Activation of Marck-like Genes and Proteins During Initial Phases of Regeneration in the Amputated Tail and Limb of the Lizard Podarcis muralis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Tissue Preparation
2.2. Microscopical Methods
3. Results
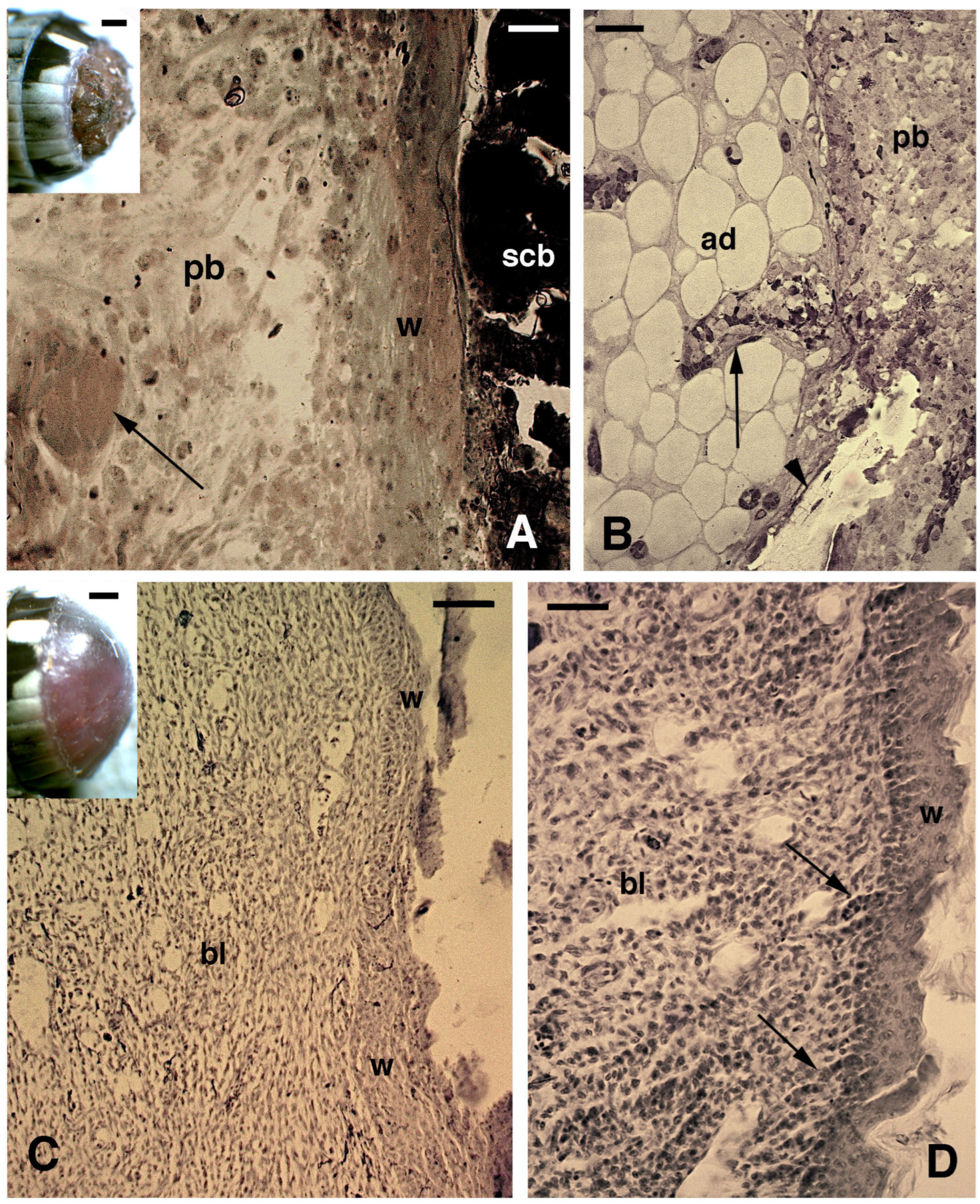
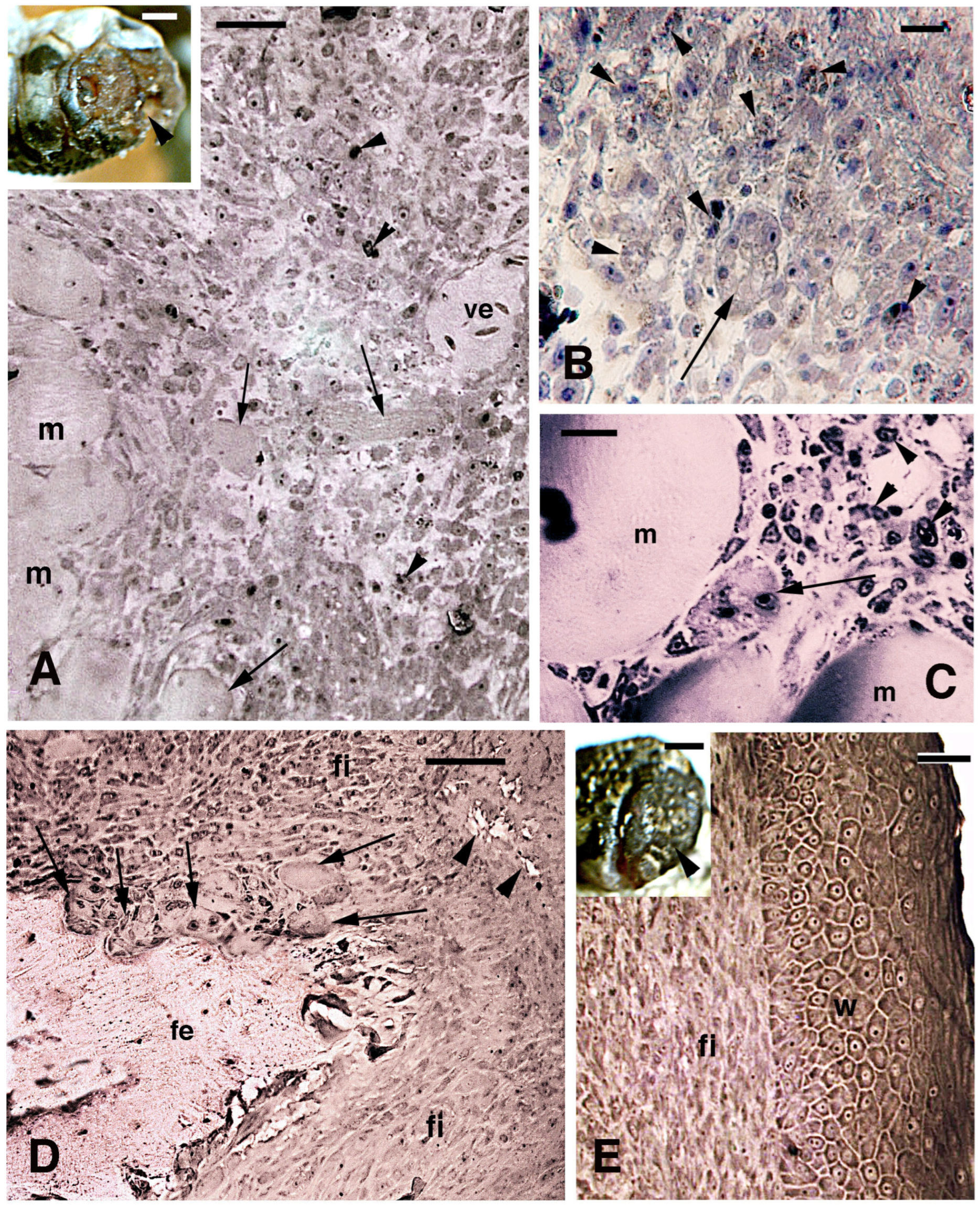
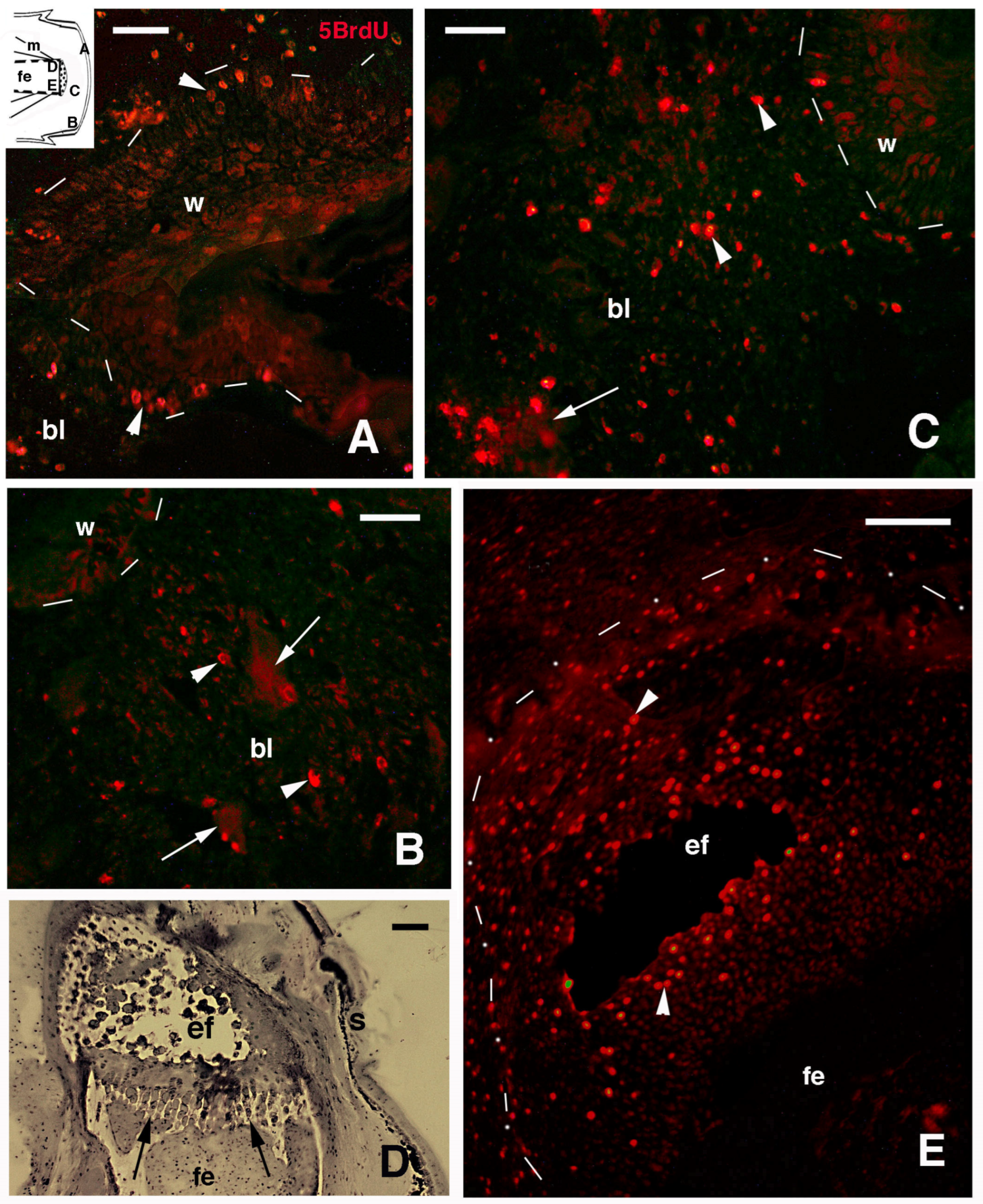
3.1. Histology and 5BrdU-Immunolabeling in the Tail and Limb Initial Blastemas
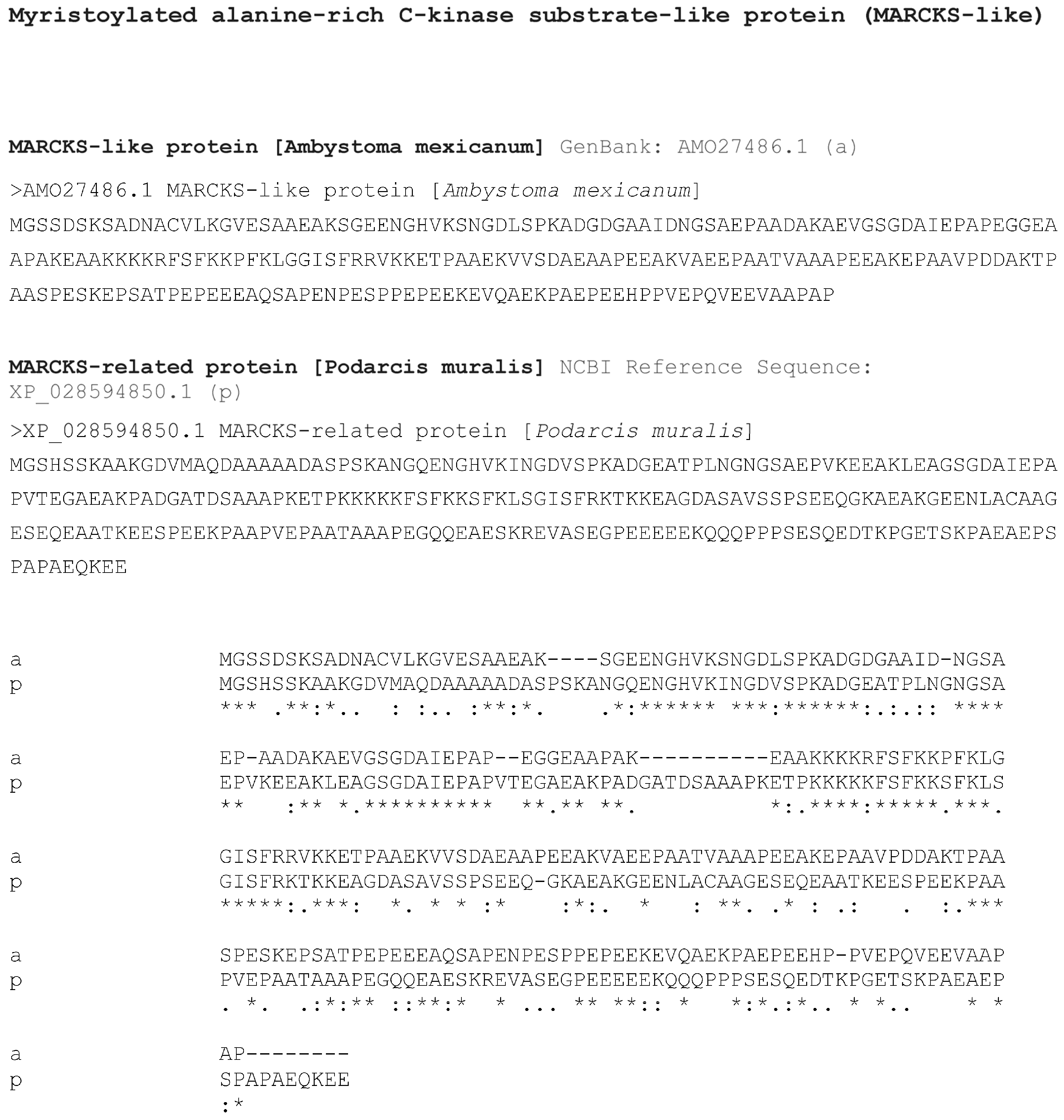
3.2. Bioinformatics Analysis
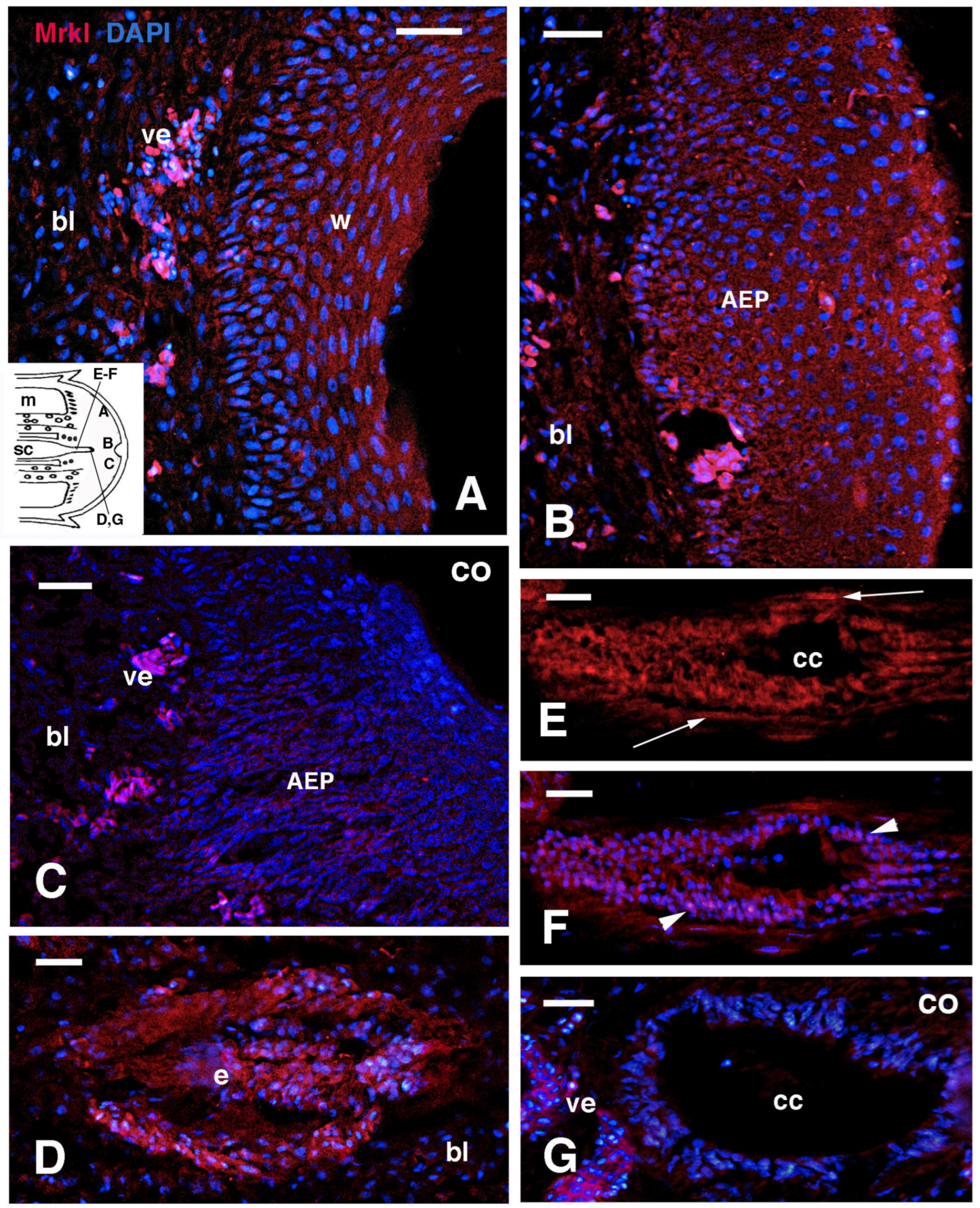
3.3. MARCK-like Immunolocalization
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichman, O.J. Evolution of regenerative capabilities. Amer. Natur. 1984, 123, 752–763. [Google Scholar] [CrossRef]
- Carlson, B.M. Principles of Regenerative Biology; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Cox, P.G. Some aspects of tail regeneration in the lizard, Anolis carolinensis. I. A description based on histology and autoradiography. J. Exp. Zool. 1969, 171, 127–150. [Google Scholar] [CrossRef]
- Bellairs, D.A.; Bryant, S.V. Autotomy and regeneration in reptiles. In Biology of the Reptilia; Billet, G.C.F., Maderson, P.F.A., Eds.; John Wiley & Sons: New York, NY, USA, 1985; Volume 15B, pp. 302–410. [Google Scholar]
