Molecular Control of Interdigital Cell Death and Cell Differentiation by Retinoic Acid during Digit Development
Abstract
:1. Introduction
2. Digit Development
2.1. Cartilage Differentiation
3. Programmed Cell Death in the Limb Mesoderm
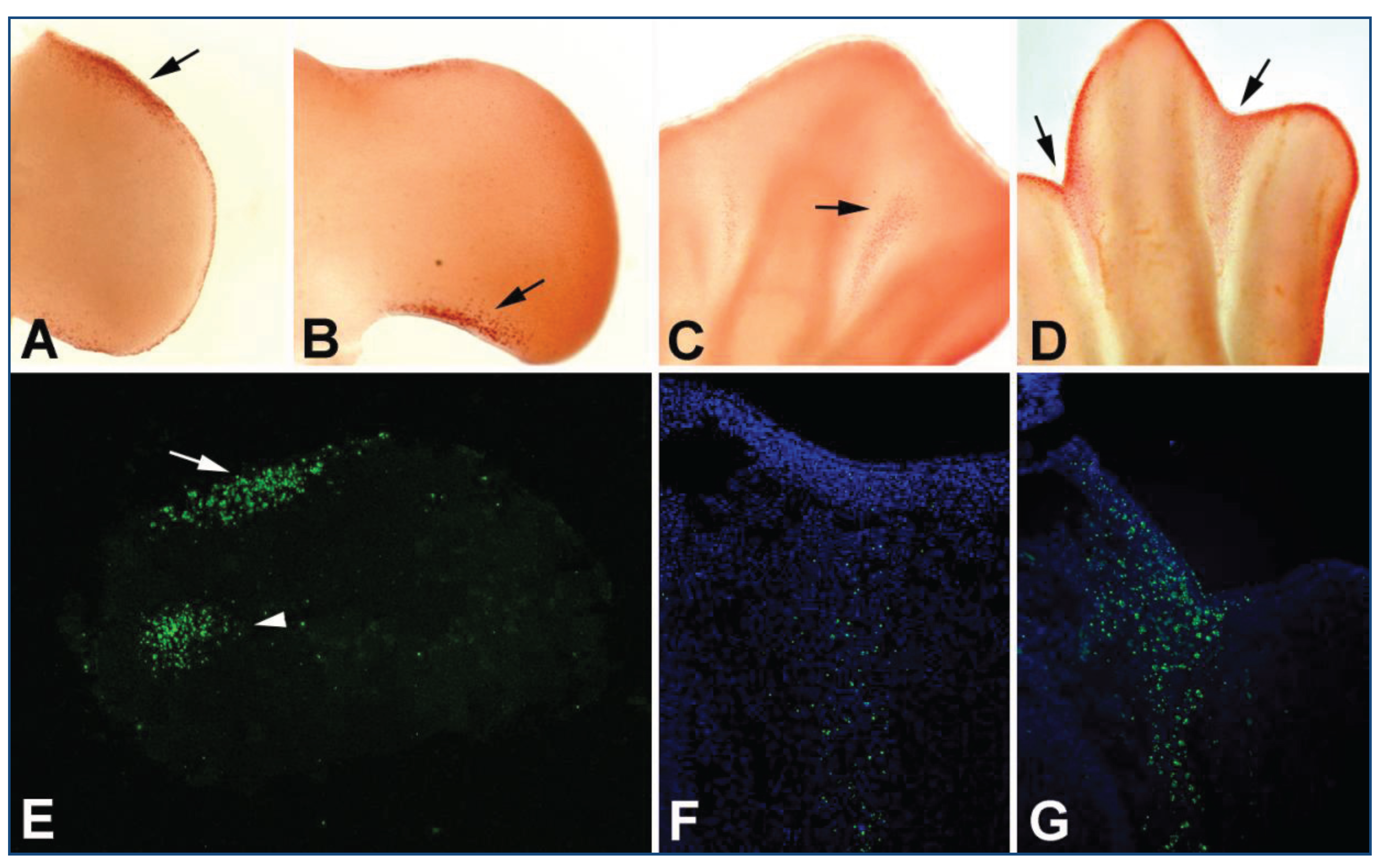
| Mutant /Treatment | Model | Phenotype | Reference | |
|---|---|---|---|---|
| Bax−/− Bak−/− | Mouse | Syndactyly | [32] | |
| Bim−/− Bid−/− Puma−/− | Mouse | Syndactyly | [32] | |
| Apaf−/− | Mouse | Normal apoptosis | [35] | |
| Z-VAD-FMK treatment | Chicken | Normal apoptosis | [40] | |
| Inhibition of cathepsin | Chicken | Normal apoptosis | [44] | |
| Inhibition of cathepsin and caspases | Chicken | Syndactyly | [44] |
| Mutant/Treatment | Model | Phenotype | Reference | |
|---|---|---|---|---|
| Adamts5−/− | Mouse | Syndactyly | [46] | |
| Versican treatment | Mouse | Rescues normal phenotype | [46] | |
| Fibrillin2−/− | Mouse | Syndactyly | [47] | |
| Nidogen1−/− | Mouse | Syndactyly | [48] | |
| Nidogen2−/− | Mouse | Syndactyly | [48] | |
| Fibulin+/− | Mouse | Synpolydactyly | [46,50] | |
| Reelin silencing | Chicken | Promotes cell death | [49,51] | |
| Dab-1 silencing | Chicken | Promotes cell death | [49] |
4. Retinoic Acid Signaling

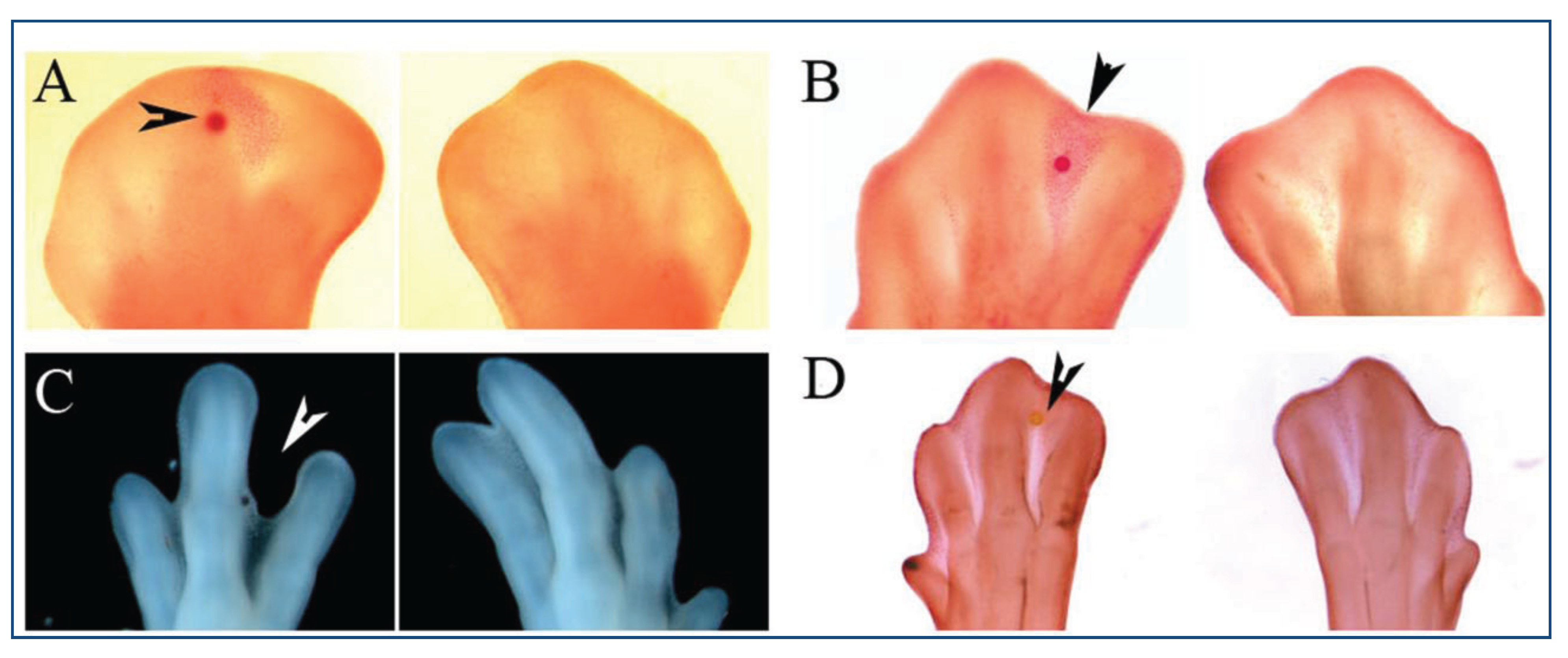
| Mutant/Treatment | Model | Phenotype | Reference | |
|---|---|---|---|---|
| Hammertoe mutant treated with RA | Mouse | Rescues normal phenotype | [52] | |
| Rdh10−/− | Mouse | Syndactyly | [49] | |
| Raldh2−/− | Mouse | Syndactyly | [54] | |
| Rarγ−/− Rxrβ−/− | Mouse | Syndactyly | [57,58] | |
| Rxrα+/− Rarα+/− | Mouse | Mild syndactyly | [57,58] | |
| Rxrα+/− Rarγ+/− | Mouse | Mild syndactyly | [57,58] | |
| Rxrα+/−Rarα+/− Rarγ+/− | Mouse | Severe syndactyly | [57,58] | |
| HoxD13−/− | Mouse | Synpolydactyly | [60] | |
| HoxD13−/− intrauterin treatment with RA | Mouse | Rescues normal phenotype | [60] | |
| pan-RAR antagonist | Chicken | Inhibition of interdigital cell death | [59] | |
| Interdigital treatment with RA | Mouse/Chicken | Promotes cell death | [29,59,70] | |
| Fused toes+/− | Mouse | Massive cell death | [71] |
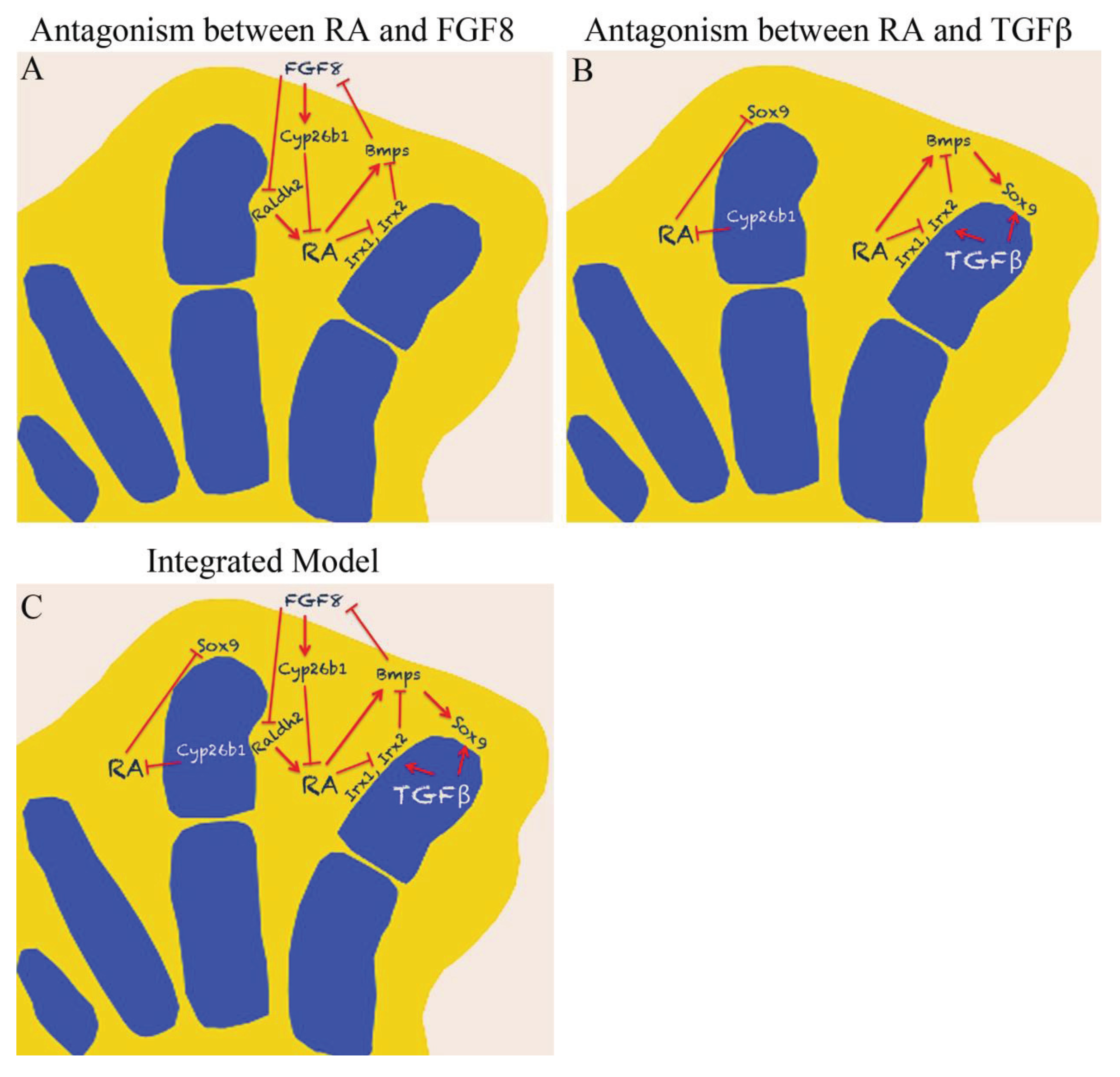
5. Antagonism between RA and FGF
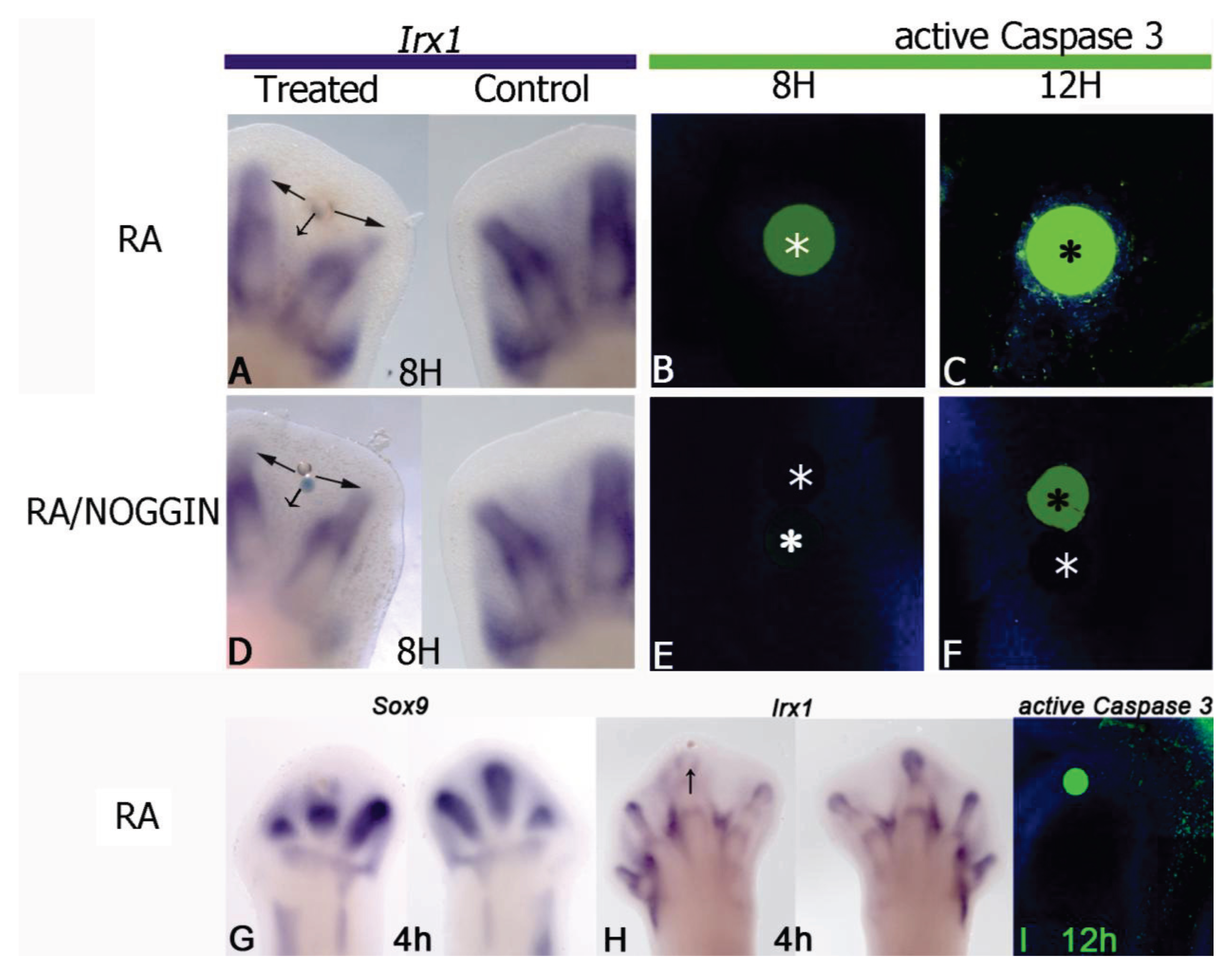
6. Antagonism between RA and TGFβ
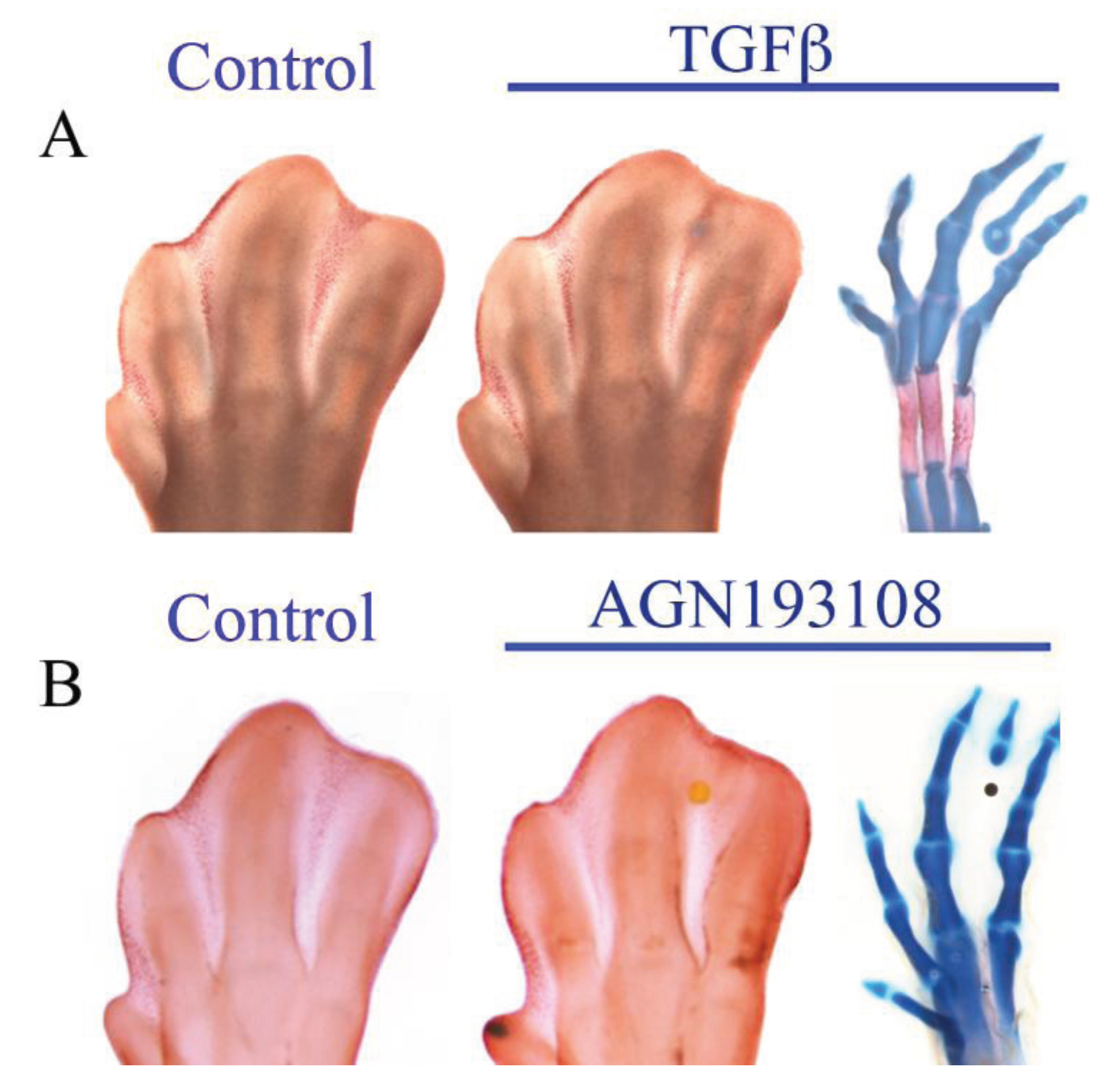
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Tabin, C.; Wolpert, L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007, 21, 1433–1442. [Google Scholar] [CrossRef]
- Fernandez-Teran, M.; Ros, M.A. The apical ectodermal ridge: Morphological aspects and signaling pathways. Int. J. Dev. Biol. 2008, 52, 857–871. [Google Scholar] [CrossRef] [Green Version]
