Matrix Metalloproteinase 13 Activity is Required for Normal and Hypoxia-Induced Precocious Hatching in Zebrafish Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Environmental Hypoxia Experiment
2.3. MMP-13 Protease Inhibitor (Mmp13PI) Experiment
2.4. Immunohistochemistry
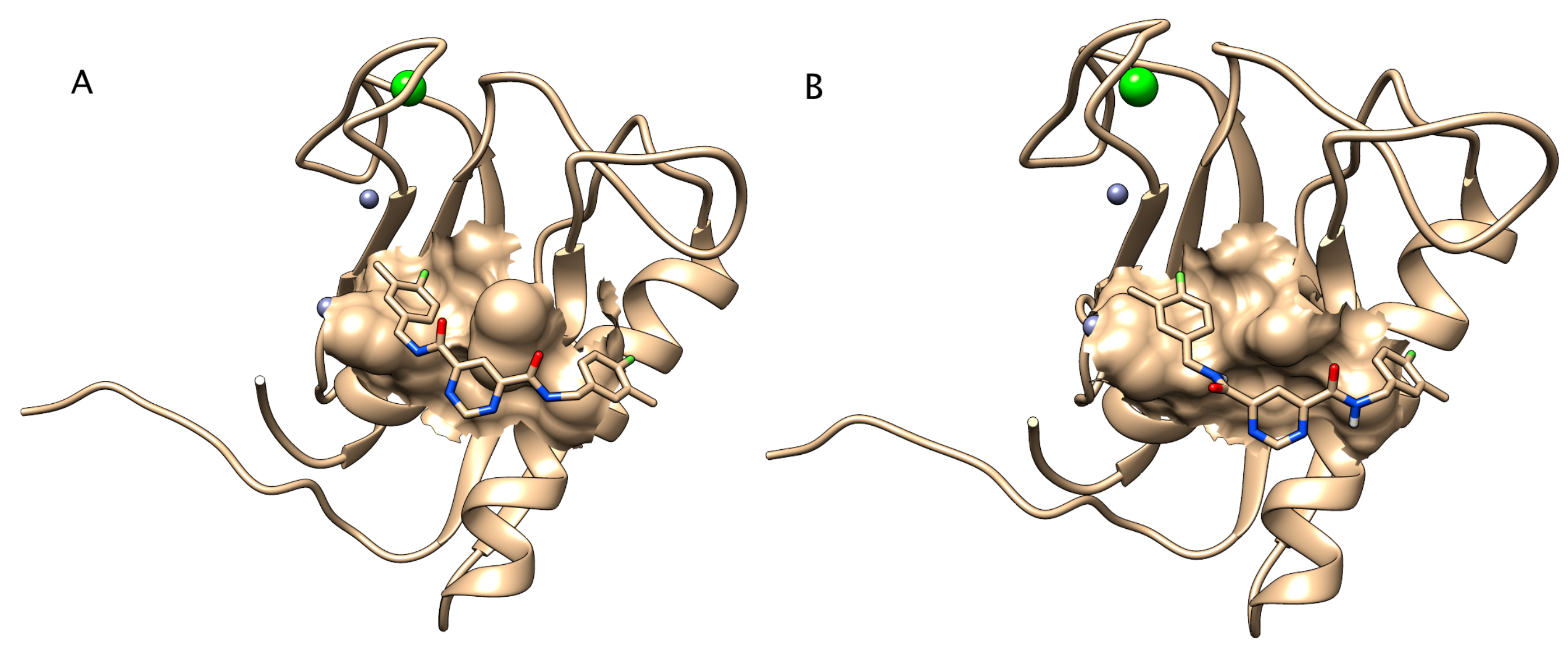
2.5. Molecular Modelling
2.5.1. Self-Docking Test
2.5.2. Homology Modelling of Zebrafish Mmp13a
2.5.3. Docking of Inhibitor to Zebrafish Mmp13a Homology Model
2.6. SDS-PAGE
2.7. In Vivo Zymography
2.8. Statistical Analysis
3. Results
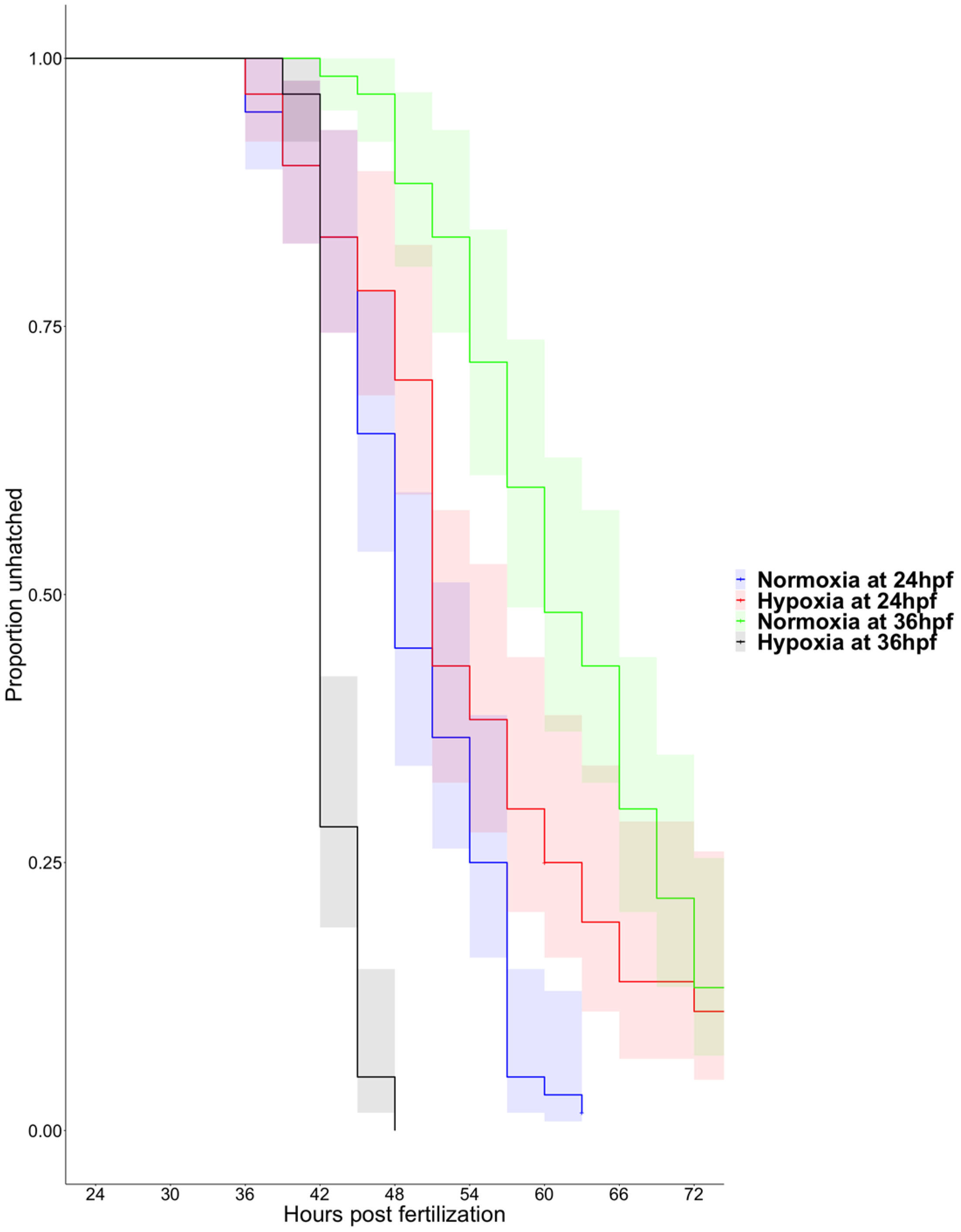
3.1. Hypoxia Induces Precocious Hatching at 36 hpf
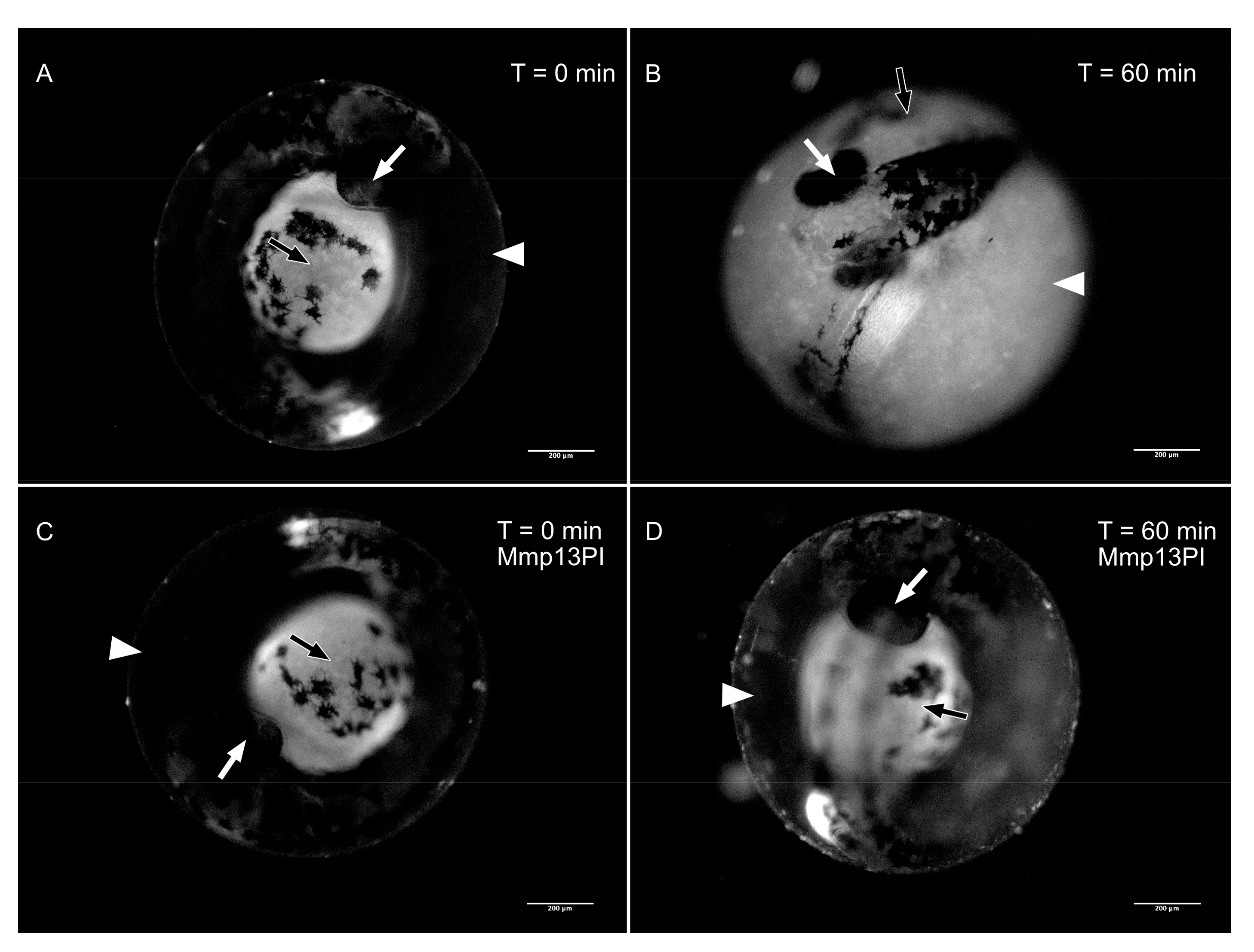
3.2. Matrix Metalloproteinase 13a Activity is Necessary for Hatching
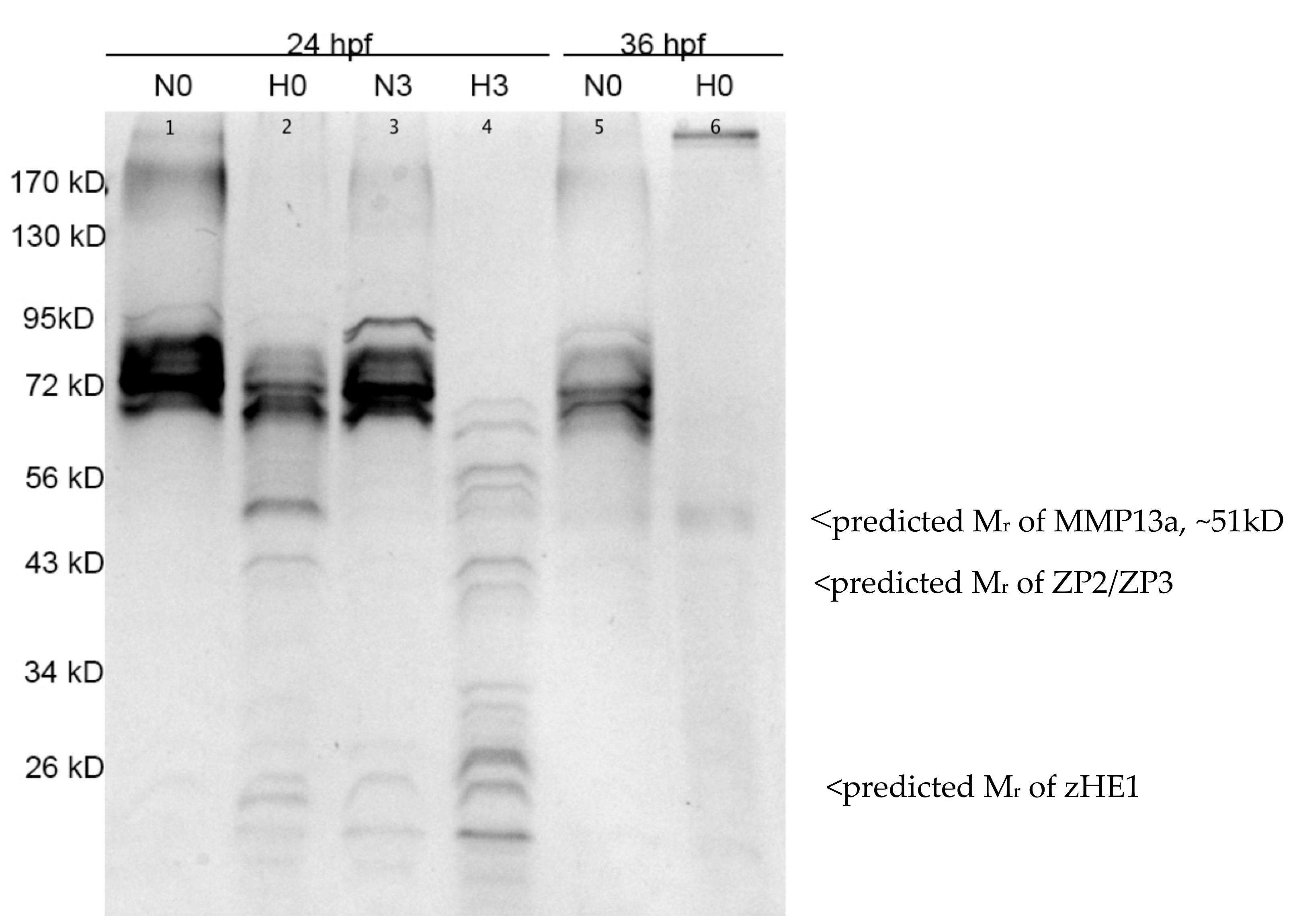
3.3. Hypoxia-Induced Proteolysis in the Chorionic Fluid is Apparent at Both 24 and 36 hpf
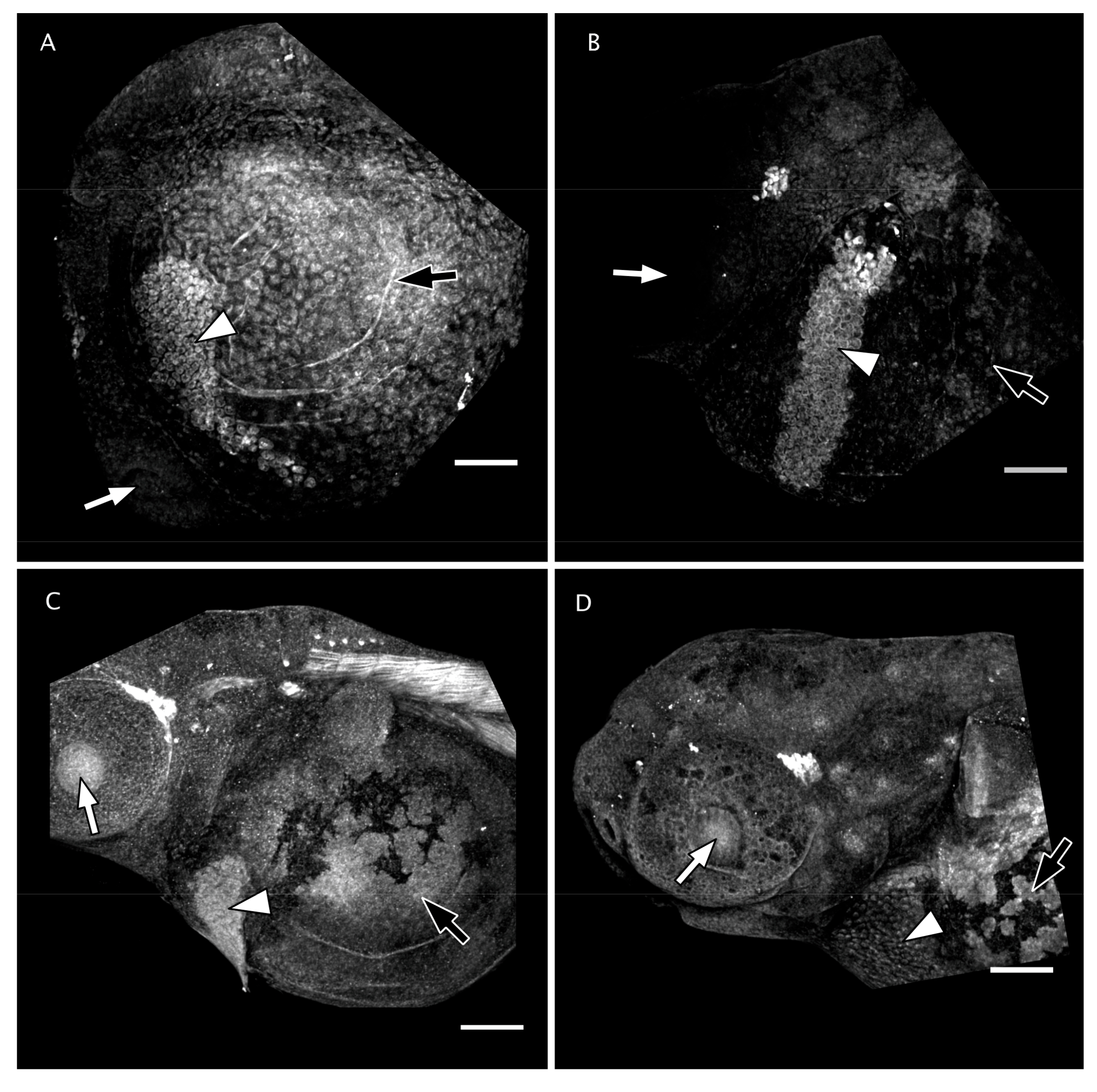
3.4. Matrix Metalloproteinase 13 Activity in the Perichorionic Fluid of Embryos at 36 hpf
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seshagiri, P.B.; Vani, V.; Madhulika, P. Cytokines and blastocyst hatching. Am. J. Reprod. Immunol. 2016, 75, 208–217. [Google Scholar] [CrossRef]
- Thome, C.; Mitz, C.; Somers, C.; Manzon, R.; Boreham, D.; Wilson, J.Y. Incubation of lake whitefish (Coregonus clupeaformis) embryos in cooling water discharge and the impacts of fluctuating thermal regimes on development. Can. J. Fish. Aquat. Sci. 2016, 73, 1213–1221. [Google Scholar] [CrossRef]
