Abstract
Retinoids function as important regulatory signaling molecules during development, acting in cellular growth and differentiation both during embryogenesis and in the adult animal. In 1953, Fell and Mellanby first found that excess vitamin A can induce transdifferentiation of chick embryonic epidermis to a mucous epithelium (Fell, H.B.; Mellanby, E. Metaplasia produced in cultures of chick ectoderm by high vitamin A. J. Physiol. 1953, 119, 470–488). However, the molecular mechanism of this transdifferentiation process was unknown for a long time. Recent studies demonstrated that Gbx1, a divergent homeobox gene, is one of the target genes of all-trans retinoic acid (ATRA) for this transdifferentiation. Furthermore, it was found that ATRA can induce the epidermal transdifferentiation into a mucosal epithelium in mammalian embryonic skin, as well as in chick embryonic skin. In the mammalian embryonic skin, the co-expression of Tgm2 and Gbx1 in the epidermis and an increase in TGF-β2 expression elicited by ATRA in the dermis are required for the mucosal transdifferentiation, which occurs through epithelial-mesenchymal interaction. Not only does retinoic acid (RA) play an important role in mucosal transdifferentiation, periderm desquamation, and barrier formation in the developing mammalian skin, but it is also involved in hair follicle downgrowth and bending by its effect on the Wnt/β-catenin pathway and on members of the Runx, Fox, and Sox transcription factor families.
1. Introduction
Skin is composed of an epidermis, which is an epithelium derived from ectodermal tissue, and an underlying dermis, which is a connective tissue derived from mesenchyme of mesodermal origin. During the formation of skin and its appendages, e.g., feathers, scales, and hair, the epithelium, and mesenchyme are inducers and targets of each other [1]. Retinoic acids (RAs) including all-trans, 9-cis, and other derivatives, an active metabolite of retinol, regulate cell proliferation, differentiation, and morphogenesis during normal development of the skin [2,3,4,5,6,7]. When human keratinocytes are grown on their dermal equivalent (fabricated collagen lattices), physiologic concentrations (1–10 nm) of RA result in an epithelium very similar to that in normally keratinized epidermis; but a higher concentration (>0.1 μm) of RA reduces epidermal maturation and produces parakeratosis, and a deficiency of RA leads to hyperkeratosis [8]. Similar to the results obtained from keratinocyte cultures, retinol deficiency in the rat can cause squamous metaplasia and keratinization in a wide variety of nonkeratinized and secretory epithelia [9,10]; and excess retinol can induce epidermal mucous metaplasia in skin cultured from chick embryos [11]. An amount of 16.7 μm of ATRA induces glandular metaplasia in mouse embryonic upper-lip skin instead of hair vibrissa follicle [12,13]. Many of the abnormalities in pattern formation and organ formation that result from the addition of exogenous RA during embryogenesis are related in part to the ability of retinoids to change the pattern of expression of the clusters of homeobox genes in the embryo [14,15,16,17]. Many homeobox genes have been shown to change their expression in the skin as a response to RA [18,19,20]. It was demonstrated over a decade ago that Gbx1 (gastrulation brain homeobox 1), a divergent homeobox gene, is one of the target genes of RA for the mucous transdifferentiation [21]. Our recent study found that 1 μm ATRA can induce mucous transdifferentiation in the mammalian embryonic skin as well as in chick embryonic skin. The molecular mechanism of this transdifferentiation was examined in the mammalian embryonic skin. Interestingly, ATRA-induced expression of Tgm2 (transglutaminase 2) and Gbx1 in the epidermis and that of TGF-β2 expression in the dermis are required for the mucosal transdifferentiation [22]. Other than the transdifferentiation, RA also plays an important role in barrier formation and hair follicle formation in skin [6,7,23,24,25,26,27]. This review highlights our current understanding of the role of retinoic acid in the epidermal transdifferentiation of skin and its appendages.
2. Effects of RA on Morphogenesis of Chick Embryonic Skin
RA is known to regulate cell proliferation, differentiation, and morphogenesis during the normal development of many tissues [16,28]. Two cutaneous structures of chick, i.e., scales and feathers, have been studied extensively to define the mechanism of retinoid-induced morphogenetic change [11,14,15,20,29,30,31]. Dhouailly et al. (1980) showed that the single injection of 125 μg (417 μm) of ATRA into the amniotic cavity of chick embryos at embryonic Hamburger-Hamilton (HH) stage 36 (day 10), which correspond to the beginning of scale morphogenesis, leads to the formation of feathers on chick foot scales [29]. An amount of 2.5 μm of RA also modulates axis orientation and phenotypes of skin appendages [14]. Using chick embryonic skin cultured in vitro, we examined the mechanism of the mucous transdifferentiation induced by RA and showed that in chick embryonic tarsometatarsal skin, this transdifferentiation to a mucous epithelium is induced by the interaction of the epidermis with the dermis, when dermal fibroblasts are pretreated with retinol (Figure 1) [21,32,33,34,35]. Furthermore, 1 μm ATRA inhibits feather bud formation and induces the transdifferentiation of the epidermis of chick embryonic dorsal skin to a mucous one [36]. In the mucous transdifferentiated epidermis, PAS-positive materials, mucous granules, and a discontinuous basement membrane are observed (Figure 1). The discrepancy between the results of Dhouailly et al. (1980) and ours may be ascribed to the difference in the RA concentrations used, the processing time, chicken embryonic stages, and culture conditions (in ovo or in vitro).
3. Effects of RA on the Expression of Homeobox Genes in Chick Embryonic Skin
Many of the abnormalities in pattern formation and organ formation that result from the addition of exogenous RA during embryogenesis are related in part to the ability of retinoids to change the pattern of expression of the clusters of homeobox (Hox) genes in the embryo [14,17]. Hox genes are master control genes that specify the body plan and regulate the development and morphogenesis of higher organisms [37]. Apart from these classic homeobox genes, there are other groups of homeobox genes (divergent homeobox genes), which are located outside the Hox loci and also play an important role in regulating growth and differentiation during the development of many organs. Using a degenerate RT-PCR (reverse transcriptase-polymerase chain reaction)-based screening method, we previously isolated divergent homeobox genes Gbx1 [21], Hex (currently Hhex) [38,39,40], and HB9 (currently Mnx1) [41,42] from chick embryonic tarsometatarsal scale skin in addition to Hox genes. Furthermore, among the many homeobox genes isolated, Gbx1 shows a strong increase in expression in the epidermis of tarsometatarsal scale skin during the course of retinol-induced epidermal transdifferentiation to mucosal epithelium; and its expression is induced by the interaction of the epidermis with dermal fibroblasts pretreated with retinol [21].
In the feather bud, homeobox genes other than Gbx1 are reported to be expressed, such as Gbx2 [43]; Msx-1 and Msx-2 [19], HB9 [42]; Hox b-4, Hox a-7, and Hox c-8 [44]; Dlx 2, 3, 5 [45]; and Hex [39,40]. Gbx2 is a homeobox gene closely related to Gbx1 and is required for proper segregation of early regional identities anterior and posterior to the mid-hindbrain boundary in the case of vertebrates [46].