- ten Berge, D.; Brugmann, S.A.; Helms, J.A.; Nusse, R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development 2008, 135, 3247–3257. [Google Scholar] [CrossRef]
- Mariani, F.V.; Ahn, C.P.; Martin, G.R. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 2008, 453, 401–405. [Google Scholar] [CrossRef]
- Montero, J.A.; Hurle, J.M. Deconstructing digit chondrogenesis. BioEssays 2007, 29, 725–737. [Google Scholar] [CrossRef]
- Montero, J.A.; Hurle, J.M. Sculpturing digit shape by cell death. Apoptosis 2010, 15, 365–375. [Google Scholar] [CrossRef]
- Montero, J.A.; Lorda-Diez, C.I.; Ganan, Y.; Macias, D.; Hurle, J.M. Activin/tgfbeta and bmp crosstalk determines digit chondrogenesis. Dev. Biol. 2008, 321, 343–356. [Google Scholar] [CrossRef]
- Towers, M.; Wolpert, L.; Tickle, C. Gradients of signalling in the developing limb. Curr. Opin. Cell Biol. 2012, 24, 181–187. [Google Scholar] [CrossRef]
- Towers, M.; Tickle, C. Growing models of vertebrate limb development. Development 2009, 136, 179–190. [Google Scholar] [CrossRef]
- Tickle, C. Making digit patterns in the vertebrate limb. Nat. Rev. Mol. Cell Biol. 2006, 7, 45–53. [Google Scholar] [CrossRef]
- Charite, J.; de Graaff, W.; Shen, S.; Deschamps, J. Ectopic expression of hoxb-8 causes duplication of the zpa in the forelimb and homeotic transformation of axial structures. Cell 1994, 78, 589–601. [Google Scholar] [CrossRef]
- Charite, J.; McFadden, D.G.; Olson, E.N. The bhlh transcription factor dhand controls sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development 2000, 127, 2461–2470. [Google Scholar]
- Noji, S.; Nohno, T.; Koyama, E.; Muto, K.; Ohyama, K.; Aoki, Y.; Tamura, K.; Ohsugi, K.; Ide, H.; Taniguchi, S.; et al. Retinoic acid induces polarizing activity but is unlikely to be a morphogen in the chick limb bud. Nature 1991, 350, 83–86. [Google Scholar] [CrossRef]
- Wanek, N.; Gardiner, D.M.; Muneoka, K.; Bryant, S.V. Conversion by retinoic acid of anterior cells into zpa cells in the chick wing bud. Nature 1991, 350, 81–83. [Google Scholar] [CrossRef]
- Riddle, R.D.; Johnson, R.L.; Laufer, E.; Tabin, C. Sonic hedgehog mediates the polarizing activity of the zpa. Cell 1993, 75, 1401–1416. [Google Scholar] [CrossRef]
- Wang, B.; Fallon, J.F.; Beachy, P.A. Hedgehog-regulated processing of gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 2000, 100, 423–434. [Google Scholar] [CrossRef]
- Litingtung, Y.; Dahn, R.D.; Li, Y.; Fallon, J.F.; Chiang, C. Shh and gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 2002, 418, 979–983. [Google Scholar] [CrossRef]
- te Welscher, P.; Zuniga, A.; Kuijper, S.; Drenth, T.; Goedemans, H.J.; Meijlink, F.; Zeller, R. Progression of vertebrate limb development through shh-mediated counteraction of gli3. Science 2002, 298, 827–830. [Google Scholar] [CrossRef]