- Alibardi, L. Ultrastructural features of the process of wound healing after tail and limb amputation in lizard. Acta Zool. 2010, 91, 306–318. [Google Scholar] [CrossRef]
- Fisher, R.E.; Geiger, L.A.; Stroik, L.K.; Hutchins, E.D.; George, R.M.; DeNardo, D.F.; Kosumi, K.; Rawls, J.A.; Wilson-Rawls, J. A histological comparison of the original and regenerated tail in the green anole, Anolis carolinensis. Anat. Rec. 2012, 295, 1609–1619. [Google Scholar] [CrossRef]
- Gilbert, E.A.B.; Payne, S.L.; Vickaryous, M.K. The anatomy and histology of caudal autotomy and regeneration in lizards. Physiol. Bioch. Zool. 2013, 86, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Tuan, R.S. Lizard tail regeneration as an instructive model of enhanced healing capabilities in an adult amniote. Connect. Tiss. Res. 2016, 58, 145–154. [Google Scholar] [CrossRef]
- Jacyniak, K.; McDonald, R.P.; Vickaryous, M.K. Tail regeneration and other phenomena of wound healing and tissue restoration in lizards. J. Exp. Biol. 2017, 220, 2858–2869. [Google Scholar] [CrossRef]
- Londono, R.; Wenzhong, W.; Wang, B.; Tuan, R.S.; Lozito, T.P. Cartilage and muscle cell fate and origin during lizard tail regeneration. Front. Bioeng. Biotechnol. 2017, 5, 70. [Google Scholar] [CrossRef]