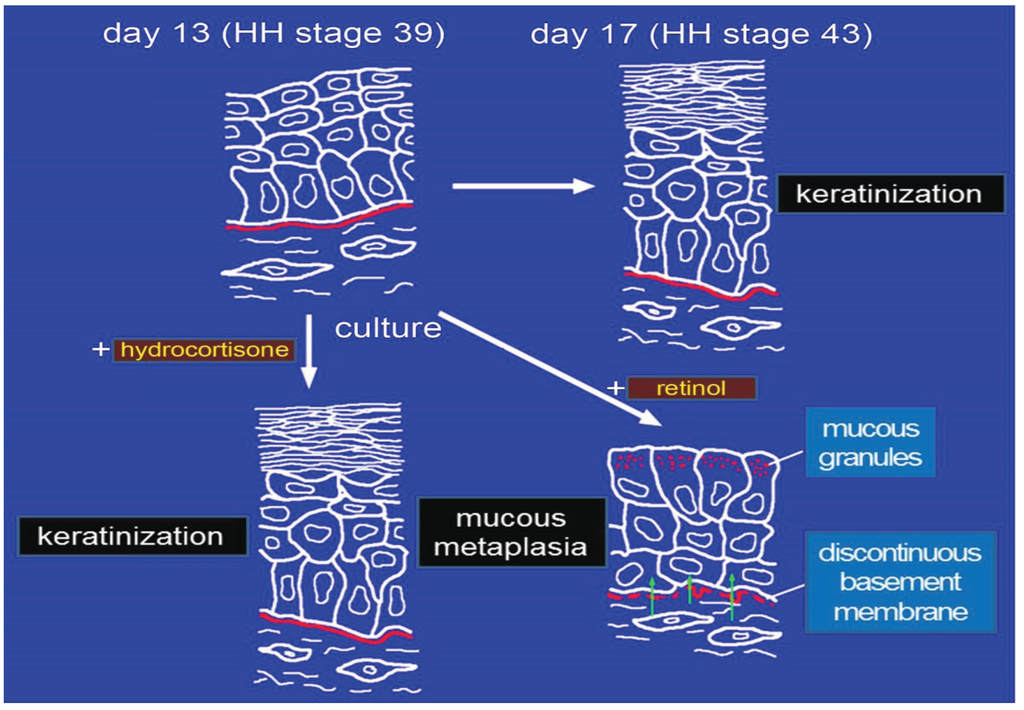
Figure 1.
Schematic diagram summarizing the effect of RA on epidermal differentiation of tarsometatarsal chick embryonic skin. Excess retinol can induce epidermal mucous metaplasia (transdifferentiation) in cultured skin obtained from chick embryos [11]. Epidermal mucous metaplasia of 13-day-old chick embryonic tarsometatarsal skin (HH stage 39) can be inducedby culturing the skin with excess retinol for only 8 h and then without retinol for six days [34], after which the mucous granules and a discontinuous basement membrane are observed. Retinol primarily affects the dermal cells [32,35], which then transform the epidermal cells into mucus-secreting cells [33,34], suggesting the importance of epithelial–mesenchymal interaction in retinol-induced epidermal mucous metaplasia.
Figure 1.
Schematic diagram summarizing the effect of RA on epidermal differentiation of tarsometatarsal chick embryonic skin. Excess retinol can induce epidermal mucous metaplasia (transdifferentiation) in cultured skin obtained from chick embryos [11]. Epidermal mucous metaplasia of 13-day-old chick embryonic tarsometatarsal skin (HH stage 39) can be inducedby culturing the skin with excess retinol for only 8 h and then without retinol for six days [34], after which the mucous granules and a discontinuous basement membrane are observed. Retinol primarily affects the dermal cells [32,35], which then transform the epidermal cells into mucus-secreting cells [33,34], suggesting the importance of epithelial–mesenchymal interaction in retinol-induced epidermal mucous metaplasia.
Studies on Gbx1 have concentrated on the brain or neurons. In mice, during development Gbx1 is expressed in the central nervous system [47,48]; and combined expression of Lhx7 and Gbx1 plays a role in the development of the cholinergic system of the basal forebrain [46]. In zebrafish, Gbx1 and Otx2 are expressed in the neuroectoderm [48]. It was recently shown for the first time that the Gbx1 gene is also expressed in the feather bud of chick embryonic dorsal skin [36]. Treatment of organ cultures of chick embryonic dorsal skin with 1 μm ATRA for 24 h induces transdifferentiation of the epidermis to mucosal epithelium with a concomitant increase in Gbx1 mRNA expression in the epidermis.
Gbx1 is expressed mainly in the epithelium of the feather bud [36]. In contrast, Gbx2 is expressed in the mesenchyme of it [43]. The gene expression pattern of Gbx1 in the epidermis of feather buds is almost the same as that of the HB9 homeobox gene, as we reported previously [42]. Other than homeobox genes, the genes encoding morphogenetic proteins such as fibroblast growth factors (FGFs) [49], bone morphogenetic proteins (BMPs) [50], sonic hedgehog (Shh) [51,52,53], Wnt 7a [54], Notch, Delta, Serrate [55], and β-catenin [56] are involved in the early stage of feather development [20]. Especially, FGF-, BMP-, Shh, and Wnt-signaling pathways play important roles in the morphogenesis of feather [20,49,50,53,54].
We showed that treatment of organ cultures of chick embryonic dorsal skin with 1 μm ATRA at HH stage 31 or 33 inhibits feather-bud formation and induces transdifferentiation of the epidermis to a non-keratinized stratified mucous epithelium concomitant with an increase in Gbx1 expression as microvilli develop on the upper surface of the epithelium [36]. In addition, a well-developed Golgi apparatus and PAS-positive materials are observed in the treated epidermis. These results are consistent with our previous reports stating that epidermal mucous transdifferentiation is induced by RA in chick embryonic tarsometatarsal skin at HH stage 39 along with an increase in Gbx1 expression and that typical goblet cells are induced in it by retinol [21,32,34].
Modulation of the axis orientation of feather buds by treatment with 1 μm ATRA throughout the culture period results in a higher frequency of small feather buds and many buds showing random orientations [14]. However, when 1 μm ATRA was used for treatment for only one day, along with 10 nm hydrocortisone which induces the differentiation (keratinization) of epidermis [57], and the skin was cultured for an additional four days without these chemicals, a different result was obtained. ATRA did not modulate the axis orientation, but rather caused the transdifferentiation of the epidermis to a mucous epithelium [36]. Thus RA has important roles in transdifferentiation, as well as in modulation of pattern formation.
4. Effects of Gbx1 on Chick Skin
As ATRA induces Gbx1 mRNA expression in the epidermis, we also examined whether Gbx1 alone (without ATRA) could induce epidermal transdifferentiation. Feather buds were elongated by transient transfection of the dorsal epidermis with Gbx1 cDNA, indicating stimulation of epidermal cell proliferation by Gbx1; however, no transdifferentiation was induced [36]. These results suggest that Gbx1 alone does not induce mucous transdifferentiation and that other signals playing a key role cooperatively with Gbx1 are involved in the epidermal to mucous transdifferentiation.
