Google Scholar] [CrossRef]
- DeMars, K.M.; Yang, C.; Candelario-Jalil, E. Neuroprotective effects of targeting BET proteins for degradation with dBET1 in aged mice subjected to ischemic stroke. Neurochem. Int. 2019, 127, 94–102. [Google Scholar] [CrossRef]
- Velíšek, L.; Shang, E.; Velíšková, J.; Chachua, T.; Macchiarulo, S.; Maglakelidze, G.; Wolgemuth, D.J.; Greenberg, D.A. GABAergic neuron deficit as an idiopathic generalized epilepsy mechanism: The role of BRD2 haploinsufficiency in juvenile myoclonic epilepsy. PLoS ONE 2011, 6, e23656. [Google Scholar] [CrossRef] [PubMed]
- McGrail, M.; Hatler, J.M.; Kuang, X.; Liao, H.K.; Nannapaneni, K.; Noack Watt, K.E.; Uhl, J.D.; Largaespada, D.A.; Vollbrecht, E.; Scheetz, T.E.; et al. Somatic mutagenesis with a sleeping beauty transposon system leads to solid tumor formation in zebrafish. PLoS ONE 2011, 6, e18826. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development in the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Cheung, K.L.; Kim, C.; Zhou, M.M. The functions of BET proteins in gene transcription of biology and diseases. Front. Mol. Biosci. 2021, 8, 728777. [Google Scholar] [CrossRef]
- Chen, A.T.; Zon, L.I. Zebrafish blood stem cells. J. Cell. Biochem. 2009, 108, 35–42. [Google Scholar] [CrossRef]
- Robu, M.E.; Larson, J.D.; Nasevicius, A.; Beiragi, S.; Brenner, C. p53 activation by knockdown technologies. PloS Genet. 2007, 3, e78. [Google Scholar] [CrossRef]
- Lewis, K.E.; Eisen, J.F. From cells to circuits: Development of the zebrafish spinal cord. Prog. Neurobiol. 2003, 69, 419–449. [Google Scholar] [CrossRef]
- Mudumana, S.P.; Hentschel, D.; Liu, Y.; Vasilyev, A.; Drummond, I.A. Odd skipped related1 reveals a novel role for endoderm in regulating kidney vs. vascular cell fate. Development 2008, 135, 3355–3367. [Google Scholar] [CrossRef]
- Olsen, K.S. Role of Brd2 in the Transient Wave of Hematopoiesis within the Posterior Blood Island of the Developing Zebrafish Embryo. Master’s Thesis, Villanova University, Villanova, PA, USA, May 2017. [Google Scholar]
- Garcia-Gutierrez, P.; Mundi, M.; Garcia-Dominguez, M. Association of bromodomain BET proteins with chromatin requires dimerization through the conserved motif B. J. Cell Sci. 2012, 125, 3671–3680. [Google Scholar] [CrossRef] [PubMed]
- Hnilicová, J.; Hozeifi, S.; Stejskalová, E.; Dušková, E.; Poser, I.; Humpolíčková, J.; Hof, M.; Staněk, D. The C-terminal domain of Brd2 is important for chromatin interaction and regulation of transcription and alternative splicing. Mol. Biol. Cell 2013, 24, 3557–3568. [Google Scholar] [CrossRef] [PubMed]