- Suzuki, T.; Hasso, S.M.; Fallon, J.F. Unique smad1/5/8 activity at the phalanx-forming region determines digit identity. Proc. Natl. Acad. Sci. USA 2008, 105, 4185–4190. [Google Scholar] [CrossRef]
- Merino, R.; Ganan, Y.; Macias, D.; Economides, A.N.; Sampath, K.T.; Hurle, J.M. Morphogenesis of digits in the avian limb is controlled by fgfs, tgfbetas, and noggin through bmp signaling. Dev. Biol. 1998, 200, 35–45. [Google Scholar] [CrossRef]
- Ganan, Y.; Macias, D.; Duterque-Coquillaud, M.; Ros, M.A.; Hurle, J.M. Role of tgf beta s and bmps as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development 1996, 122, 2349–2357. [Google Scholar]
- Merino, R.; Macias, D.; Ganan, Y.; Rodriguez-Leon, J.; Economides, A.N.; Rodriguez-Esteban, C.; Izpisua-Belmonte, J.C.; Hurle, J.M. Control of digit formation by activin signalling. Development 1999, 126, 2161–2170. [Google Scholar]
- Chimal-Monroy, J.; Rodriguez-Leon, J.; Montero, J.A.; Ganan, Y.; Macias, D.; Merino, R.; Hurle, J.M. Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: Sox genes and bmp signaling. Dev. Biol. 2003, 257, 292–301. [Google Scholar] [CrossRef]
- Hernandez-Martinez, R.; Covarrubias, L. Interdigital cell death function and regulation: New insights on an old programmed cell death model. Dev. Growth Differ. 2011, 53, 245–258. [Google Scholar] [CrossRef]
- Zuzarte-Luis, V.; Hurle, J.M. Programmed cell death in the embryonic vertebrate limb. Semin. Cell Dev. Biol. 2005, 16, 261–269. [Google Scholar] [CrossRef]
- Dahn, R.D.; Fallon, J.F. Interdigital regulation of digit identity and homeotic transformation by modulated bmp signaling. Science 2000, 289, 438–441. [Google Scholar] [CrossRef]
- Ros, M.A.; Piedra, M.E.; Fallon, J.F.; Hurle, J.M. Morphogenetic potential of the chick leg interdigital mesoderm when diverted from the cell death program. Dev. Dynam. 1997, 208, 406–419. [Google Scholar] [CrossRef]
- Fernandez-Teran, M.A.; Hinchliffe, J.R.; Ros, M.A. Birth and death of cells in limb development: A mapping study. Dev. Dynam. 2006, 235, 2521–2537. [Google Scholar] [CrossRef]
- Diaz-Hernandez, M.E.; Bustamante, M.; Galvan-Hernandez, C.I.; Chimal-Monroy, J. Irx1 and irx2 are coordinately expressed and regulated by retinoic acid, tgfbeta and fgf signaling during chick hindlimb development. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Abarca-Buis, R.F.; Bustamante, M.; Cuervo, R.; Aguilar-Fernandez-de-Lara, D.; Chimal-Monroy, J. Smad8 is expressed in the anterior necrotic zone: Evidence for a role of bone morphogenetic proteins/smad signaling in the activation of a molecular cascade that culminates in cell death. Dev. Growth Differ. 2011, 53, 780–792. [Google Scholar]
