Regulation of the Brain Neural Niche by Soluble Molecule Akhirin
Abstract
1. Introduction
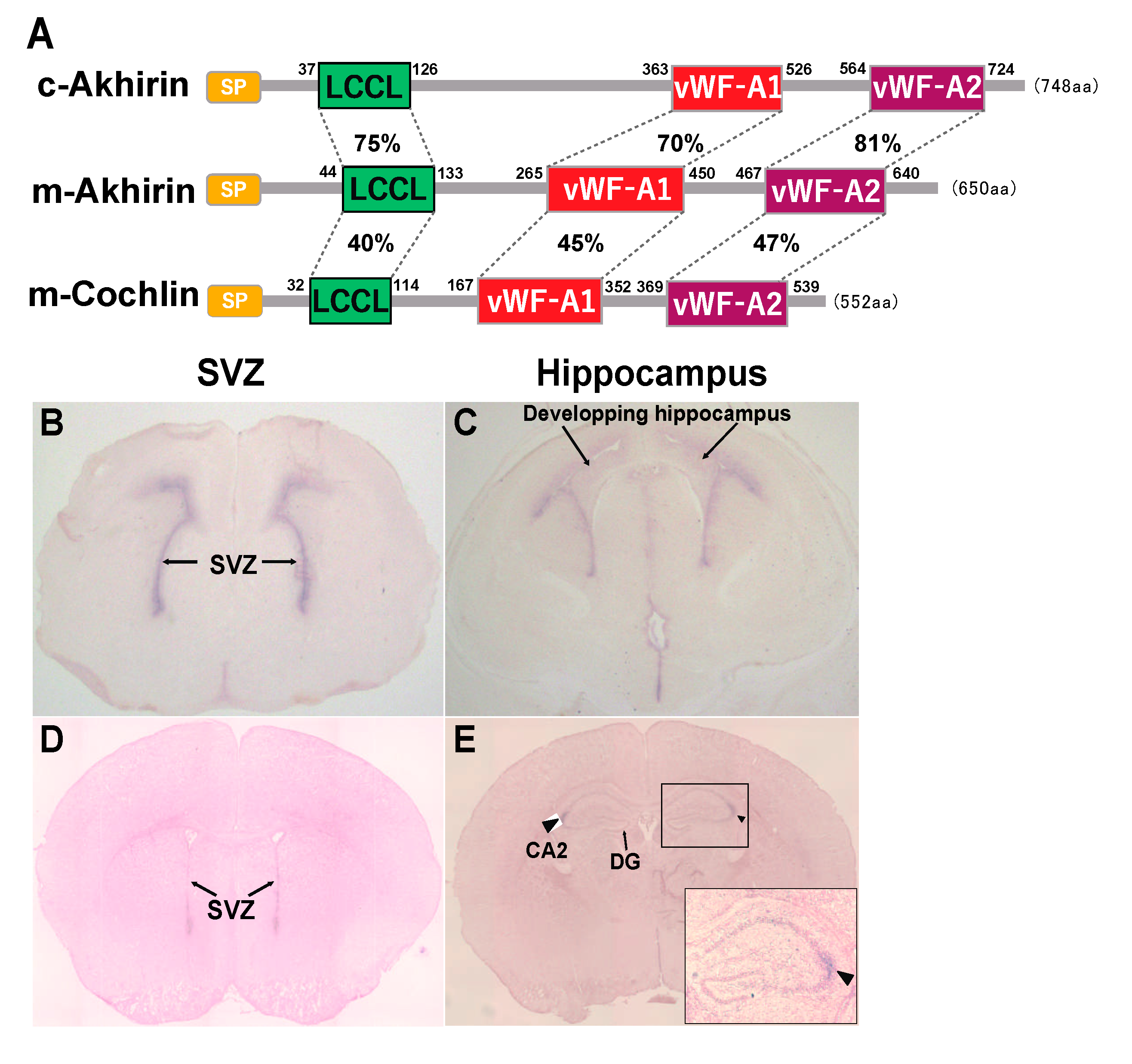
2. Possible Roles of the vWF-A and LCCL Domains in AKH
3. AKH Localizes in the Niche of the Eye
4. AKH Localizes in the Niche of the Spinal Cord
5. AKH Is Exclusively Localized in Brain Neurogenic Niches
6. Effects of AKH Knockout on Neurogenesis and Neuronal Differentiation in the Brain NSC Niche
7. Relationship between AKH and Hydrocephalus
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohta, K.; Ito, A.; Tanaka, H. Neuronal stem/progenitor cells in the vertebrate eye. Dev. Growth Differ. 2008, 50, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Tang, L.; Pham, H. Identification of Neural Progenitors in the Adult Mammalian Eye. Biochem. Biophys. Res. Commun. 2000, 270, 517–521. [Google Scholar] [CrossRef]
- Tropepe, V.; Coles, B.L.K.; Chiasson, B.J.; Horsford, D.J.; Elia, A.J.; McInnes, R.R.; van der Kooy, D. Retinal Stem Cells in the Adult Mammalian Eye. Science 2000, 287, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Kosaka, M.; Kanegae, Y.; Saito, I.; Inoue, T.; Kageyama, R.; Nishida, A.; Honda, Y.; Takahashi, M. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat. Neurosci. 2001, 4, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.; Reh, T.A. Persistent Progenitors at the Retinal Margin of ptc+/− Mice. J. Neurosci. 2004, 24, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Reh, T.A. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001, 4, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Takahashi, M. Neurogenic potential of Mueller glia in the adult mammalian retina. Inflamm. Regen. 2007, 27, 499–505. [Google Scholar] [CrossRef]
- Hamilton, L.; Truong, M.; Bednarczyk, M.; Aumont, A.; Fernandes, K. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience 2009, 164, 1044–1056. [Google Scholar] [CrossRef]
- Namiki, J.; Tator, C.H. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J. Neuropathol. Exp. Neurol. 1999, 58, 489–498. [Google Scholar] [CrossRef]