5. ATRA Induces Transdifferentiation of Rat Embryonic Epidermis to Mucosal Epithelium with Up-Regulation of Esophageal Markers MUC4 and Keratin 4
To determine the molecular mechanism of the transdifferentiation induced by ATRA, we established a culture system using rat embryonic skin in which ATRA induces epidermal transdifferentiation that is accompanied by the expression of markers of esophageal epithelium. We examined what genes are up-regulated and down-regulated in the 16E rat embryonic skin by ATRA. Representative genes up-regulated after 24 h ATRA treatment are shown in Table 1. The remainder of the up-regulated genes is shown in Supplementary Table 1. The down-regulated genes are shown in Supplementary Table 2. Bioinformatic pathway analysis of the microarray data identifies the up-regulated and down-regulated genes involved in retinol signaling pathway. Stra6 (stimulated by retinoic acid gene 6), Cyp2b3, d1 and Cyp26a1, b1 (cytochrome P450, family 2 and 26), Lrat (lecithin-retinol acyltransferase), and Ugt2b (UDP glycosyltransferase 2 family, polypeptide B) are increased in expression at the mRNA level (Table 1 and Supplementary Table 1), while Adh7 (alcohol dehydrogenase 7), Cyp1a1, Cyp2c12, Cyp3a23/3a1, and Ugt1a1(UDP glucuronosyltransferase 1 family, polypeptide A1) are decreased (Supplementary Table 2). Other than these up-regulated genes, not only Tgm2 but also Mucin 4 (MUC4) and keratin 4 (K4), which are markers of esophageal cells, are increased in expression at the mRNA level [22]. K4 and MUC4 are expressed in non-keratinized squamous stratified epithelium and in respiratory and digestive epithelium, respectively [58,59]. Cytokeratin, an intermediate filament observed mainly in epithelial cells, is an essential cytoskeleton component involved in the maintenance of cell morphology. The cytoskeleton of epithelia is formed by 20 subtypes of cytokeratins whose expression depends primarily on the epithelial cell type and degree of differentiation [60]. As keratin filaments, e.g., tonofibrils, K1 and K10 are not at all observed throughout the epidermis; but both K4 and MUC4 proteins are expressed in RA-induced transdifferenti ated epithelial cells, but not in control epidermal cells [22]. According to the results by electron microscopic and immunofluorescent analyses on tonofibrils, we consider that all the cells in the epidermis are induced by ATRA to undergo transdifferentiation. This transdifferentiation of whole epidermal tissue to mucosal tissue occurs without any gene transfection and requires only a one-day treatment with ATRA. Thus, this is an exciting model for induction of tissue organization, regeneration, and transdifferentiation because the generation of these transdifferentiated cells is fast and efficient.

Table 1.
List of representative genes up-regulated in 16E rat embryonic dorsal skin after 24 h incubation with 1 μm retinoic acid (RA) (Fold change).
| Fold | Gene Symbol | Gene Title | Public ID |
|---|---|---|---|
| 22.07 | Stra6 | stimulated by retinoic acid gene 6 | BI284420 |
| 17.40 | LOC363060 | similar to RIKEN cDNA 1600029D21 | AI599133 |
| 11.37 | Lrat | lecithin-retinol acyltransferase | NM_022280 |
| 9.18 | A2m | alpha-2-macroglobulin | NM_012488 |
| 8.61 | Amy1a | amylase, alpha 1A (salivary) | AB057450 |
| 8.43 | Crisp1 | cysteine-rich secretory protein 1 | NM_022859 |
| 8.04 | Gpr85 | G protein-coupled receptor 85 | AF203907 |
| 7.94 | Cldn7 | claudin 7 | AJ011811 |
| 7.41 | Dhrs3 | dehydrogenase/reductase (SDR family) member 3 | BI276935 |
| 7.12 | Nupl1 | nucleoporin like 1 | AF000901 |
| 7.10 | Dusp14 | dual specificity phosphatase 14 | AI236997 |
| 6.83 | Il6r | interleukin 6 receptor | NM_017020 |
| 6.66 | Ces1d | carboxylesterase 1D | L46791 |
| 6.57 | Tmprss2 | transmembrane protease, serine 2 | AI412136 |
| 6.35 | LOC685158 | similar to CG8138-PA | AI639305 |
| 6.24 | Igfbp5 | insulin-like growth factor binding protein 5 | BE113270 |
| 5.62 | Zfp667 | zinc finger protein 667 | BF402458 |
| 5.51 | Cyp26b1 | cytochrome P450, family 26, subfamily b, polypeptide 1 | BE105541 |
| 5.37 | Tgm2 | transglutaminase 2, C polypeptide | BI275994 |
| 5.27 | Amy2 | amylase 2, pancreatic | NM_031502 |
| 5.17 | Trdn | triadin | AF220558 |
| 5.14 | Sorl1 | sortilin-related receptor, LDLR class A repeats-containing | AI177589 |
| 5.11 | Cyp26a1 | cytochrome P450, family 26, subfamily a, polypeptide 1 | NM_130408 |
| 4.95 | Akap5 | A kinase (PRKA) anchor protein 5 | NM_133515 |
| 4.69 | Mcpt10 | mast cell protease 10 | X68657 |
| 4.59 | Klf2 | Kruppel-like factor 2 (lung) | BF288243 |
| 4.50 | Ush1c | Usher syndrome 1C homolog (human) | BI291932 |
| 4.50 | Cfi | complement factor I | NM_024157 |
| 4.48 | Tpc1808 | tropic 1808 | NM_022625 |
| 4.46 | Car5b | carbonic anhydrase 5b, mitochondrial | AI411132 |
| 4.45 | Scgb1d2 | secretoglobin, family 1D, member 2 | BI285057 |
| 4.37 | Srpx2 | sushi-repeat-containing protein, X-linked 2 | AA818334 |
| 4.37 | Synpr | synaptoporin | BG666364 |
| 4.36 | Slc6a1 | solute carrier family 6 (neurotransmitter transporter, GABA), member 1 | NM_024371 |
| 4.34 | St6gal1 | ST6 beta-galactosamide alpha-2,6-sialyltranferase 1 | M83143 |
| 4.32 | Oprk1 | opioid receptor, kappa 1 | L22536 |
| 4.15 | Mmp11 | matrix metallopeptidase 11 | NM_012980 |
| 4.11 | Dcx | doublecortin | NM_053379 |
| 4.08 | Kalrn | kalirin, RhoGEF kinase | AI639313 |
| 4.05 | Wfdc1 | WAP four-disulfide core domain 1 | BI279661 |
| 4.04 | Scaper | S-phase cyclin A-associated protein in the ER | BF405311 |
| 4.03 | Igfbp6 | insulin-like growth factor binding protein 6 | NM_013104 |
| 3.95 | Prelp | proline/arginine-rich end leucine-rich repeat protein | AI011747 |
| 3.90 | Aoc3 | amine oxidase, copper containing 3 (vascular adhesion protein 1) | AI070137 |
| 3.88 | Tmem176a | transmembrane protein 176A | BM388911 |
| 3.83 | Egr4 | early growth response 4 | NM_019137 |
| 3.75 | Naaa | N-acylethanolamine acid amidase | AI412627 |
| 3.74 | RGD1562533 | similar to mKIAA0774 protein | BI299098 |
| 3.74 | Slc19a1 | solute carrier family 19 (folate transporter), member 1 | NM_017299 |
| 3.69 | Rnf207 | ring finger protein 207 | BF408540 |
| 3.68 | Il17d | Interleukin 17D | AI407169 |
| 3.67 | Pkia | Protein kinase (cAMP-dependent, catalytic) inhibitor alpha | AA996685 |
| 3.66 | Klk8 | kallikrein related-peptidase 8 | BI282567 |
| 3.62 | Map7d1 | MAP7 domain containing 1 | AW253217 |
| 3.62 | Mapk11 | mitogen-activated protein kinase 11 | BF414412 |
| 3.61 | Muc4 | mucin 4, cell surface associated | BM391100 |
6. ATRA Increases Expression of Tgm2 and Gbx1 mRNA and Protein in Rat Embryonic Epidermis
We focused on three genes, i.e., Tgm2, Gbx1, and TGF-β, because we [21] and others [61,62] showed these genes are induced by RA. At first we examined the expression of two of them, Tgm2 and Gbx1, in rat embryonic skin. The expression of both Tgm2 and Gbx1 mRNAs is increased in the epidermis of the skin cultured for one day with ATRA and then for four days without RA [22]. The expression of both Tgm2 and Gbx1 proteins is also increased throughout the epidermis cultured for one day in the presence of ATRA and then for an additional four days in its absence. Tgm2 protein is detected in the cytoplasm, nuclei, and the area of cell adhesion in the intermediate and upper layers of the epidermis, reflecting the multifunctional nature of this protein [63,64,65,66]; whereas the expression of Gbx1 protein is seen mainly in the nuclei of the epidermal skin cells. Interestingly, in situ transamidase activity of Tgm2 is not detected in RA-treated Tgm2 protein [22], suggesting that the enzymatic activity might be dispensable and that other potential functions of Tgm2 [63] (discussed below) might be involved in transdifferentiation. In fact, RA-induced transdifferentiation is not inhibited by a transamidase inhibitor, ZM449829 [67], in the range of 100–300 nm (unpublished data).