- Hutchins, E.D.; Markov, G.J.; Eckalbar, W.L.; Gorge, R.M.; King, J.M.; Tokuyama, M.A.; Geiger, L.A.; Emmert, N.; Ammar, M.J.; Allen, A.P.; et al. Transcriptomic analysis of tail regeneration in the lizard Anolis carolinensis reveals activation of conserved vertebrate developmental and repair mechanisms. PLoS ONE 2014, 9, e105004. [Google Scholar] [CrossRef]
- Vitulo, N.; Dalla Valle, L.; Skobo, T.; Valle, G.; Alibardi, L. Transcriptome analysis of the regenerating tail versus the scarring limb in lizard reveals pathways leading to successful versus unsuccessful organ regeneration in amniotes. Dev. Dyn. 2017, 246, 116–134. [Google Scholar] [CrossRef]
- Vitulo, N.; Dalla Valle, L.; Skobo, T.; Valle, G.; Alibardi, L. Down-regulation of lizard immuno-genes in the regenerating tail and myo-genes in the scarring limb suggests that tail regeneration occurs in an immuno-privileged organ. Protoplasma 2017, 254, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Original and regenerating lizard tail cartilage contain putative resident stem/progenitor cells. Micron 2015, 78, 10–18. [Google Scholar] [CrossRef]
- Alibardi, L. Immunolocalization indicates that both original and regenerated lizard tail tissues contain populations of long retaining cells, putative stem/progenitor cells. Micr. Res. Tech. 2015, 78, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Temporal distribution of 5BrdU-labeled cells suggests that most injured tissues contribute proliferating cells for the regeneration of the tail and limb in lizard. Acta Zool. 2019, 100, 303–319. [Google Scholar] [CrossRef]