- Orrenius, S.; Nicotera, P.; Zhivotovsky, B. Cell death mechanisms and their implications in toxicology. Toxicol. Sci. 2011, 119, 3–19. [Google Scholar] [CrossRef]
- Ren, D.; Tu, H.C.; Kim, H.; Wang, G.X.; Bean, G.R.; Takeuchi, O.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.; Cheng, E.H. Bid, bim, and puma are essential for activation of the bax- and bak-dependent cell death program. Science 2010, 330, 1390–1393. [Google Scholar] [CrossRef]
- Mace, P.D.; Shirley, S.; Day, C.L. Assembling the building blocks: Structure and function of inhibitor of apoptosis proteins. Cell Death Differ. 2010, 17, 46–53. [Google Scholar] [CrossRef]
- Hui, K.K.; Kanungo, A.K.; Elia, A.J.; Henderson, J.T. Caspase-3 deficiency reveals a physiologic role for smac/diablo in regulating programmed cell death. Cell Death Differ. 2011, 18, 1780–1790. [Google Scholar]
- Repnik, U.; Stoka, V.; Turk, V.; Turk, B. Lysosomes and lysosomal cathepsins in cell death. Biochim. Biophys. Acta 2012, 1824, 22–33. [Google Scholar]
- Lindsten, T.; Ross, A.J.; King, A.; Zong, W.X.; Rathmell, J.C.; Shiels, H.A.; Ulrich, E.; Waymire, K.G.; Mahar, P.; Frauwirth, K.; et al. The combined functions of proapoptotic bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 2000, 6, 1389–1399. [Google Scholar] [CrossRef]
- Yoshida, H.; Kong, Y.Y.; Yoshida, R.; Elia, A.J.; Hakem, A.; Hakem, R.; Penninger, J.M.; Mak, T.W. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 1998, 94, 739–750. [Google Scholar] [CrossRef]
- Cecconi, F.; Alvarez-Bolado, G.; Meyer, B.I.; Roth, K.A.; Gruss, P. Apaf1 (ced-4 homolog) regulates programmed cell death in mammalian development. Cell 1998, 94, 727–737. [Google Scholar] [CrossRef]
- Chautan, M.; Chazal, G.; Cecconi, F.; Gruss, P.; Golstein, P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 1999, 9, 967–970. [Google Scholar] [CrossRef]
- Zuzarte-Luis, V.; Berciano, M.T.; Lafarga, M.; Hurle, J.M. Caspase redundancy and release of mitochondrial apoptotic factors characterize interdigital apoptosis. Apoptosis 2006, 11, 701–715. [Google Scholar] [CrossRef]
- Huang, C.; Hales, B.F. Role of caspases in murine limb bud cell death induced by 4-hydroperoxycyclophosphamide, an activated analog of cyclophosphamide. Teratology 2002, 66, 288–299. [Google Scholar] [CrossRef]
- Milligan, C.E.; Prevette, D.; Yaginuma, H.; Homma, S.; Cardwell, C.; Fritz, L.C.; Tomaselli, K.J.; Oppenheim, R.W.; Schwartz, L.M. Peptide inhibitors of the ice protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron 1995, 15, 385–393. [Google Scholar] [CrossRef]