To examine the function of Tgm2 and Gbx1 in the epidermis, we transfected the epidermis with these genes by electroporation. Epidermal keratinization and expression of K10, which is specifically expressed in the epidermis, are inhibited. In addition, rounded cells in the upper layer of the epidermis are observed in the skin by overexpression of both Tgm2 and Gbx1 [22]. However, epidermal keratinization is observed in the skin by overexpression of either Tgm2 or Gbx1 [22]. However, neither K4 nor MUC4 expression is observed in the skin overexpressing both Tgm2 and Gbx1 genes [22]. Thus these findings indicated that co-expression of Tgm2 and Gbx1 in the epidermis is required for ATRA-induced mucous transdifferentiation but that Tgm2 or Gbx1 alone cannot induce esophagus-like mucosal transdifferentiation. RA is a consistent inducer of Tgm2 expression in various cells and tissues [68,69,70]. In mammalian epidermal cells, 3 μM RA induces tissue transglutaminase (Tgm2) [71]. Tgm2 knock-out mice do not have any defects in their keratinocyte differentiation program [72]. Tgm2 is the most diverse and ubiquitous enzyme of the transglutaminase family [73] and has been implicated in diverse processes such as inflammation, wound healing, apoptosis, neurodegenerative disorders, and cancer [63,74,75]. The most characteristic enzyme function of the transglutaminase family is calcium-dependent transamidation activity, resulting in the formation of ε-(g-glutamyl) lysine cross-links between proteins and, thus, polymerization. Tgm1, Tgm3, and Tgm5 of the transglutaminase family are found in mammalian keratinocytes and play an important role in the formation of the stratum corneum in the skin by the introduction of cross-links between proteins [76,77,78]. In addition to its transamidation activity, Tgm2 functions as a signal-transducing GTP-binding protein [64] and as a protein-disulfide isomerase that regulates adenylate cyclase [66].
Hence, Gbx1 may be a key regulator of epithelial differentiation in addition to its known role in brain development. We showed immunohistochemically that the expression of Gbx1 protein starts to increase in the nuclei of all cells of the epidermis after one day in culture. The expression of Gbx1 mRNA in the epidermis is up-regulated not only after 8 h of treatment with RA, but also by the physical interaction of untreated skin with the RA-pretreated dermal fibroblasts in which these fibroblasts actively migrate into the dermis, suggesting that Gbx1 expression would appear to be regulated by some unidentified factor in the dermis (Figure 2) [21,22].
7. Involvement of TGF-β Signaling Pathway in ATRA-Induced Epidermal Transdifferentiation to Mucous Epithelium in Rat Embryonic Skin
RA added to cultures of human scalp hair follicles increases the expression of TGF-β in the dermal sheath, dermal papilla, and hair follicle [62]. In addition to Tgm2 and Gbx1, ATRA also increases the expression of both TGF-β mRNA and protein in the dermis of rat embryonic skin, but does so only slightly in the epidermis [22]. As ATRA increases the expression of TGF-β in the dermis, this finding suggests that the interaction between epidermis and dermis might have an important role in epidermal transdifferentiation to a mucous epithelium. Furthermore, SB431542, a specific inhibitor of ser/thr kinase of the TGF-β type II receptor [79], and pan-TGF-β1, -β2, -β3 mAb, a neutralizing antibody, partially suppress the expression of K4 and MUC4, indicating that the TGF-β signaling pathway is involved in ATRA-induced epidermal transdifferentiation to a mucous epithelium (Figure 2).
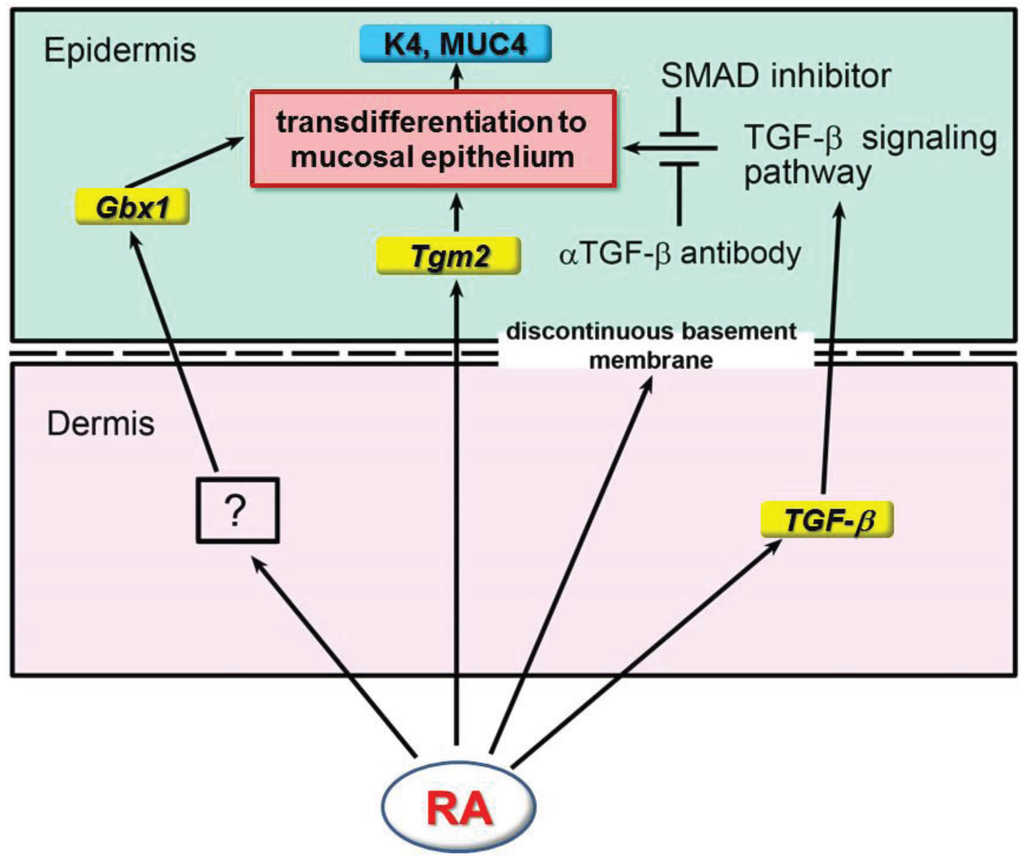
Figure 2.
