Selecting Normalizers for MicroRNA RT-qPCR Expression Analysis in Murine Preimplantation Embryos and the Associated Conditioned Culture Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Preimplantation Embryo Collection
2.2. Embryo Culture for Developmental Series Sample Preparation
2.3. Embryo Culture for Media Conditioning Sample Preparation
2.4. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.5. Reverse Transcription Droplet Digital PCR (RT-ddPCR)
2.6. Data Analysis
3. Results
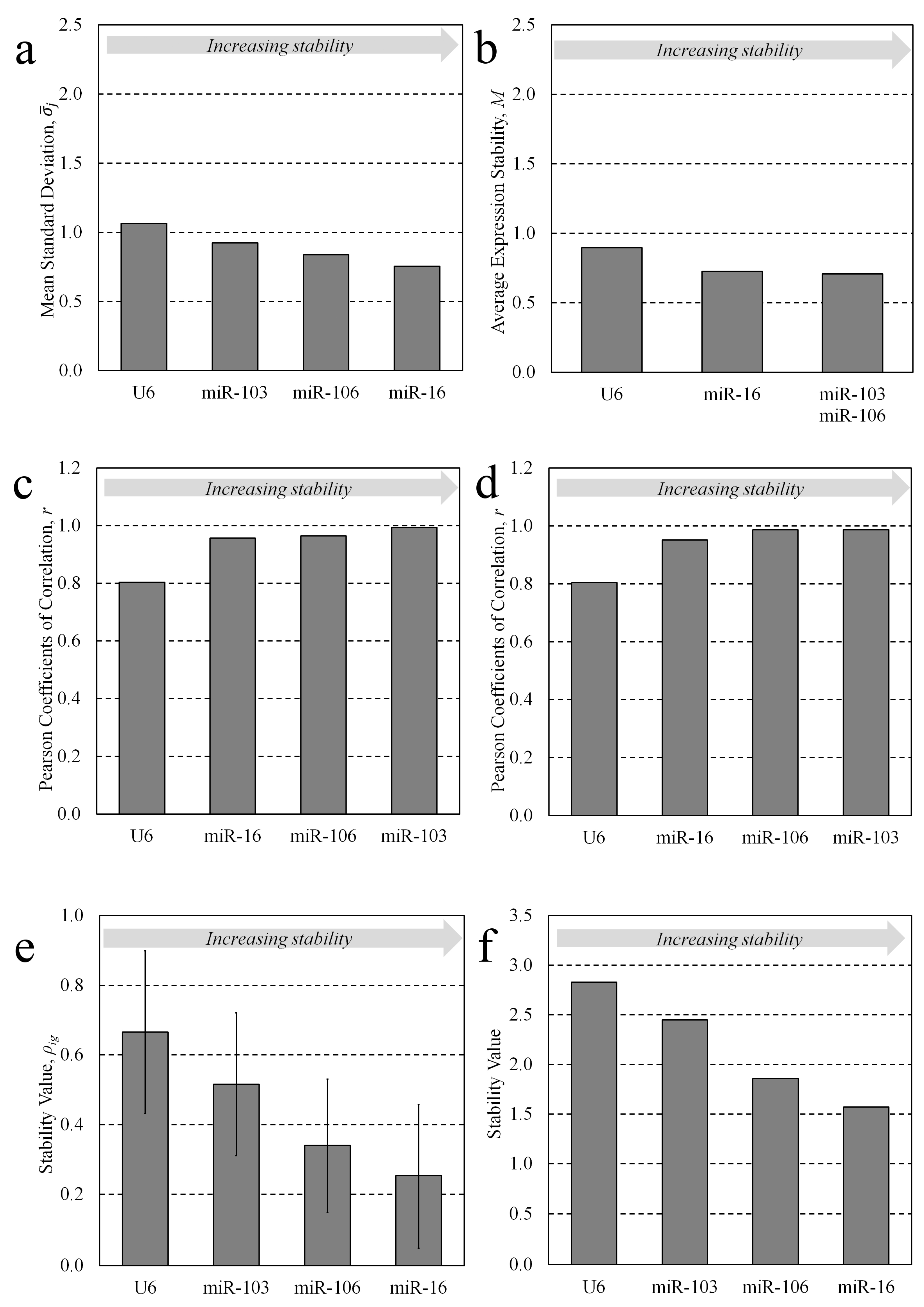
3.1. Candidate Stability Analysis
3.2. Multiple Candidate Stability Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Lai, E. Micro RNAs Are Complementary to 3′ UTR Sequence Motifs That Mediate Negative Post-Transcriptional Regulation. Nat. Genet. 2002, 30, 363–364. [Google Scholar] [CrossRef]
- Tribolet, L.; Kerr, E.; Cowled, C.; Bean, A.G.D.; Stewart, C.R.; Dearnley, M.; Farr, R.J. MicroRNA Biomarkers for Infectious Diseases: From Basic Research to Biosensing. Front. Microbiol. 2020, 11, 1197. [Google Scholar] [CrossRef]
- Rosenbluth, E.M.; Shelton, D.N.; Sparks, A.E.T.; Devor, E.; Christenson, L.; Van Voorhis, B.J. MicroRNA expression in the human blastocyst. Fertil. Steril. 2013, 99, 855–861. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, W.; Zhang, L.; Yin, G.; Wang, X.; Wang, J.; Zhao, H.; Han, Y.; Yao, Y.-Q. Determination of microRNAs in mouse preimplantation embryos by microarray. Dev. Dyn. 2008, 237, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Nabih, A.; Spies, D.; Hermes, V.; Bodak, M.; Wischnewski, H.; Stalder, P.; Ngondo, R.P.; Liechti, L.A.; Sajic, T.; et al. Global and Precise Identification of Functional MIRNA Targets in MESCS by Integrative Analysis. EMBO Rep. 2022, 23, e54762. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Ai, W.-B.; Wan, L.-Y.; Tan, X.; Wu, J.-F. The miR-290-295 cluster as multi-faceted players in mouse embryonic stem cells. Cell Biosci. 2017, 7, 38. [Google Scholar] [CrossRef]
- Hawke, D.C.; Watson, A.J.; Betts, D.H. Extracellular Vesicles, MicroRNA and the Preimplantation Embryo: Non-Invasive Clues of Embryo Well-Being. Reprod. BioMedicine Online 2020, 42, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Forero, D.A.; González-Giraldo, Y.; Castro-Vega, L.J.; Barreto, G.E. qPCR-based methods for expression analysis of miRNAs. BioTechniques 2019, 67, 192–199. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Silver, N.; Best, S.; Jiang, J.; Thein, S. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Goossens, K.; Van Poucke, M.; Van Soom, A.; Vandesompele, J.; Van Zeveren, A.; Peelman, L.J. Selection of reference genes for quantitative real-time PCR in bovine preimplantation embryos. BMC Dev. Biol. 2005, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Mahdipour, M.; van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Validating reference microRNAs for normalizing qRT-PCR data in bovine oocytes and preimplantation embryos. BMC Dev. Biol. 2015, 15, 25. [Google Scholar] [CrossRef]