- Zuzarte-Luis, V.; Montero, J.A.; Torre-Perez, N.; Garcia-Porrero, J.A.; Hurle, J.M. Cathepsin d gene expression outlines the areas of physiological cell death during embryonic development. Dev. Dynam. 2007, 236, 880–885. [Google Scholar] [CrossRef]
- Zuzarte-Luis, V.; Montero, J.A.; Kawakami, Y.; Izpisua-Belmonte, J.C.; Hurle, J.M. Lysosomal cathepsins in embryonic programmed cell death. Dev. Biol. 2007, 301, 205–217. [Google Scholar] [CrossRef]
- Montero, J.A.; Lorda-Diez, C.I.; Certal, A.C.; Moreno, N.; Rodriguez-Leon, J.; Torriglia, A.; Hurle, J.M. Coordinated and sequential activation of neutral and acidic dnases during interdigital cell death in the embryonic limb. Apoptosis 2010, 15, 1197–1210. [Google Scholar] [CrossRef]
- McCulloch, D.R.; Nelson, C.M.; Dixon, L.J.; Silver, D.L.; Wylie, J.D.; Lindner, V.; Sasaki, T.; Cooley, M.A.; Argraves, W.S.; Apte, S.S. Adamts metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell 2009, 17, 687–698. [Google Scholar] [CrossRef]
- Arteaga-Solis, E.; Gayraud, B.; Lee, S.Y.; Shum, L.; Sakai, L.; Ramirez, F. Regulation of limb patterning by extracellular microfibrils. J. Cell Biol. 2001, 154, 275–281. [Google Scholar] [CrossRef]
- Bose, K.; Nischt, R.; Page, A.; Bader, B.L.; Paulsson, M.; Smyth, N. Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J. Biol. Chem. 2006, 281, 39620–39629. [Google Scholar] [CrossRef]
- Diaz-Mendoza, M.J.; Lorda-Diez, C.I.; Montero, J.A.; Garcia-Porrero, J.A.; Hurle, J.M. Interdigital cell death in the embryonic limb is associated with depletion of reelin in the extracellular matrix. Cell Death Dis. 2013, 4. [Google Scholar] [CrossRef]
- Debeer, P.; Schoenmakers, E.F.; Twal, W.O.; Argraves, W.S.; De Smet, L.; Fryns, J.P.; Van De Ven, W.J. The fibulin-1 gene (fbln1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J. Med. Genet. 2002, 39, 98–104. [Google Scholar] [CrossRef]
- Ohkubo, N.; Vitek, M.P.; Morishima, A.; Suzuki, Y.; Miki, T.; Maeda, N.; Mitsuda, N. Reelin signals survival through src-family kinases that inactivate bad activity. J. Neurochem. 2007, 103, 820–830. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef]
- Farjo, K.M.; Moiseyev, G.; Nikolaeva, O.; Sandell, L.L.; Trainor, P.A.; Ma, J.X. Rdh10 is the primary enzyme responsible for the first step of embryonic vitamin a metabolism and retinoic acid synthesis. Dev. Biol. 2011, 357, 347–355. [Google Scholar] [CrossRef]
- Niederreither, K.; Dolle, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553. [Google Scholar] [CrossRef]
- Fujii, H.; Sato, T.; Kaneko, S.; Gotoh, O.; Fujii-Kuriyama, Y.; Osawa, K.; Kato, S.; Hamada, H. Metabolic inactivation of retinoic acid by a novel p450 differentially expressed in developing mouse embryos. EMBO J. 1997, 16, 4163–4173. [Google Scholar] [CrossRef]
