Abstract
Nephrons are the functional units which comprise the kidney. Each nephron contains a number of physiologically unique populations of specialized epithelial cells that are organized into discrete domains known as segments. The principles of nephron segment development have been the subject of many studies in recent years. Understanding the mechanisms of nephrogenesis has enormous potential to expand our knowledge about the basis of congenital anomalies of the kidney and urinary tract (CAKUT), and to contribute to ongoing regenerative medicine efforts aimed at identifying renal repair mechanisms and generating replacement kidney tissue. The study of the zebrafish embryonic kidney, or pronephros, provides many opportunities to identify the genes and signaling pathways that control nephron segment development. Here, we describe recent advances of nephron segment patterning and differentiation in the zebrafish, with a focus on distal segment formation.
1. Introduction
1.1. Essential Functions of the Kidney
The kidney performs various vital functions in the body, such as removing metabolic waste products, monitoring blood pressure, secreting hormones and maintaining pH, electrolyte and water balance [1]. Nephron functional units in the kidney consist of subdomains, or segments, that are dedicated to particular physiological tasks (Figure 1) [2,3]. These segment regions include the renal corpuscle that filters the blood; tubule segments that modify the filtrate, including the proximal tubule, loop of Henle, distal tubule and connecting tubule; and lastly, the collecting duct, which performs the final modifications on urine and conveys it out of the kidney [4,5,6,7,8,9,10,11,12]. Within the renal corpuscle, the podocytes secrete collagen and growth factors to help maintain glomerular basement membrane and endothelial cell fenestration, respectively [4,5]. Additionally, the ultrastructural features of the podocytes are crucial components in establishing glomerular filtration [4,5]. The proximal tubule is important for reabsorbing nutrients, electrolytes and water: in total, it reabsorbs about two thirds of most water and important electrolytes, such as Na+, Cl− and HCO3−, as well as 99.8% of glucose and amino acids filtered by the human kidney [6,7]. The loop of Henle is further divided into the descending thin limb (DTL), the ascending thin limb (ATL) and the thick ascending limb (TAL), which together play critical roles in water homeostasis [8]. This is achieved by the expression of the Na+/K+/2Cl− cotransporter in the TAL, which helps the reabsorption of 25% of filtered Na+ [9]. Additionally, the TAL also reabsorbs filtered Ca2+ and Mg2+ [9]. The distal tubule is important in regulating electrolytes such as Na+, K+ and Ca2+ and pH levels [10]. The distal tubule reabsorbs approximately 5–10% of filtered Na+, as well as 7–10% of filtered calcium [10]. The collecting duct originates from the ureteric bud, and is essential in regulating water and electrolytes such as Na+ and Cl− [11,12].
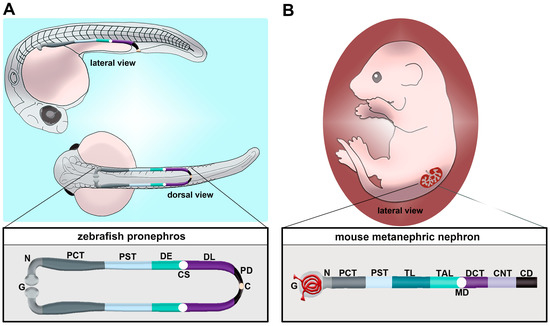
Figure 1.
Comparison of nephron segment composition between the zebrafish and the mouse. (A) The zebrafish embryo forms a pronephros that consists of two nephrons located on either side of the midline, which form by the 24 h post fertilization (hpf) stage and quickly undergo morphogenesis events that enable them to begin blood filtration by approximately 48 hpf. (B) Nephron composition in the mouse metanephros; note that the nephron is drawn here in a linear configuration, but it would display a folded/convoluted anatomical configuration within the native kidney. There is a striking conservation of proximal and distal segments, but the thin limb is one notable distinction between these nephron forms. Abbreviations are as follows: P = podocytes; N = neck; PCT = proximal convoluted tubule; PST = proximal straight tubule; DE = distal early; CS = Corpuscle of Stannius; DL = distal late; CD = collecting duct; TL = thin limb; TAL = thick ascending limb; MD = macula densa; DCT = distal convoluted tubule; CNT = connecting tubule.
Given the crucial tasks accomplished by kidney nephrons, their proper formation is vital for normal renal function [13,14,15]. Alterations in the number and composition of nephrons have significant ramifications for kidney health, and can lead to congenital anomalies of the kidney and urinary tract (CAKUT) [16,17,18,19,20,21,22]. Understanding the mechanisms of nephrogenesis has many potential applications to treat both inherited and acquired renal diseases [23,24,25].
1.2. Vertebrate Kidney Forms Are Comprised of Nephrons
Several versions of the kidney organ are made and degraded during vertebrate ontogeny [26,27]. They develop in a prototypical sequence: the first form is termed the pronephros, the second form is termed the mesonephros and the third possible form is termed the metanephros. This last form is typically made in reptiles, birds and mammals. Across all forms, the nephron is the common structural and functional unit. In general, each version of the kidney in a given species exhibits increasing complexity with regard to the absolute number and arrangement of its nephrons. There is broad conservation of nephron segment composition. However, there are notable variations across the phylogenetic spectrum, such as the aglomerular nephrons in some species, and differences in whether nephrons possess a loop of Henle segment [23,28,29,30,31,32,33,34]. In this review, we specifically discuss kidney form and development in the zebrafish, Danio rerio, which has been used extensively over the past 25 years to study the mechanisms of nephron ontogeny, physiology and regeneration as well [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. In the following sections, we provide an overview of the kidney forms, and then discuss the recent advances in understanding the genetic mechanisms of distal segment patterning and differentiation.