Schematic diagram summarizing the effect of RA on epidermal mucosal transdifferentiation. RA up-regulates the expression of Tgm2 and TGF-β in the epidermis and dermis, respectively, after 8–24 h of treatment, which is followed by the appearance of a discontinuous basement membrane. On the other hand, Gbx1 expression is up-regulated in the epidermis after 8 h by an unknown factor, which is induced in the dermis by RA [21]. The TGF-β signaling pathway activated by TGF-β and acting together with Gbx1 induces epidermal transdifferentiation to an esophagus-like mucosal epithelium [22] (reproduced with permission from Int. J. Dev. Biol. 2011, 55, 933–943).
Figure 2.
Schematic diagram summarizing the effect of RA on epidermal mucosal transdifferentiation. RA up-regulates the expression of Tgm2 and TGF-β in the epidermis and dermis, respectively, after 8–24 h of treatment, which is followed by the appearance of a discontinuous basement membrane. On the other hand, Gbx1 expression is up-regulated in the epidermis after 8 h by an unknown factor, which is induced in the dermis by RA [21]. The TGF-β signaling pathway activated by TGF-β and acting together with Gbx1 induces epidermal transdifferentiation to an esophagus-like mucosal epithelium [22] (reproduced with permission from Int. J. Dev. Biol. 2011, 55, 933–943).
8. Development of Skin in Retinoid Signaling Deficient Mice
RA is commonly used in the treatment of skin diseases such as acne and psoriasis, as well as in chemotherapy for leukemia. A frequent adverse effect of these therapies is RA-induced hair loss [62,80]. The mechanisms to explain how excess RA arrests hair follicle growth have been explored [27,62]. ATRA induces a catagen-like stage in human hair follicles, presumably via up-regulation of TGF-β2 in the dermal papilla, resulting in hair loss [62]. In a mouse model which knocked out cytochrome P450, family 26, subfamily b, polypeptide 1 (Cyp26b1), which encodes an RA-degrading enzyme, the in vivo administration of RA to pregnant mice or its addition to skin cultures demonstrated that appropriate RA levels are important for periderm desquamation, embryonic skin differentiation, and barrier formation in the developing mammalian skin [26]. Furthermore knock-out mouse lacking Cyp26b1 in their skin exhibit major hair follicle development defects [27]. By acting on the Wnt/β-catenin pathway and on members of the Runx, Fox, and Sox transcription factor families, RA modulates pathways and factors implicated in hair follicle downgrowth and bending [27]. In the human and mouse cicatricial alopecia and alopecia areata, retinoid metabolism is altered and genes involved in RA synthesis increases, while RA degradation genes are decreased [81,82]. Appropriate RA metabolism is needed for the normal formation of the hair follicle [6,27,81,82].
Retinoic acid receptors (RARα, β, γ) and retinoic X receptors (RXRα, β, γ) are expressed in the skin and play an important role in the development of epidermis [2,3,16,25,83,84,85,86]. In epidermis of skin, RARα, RARγ, RXRα, RXRβ, and RXRγ are highly expressed, while expression of RARβ is weak or absent [84]. In dermis of skin, RARβ, RXRα, RXRβ, and RXRγ are weakly expressed, while RARα and RARγ are moderately expressed [84]. In the mouse lacking RXRα selectively in adult keratinocytes, hair follicle degeneration, marked hair loss, and hyperplastic interfollicular epidermis are observed [3,85]. It was shown that RXRα has key roles in hair development and in epidermal keratinocyte proliferation and differentiation [3,84]. The knock-out of the RARα, RARβ, or RARγ genes does not prevent RA-induced mouse upper-lip skin glandular metaplasia, but RARγ knock-out dramatically decreases its ratio [87]. Both RARα and RARγ can initiate a glandular metaplasia, whereas RARβ cannot give rise to any metaplasia, but the following progression of metaplasia requires RARβ, indicating that these receptors have both redundant and unique functions [87].
Neutral lipid synthesis enzyme acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) functions as the major acyl-CoA:retinol acyltransferase (ARAT) in murine skin. When dietary retinol is abundant, DGAT1 deficiency results in elevated levels of RA in skin and cyclical hair loss [88]. Deletion of DGAT1 specifically in the epidermis causes alopecia. DGAT1 functions as an ARAT in the skin, where it acts to maintain retinoid homeostasis and modulate RA signaling [88].
9. Concluding Remarks
RA plays important roles in skin development by affecting signaling pathways and the expression of many genes including homeobox genes. RA induces epidermal transdifferentiation in chick and rodent embryonic skin. We identified Gbx-1, Tgm2 and TGF-β as the key target genes regulated by ATRA during transdifferentiation. Thus far we don’t have any information on which isoforms of RARs and RXRs are directly involved in the ATRA-induced transdifferentiation, and to what extent are 9-cis retinoic acid and non-genomic signals, i.e., posttranslational modification, important to the transdifferentiation. These issues remain to be explored. An embryonic skin culture system where RA induces epidermal transdifferentiation provides a useful model in which RA can direct cell reprogramming to another cell. As the generation of these transdifferentiated cells is fast, efficient, and devoid of tumorigenic pluripotent stem cells, these cells can provide a novel system for regenerative medicine.
Further study is required to elucidate the role of RA in the skin and skin appendage development by identifying additional developmental signaling pathways and transcriptional regulatory proteins regulated by RA.