- Eme, J.; Mueller, C.A.; Leeb, A.H.; Melendez, C.; Richard, G.; Manzon, R.G.; Somers, C.M.; Boreham, D.R.; Wilson, J.Y. Daily, repeating fluctuations in embryonic incubation temperature alter metabolism and growth of Lake whitefish (Coregonus clupeaformis). Comp. Biochem. Physiol. A 2018, 226, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rombough, P.J. Respiratory Gas Exchange, Aerobic Metabolism, and Effects of Hypoxia during Early Life. In Fish Physiology, Part A; Hoar, W.S., Randall, D.J., Eds.; Academic Press: San Diego, CA, USA, 1988; Volume 11, pp. 59–161. [Google Scholar]
- Coble, D.W. Influence of water exchange and dissolved oxygen in redds on survival of steelhead trout embryos. Trans. Am. Fish. Soc. 1961, 90, 469–474. [Google Scholar] [CrossRef]
- Sear, D.A.; Pattison, I.; Collins, A.L.; Newson, M.D.; Jones, J.I.; Naden, P.S.; Carling, P.A. Factors controlling the temporal variability in dissolved oxygen regime of salmon spawning gravels. Hydrol. Process. 2012, 28, 86–103. [Google Scholar] [CrossRef]
- Davenport, J. Synopsis of Biological Data on the Lumpsucker, Cyclopterus lumpus (Linnaeus, 1758); FAO Fisheries Synopsis No. 147; Food and Agricultural Organization of the United Nation: Rome, Italy, 1985. [Google Scholar]
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; Garcia de Leaniz, C. Use of lumpfish for sea-lice control in salmon farming: Challenges and opportunities. Rev. Aquacult. 2017, 10, 683–702. [Google Scholar] [CrossRef]