- Meletis, K.; Barnabé-Heider, F.; Carlen, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- Doetsch, F.; Caillé, I.; Lim, D.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult Mammalian Brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Furutachi, S.; Miya, H.; Watanabe, T.; Kawai, H.; Yamasaki, N.; Harada, Y.; Imayoshi, I.; Nelson, M.; I Nakayama, K.; Hirabayashi, Y.; et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 2015, 18, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Falk, S.; Bugeon, S.; Ninkovic, J.; Pilz, G.-A.; Postiglione, M.P.; Cremer, H.; Knoblich, J.A.; Götz, M. Time-Specific Effects of Spindle Positioning on Embryonic Progenitor Pool Composition and Adult Neural Stem Cell Seeding. Neuron 2017, 93, 777–791.e3. [Google Scholar] [CrossRef]
- Pilz, G.-A.; Shitamukai, A.; Reillo, I.; Pacary, E.; Schwausch, J.; Stahl, R.; Ninkovic, J.; Snippert, H.J.; Clevers, H.; Godinho, L.; et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat. Commun. 2013, 4, 2125. [Google Scholar] [CrossRef]
- Mu, H.; Ohta, K.; Kuriyama, S.; Shimada, N.; Tanihara, H.; Yasuda, K.; Tanaka, H. Equarin, a novel soluble molecule expressed with polarity at chick embryonic lens equator, is involved in eye formation. Mech. Dev. 2003, 120, 143–155. [Google Scholar] [CrossRef]
- Ahsan, M.; Ohta, K.; Kuriyama, S.; Tanaka, H. Novel soluble molecule, Akhirin, is expressed in the embryonic chick eyes and exhibits heterophilic cell-adhesion activity. Dev. Dyn. 2005, 233, 95–104. [Google Scholar] [CrossRef]
- Abdulhaleem, M.F.A.; Song, X.; Kawano, R.; Uezono, N.; Ito, A.; Ahmed, G.; Hossain, M.; Nakashima, K.; Tanaka, H.; Ohta, K. Akhirin regulates the proliferation and differentiation of neural stem cells in intact and injured mouse spinal cord. Dev. Neurobiol. 2014, 75, 494–504. [Google Scholar] [CrossRef]
- Anam, M.B. Akhirin regulates the proliferation and differentiation of neural stem cells/progenitor cells at neurogenic niches in mouse brain. Dev. Growth Differ. 2020, 62, 97–107. [Google Scholar] [CrossRef]
- Mayne, R.; Ren, Z.X.; Liu, J.; Cook, T.; Carson, M.; Narayana, S. VIT-1: The second member of a new branch of the von willebrand factor A do-main superfamily. Biochem. Soc. Trans. 1999, 27, 832–835. [Google Scholar] [CrossRef]
- Heller, S.; Sheane, C.A.; Javed, Z.; Hudspeth, A.J. Molecular Markers for Cell Types of the Inner Ear and Candidate Genes for Hearing Disorders. 1998. Available online: https://www.pnas.org/content/95/19/11400 (accessed on 25 July 2021).