- Schulz, K.N.; Harrison, M.M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 2019, 20, 221–234. [Google Scholar] [CrossRef]
- Svoboda, P. Mammalian Zygotic Genome Activation. Semin. Cell Dev. Biol. 2018, 84, 118–126. [Google Scholar] [CrossRef]
- Mondou, E.; Dufort, I.; Gohin, M.; Fournier, E.; Sirard, M.-A. Analysis of microRNAs and their precursors in bovine early embryonic development. Mol. Hum. Reprod. 2012, 18, 425–434. [Google Scholar] [CrossRef]
- Rosenbluth, E.M.; Shelton, D.N.; Wells, L.M.; Sparks, A.E.T.; Van Voorhis, B.J. Human embryos secrete microRNAs into culture media—A potential biomarker for implantation. Fertil. Steril. 2014, 101, 1493–1500. [Google Scholar] [CrossRef]
- Cuman, C.; Van Sinderen, M.; Gantier, M.P.; Rainczuk, K.; Sorby, K.; Rombauts, L.; Osianlis, T.; Dimitriadis, E. Human Blastocyst Secreted microRNA Regulate Endometrial Epithelial Cell Adhesion. EBioMedicine 2015, 2, 1528–1535. [Google Scholar] [CrossRef]
- Heidari, F.; Hosseini, S.; Yeganeh, S.M.; Salehi, M. Expression of miR-Let-7a, miR-15a, miR-16-1, and their target genes in fresh and vitrified embryos and its surrounding culture media for noninvasive embryo assessment. J. Cell. Biochem. 2019, 120, 19691–19698. [Google Scholar] [CrossRef]
- Lin, X.; Beckers, E.; Mc Cafferty, S.; Gansemans, Y.; Joanna Szymańska, K.; Chaitanya Pavani, K.; Catani, J.P.; Van Nieuwerburgh, F.; Deforce, D.; De Sutter, P.; et al. Bovine Embryo-Secreted microRNA-30c Is a Potential Non-invasive Biomarker for Hampered Preimplantation Developmental Competence. Front. Genet. 2019, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Häusler, S.; Backes, C.; Fehlmann, T.; Staib, C.; Nestel, S.; Nazarenko, I.; Meese, E.; Keller, A. Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing In Vitro Fertilization. Sci. Rep. 2017, 7, 13525. [Google Scholar] [CrossRef] [PubMed]
- Tripurani, S.K.; Xiao, C.; Salem, M.; Yao, J. Cloning and analysis of fetal ovary microRNAs in cattle. Anim. Reprod. Sci. 2010, 120, 16–22. [Google Scholar] [CrossRef]
- Tripurani, S.K.; Lee, K.-B.; Wee, G.; Smith, G.W.; Yao, J. MicroRNA-196a regulates bovine newborn ovary homeobox gene (NOBOX) expression during early embryogenesis. BMC Dev. Biol. 2011, 11, 25. [Google Scholar] [CrossRef]
- Tesfaye, D.; Worku, D.; Rings, F.; Phatsara, C.; Tholen, E.; Schellander, K.; Hoelker, M. Identification and expression profiling of microRNAs during bovine oocyte maturation using heterologous approach. Mol. Reprod. Dev. 2009, 76, 665–677. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; da Silva, A.M.; Calin, G.; Pantel, K. Data Normalization Strategies for MicroRNA Quantification. Clin. Chem. 2015, 61, 1333–1342. [Google Scholar] [CrossRef]
- Tang, F.; Kaneda, M.; O’Carroll, D.; Hajkova, P.; Barton, S.C.; Sun, Y.A.; Lee, C.; Tarakhovsky, A.; Lao, K.; Surani, M.A. Maternal microRNAs are essential for mouse zygotic development. Genes Dev. 2007, 21, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-M.; Pang, R.T.K.; Chiu, P.C.N.; Wong, B.P.C.; Lao, K.; Lee, K.-F.; Yeung, W.S.B. Sperm-borne microRNA-34c is required for the first cleavage division in mouse. Proc. Natl. Acad. Sci. USA 2012, 109, 490–494. [Google Scholar] [CrossRef]