- Haque, M.; Anreola, F. The cloning and characterization of a novel cytochrome p450 family, cyp26, with specificity toward retinoic acid. Nutr. Rev. 1998, 56, 84–85. [Google Scholar] [CrossRef]
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dolle, P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 2002, 129, 3563–3574. [Google Scholar]
- Cunningham, T.J.; Chatzi, C.; Sandell, L.L.; Trainor, P.A.; Duester, G. Rdh10 mutants deficient in limb field retinoic acid signaling exhibit normal limb patterning but display interdigital webbing. Dev. Dynam. 2011, 240, 1142–1150. [Google Scholar] [CrossRef]
- Sandell, L.L.; Sanderson, B.W.; Moiseyev, G.; Johnson, T.; Mushegian, A.; Young, K.; Rey, J.P.; Ma, J.X.; Staehling-Hampton, K.; Trainor, P.A. Rdh10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007, 21, 1113–1124. [Google Scholar] [CrossRef]
- Ahuja, H.S.; James, W.; Zakeri, Z. Rescue of the limb deformity in hammertoe mutant mice by retinoic acid-induced cell death. Dev. Dynam. 1997, 208, 466–481. [Google Scholar] [CrossRef]
- Zakeri, Z.; Quaglino, D.; Ahuja, H.S. Apoptotic cell death in the mouse limb and its suppression in the hammertoe mutant. Dev. Biol. 1994, 165, 294–297. [Google Scholar] [CrossRef]
- Zhao, X.; Brade, T.; Cunningham, T.J.; Duester, G. Retinoic acid controls expression of tissue remodeling genes hmgn1 and fgf18 at the digit-interdigit junction. Dev. Dynam. 2010, 239, 665–671. [Google Scholar] [CrossRef]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef]
- Campo-Paysaa, F.; Marletaz, F.; Laudet, V.; Schubert, M. Retinoic acid signaling in development: Tissue-specific functions and evolutionary origins. Genesis 2008, 46, 640–656. [Google Scholar] [CrossRef]
- Kastner, P.; Mark, M.; Ghyselinck, N.; Krezel, W.; Dupe, V.; Grondona, J.M.; Chambon, P. Genetic evidence that the retinoid signal is transduced by heterodimeric rxr/rar functional units during mouse development. Development 1997, 124, 313–326. [Google Scholar]
- Lohnes, D.; Mark, M.; Mendelsohn, C.; Dolle, P.; Dierich, A.; Gorry, P.; Gansmuller, A.; Chambon, P. Function of the retinoic acid receptors (rars) during development (I). Craniofacial and skeletal abnormalities in rar double mutants. Development 1994, 120, 2723–2748. [Google Scholar]
- Rodriguez-Leon, J.; Merino, R.; Macias, D.; Ganan, Y.; Santesteban, E.; Hurle, J.M. Retinoic acid regulates programmed cell death through bmp signalling. Nat. Cell Biol. 1999, 1, 125–126. [Google Scholar] [CrossRef]