- Nourhidayat, L.; Benes, V.; Blom, S.; Gomes, I.; Firdausi, N.; de Bakker, M.A.G.; Spaink, H.P.; Richardson, M.K. Tokay gecko tail regeneration involves temporally collinear expression of HOXC genes and early expression of satellite cell markers. BMC Biol. 2025, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Ultrastructural analysis of early regenerating tail suggests that a process of dedifferentiation is involved in the formation of the regenerative blastema. J. Morphol. 2018, 279, 1171–1184. [Google Scholar] [CrossRef]
- Simon, A.; Tanaka, E.M. Limb regeneration. WIREs Dev. Biol. 2013, 2, 291–300. [Google Scholar] [CrossRef]
- Daponte, V.; Tylzanowski, P.; Forlino, A. Appendage regeneration in vertebrates: What makes this possible? Cells 2021, 10, 242. [Google Scholar] [CrossRef]
- Grigoryan, E. Study on natural longlife juvenility and tissue regeneration in caudate amphibians and potential application of resulting data in biomedicine. J. Dev. Biol. 2021, 9, 2. [Google Scholar] [CrossRef]
- Sugiura, T.; Wang, H.; Barsacchi, R.; Simon, A.; Tanaka, E.M. MARCKS-Like proteins is an initiator in axolotl appendage regeneration. Nature 2016, 531, 237–240. [Google Scholar] [CrossRef]