- Tam, O.H.; Aravin, A.A.; Stein, P.; Girard, A.; Murchison, E.P.; Cheloufi, S.; Hodges, E.; Anger, M.; Sachidanandam, R.; Schultz, R.M.; et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 2008, 453, 534–538. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, F.; Xiang, G.; Jiang, D.; Pu, X. Identification of Endogenous Controls for Analyzing Serum Exosomal miRNA in Patients with Hepatitis B or Hepatocellular Carcinoma. Dis. Markers 2015, 2015, 893594. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bai, Z.; Han, W.; Zhang, J.; Meng, H.; Bi, J.; Ma, X.; Han, S.; Zhang, Z. Identification of Suitable Reference Genes for qPCR Analysis of Serum microRNA in Gastric Cancer Patients. Dig. Dis. Sci. 2012, 57, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q. Different Normalization Strategies Might Cause Inconsistent Variation in Circulating microRNAs in Patients with Hepatocellular Carcinoma. Med. Sci. Monit. 2015, 21, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Gade, S.; Steinbach, B.; Loibl, S.; von Minckwitz, G.; Untch, M.; Schwedler, K.; Lübbe, K.; Schem, C.; Fasching, P.A.; et al. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: A translational research project within the Geparquinto trial. Breast Cancer Res. Treat. 2014, 147, 61–68. [Google Scholar] [CrossRef]
- McDermott, A.M.; Kerin, M.J.; Miller, N. Identification and Validation of miRNAs as Endogenous Controls for RQ-PCR in Blood Specimens for Breast Cancer Studies. PLoS ONE 2013, 8, e83718. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Du, L.; Li, J.; Jiang, X.; Zheng, G.; Qu, A.; Wang, H.; Wang, L.; Zhang, X.; et al. Identification and validation of reference genes for the detection of serum microRNAs by reverse transcription-quantitative polymerase chain reaction in patients with bladder cancer. Mol. Med. Rep. 2015, 12, 615–622. [Google Scholar] [CrossRef]
- Peltier, H.J.; Latham, G.J. Normalization of microRNA expression levels in quantitative RT-PCR assays: Identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 2008, 14, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-T.; Dong, Q.-Z.; Wang, G.; Zhou, H.-J.; Ren, N.; Jia, H.-L.; Ye, Q.-H.; Qin, L.-X. Identification of Suitable Reference Genes for qRT-PCR Analysis of Circulating microRNAs in Hepatitis B Virus-Infected Patients. Mol. Biotechnol. 2012, 50, 49–56. [Google Scholar] [CrossRef]
- Wotschofsky, Z.; Meyer, H.-A.; Jung, M.; Fendler, A.; Wagner, I.; Stephan, C.; Busch, J.; Erbersdobler, A.; Disch, A.C.; Mollenkopf, H.-J.; et al. Reference genes for the relative quantification of microRNAs in renal cell carcinomas and their metastases. Anal. Biochem. 2011, 417, 233–241. [Google Scholar] [CrossRef]