- Kuss, P.; Villavicencio-Lorini, P.; Witte, F.; Klose, J.; Albrecht, A.N.; Seemann, P.; Hecht, J.; Mundlos, S. Mutant hoxd13 induces extra digits in a mouse model of synpolydactyly directly and by decreasing retinoic acid synthesis. J. Clin. Investig. 2009, 119, 146–156. [Google Scholar]
- Merino, R.; Rodriguez-Leon, J.; Macias, D.; Ganan, Y.; Economides, A.N.; Hurle, J.M. The bmp antagonist gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development 1999, 126, 5515–5522. [Google Scholar]
- Grotewold, L.; Ruther, U. The fused toes (ft) mouse mutation causes anteroposterior and dorsoventral polydactyly. Dev. Biol. 2002, 251, 129–141. [Google Scholar] [CrossRef]
- Sun, X.; Mariani, F.V.; Martin, G.R. Functions of fgf signalling from the apical ectodermal ridge in limb development. Nature 2002, 418, 501–508. [Google Scholar] [CrossRef]
- Pajni-Underwood, S.; Wilson, C.P.; Elder, C.; Mishina, Y.; Lewandoski, M. Bmp signals control limb bud interdigital programmed cell death by regulating fgf signaling. Development 2007, 134, 2359–2368. [Google Scholar] [CrossRef]
- Merino, R.; Ganan, Y.; Macias, D.; Rodriguez-Leon, J.; Hurle, J.M. Bone morphogenetic proteins regulate interdigital cell death in the avian embryo. Ann. N. Y. Acad. Sci. 1999, 887, 120–132. [Google Scholar]
- Montero, J.A.; Ganan, Y.; Macias, D.; Rodriguez-Leon, J.; Sanz-Ezquerro, J.J.; Merino, R.; Chimal-Monroy, J.; Nieto, M.A.; Hurle, J.M. Role of fgfs in the control of programmed cell death during limb development. Development 2001, 128, 2075–2084. [Google Scholar]
- Hernandez-Martinez, R.; Castro-Obregon, S.; Covarrubias, L. Progressive interdigital cell death: Regulation by the antagonistic interaction between fibroblast growth factor 8 and retinoic acid. Development 2009, 136, 3669–3678. [Google Scholar] [CrossRef]
- Gomez-Skarmeta, J.L.; Modolell, J. Iroquois genes: Genomic organization and function in vertebrate neural development. Curr. Opin. Genet. Dev. 2002, 12, 403–408. [Google Scholar] [CrossRef]
- Villa-Cuesta, E.; Modolell, J. Mutual repression between msh and iro-c is an essential component of the boundary between body wall and wing in drosophila. Development 2005, 132, 4087–4096. [Google Scholar] [CrossRef]
- Gomez-Skarmeta, J.; de La Calle-Mustienes, E.; Modolell, J. The wnt-activated xiro1 gene encodes a repressor that is essential for neural development and downregulates bmp4. Development 2001, 128, 551–560. [Google Scholar]
- Glavic, A.; Gomez-Skarmeta, J.L.; Mayor, R. Xiro-1 controls mesoderm patterning by repressing bmp-4 expression in the spemann organizer. Dev. Dynam. 2001, 222, 368–376. [Google Scholar] [CrossRef]
- Feijoo, C.G.; Saldias, M.P.; De la Paz, J.F.; Gomez-Skarmeta, J.L.; Allende, M.L. Formation of posterior cranial placode derivatives requires the iroquois transcription factor irx4a. Mol. Cell. Neurosci. 2009, 40, 328–337. [Google Scholar] [CrossRef]