- Alibardi, L. Immunolocalization of 5BrdU long retaining labeled cells and macrophage infiltration in the scarring limb of lizard after limb amputation. Tiss. Cell 2016, 48, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, C.C.; Wang, J.K.T.; Walass, S.I.; Albert, K.A.; Greengard, P. Localization of the MARCKS (87 kDa) protein, a major specific substrate for protein kinase C, in rat brain. J. Neurosci. 1990, 10, 1683–1698. [Google Scholar] [CrossRef] [PubMed]
- El Amri, M.; Fitzgerald, U.; Schlosser, G. MARCKS and MARCKS-like proteins in development and regeneration. J. Biomed. Sci. 2018, 25, 43. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Selmi, C.; Ridgway, W.M.; Leung, P.S.C.; Zhang, F.; Gershwin, M.E. The myristoylated alanine-rich C-kinase substrates (MARCKs): A membrane-anchored mediator of the cell function. Autoimmun. Rev. 2021, 20, 102942. [Google Scholar] [CrossRef]
- McNamara, R.K.; Jiang, Y.; Streit, W.J.; Lenox, R.H. Facial motor neuron regeneration induces a unique spatial and temporal pattern of myristoylated alanine-rich C kinase substrate expression. Neuroscience 2000, 97, 581–589. [Google Scholar] [CrossRef]
- Weimer Weimer, J.M.; Yokota, Y.; Stanco, A.; Stumpo, D.J.; Blackshear, P.J.; Anton, E.S. MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development 2009, 136, 2965–2975. [Google Scholar] [CrossRef]