- Shang, E.; Cui, Q.; Wang, X.; Beseler, C.; Greenberg, D.A.; Wolgemuth, D.J. The bromodomain-containing gene BRD2 is regulated at transcription, splicing, and translation levels. J. Cell Biochem. 2011, 112, 2784–2793. [Google Scholar] [CrossRef]
- Thorpe, K.L.; Beck, S. DNA sequence and structure of the mouse RING3 gene: Identification of variant RING3 transcripts. Immunogenetics 1998, 48, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Collombet, S.; Ranisavljevic, N.; Nagano, T.; Varnai, C.; Shisode, T.; Leung, W.; Piolot, T.; Galupa, R.; Borensztein, M.; Servant, N.; et al. Parental-to-embryo switch of chromosome organization in early embryogenesis. Nature 2020, 580, 142–146. [Google Scholar] [CrossRef]
- Hug, C.B.; Vaquerizas, J.M. The birth of the 3D genome during early embryonic development. Trends Genet. 2018, 34, 903–914. [Google Scholar] [CrossRef]
- Surface, L.E.; Fields, P.A.; Subramanian, V.; Behmer, R.; Udeshi, N.; Peach, S.E.; Carr, S.A.; Jaffe, J.D.; Boyer, L.A. H2A.Z.1 monoubiquitylation antagonizes BRD2 to maintain poised chromatin in ESCs. Cell Rep. 2016, 14, 1142–1155. [Google Scholar] [CrossRef]
- Hsu, S.C.; Gilgenast, T.G.; Bartman, C.R.; Edwards, C.R.; Stonestrom, A.J.; Huang, P.; Emerson, D.J.; Evans, P.; Werner, M.T.; Keller, C.A.; et al. The BET protein BRD2 cooperates with CTCF to enforce transcriptional and architectural boundaries. Mol. Cell. 2017, 66, 102–116.e7. [Google Scholar] [CrossRef] [PubMed]
- Magella, B.; Mahoney, R.; Adam, M.; Potter, S.S. Reduced Abd-B Hox function during kidney development results in lineage infidelity. Dev. Biol. 2018, 438, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.A.; Yan, Z.; Zhang, W.; Wildonger, J.; Jan, L.Y.; Jan, Y.N. Double-bromo and extraterminal (BET) domain proteins regulate dendrite morphology and mechanosensory function. Genes Dev. 2014, 28, 1940–1956. [Google Scholar] [CrossRef]
- Bandopadhayay, P.; Piccioni, F.; O’Rourke, R.; Ho, P.; Gonzalez, E.M.; Buchan, G.; Qian, K.; Gionet, G.; Girard, E.; Coxon, M.; et al. Neuronal differentiation and cell-cycle programs mediate response to BET-bromodomain inhibition in MYC-driven medulloblastoma. Nat. Commun. 2019, 10, 2400. [Google Scholar] [CrossRef] [PubMed]
- Luna-Peláez, N.; García-Domínguez, M. Lyar-mediated recruitment of Brd2 to the chromatin attenuates Nanog downregulation following induction of differentiation. J. Mol. Biol. 2018, 430, 1084–1097. [Google Scholar] [CrossRef]
- Fernandez-Alonso, R.; Davidson, L.; Hukelmann, J.; Zengerle, M.; Prescott, A.R.; Lamond, A.; Ciulli, A.; Sapkota, G.P.; Findlay, G.M. Brd4-Brd2 isoform switching coordinates pluripotent exit and Smad2-dependent lineage specification. EMBO Rep. 2017, 18, 1108–1122. [Google Scholar] [CrossRef]