Acknowledgments
This work was supported, in part, by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Japan (O.A., Y.A.), and from the Kazato Research Foundation (Y.A.). We thank Hayato Kawakami, Akihiko Kudo and Masuo Obinata for valuable discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sengel, P. Morphogenesis of Skin; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Fisher, G.J.; Voorhees, J.J. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996, 10, 1002–1013. [Google Scholar]
- Li, M.; Indra, A.K.; Warot, X.; Brocard, J.; Messaddeq, N.; Kato, S.; Metzger, D.; Chambon, P. Skin abnormalities generated by temporally controlled RXRα mutations in mouse epidermis. Nature 2000, 407, 633–636. [Google Scholar] [CrossRef]
- Chapellier, B.; Mark, M.; Messaddeq, N.; Calléja, C.; Warot, X.; Brocard, J.; Gérard, C.; Li, M.; Metzger, D.; Ghyselinck, N.B.; et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002, 21, 3402–3413. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Chapellier, B.; Calléja, C.; Indra, A.K.; Li, M.; Messaddeq, N.; Mark, M.; Metzger, D.; Chambon, P. Genetic dissection of retinoic acid function in epidermis physiology. Ann. Dermatol. Venereol. 2002, 129, 793–799. [Google Scholar]
- García-Fernández, R.A.; Pérez-Martínez, C.; Escudero-Diez, A.; García-Iglesias, M.J. Effects of in utero retinoic acid exposure on mouse pelage hair follicle development. Vet. Dermatol. 2002, 13, 157–163. [Google Scholar] [CrossRef]
- García-Fernández, R.A.; Pérez-Martínez, C.; Alvarez, J.E.; Navarrete, A.J.; García-Iglesias, M.J. Mouse epidermal development: Effects of retinoic acid exposure in utero. Vet. Dermatol. 2006, 17, 36–44. [Google Scholar] [CrossRef]
- Asselineau, D.; Bernard, B.A.; Bailly, C.; Darmon, M. Retinoic acid improves epidermal morphogenesis. Dev. Biol. 1989, 133, 322–335. [Google Scholar] [CrossRef]
- Mori, S. The changes in the para-ocular glands which follow the administration of diets low in fat-soluble vitamin A; with notes on the effect of the same diets on the salivary glands and the mucosa of the larynx and trachea. Bull. Johns Hopkins Hosp. 1922, 33, 357–359. [Google Scholar]
- Wolbach, S.B.; Howe, P.R. Tissue changes following deprivation of fat-soluble vitamin A. J. Exp. Med. 1925, 42, 753–777. [Google Scholar] [CrossRef]
- Fell, H.B.; Mellanby, E. Metaplasia produced in cultures of chick ectoderm by high vitamin A. J. Physiol. 1953, 119, 470–488. [Google Scholar]
- Viallet, J.P.; Ruberte, E.; du Manoir, S.; Krust, A.; Zelent, A.; Dhouailly, D. Retinoic acid-induced glandular metaplasia in mouse skin is linked to the dermal expression of retinoic acid receptor beta mRNA. Dev. Biol. 1991, 144, 424–428. [Google Scholar] [CrossRef]
- Viallet, J.P.; Dhouailly, D. Retinoic acid and mouse skin morphogenesis. II. Role of epidermal competence in hair glandular metaplasia. Dev. Biol. 1994, 166, 277–288. [Google Scholar] [CrossRef]
- Chuong, C.-M.; Ting, S.A.; Widelitz, R.B.; Lee, Y.-S. Mechanism of skin morphogenesis. II. Retinoic acid modulates axis orientation and phenotypes of skin appendages. Development 1992, 115, 839–852. [Google Scholar]
- Dhouailly, D. Genetic expression and morphogenesis of the skin in vertebrates. Ann. Genet. 1993, 36, 47–55. [Google Scholar]
- Gudas, L.J. Retinoids and vertebrate development. J. Biol. Chem. 1994, 269, 15399–15402. [Google Scholar]
- Cardoso, W.V.; Mitsialis, S.A.; Brody, J.S.; Williams, M.C. Retinoic acid alters the expression of pattern-related genes in the developing rat lung. Dev. Dyn. 1996, 207, 47–59. [Google Scholar] [CrossRef]
- Kanzler, B.; Prin, F.; Thelu, J.; Dhouailly, D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev. Dyn. 1997, 210, 274–287. [Google Scholar] [CrossRef]
- Noveen, A.; Jiang, T.X.; Ting-Berreth, S.A.; Chuong, C.M. Homeobox genes Msx-1 and Msx-2 are associated with induction and growth of skin appendages. J. Invest. Dermatol. 1995, 104, 711–719. [Google Scholar]
- Lin, C.M.; Jiang, T.X.; Widelitz, R.B.; Chuong, C.M. Molecular signaling in feather morphogenesis. Curr. Opin. Cell Biol. 2006, 18, 730–741. [Google Scholar] [CrossRef]
- Obinata, A.; Akimoto, Y.; Omoto, Y.; Hirano, H. Increase in expression of the homeobox gene, Gbx1, in retinol-induced epidermal mucous metaplasia. Biochem. Biophys. Res. Commun. 2001, 280, 1055–1061. [Google Scholar] [CrossRef]
- Obinata, A.; Osakabe, K.; Yamaguchi, M.; Morimoto, R.; Akimoto, Y. Tgm2/Gh, Gbx1 and TGF-β are involved in retinoic acid-induced transdifferentiation from epidermis to mucosal epithelium. Int. J. Dev. Biol. 2011, 55, 933–943. [Google Scholar] [CrossRef]
- Imakado, S.; Bickenbach, J.R.; Bundman, D.S.; Rothnagel, J.A.; Attar, P.S.; Wang, X.J.; Walczak, V.R.; Wisniewski, S.; Pote, J.; Gordon, J.S.; Heyman, R.A.; et al. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995, 9, 317–329. [Google Scholar] [CrossRef]
- Attar, P.S.; Wertz, P.W.; McArthur, M.; Imakado, S.; Bickenbach, J.R.; Roop, D.R. Inhibition of retinoid signaling in transgenic mice alters lipid processing and disrupts epidermal barrier function. Mol. Endocrinol. 1997, 11, 792–800. [Google Scholar] [CrossRef]
- Calléja, C.; Messaddeq, N.; Chapellier, B.; Yang, H.; Krezel, W.; Li, M.; Metzger, D.; Mascrez, B.; Ohta, K.; Kagechika, H.; et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006, 20, 1525–1538. [Google Scholar] [CrossRef]
- Okano, J.; Lichti, U.; Mamiya, S.; Aronova, M.; Zhang, G.; Yuspa, S.H.; Hamada, H.; Skai, Y,; Morasso, M.I. Increased retinoic acid levels through ablation of Cyp26b1 determine the processes of embryonic skin barrier formation and peridermal development. J. Cell Sci. 2012, 125, 1827–1836. [Google Scholar] [CrossRef]
- Okano, J.; Levy, C.; Lichti, U.; Sun, H.W.; Yuspa, S.H.; Sakai, Y.; Morasso, M.I. Cutaneous retinoic acid levels determine hair follicle development and downgrowth. J. Biol. Chem. 2012, 287, 39304–39315. [Google Scholar]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Dhouailly, D.; Hardy, M.H.; Sengel, P. Formation of feathers on chick foot scales: A stage-dependent morphogenetic response to retinoic acid. J. Embryol. Exp. Morphol. 1980, 58, 63–78. [Google Scholar]
- Cadi, R.; Dhouailly, D.; Sengel, P. Use of retinoic acid for the analysis of dermal-epidermal interactions in the tarsometatarsal skin of the chick embryo. Dev. Biol. 1983, 100, 489–495. [Google Scholar] [CrossRef]