- Chuang, H.N.; Cheng, H.Y.; Hsiao, K.M.; Lin, C.W.; Lin, M.L.; Pan, H. The zebrafish homeobox gene irxl1 is required for brain and pharyngeal arch morphogenesis. Dev. Dynam. 2010, 239, 639–650. [Google Scholar] [CrossRef]
- van Tuyl, M.; Liu, J.; Groenman, F.; Ridsdale, R.; Han, R.N.; Venkatesh, V.; Tibboel, D.; Post, M. Iroquois genes influence proximo-distal morphogenesis during rat lung development. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L777–L789. [Google Scholar] [CrossRef]
- Chimal-Monroy, J.; Abarca-Buis, R.F.; Cuervo, R.; Diaz-Hernandez, M.; Bustamante, M.; Rios-Flores, J.A.; Romero-Suarez, S.; Farrera-Hernandez, A. Molecular control of cell differentiation and programmed cell death during digit development. IUBMB Life 2011, 63, 922–929. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of sox5 and sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Dupe, V.; Ghyselinck, N.B.; Thomazy, V.; Nagy, L.; Davies, P.J.; Chambon, P.; Mark, M. Essential roles of retinoic acid signaling in interdigital apoptosis and control of bmp-7 expression in mouse autopods. Dev. Biol. 1999, 208, 30–43. [Google Scholar] [CrossRef]
- Crocoll, A.; Herzer, U.; Ghyselinck, N.B.; Chambon, P.; Cato, A.C. Interdigital apoptosis and downregulation of bag-1 expression in mouse autopods. Mech. Dev. 2002, 111, 149–152. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Díaz-Hernández, M.E.; Rios-Flores, A.J.; Abarca-Buis, R.F.; Bustamante, M.; Chimal-Monroy, J. Molecular Control of Interdigital Cell Death and Cell Differentiation by Retinoic Acid during Digit Development. J. Dev. Biol. 2014, 2, 138-157. https://doi.org/10.3390/jdb2020138
Díaz-Hernández ME, Rios-Flores AJ, Abarca-Buis RF, Bustamante M, Chimal-Monroy J. Molecular Control of Interdigital Cell Death and Cell Differentiation by Retinoic Acid during Digit Development. Journal of Developmental Biology. 2014; 2(2):138-157. https://doi.org/10.3390/jdb2020138
Chicago/Turabian StyleDíaz-Hernández, Martha Elena, Alberto Jesús Rios-Flores, René Fernando Abarca-Buis, Marcia Bustamante, and Jesús Chimal-Monroy. 2014. "Molecular Control of Interdigital Cell Death and Cell Differentiation by Retinoic Acid during Digit Development" Journal of Developmental Biology 2, no. 2: 138-157. https://doi.org/10.3390/jdb2020138
APA StyleDíaz-Hernández, M. E., Rios-Flores, A. J., Abarca-Buis, R. F., Bustamante, M., & Chimal-Monroy, J. (2014). Molecular Control of Interdigital Cell Death and Cell Differentiation by Retinoic Acid during Digit Development. Journal of Developmental Biology, 2(2), 138-157. https://doi.org/10.3390/jdb2020138