- Caputo, V.S.; Trasanidis, N.; Xiao, X.; Robinson, M.E.; Katsarou, A.; Ponnusamy, K.; Prinjha, R.K.; Smithers, N.; Chaidos, A.; Auner, H.W.; et al. Brd2/4 and Myc regulate alternative cell lineage programmes during early osteoclast differentiation in vitro. iScience 2020, 24, 101989. [Google Scholar] [CrossRef]
- Stonestrom, A.J.; Hsu, S.C.; Jahn, K.S.; Huang, P.; Keller, C.A.; Giardine, B.M.; Kadauke, S.; Campbell, A.E.; Evans, P.; Hardison, R.C.; et al. Functions of BET proteins in erythroid gene expression. Blood 2015, 125, 2825–2834. [Google Scholar] [CrossRef]
- Andrieu, G.P.; Denis, G.V. BET proteins exhibit transcriptional and functional opposition in the epithelial-to-mesenchymal transition. Mol. Cancer Res. 2018, 16, 580–586. [Google Scholar] [CrossRef]
- Shum, E.Y.; Jones, S.H.; Shao, A.; Dumdie, J.; Krause, M.D.; Chan, W.K.; Lou, C.H.; Espinoza, J.L.; Song, H.W.; Phan, M.H.; et al. The antagonistic gene paralogs Upf3a and Upf3b govern nonsense-mediated RNA decay. Cell 2016, 165, 382–395. [Google Scholar] [CrossRef]
- Reddy, K.C.; Dror, T.; Underwood, R.S.; Osman, G.A.; Elder, C.R.; Desjardins, C.A.; Cuomo, C.A.; Barkoulas, M.; Troemel, E.R. Antagonistic paralogs control a switch between growth and pathogen resistance in C. elegans. PLoS Pathog. 2019, 15, e1007528. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.; Chowdhury, A.H.; Vaidyanathan, B.; Kim, D.; Saleque, S. Antagonistic actions of Rcor proteins regulate LSD1 activity and cellular differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Mazet, F.; Shimeld, S.M. Gene duplication and divergence in the early evolution of vertebrates. Curr. Opin. Genet. Dev. 2002, 12, 393–396. [Google Scholar] [CrossRef]
- Li, W.H.; Yang, J.; Gu, X. Expression divergence between duplicate genes. Trends Genet. 2005, 21, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Modos, D.; Brooks, J.; Fazekas, D.; Ari, E.; Vellai, T.; Csermely, P.; Korcsmaros, T.; Lenti, K. Identification of critical paralog groups with indispensable roles in the regulation of signal flow. Sci. Rep. 2016, 6, 38588. [Google Scholar] [CrossRef]



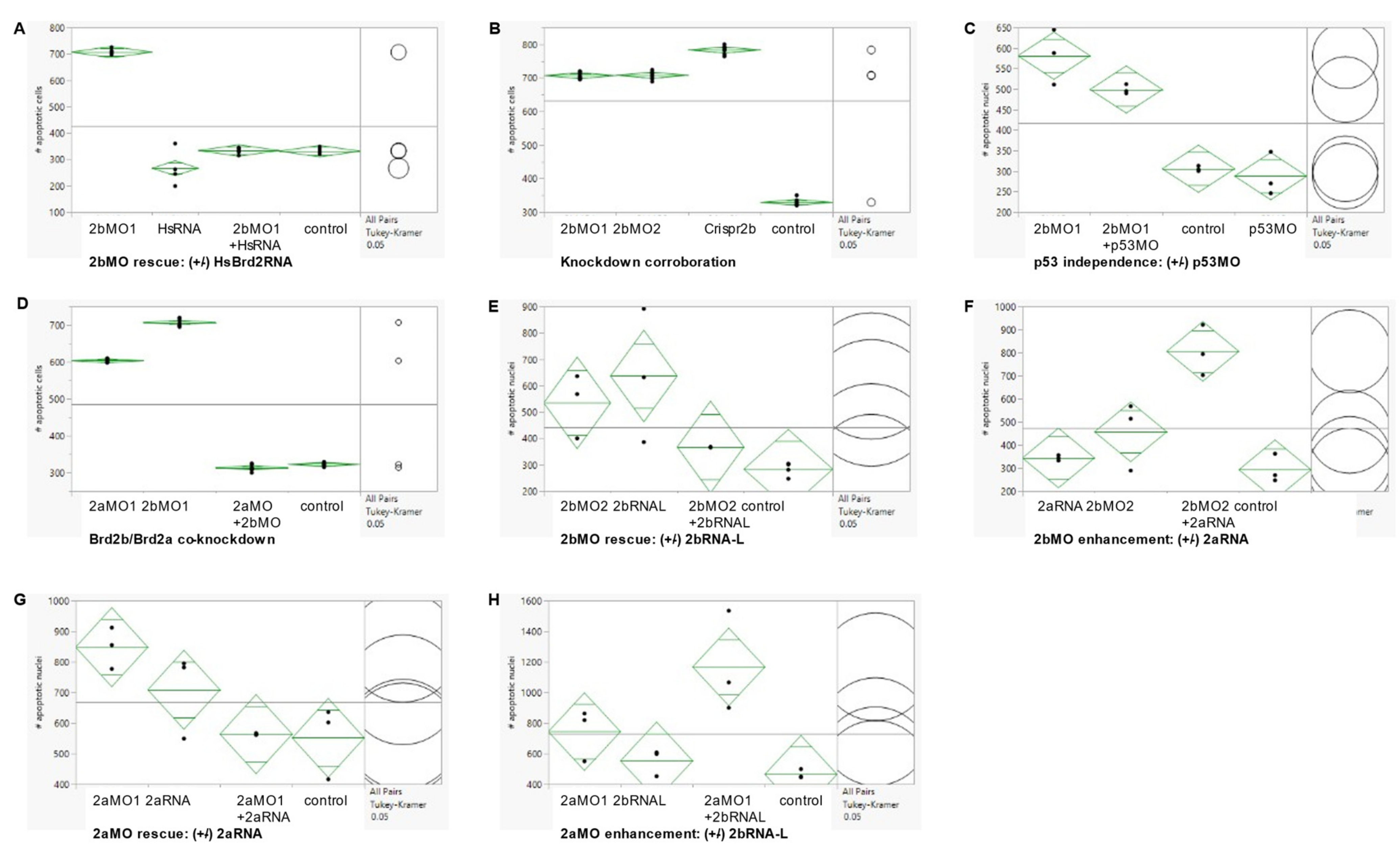


| Treatment | % Brain Defect a | % Pbi Defect b | % Duct Defect c | % Circ Defects d |
|---|---|---|---|---|
| brd2bMO1 +/− HsBrd2RNA rescue e | ||||
| control | 12.5 | 0 | 0 | 0 |
| HsBrd2RNA | 11.5 | 0 | 7.7 | 0 |
| brd2bMO1 | 100 | 100 | 100 | 50 |
| brd2bMO1 + HsBrd2RNA | 20 | 8.6 | 17.1 | 5.7 |
| Chi-square Contingency, Fisher’s Exact Test: p < 0.0001 for all four defects. Correspondence Analysis: [wildtype: control, RNA, brd2bMO+HsRNA] [defect: 2bMO], for all four parameters. | ||||
| brd2bMO single knockdown corroboration f | ||||
| control | 0 | 0 | 0 | 0 |
| mis5 | 0 | 0 | 0 | 0 |
| 2bMO1 | 100 | 25.8 | 88.7 | 93.7 |
| 2bMO2 | 100 | 24.2 | 100 | 100 |