- Di Marco, B.; Crouch, E.E.; Shah, B.; Duman, C.; Paredes, M.F.; de Almodovar, C.R.; Huang, E.J.; Alfonso, J. Reciprocal Interaction between Vascular Filopodia and Neural Stem Cells Shapes Neurogenesis in the Ventral Telencephalon. Cell Rep. 2020, 33, 108256. [Google Scholar] [CrossRef]
- Funa, K.; Sasahara, M. The Roles of PDGF in Development and During Neurogenesis in the Normal and Diseased Nervous System. J. Neuroimmune Pharmacol. 2014, 9, 168–181. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Merkle, F.; Soriano-Navarro, M.; García-Verdugo, J.M.; Alvarez-Buylla, A. Neural Stem Cells Confer Unique Pinwheel Architecture to the Ventricular Surface in Neurogenic Regions of the Adult Brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; García-Verdugo, J.M.; Doetsch, F. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef]
- Robertson, N.G.; Lu, L.; Heller, S.; Merchant, S.N.; Eavey, R.D.; McKenna, M.; Nadol, J.B.; Miyamoto, R.T.; Linthicum, F.H.; Neto, J.F.L.; et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat. Genet. 1998, 20, 299–303. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Jung, J.; Yoo, J.E.; Choe, Y.H.; Park, S.C.; Lee, H.J.; Lee, H.J.; Noh, B.; Kim, S.H.; Kang, G.-Y.; Lee, K.-M.; et al. Cleaved Cochlin Sequesters Pseudomonas aeruginosa and Activates Innate Immunity in the Inner Ear. Cell Host Microbe 2019, 25, 513–525.e6. [Google Scholar] [CrossRef]
- Pickard, J. Physiology and Pathophysiology of the Cerebrospinal Fluid. J. Neurol. Neurosurg. Psychiatry 1988, 51, 469–470. [Google Scholar] [CrossRef][Green Version]
- Cui, J.; Shipley, F.B.; Shannon, M.L.; Alturkistani, O.; Dani, N.; Webb, M.D.; Sugden, A.U.; Andermann, M.L.; Lehtinen, M.K. Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation. Dev. Cell 2020, 55, 617–628.e6. [Google Scholar] [CrossRef]
- Ozaki, K.; Kato, D.; Ikegami, A.; Hashimoto, A.; Sugio, S.; Guo, Z.; Shibushita, M.; Tatematsu, T.; Haruwaka, K.; Moorhouse, A.J.; et al. Maternal immune activation induces sustained changes in fetal microglia motility. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, F.; Suzuki, I.K.; Shitamukai, A.; Sakaguchi, H.; Iwashita, M.; Kobayashi, T.; Toné, S.; Toida, K.; Vanderhaeghen, P.; Kosodo, Y. Novel and Robust Transplantation Reveals the Acquisition of Polarized Processes by Cortical Cells Derived from Mouse and Human Pluripotent Stem Cells. Stem Cells Dev. 2014, 23, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.O.; Mullick, L.H.; Wingerd, K.L.; Lin, H.; Atienza, J.W.; Bradshaw, A.D.; Gervin, D.B.; Cann, G.M. Adhesive events in retinal development and function: The role of integrin receptors. In Chemistry and Biology of Pteridines and Folates; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2000; Volume 31, pp. 141–156. [Google Scholar]
- Zhao, X.; Das, A.V.; Soto-Leon, F.; Ahmad, I. Growth factor-responsive progenitors in the postnatal mammalian retina. Dev. Dyn. 2005, 232, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS Stem Cells Are Present in the Adult Mammalian Spinal Cord and Ventricular Neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, J.-C.; Ackema, K.B.; Ohayon, D.; Guichet, P.-O.; Perrin, F.E.; Garcès, A.; Ripoll, C.; Charitã, J.; Simonneau, L.; Kettenmann, H.; et al. A Mesenchymal-Like ZEB1+Niche Harbors Dorsal Radial Glial Fibrillary Acidic Protein-Positive Stem Cells in the Spinal Cord. Stem Cells 2009, 27, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, U.K.; Felemban, A.A.; Riyadh, A.M.; Ohta, K. Regulation of the neural niche by the soluble molecule Akhirin. Dev. Growth Differ. 2016, 58, 463–468. [Google Scholar] [CrossRef]
- Wintzer, M.E.; Boehringer, R.; Polygalov, D.; McHugh, T.J. The Hippocampal CA2 Ensemble Is Sensitive to Contextual Change. J. Neurosci. 2014, 34, 3056–3066. [Google Scholar] [CrossRef]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, C.J.; Tonegawa, S. Crucial role for CA2 inputs in the sequential organization of CA1 time cells supporting memory. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- McAllister, T.W. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, D.; Harris, L.; Harvey, T.J.; Heng, Y.H.E.; Smith, A.G.; Osinski, J.; Hughes, J.; Thomas, P.; Gronostajski, R.M.; Bailey, T.L.; et al. Expansion of the lateral ventricles and ependymal deficits underlie the hydrocephalus evident in mice lacking the transcription factor NFIX. Brain Res. 2015, 1616, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Moon, U.Y.; Park, J.Y.; Hughes, L.J.; Johnson, R.L.; Cho, S.-H.; Kim, S. Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nat. Commun. 2016, 7, 10329. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, M.; Ohta, K. Regulation of the Brain Neural Niche by Soluble Molecule Akhirin. J. Dev. Biol. 2021, 9, 29. https://doi.org/10.3390/jdb9030029
Kudo M, Ohta K. Regulation of the Brain Neural Niche by Soluble Molecule Akhirin. Journal of Developmental Biology. 2021; 9(3):29. https://doi.org/10.3390/jdb9030029
Chicago/Turabian StyleKudo, Mikiko, and Kunimasa Ohta. 2021. "Regulation of the Brain Neural Niche by Soluble Molecule Akhirin" Journal of Developmental Biology 9, no. 3: 29. https://doi.org/10.3390/jdb9030029
APA StyleKudo, M., & Ohta, K. (2021). Regulation of the Brain Neural Niche by Soluble Molecule Akhirin. Journal of Developmental Biology, 9(3), 29. https://doi.org/10.3390/jdb9030029