- Prin, F.; Dhouailly, D. How and when the regional competence of chick epidermis is established: feathers vs. scutate and reticulate scales, a problem en route to a solution. Int. J. Dev. Biol. 2004, 48, 137–148. [Google Scholar] [CrossRef]
- Obinata, A.; Kawada, M.; Endo, H. Induction of epidermal mucous metaplasia by culture of recombinants of undifferentiated epidermis and retinol-treated dermis in a chemically defined medium. Dev. Biol. 1987, 123, 59–62. [Google Scholar] [CrossRef]
- Obinata, A.; Akimoto, Y.; Hirano, H.; Endo, H. Stimulation by Bt2cAMP of epidermal mucous metaplasia in retinol-pretreated chick embryonic cultured skin, and its inhibition by herbimycin A, an inhibitor for protein tyrosine kinase. Exp. Cell Res. 1991, 193, 36–44. [Google Scholar] [CrossRef]
- Obinata, A.; Akimoto, Y.; Hirano, H.; Endo, H. Short-term retinol treatment in vitro induces stable transdifferentiation of chick epidermal cells into mucus-secreting cells. Roux’s Arch. Dev. Biol. 1991, 200, 289–295. [Google Scholar] [CrossRef]
- Obinata, A.; Akimoto, Y.; Kawamata, T.; Hirano, H. Induction of mucous metaplasia in chick embryonic skin by retinol-pretreated embryonic chick or quail dermal fibroblasts through cell-cell interaction: Correlation of a transient increase in retinoic acid receptor β mRNA in retinol-treated dermal fibroblasts with their competence to induce epidermal mucous metaplasia. Dev. Growth Differ. 1994, 36, 579–587. [Google Scholar]
- Obinata, A.; Akimoto, Y. Effects of retinoic acid and Gbx1 on feather-bud formation and epidermal transdifferentiation in chick embryonic cultured dorsal skin. Dev. Dyn. 2012, 241, 1405–1412. [Google Scholar]
- Gehring, W.J.; Affolter, M.; Bürglin, T. Homeodomain proteins. Annu. Rev. Biochem. 1994, 63, 487–526. [Google Scholar] [CrossRef]
- Obinata, A.; Akimoto, Y.; Omoto, Y.; Hirano, H. Expression of Hex homeobox gene during skin development: Increase in epidermal cell proliferation by transfecting the Hex to the dermis. Dev. Growth Differ. 2002, 44, 281–292. [Google Scholar]
- Obinata, A.; Akimoto, Y. Expression of Hex during feather bud development. Int. J. Dev. Biol. 2005, 49, 885–890. [Google Scholar] [CrossRef]
- Obinata, A.; Akimoto, Y. Involvement of Hex in the initiation of feather morphogenesis. Int. J. Dev. Biol. 2005, 49, 953–960. [Google Scholar] [CrossRef]
- Kosaka, Y.; Akimoto, Y.; Omoto, Y.; Obinata, A.; Hirano, H. Expression of the HB9 homeobox gene concomitant with proliferation accompanying epidermal stratification during development of chick embryonic tarsometatarsal skin. Histochem. J. 2000, 32, 275–280. [Google Scholar] [CrossRef]
- Kosaka, Y.; Akimoto, Y.; Obinata, A.; Hirano, H. Localization of HB9 homeobox gene mRNA and protein during the early stages of chick feather development. Biochem. Biophys. Res. Commun. 2000, 276, 1112–1117. [Google Scholar] [CrossRef]
- Niss, K.; Leutz, A. Expression of the homeobox gene GBX2 during chicken development. Mech. Dev. 1998, 76, 151–155. [Google Scholar] [CrossRef]
- Reid, A.I.; Gaunt, S.J. Colinearity and non-colinearity in the expression of Hox genes in developing chick skin. Int. J. Dev. Biol. 2002, 46, 209–215. [Google Scholar]
- Rouzankina, I.; Abate-Shen, C.; Niswander, L. Dlx genes integrate positive and negative signals during feather bud development. Dev. Biol. 2004, 265, 219–233. [Google Scholar]
- Asbreuk, C.H.J.; van Schaick, H.S.A.; Cox, J.J.; Kromkamp, M.; Smidt, M.P.; Burbach, J.P.H. The homeobox genes Lhx7 and Gbx1 are expressed in the basal forebrain cholinergic system. Neuroscience 2002, 109, 287–298. [Google Scholar] [CrossRef]
- Waters, S.T.; Wilson, C.P.; Lewandoski, M. Cloning and embryonic expression analysis of the mouse Gbx1 gene. Gene Expr. Patterns 2003, 3, 313–317. [Google Scholar] [CrossRef]
- Rhinn, M.; Lun, K.; Werner, M.; Simeone, A.; Brand, M. Isolation and expression of the homeobox gene Gbx1 during mouse development. Dev. Dyn. 2004, 229, 334–339. [Google Scholar] [CrossRef]
- Widelitz, R.B.; Jiang, T.X.; Noveen, A.; Chen, C.W.; Chuong, C.M. FGF induces new feather buds from developing avian skin. J. Invest. Dermatol. 1996, 107, 797–803. [Google Scholar]
- Noramly, S.; Morgan, B.A. BMPs mediate lateral inhibition at successive stages in feather tract development. Development 1998, 125, 3775–3787. [Google Scholar]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 1995, 172, 126–138. [Google Scholar] [CrossRef]
- Noveen, A.; Jiang, T.X.; Chuong, C.M. cAMP, an activator of protein kinase A, suppresses the expression of sonic hedgehog. Biochem. Biophys. Res. Commun. 1996, 219, 180–185. [Google Scholar] [CrossRef]
- Ting-Berreth, S.A.; Chuong, C.M. Sonic Hedgehog in feather morphogenesis: Induction of mesenchymal condensation and association with cell death. Dev. Dyn. 1996, 207, 157–170. [Google Scholar] [CrossRef]
- Widelitz, R.B.; Jiang, T.X.; Chen, C.W.; Stott, N.S.; Jung, H.S.; Chuong, C.M. Wnt-7a in feather morphogenesis: Involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model. Development 1999, 126, 2577–2587. [Google Scholar]
- Chen, C.W.; Jung, H.S.; Jiang, T.X.; Chuong, C.M. Asymmetric expression of Notch/Delta/Serrate is associated with the anterior-posterior axis of feather buds. Dev. Biol. 1997, 188, 181–187. [Google Scholar] [CrossRef]
- Noramly, S.; Freeman, A.; Morgan, B.A. β-catenin signaling can initiate feather bud development. Development 1999, 126, 3509–3521. [Google Scholar]
- Takata, K.; Obinata, A.; Endo, H.; Hirano, H. Induction of the α-type keratinization by hydrocortisone in embryonic chick skins grown in a chemically defined medium. An electron microscopic study. Dev. Biol. 1981, 85, 370–379. [Google Scholar] [CrossRef]
- Ness, S.L.; Edelmann, W.; Jenkins, T.D.; Liedtke, W.; Rustgi, A.K.; Kucherlapati, R. Mouse keratin 4 is necessary for internal epithelial integrity. J. Biol. Chem. 1998, 273, 23904–23911. [Google Scholar] [CrossRef]
- Guillem, P.; Billeret, V.; Buisine, M.-P.; Flejou, J.-F.; Lecomte-Houcke, M.; Degand, P.; Aubert, J.-P.; Triboulet, J.-P.; Porchet, N. Mucin gene expression and cell differentiation in human normal, premalignant and malignant esophagus. Int. J. Cancer 2000, 88, 856–861. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Lichti, U.; Yuspa, S.H. Inhibition of epidermal terminal differentiation and tumour promotion by retinoids. Ciba Found. Symp. 1985, 113, 77–89. [Google Scholar]