- El Amri, M.; Pandit, A.; Schlosser, G. Marcks and Marcks like-1 proteins promote spinal cord development and regeneration in Xenopus. eLife 2024, 13, e98277. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.F.; Costa, C.M.; Lorena, J.; Moreira, R.N.; Frota-Lima, G.N.; Furtado, C.; Robinson, M.; Amemiya, C.T.; Darnet, S.; Schneider, I. Tetrapod limb and sarcopterygian fin regeneration share a core genetic program. Nat. Commun. 2016, 7, 13364. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, J.H.; Kim, H.S.; Park, D.E.; Chung, C.H. Involvement of the O-type protein kinase C in translocation of myristoylated alanine-rich C kinase substrate (MARCKS) during myogenesis of chick embryonic myoblasts. Bioch. J. 2000, 347, 139–146. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, J.H.; Lee, S.H.; Chung, S.S.; Bang, O.S.; Park, D.; Chung, C.H. Involvement of protein phosphatase-1-mediated MARCKS translocation in myogenic differentiation of embryonic muscle cells. J. Cell Sci. 2002, 115, 2465–2473. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Song, H.; Zhang, X.; Wang, W.; Du, W.; Song, T.; Liang, H.L.; Chen, X.; Wang, Y. Macrophage migration inhibitory factor derived from spinal cord is involved in activation of macrophages following gecko tail amputation. FASEB J. 2019, 33, 14798–14810. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Song, H.; Wang, Y. Self-control of inflammation during tail regeneration of lizards. J. Dev. Biol. 2021, 9, 48. [Google Scholar] [CrossRef]