| Crispr2b | 100 | 21 | 100 | 100 |
| Chi-square Contingency, Fisher’s Exact Test: p < 0.0001 for all four defects. Correspondence Analysis: [wildtype: control, mis5] [defect: MOs, Crispr] for brain; [wildtype: control, mis5] [defect: MOs, Crispr] for PBI, duct, circulation | ||||
| brd2aMO/brd2bMO co-knockdown f | ||||
| control | 0 | 0 | 0 | 0 |
| 2aMO1 | 100 | 80 | 0 | 0 |
| 2bMO1 | 100 | 26.7 | 91.7 | 96.7 |
| 2aMO1 + 2bMO1 | 5 | 0 | 0 | 0 |
| Chi-square Contingency, Fisher’s Exact Test: p < 0.0001 for all four defects Correspondence Analysis: [Wildtype: control, 2aMO+2bMO] [Defect: 2aMO, 2bMO] for brain; | ||||
| [Wildtype: control, 2aMO+2bMO, 2bMO] [defect: 2aMO] For PBI; [Wildtype: control, 2aMO+2bMO, 2aMO] [defect: 2bMO] for duct, circulation | ||||
| Rescue Treatment a | % Defects b |
|---|---|
| 2bMO rescue-2bRNA-L_brain c | |
| control | 13.0 |
| 2bMO2 | 40.4 |
| 2bRNA-L | 39.7 |
| brd2bMO2 + 2bRNA-L | 24.6 |
| Chi-square Contingency, Fisher’s Exact Test: p = 0.0026 Correspondence Analysis: [wildtype: control, 2bMO2+2bRNA-L] [defect: 2bMO2, 2bRNA-L] | |
| 2aMO rescue-2aRNA_brain d | |
| Control | 5 |
| 2aMO1 | 35 |
| 2aRNA | 20 |
| 2aMO1 + 2aRNA | 20 |
| Chi-square Contingency, Fisher’s Exact Test: p = 0.1416 Correspondence Analysis: [wildtype: control, 2aMO1+2aRNA, 2aRNA] [defect: 2aMO1] | |
| 2bMO rescue-2bRNA-S_brain e | |
| control | 5 |
| 2bMO2 | 33.3 |
| 2bRNA-S | 25 |
| 2bMO2 + 2bRNA-S | 33 |
| Chi-square Contingency, Fisher’s Exact Test: p = 0.0840 Correspondence Analysis: [wildtype: control] [defect: 2bRNA-S, 2bMO2+2bRNA-S, 2bMO2] | |
| 2bMO rescue-2bRNA-L_duct f | |
| control | 40 |
| 2bMO2 | 80 |
| 2bRNA-S | 55 |
| 2bMO2 + 2bRNA-S | 29.6 |
| Chi-square contingency, Fisher’s Exact Test: p = 0.0128 Correspondence Analysis: [wildtype: control, 2bMO2+2bRNA-S] [defect: 2bRNA-S, 2bMO] | |
| Enhancement Treatment g | % Defects b |
| 2bMO enhancement-2aRNA_brainf | |
| control | 11.1 |
| 2bMO2 | 30.8 |
| 2aRNA | 25 |
| 2bMO2 + 2aRNA | 42.9 |
| Chi-square contingency, Fisher’s Exact Test: p = 0.0059 Correspondence Analysis: [wildtype: control, 2aRNA][2bMO][defect: 2bMO2+2aRNA] | |
| 2aMO enhancement- 2bRNA-L_brain f | |
| control | 11.5 |
| 2aMO1 | 41.5 |
| 2bRNA-L | 30.4 |
| 2aMO1 + 2bRNA-L | 61.4 |
| Chi-square contingency, Fisher’s Exact Test: p = 0.0109 Correspondence Analysis: [wildtype: control, 2bRNA-L][2aMO][defect: 2aMO1+2bRNA-L] | |
| 2aMO enhancement 2bRNA-S_braine control 2aMO1 2bRNA-S 2aMO1 + 2bRNA-S | |
| 0 | |
| 63.6 | |
| 50 | |
| 71.4 | |
| Chi-square contingency, Fisher’s exact test: p < 0.0001 Correspondence analysis: [wildtype: control][2bRNA-S][defect: 2aMO, 2aMO1+2bRNA-L] | |
| 2bMO enhancement-2aRNA_ductc Control 2bMO2 2aRNA 2bMO2 + 2aRNA | |
| 16.2 | |