- Ciuhandu, C.S.; Wright, P.A.; Goldberg, J.I.; Stevens, E.D. Parameters influencing the dissolved oxygen in the boundary layer of rainbow trout (Oncorhynchus mykiss) embryos and larvae. J. Exp. Biol. 2007, 210, 1435–1445. [Google Scholar] [CrossRef]
- Miller, S.C.; Reeb, S.E.; Wright, P.A.; Gillis, T.E. Oxygen concentration in the water boundary layer next to rainbow trout (Oncorhynchus mykiss) embryos is influenced by hypoxia exposure time, metabolic rate, and water flow. Can. J. Fish. Aquat. Sci. 2008, 65, 2170–2177. [Google Scholar] [CrossRef]
- DiMichele, L.; Powers, D.A. The mechanism of hatching in Fundulus heteroclitus: Development and physiology. J. Exp. Zool. 1981, 217, 73–79. [Google Scholar] [CrossRef]
- DiMichele, L.; Powers, D.A. Developmental and Oxygen Consumption Rate Differences between Lactate Dehydrogenase-B Genotypes of Fundulus heteroclitus and Their Effect on Hatching Time. Physiol. Zool. 1984, 57, 52–56. [Google Scholar] [CrossRef]
- Czerkies, P.; Brzuzan, P.; Kordalski, K.; Luczynski, L. Critical partial pressures of oxygen causing precocious hatching in Coregonus lavaretus and C. albula embryos. Aquaculture 2001, 196, 151–158. [Google Scholar] [CrossRef]
- Robertson, C.E.; Wright, P.A.; Koblitz, L.; Bernier, N.J. Hypoxia-inducible factor-1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish, Danio rerio. Proc. Royal Soc. Lond. B 2014, 281, 20140637. [Google Scholar] [CrossRef] [PubMed]