- Park, J.; Fang, S.; Crews, A.L.; Lin, K.W.; Adler, K.B. MARCKS regulation of mucin secretion by airway epithelium in Vitro. Am. J. Respir. Cell Mol. Biol. 2008, 39, 68–76. [Google Scholar] [CrossRef]
- Rombouts, K.; Mello, T.; Liotta, F.; Galli, A.; Caligiuri, A.; Annunziato, F.; Pinzani, M. MARCKS actin binding capacity mediates actin filament assembly during mitosis in human hepatic stellate cells. Amer. J. Physiol. 2012, 303, C357–C367. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, T.D.; Shah, N.; Jackson, W.P.; Feeney, E.V.; Scanlon, S.; Gish, R.; Khodadadi, R.; Hyde, S.O.; Hicks, P.H.; Anderson, J.C.; et al. The effector domain of MARCKS is a nuclear localization signal that regulates cellular PIP2 levels and nuclear PIP2 localization. PLoS ONE 2015, 10, e0140870. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.E.; Vickaryous, M.K. A novel amniote model of epimorphic regeneration: The leopard gecko, Eublepharis macularius. BMC Dev. Biol. 2011, 11, 50–74. [Google Scholar] [CrossRef]
- Simpson, S.B.; Duffy, M.T. The lizard spinal cord: A model system for the study of spinal cord injury and repair. Progr. Brain Res. 1994, 103, 229–241. [Google Scholar]
- Tokuyama, M.A.; Xu, C.; Fisher, R.E.; Wilson-Rawls, J.; Kusumi, K.; Newbern, J.M. Developmental and adult-specific processes contribute to de novo neuromuscular regeneration in the lizard tail. Dev. Biol. 2018, 43, 287–296. [Google Scholar] [CrossRef]