| 48.2 | |
| 50 | |
| 69.2 | |
| >Chi square contingency, Fisher’s exact test: p < 0.0001 Correspondence analysis: [wildtype: control][2aRNA, 2bMO] [defect: 2bMO2+2aRNA] | |
| Morphant Defects | Brd2a Deficiency | Brd2b Deficiency | Suppression | Skew from WT | Path a | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2AMO | 2BRNA-L | 2BRNA-S | 2BMO | 2ARNA | 2AMO + 2BMO | AA:AB:BB | AA:AB:BB | ||
 |  | ||||||||
| CNS | |||||||||
| Brain Morphology/PCD | X | X | X | X | WT | AA < AB < BB or AA > AB > BB | pcd+ | ||
| MHB Genes | X | WT | WT | AA < AB < BB | hox | ||||
| VNC Interneurons | X | X | WT | AA < AB < BB or AA > AB > BB | hox | ||||
| PBI | |||||||||
| Morphology/PCD | X | X | WT | AA < AB < BB or AA > AB > BB | pcd+ | ||||
| Pronephric Duct | |||||||||
| Tube PAX2a(+) | X (+) | X (−) | AA < AB < BB | lin+ m | |||||
| AA > AB > BB | lin− m | ||||||||
| Cloaca | |||||||||
| Plug | WT | X b | X | X | WT | AA > AB > BB b | pcd+ m | ||
| PCD | WT | X (−) | WT | AA > AB > BB | pcd− | ||||
| PAX2a (+) | WT | X (+) | WT | AA > AB > BB | pcd− m | ||||
| Circ/Lethality | X | WT | AA > AB > BB | ??? | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branigan, G.L.; Olsen, K.S.; Burda, I.; Haemmerle, M.W.; Ho, J.; Venuto, A.; D’Antonio, N.D.; Briggs, I.E.; DiBenedetto, A.J. Zebrafish Paralogs brd2a and brd2b Are Needed for Proper Circulatory, Excretory and Central Nervous System Formation and Act as Genetic Antagonists during Development. J. Dev. Biol. 2021, 9, 46. https://doi.org/10.3390/jdb9040046
Branigan GL, Olsen KS, Burda I, Haemmerle MW, Ho J, Venuto A, D’Antonio ND, Briggs IE, DiBenedetto AJ. Zebrafish Paralogs brd2a and brd2b Are Needed for Proper Circulatory, Excretory and Central Nervous System Formation and Act as Genetic Antagonists during Development. Journal of Developmental Biology. 2021; 9(4):46. https://doi.org/10.3390/jdb9040046
Chicago/Turabian StyleBranigan, Gregory L., Kelly S. Olsen, Isabella Burda, Matthew W. Haemmerle, Jason Ho, Alexandra Venuto, Nicholas D. D’Antonio, Ian E. Briggs, and Angela J. DiBenedetto. 2021. "Zebrafish Paralogs brd2a and brd2b Are Needed for Proper Circulatory, Excretory and Central Nervous System Formation and Act as Genetic Antagonists during Development" Journal of Developmental Biology 9, no. 4: 46. https://doi.org/10.3390/jdb9040046
APA StyleBranigan, G. L., Olsen, K. S., Burda, I., Haemmerle, M. W., Ho, J., Venuto, A., D’Antonio, N. D., Briggs, I. E., & DiBenedetto, A. J. (2021). Zebrafish Paralogs brd2a and brd2b Are Needed for Proper Circulatory, Excretory and Central Nervous System Formation and Act as Genetic Antagonists during Development. Journal of Developmental Biology, 9(4), 46. https://doi.org/10.3390/jdb9040046