- Engeszer, R.E.; Patterson, L.B.; Rao, A.A.; Parichy, D.M. Zebrafish in The Wild: A Review of Natural History And New Notes from The Field. Zebrafish 2007, 4, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2007, 83, 13–34. [Google Scholar] [CrossRef]
- Padilla, P.A.; Roth, M.B. Oxygen deprivation causes suspended animation in the zebrafish embryo. PNAS 2001, 98, 7331–7335. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. The Fish Egg’s Zona Pellucida. In Extracellular Matrix and Egg Coats, 1st ed.; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 130, pp. 275–305. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; Univ. of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Kim, D.K.; Sun, Y.; Yun, S.; Kim, B.; Hwang, C.N.; Lee, S.H.; Nelson, B.J. Mechanical property characterization of the zebrafish embryo chorion. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5, September 2004; IEEE: San Francisco, CA, USA. [Google Scholar] [CrossRef]
- Yamagami, K. Studies on the Hatching Enzyme (Choriolysin) and Its Substrate, Egg Envelope, Constructed of the Precursors (Choriogenins) in Oryzias latipes: A Sequel to the Information In 1991/1992. Zool. Sci. 1996, 13, 331–340. [Google Scholar] [CrossRef]
- Sano, K.; Inohaya, K.; Kawaguchi, M.; Yoshizaki, N.; Iuchi, I.; Yasumasu, S. Purification and characterization of zebrafish hatching enzyme—An evolutionary aspect of the mechanism of egg envelope digestion. FEBS J. 2008, 275, 5934–5946. [Google Scholar] [CrossRef]