- Hu, Z.; Dong, J.; Wang, L.-E.; Ma, H.; Liu, J.; Zhao, Y.; Tang, J.; Chen, X.; Dai, J.; Wei, Q.; et al. Serum microRNA profiling and breast cancer risk: The use of miR-484/191 as endogenous controls. Carcinogenesis 2012, 33, 828–834. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, H.; Zhang, X.; Yang, Y.; Wang, L.; Du, L.; Li, W.; Li, J.; Qu, A.; Liu, Y.; et al. Identification and Validation of Reference Genes for qPCR Detection of Serum microRNAs in Colorectal Adenocarcinoma Patients. PLoS ONE 2013, 8, e83025. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, Y.; Ye, F.; Wang, F.; Wan, X.; Lu, W.; Xie, X. Identification of miR-23a as a novel microRNA normalizer for relative quantification in human uterine cervical tissues. Exp. Mol. Med. 2011, 43, 358. [Google Scholar] [CrossRef] [PubMed]
- Olds, P.J.; Stern, S.; Biggers, J.D. Chemical estimates of the RNA and DNA contents of the early mouse embryo. J. Exp. Zool. 1973, 186, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.P.M.; Lintern-Moore, S.; Scott-Murphy, L. Relative changes in the RNA contents of oocytes and preimplantation embryos of the mouse. Reprod. Nutr. Dévelop. 1981, 21, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Hawke, D.C.; Ahmed, D.B.; Watson, A.J.; Betts, D.H. Murine Blastocysts Release Mature MicroRNAs Into Culture Media That Reflect Developmental Status. Front. Genet. 2021, 12, 655882. [Google Scholar] [CrossRef]
- Maraghechi, P.; Hiripi, L.; Toth, G.; Bontovics, B.; Bosze, Z.; Gocza, E. Discovery of pluripotency-associated microRNAs in rabbit preimplantation embryos and embryonic stem-like cells. Reproduction 2013, 145, 421–437. [Google Scholar] [CrossRef][Green Version]
- Álvarez, C.; García-Garrido, C.; Taronger, R.; de Merlo, G.G. In vitro maturation, fertilization, embryo development & clinical outcome of human metaphase-I oocytes retrieved from stimulated intracytoplasmic sperm injection cycles. Indian J. Med. Res. 2013, 8, 331–338. [Google Scholar]
- Ko, D.S.; Lee, S.-H.; Park, D.-W.; Yang, K.M.; Lim, C.K. Pregnancy and fertilization potential of immature oocytes retrieved in intracytoplasmic sperm injection cycles. Clin. Exp. Reprod. Med. 2015, 42, 118. [Google Scholar] [CrossRef]
- Gilchrist, G.; Tscherner, A.; Nalpathamkalam, T.; Merico, D.; LaMarre, J. MicroRNA Expression during Bovine Oocyte Maturation and Fertilization. Int. J. Mol. Sci. 2016, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Mihalas, B.P.; Camlin, N.J.; Xavier, M.J.; Peters, A.E.; Holt, J.E.; Sutherland, J.M.; McLaughlin, E.A.; Eamens, A.L.; Nixon, B. The small non-coding RNA profile of mouse oocytes is modified during aging. Aging 2019, 11, 2968–2997. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.; Vento, M.E.; Ragusa, M.; Barbagallo, D.; La Ferlita, A.; Di Emidio, G.; Borzi, P.; Artini, P.G.; Scollo, P.; Tatone, C.; et al. MicroRNAs Are Stored in Human MII Oocyte and Their Expression Profile Changes in Reproductive Aging. Biol. Reprod. 2016, 95, 131. [Google Scholar] [CrossRef]