- Alibardi, L.; Boschetti, F. Immunolabeling for RhoV and Actin in early regenerating tail of the lizard Podarcis muralis suggests involvement in epithelial and mesenchymal cell motility. Acta Zool. 2021, 102, 51–62. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibardi, L. Activation of Marck-like Genes and Proteins During Initial Phases of Regeneration in the Amputated Tail and Limb of the Lizard Podarcis muralis. J. Dev. Biol. 2025, 13, 12. https://doi.org/10.3390/jdb13020012
Alibardi L. Activation of Marck-like Genes and Proteins During Initial Phases of Regeneration in the Amputated Tail and Limb of the Lizard Podarcis muralis. Journal of Developmental Biology. 2025; 13(2):12. https://doi.org/10.3390/jdb13020012
Chicago/Turabian StyleAlibardi, Lorenzo. 2025. "Activation of Marck-like Genes and Proteins During Initial Phases of Regeneration in the Amputated Tail and Limb of the Lizard Podarcis muralis" Journal of Developmental Biology 13, no. 2: 12. https://doi.org/10.3390/jdb13020012
APA StyleAlibardi, L. (2025). Activation of Marck-like Genes and Proteins During Initial Phases of Regeneration in the Amputated Tail and Limb of the Lizard Podarcis muralis. Journal of Developmental Biology, 13(2), 12. https://doi.org/10.3390/jdb13020012