- Sano, K.; Kawaguchi, M.; Watanabe, S.; Yasumasu, S. Neofunctionalization of a duplicate hatching enzyme gene during the evolution of teleost fishes. BMC Evol. Biol. 2014, 14, 221. [Google Scholar] [CrossRef]
- Okada, A.; Sano, K.; Nagata, K.; Yasumasu, S.; Ohtsuka, J.; Yamamura, A.; Kubota, K.; Iuchi, I.; Tanokura, M. Crystal Structure of Zebrafish Hatching Enzyme 1 from the Zebrafish Danio rerio. J. Mol. Biol. 2010, 402, 865–878. [Google Scholar] [CrossRef]
- Yasumasu, S.; Yamada, K.; Akasaka, K.; Mitsunaga, K.; Iuchi, I.; Shimada, H.; Yamagami, K. Isolation of cDNAs for LCE and HCE, two constituent proteases of the hatching enzyme of Oryzias latipes, and concurrent expression of their mRNAs during development. Dev. Biol. 1992, 153, 250–258. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kawaguchi, M.; Yano, T.; Sano, K.; Okabe, M.; Yasumasu, S. Evolutionary Changes in the Developmental Origin of Hatching Gland Cells in Basal Ray-Finned Fishes. Zool. Sci. 2016, 33, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yasumasu, S.; Katow, S.; Hamazaki, T.S.; Iuchi, I.; Yamagami, K. Two constituent proteases of a teleostean hatching enzyme: Concurrent syntheses and packaging in the same secretory granules in discrete arrangement. Dev. Biol. 1992, 149, 349–356. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Dzamba, B.J.; DeSimone, D.W. Extracellular Matrix (ECM) and the Sculpting of Embryonic Tissues. In Extracellular Matrix and Egg Coats, 1st ed.; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press: Cambridge, CA, USA, 2018; Volume 130, pp. 245–274. [Google Scholar] [CrossRef]