- Sánchez-Ribas, I.; Diaz-Gimeno, P.; Quiñonero, A.; Ojeda, M.; Larreategui, Z.; Ballesteros, A.; Domínguez, F. NGS Analysis of Human Embryo Culture Media Reveals miRNAs of Extra Embryonic Origin. Reprod. Sci. 2019, 26, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Max, K.E.A.; Bertram, K.; Akat, K.M.; Bogardus, K.A.; Li, J.; Morozov, P.; Ben-Dov, I.Z.; Li, X.; Weiss, Z.R.; Azizian, A.; et al. Human plasma and serum extracellular small RNA reference profiles and their clinical utility. Proc. Natl. Acad. Sci. USA 2018, 115, E5334–E5343. [Google Scholar] [CrossRef]



| Candidate Name | NCBI/miRBase Accession Number | TaqMan Assay ID | Reference(s) |
|---|---|---|---|
| U6 | NR_004394 | 001973 | [20,21,22,23,37] |
| let-7a-5p | MIMAT0000521 | 000377 | [32,33,34] |
| miR-16-5p | MIMAT0000527 | 000391 | [33,34,35,36,37,38] |
| miR-26a-5p | MIMAT0000533 | 000405 | [32,39] |
| miR-103-3p | MIMAT0000546 | 000439 | [32,40] |
| miR-106a-5p | MIMAT0000385 | 002459 | [33,38,40] |
| miR-191-5p | MIMAT0000221 | 002299 | [37,38,41,42,43] |
| Ranking Order | |||||||
|---|---|---|---|---|---|---|---|
| Method | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Delta CT | miR-16 | miR-191 | miR-106 | miR-103 | miR-26a | U6 | let-7a |
| geNorm | miR-16|miR-191 | miR-106 | miR-103 | U6 | miR-26a | let-7a | |
| BestKeeper | miR-16 | miR-103 | miR-191 | miR-106 | U6 | miR-26a | let-7a |
| BestKeeper (repeated) | miR-103 | miR-191 | miR-16 | miR-106 | U6 | miR-26a | let-7a |
| Normfinder | miR-16 | miR-191 | miR-106 | miR-103 | miR-26a | U6 | let-7a |
| RefFinder | miR-16 | miR-191 | miR-106 | miR-26a | let-7a | miR-103 | U6 |
| Ranking Order | ||||
|---|---|---|---|---|
| Method | 1 | 2 | 3 | 4 |
| Delta CT | miR-16 | miR-106 | miR-103 | U6 |
| geNorm | miR-103|miR-106 | miR-16 | U6 | |
| BestKeeper | miR-103 | miR-106 | miR-16 | U6 |
| BestKeeper (repeated) | miR 103|miR-106 | miR-16 | U6 | |
| Normfinder | miR-16 | miR-106 | miR-103 | U6 |
| RefFinder | miR-16 | miR-106 | miR-103 | U6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawke, D.C.; Watson, A.J.; Betts, D.H. Selecting Normalizers for MicroRNA RT-qPCR Expression Analysis in Murine Preimplantation Embryos and the Associated Conditioned Culture Media. J. Dev. Biol. 2023, 11, 17. https://doi.org/10.3390/jdb11020017
Hawke DC, Watson AJ, Betts DH. Selecting Normalizers for MicroRNA RT-qPCR Expression Analysis in Murine Preimplantation Embryos and the Associated Conditioned Culture Media. Journal of Developmental Biology. 2023; 11(2):17. https://doi.org/10.3390/jdb11020017
Chicago/Turabian StyleHawke, David C., Andrew J. Watson, and Dean H. Betts. 2023. "Selecting Normalizers for MicroRNA RT-qPCR Expression Analysis in Murine Preimplantation Embryos and the Associated Conditioned Culture Media" Journal of Developmental Biology 11, no. 2: 17. https://doi.org/10.3390/jdb11020017
APA StyleHawke, D. C., Watson, A. J., & Betts, D. H. (2023). Selecting Normalizers for MicroRNA RT-qPCR Expression Analysis in Murine Preimplantation Embryos and the Associated Conditioned Culture Media. Journal of Developmental Biology, 11(2), 17. https://doi.org/10.3390/jdb11020017