- Foitzik, K.; Spexard, T.; Nakamura, M.; Halsner, U.; Paus, R. Towards dissecting the pathogenesis of retinoid-induced hair loss: All-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-β2 in the dermal papilla. J. Invest. Dermatol. 2005, 124, 1119–1126. [Google Scholar]
- Fesus, L.; Piacentini, M. Transglutaminase 2: An enigmatic enzyme with diverse functions. Trends Biochem. Sci. 2002, 27, 534–539. [Google Scholar] [CrossRef]
- Nakaoka, H.; Perez, D.M.; Baek, K.J.; Das, T.; Husain, A.; Misono, K.; Im, M.-J.; Graham, R.M. Gh: A GTP-binding protein with transglutaminase activity and receptor signaling function. Science 1994, 264, 1593–1596. [Google Scholar]
- Mishra, S.; Murphy, L.J. Tissue transglutaminase has intrinsic kinase activity: Identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J. Biol. Chem. 2004, 279, 23863–23868. [Google Scholar] [CrossRef]
- Tucholski, J.; Johnson, G.V.W. Tissue transglutaminase directly regulates adenylyl cyclase resulting in enhanced cAMP-response element-binding protein (CREB) activation. J. Biol. Chem. 2003, 278, 26838–26843. [Google Scholar] [CrossRef]
- Lai, T.S.; Liu, Y.; Tucker, T.; Daniel, K.R.; Sane, D.C.; Toone, E.; Burke, J.R.; Strittmatter, W.J.; Greenberg, C.S. Identification of chemical inhibitors to human tissue transglutaminase by screening existing drug libraries. Chem. Biol. 2008, 15, 969–978. [Google Scholar] [CrossRef]
- Singh, U.S.; Pan, J.; Kao, Y.-L.; Joshi, S.; Young, K.L.; Baker, K.M. Tissue transglutaminase mediates activation of RhoA and MAP kinase pathways during retinoic acid-induced differentiation of SH-SY5Y cells. J. Biol. Chem. 2003, 278, 391–399. [Google Scholar]
- Ou, H.; Haendeler, J.; Aebly, M.R.; Kelly, L.A.; Cholewa, B.C.; Koike, J.; Kwitek-Black, A.; Jacob, H.J.; Berk, B.C.; Miano, J.M. Retinoic acid-induced tissue transglutaminase and apoptosis in vascular smooth muscle cells. Circ. Res. 2000, 87, 881–887. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Singh, U.S.; Lee, D.A.; Boehm, J.E.; Combs, C.; Zgola, M.M.; Page, R.L.; Cerione, R.A. Effects of tissue transglutaminase on retinoic acid-induced cellular differentiation and protection against apoptosis. J. Biol. Chem. 2001, 276, 33582–33587. [Google Scholar]
- Lichti, U.; Ben, T.; Yuspa, S.H. Retinoic acid-induced transglutaminase in mouse epidermal cells is distinct from epidermal transglutaminase. J. Biol. Chem. 1985, 260, 1422–1426. [Google Scholar]
- Nanda, N.; Iismaa, S.E.; Owens, W.A.; Husain, A.; Mackay, F.; Graham, R.M. Targeted inactivation of Gh/tissue transglutaminase II. J. Biol. Chem. 2001, 276, 20673–20678. [Google Scholar]
- Lorand, L.; Graham, R.M. Transglutaminases crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003, 4, 140–156. [Google Scholar] [CrossRef]
- Dalby, K.N.; Takedereli, I.; Lopez-Berestein, G.; Ozpolat, B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy 2010, 6, 322–329. [Google Scholar] [CrossRef]
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)—A wound response enzyme. Front. Biosci. 2006, 11, 867–882. [Google Scholar] [CrossRef]
- Steinert, P.M.; Kim, S.Y.; Chung, S.I.; Marecov, L.N. The transglutaminase 1 enzyme is variably acylated by myristate and palmitate during differentiation in epidermal keratinocytes. J. Biol. Chem. 1996, 271, 26242–26250. [Google Scholar]
- Zhang, J.; Zhi, H.Y.; Ding, F.; Luo, A.P.; Liu, Z.H. Transglutaminase3 expression in C57BL/6J mouse embryo epidermis and the correlation with its differentiation. Cell Res. 2005, 15, 105–110. [Google Scholar] [CrossRef]
- Candi, E.; Oddi, S.; Paradisi, A.; Terrinoni, A.; Ranalli, M.; Teofoli, P.; Citro, G.; Scarpato, S.; Puddu, P.; Melino, G. Expression of transglutaminase 5 in normal and pathologic human epidermis. J. Invest. Dermatol. 2002, 119, 670–677. [Google Scholar] [CrossRef]
- Inman, G.J.; Nicolás, F.J.; Callahan, J.F.; Harling, J.D.; Gaster, L.M.; Reith, A.D.; Laping, N.J.; Hill, C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002, 62, 65–74. [Google Scholar] [CrossRef]
- Everts, H.B. Endogenous retinoids in the hair follicle and sebaceous gland. Biochim. Biophys. Acta 2012, 1821, 222–229. [Google Scholar]
- Everts, H.B.; Silva, K.A.; Montgomery, S.; Suo, L.; Menser, M.; Valet, A.S.; King, L.E.; Ong, D.E.; Sundberg, J.P. Retinoid metabolism is altered in human and mouse cicatricial alopecia. J. Invest. Dermatol. 2013, 133, 325–333. [Google Scholar] [CrossRef]
- Duncan, F.J.; Silva, K.A.; Johnson, C.J.; King, B.L.; Szatkiewicz, J.P.; Kamdar, S.P.; Ong, D.E.; Napoli, J.L.; Wang, J.; King, L.E., Jr.; et al. Endogenous retinoids in the pathogenesis of alopecia areata. J. Invest. Dermatol. 2013, 133, 334–343. [Google Scholar] [CrossRef]
- Saitou, M.; Sugai, S.; Tanaka, T.; Shimouchi, K.; Fuchs, E.; Narumiya, S.; Kakizuka, A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature 1995, 374, 159–162. [Google Scholar] [CrossRef]
- Reichrath, J.; Mittmann, M.; Kamradt, J.; Muller, S.M. Expression of retinoid-X receptors (-α, -β, -γ) and retinoic acid receptors (-α, -β, -γ) in normal human skin: An immunohistological evaluation. Histochem. J. 1997, 29, 127–133. [Google Scholar] [CrossRef]
- Li, M.; Chiba, H.; Warot, X.; Messaddeq, N.; Gérard, C.; Chambon, P.; Metzger, D. RXRα ablation in skin keratinocytes results in alopecia and epidermal alterations. Development 2001, 128, 675–688. [Google Scholar]
- Chen, C.F.; Lohnes, D. Dominant-negative retinoic acid receptors elicit epidermal defects through a non-canonical pathway. J. Biol. Chem. 2005, 280, 3012–3021. [Google Scholar] [CrossRef]
- Blanchet, S.; Favier, B.; Chevalier, G.; Kastner, P.; Michaille, J.J.; Chambon, P.; Dhouailly, D. Both retinoic acid receptors α (RARα) and γ (RARγ) are able to initiate mouse upper-lip skin glandular metaplasia. J. Invest Dermatol. 1998, 111, 206–212. [Google Scholar] [CrossRef]
- Shih, M.Y.; Kane, M.A.; Zhou, P.; Yen, C.L.; Streeper, R.S.; Napoli, J.L.; Farese, R.V., Jr. Retinol esterification by DGAT1 is essential for retinoid homeostasis in murine skin. J. Biol. Chem. 2009, 284, 4292–4299. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