- Loffek, S.; Schilling, O.; Franzke, C.W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2010, 38, 191–208. [Google Scholar] [CrossRef]
- Young, D.; Das, N.; Anowai, A.; Dufour, A. Matrix Metalloproteases as Influencers of the Cells’ Social Media. Int. J. Mol. Sci. 2016, 20, 3847. [Google Scholar] [CrossRef]
- Fallata, A.M.; Wyatt, R.A.; Levesque, J.M.; Dufour, A.; Overall, C.M.; Crawford, B.D. Intracellular Localization in Zebrafish Muscle and Conserved Sequence Features Suggest Roles for Gelatinase A Moonlighting in Sarcomere Maintenance. Biomedicines 2019, 7, 93. [Google Scholar] [CrossRef]
- Matchett, E.F.; Wang, S.; Crawford, B.D. Paralogues of Mmp11 and Timp4 Interact during the Development of the Myotendinous Junction in the Zebrafish Embryo. J. Dev. Biol. 2019, 7, 22. [Google Scholar] [CrossRef]
- Wyatt, R.A.; Keow, J.Y.; Harris, N.D.; Haché, C.A.; Li, D.H.; Crawford, B.D. The Zebrafish Embryo: A Powerful Model System for Investigating Matrix Remodeling. Zebrafish 2009, 6, 347–354. [Google Scholar] [CrossRef]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Gen. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Ravi, V.; Venkatesh, B. The Divergent Genomes of Teleosts. Annu. Rev. Anim. Biosci. 2018, 6, 47–68. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Hillegass, J.M.; Villano, C.M.; Cooper, K.R.; White, L.A. Matrix Metalloproteinase-13 Is Required for Zebra fish (Danio rerio) Development and Is a Target for Glucocorticoids. Toxicol. Sci. 2007, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Waldron, A.L.; Schroder, P.A.; Bourgon, K.L.; Bolduc, J.K.; Miller, J.L.; Pellegrini, A.D.; Dubois, A.L.; Blaszkiewicz, M.; Townsend, K.L.; Rieger, S. Oxidative stress-dependent MMP-13 activity underlies glucose neurotoxicity. J. Diabetes Complicat. 2018, 32, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Trikić, M.Z.; Monk, P.; Roehl, H.; Partridge, L.J. Regulation of Zebrafish Hatching by Tetraspanin cd63. PLoS ONE 2011, 6, e19683. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Sammons, D.W.; Adams, L.D.; Nishizawa, E.E. Ultrasensitive silver-based color staining of polypeptides in polyacrylamide gels. Electrophoresis 1981, 2, 135–141. [Google Scholar] [CrossRef]
- Crawford, B.D.; Pilgrim, D.B. Ontogeny and regulation of matrix metalloproteinase activity in the zebrafish embryo by in vitro and in vivo zymography. Dev. Biol. 2005, 286, 405–414. [Google Scholar] [CrossRef]
- Keow, J.Y.; Herrmann, K.M.; Crawford, B.D. Differential in vivo zymography: A method for observing matrix metalloproteinase activity in the zebrafish embryo. Matrix Biol. 2011, 30, 169–177. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 31 December 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Kassambara, A.; Kosinski, M. Survminer: Drawing Survival Curves using ‘ggplot2’. R package version 0.4.4. Available online: https://CRAN.R-project.org/package=survminer (accessed on 31 December 2019).
- Engel, C.K.; Pirard, B.; Schimanski, S.; Kirsch, R.; Habermann, J.; Klingler, O.; Schlotte, V.; Weithmann, K.U.; Wendt, K.U. Structural Basis for the Highly Selective Inhibition of MMP-13. Chem. Biol. 2005, 12, 181–189. [Google Scholar] [CrossRef]
- Breau, C.; Cunjak, R.A.; Peake, S.J. Behaviour during elevated water temperatures: Can physiology explain movement of juvenile Atlantic salmon to cool water? J. Anim. Ecol. 2011, 80, 844–853. [Google Scholar] [CrossRef]
- Kurylyk, B.L.; MacQuarrie, K.T.B.; Linnansaari, T.; Cunjak, R.A.; Curry, R.A. Preserving, augmenting, and creating cold-water thermal refugia in rivers: Concepts derived from research on the Miramichi River, New Brunswick (Canada). Ecohydrology 2014, 8, 1095–1108. [Google Scholar] [CrossRef]
- Kajimura, S.; Aida, K.; Duan, C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. PNAS 2005, 102, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. CSH Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Small, C.D.; Crawford, B.D. Matrix metalloproteinases in neural development: A phylogenetically diverse perspective. Neural Regen. Res. 2016, 11, 35–362. [Google Scholar] [CrossRef]
- Lisse, T.S.; Middleton, L.J.; Pellegrini, A.D.; Marin, P.B.; Spaulding, E.L.; Lopes, O.; Brochu, E.A.; Carter, E.V.; Waldron, A.; Rieger, S. Paclitaxel-induced epithelial damage and ectopic MMP-13 expression promotes neurotoxicity in zebrafish. PNAS 2016, 113, E2189–E2198. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, J.; Sear, D.; Kemp, P. Does variation in egg structure among five populations of Atlantic salmon (Salmo salar) influence survival in low oxygen conditions? R. Soc. Open Sci. 2019, 6, 181020. [Google Scholar] [CrossRef]
- Müller, U.K.; van Leeuwen, J.L. Swimming of larval zebrafish: Ontogeny of body waves and implications for locomotory development. J. Exp. Biol. 2004, 207, 853–868. [Google Scholar] [CrossRef]
- Voesenek, C.J.; Muijres, F.T.; van Leeuwen, J.L. Biomechanics of swimming in developing larval fish. J. Exp. Biol. 2018, 221, jeb149583. [Google Scholar] [CrossRef]
- Jeffrey, E.J.; Crawford, B.D. The epitope-mediated MMP activation assay: Detection and quantification of the activation of Mmp2 in vivo in the zebrafish embryo. Histochem. Cell Biol. 2018, 149, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Hiroi, J.; Miya, M.; Nishida, M.; Iuchi, I.; Yasumasu, S. Intron-loss evolution evolution of hatching enzyme genes in Teleostei. BMC Evol. Biol. 2010, 10. [Google Scholar] [CrossRef]
- Macklon, N.S. Conception to ongoing pregnancy: The “black box” of early pregnancy loss. Hum. Reprod. Update 2002, 8, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Fukui, E.; Yoshizawa, M. Molecular and cellular events involved in the completion of blastocyst implantation. Reprod. Med. Biol. 2015, 15, 53–58. [Google Scholar] [CrossRef]
- Frum, T.; Ralston, A. Cell signaling and transcription factors regulating cell fate during formation of the mouse blastocyst. Trends Genet. 2015, 31, 402–410. [Google Scholar] [CrossRef]
- Rossant, J. Making the Mouse Blastocyst. In Essays on Developmental Biology, Part B; Wassarman, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 117, pp. 275–288. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, J.S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Chimote, N.; Chimote, N.; Mehta, B.; Nath, N. Transfer of spontaneously hatching or hatched blastocyst yields better pregnancy rates than expanded blastocyst transfer. J. Hum. Reproductive Sci. 2013, 6, 183. [Google Scholar] [CrossRef]
- Fong, C.Y.; Bongso, A.; Ng, S.C.; Kumar, J.; Trounson, A.; Ratnam, S. Blastocyst transfer after enzymatic treatment of the zona pellucida: Improving in-vitro fertilization and understanding implantation. Hum. Reprod. 1998, 13, 2926–2932. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Small, C.D.; el-Khoury, M.; Deslongchamps, G.; Benfey, T.J.; Crawford, B.D. Matrix Metalloproteinase 13 Activity is Required for Normal and Hypoxia-Induced Precocious Hatching in Zebrafish Embryos. J. Dev. Biol. 2020, 8, 3. https://doi.org/10.3390/jdb8010003
Small CD, el-Khoury M, Deslongchamps G, Benfey TJ, Crawford BD. Matrix Metalloproteinase 13 Activity is Required for Normal and Hypoxia-Induced Precocious Hatching in Zebrafish Embryos. Journal of Developmental Biology. 2020; 8(1):3. https://doi.org/10.3390/jdb8010003
Chicago/Turabian StyleSmall, Christopher D., Megan el-Khoury, Ghislain Deslongchamps, Tillmann J. Benfey, and Bryan D. Crawford. 2020. "Matrix Metalloproteinase 13 Activity is Required for Normal and Hypoxia-Induced Precocious Hatching in Zebrafish Embryos" Journal of Developmental Biology 8, no. 1: 3. https://doi.org/10.3390/jdb8010003
APA StyleSmall, C. D., el-Khoury, M., Deslongchamps, G., Benfey, T. J., & Crawford, B. D. (2020). Matrix Metalloproteinase 13 Activity is Required for Normal and Hypoxia-Induced Precocious Hatching in Zebrafish Embryos. Journal of Developmental Biology, 8(1), 3. https://doi.org/10.3390/jdb8010003