2. Using the Zebrafish to Study Nephron Development
2.1. Zebrafish Pronephros Composition and Function
Unlike mammals, which manifest the pronephros, mesonephros and metanephros during development, the zebrafish only manifests the pronephros and the mesonephros [37]. Each of these kidney structures originates from populations of mesodermally derived renal progenitors. The zebrafish pronephros is extremely simple, being comprised of two nephrons that form rapidly and function throughout the first several weeks of life [53,54,55,56,57,58,59,60,61,62,63,64]. Key transcription factors such as Pax2a, Pax8, Hand1 and Osr1 have critical roles in the specification of the renal progenitor’s fate [55,65,66,67,68,69,70,71,72]. Once this mesenchymal identity is set, which is thought to transpire from the tailbud stage or 10 h post fertilization (hpf) through to 24 hpf, the renal progenitors undergo events that transition them to form tubules with defined proximal and distal epithelial cell identities [73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90]. The zebrafish segments include the neck (N), proximal convoluted tubule (PCT), proximal straight tubule (PST), distal early (DE) tubule and distal late (DL) tubule (Figure 1A) [73]. Compared to the typical mammalian nephron, the loop of Henle is missing (Figure 1B) [73]. At approximately 48 hpf, the pronephros will become functional and begin to filter the circulation [60]. Between 24 hpf and 48 hpf, there are several morphogenesis events which happen to create this functioning pronephros [37]. For example, podocyte precursors migrate to the midline and recruit blood vessels, forming the glomerulus. The tubular segments continue to differentiate, showing the expression of additional solute transporter genes that impart unique physiological capabilities to each segment. Combined with this is the growth of the renal tubule and coiling event of the PCT.
2.2. Zebrafish Mesonephros Composition and Function: Spotlight on Renal Regeneration Studies
The adult form of the zebrafish kidney, also known as the mesonephros, develops beginning around 12–14 days post fertilization (dpf) [37,91,92]. During this process, more nephrons are created and joined with the existing pronephros tubules, forming a small collection of interconnected nephrons that drain via a pair of renal collecting ducts [37,91,92]. Eventually, this process generates between 300–500 nephrons [91,92]. The zebrafish body size positively correlates with the number of mesonephric nephrons [91,92]. After complete mesonephros generation, the zebrafish possesses more complex nephron branching, yet the segmental composition remains similar to that of the pronephros [92]. The adult mesonephros is ultimately located at the dorsal body wall surrounded by connective tissues. In the zebrafish, the mesonephros contains a head region, a saddle and a tail region. Additionally, based on the density of nephrons throughout the mesonephros, there are four further subdivisions: anterior nephron-dense region (ANDR), medial nephron-sparse region (MNSR), medial nephron-dense region (MNDR) and posterior nephron-sparse region (PNSR). Compared to mammalian kidneys, which cannot regenerate nephrons, in the zebrafish kidney, zebrafish keep adding nephrons to their kidney throughout their lifetime. In addition to nephron addition throughout development, the mesonephros can also regenerate damage to existing nephrons after injury [91,92,93,94,95,96,97,98,99,100,101,102].
3. Molecular Genetic Toolkit in the Zebrafish Animal Model
3.1. Zebrafish as a Model for Developmental Biology and Biomedical Research
The zebrafish offers a simple, yet effective, animal model to study developmental biology and biomedical research [103,104,105,106,107,108,109]. There are multiple traits that facilitate developmental research. First, the zebrafish has a simple architecture: at its embryonic stage, the embryonic kidney only comprises two nephrons [53]. Much due to their simplicity, zebrafish carry high similarity with mammalian kidneys: 70% of human genes share at least one homolog with the zebrafish [106]. The zebrafish kidney also shares most of the kidney segments with the mammalian kidney, with the exception of the mammalian loop of Henle [73]. Furthermore, zebrafish development occurs ex utero, and embryonic zebrafish are optically transparent, allowing for live imaging and visualization of organs. Next, zebrafish have high regeneration ability: new kidney nephrons keep being added to the developing kidney throughout the lifetime or after injury. Meanwhile, mammalian kidneys cannot regenerate or produce more nephrons after they are destroyed. Additionally, zebrafish demonstrate fast organogenesis, with the vital organs such as the kidney, heart and eyes available as early as 24 hpf. At 48 hpf, the kidney is fully functional, and all common organs are visible at 120–144 hpf. Coupled with this is high fecundity: zebrafish in a good laboratory environment can breed all year round, and each week they can breed up to 300 eggs.
3.2. Forward Genetics: From Random Mutagenesis Screens to Chemical Screens
In forward genetic approaches, the screening of animal populations that have random mutations in the genome can uncover the novel importance of genes in biological pathways. To start this process, the first step is creating mutagenic lesions on the genome that are heritable and phenotypically distinguishable. There are several types of random mutagenesis which have been studied. Early work used gamma irradiation to create breaks in the chromosome to create mutant phenotypes. This method has a weakness as it causes large deletions, translocations or chromosomal aberrations that complicate the process to specify the genes responsible for the mutant phenotype. Subsequently, alkylating agents were used, such as ethylnitrosourea (ENU). In this process, chemical mutagens were exposed to adult males, causing random point mutations in their DNA [53]. The adult males were then bred with WT females, generating heterozygous F1. To expand heterozygous populations, F1 heterozygotes were then outcrossed again with WT to generate F2 heterozygous generation. F2 heterozygotes were then incrossed with each other to generate the desired homozygous mutants. This method provides more advantages over gamma irradiation, such as high mutagenic loads in the zebrafish genome, as well as the induced phenotypes being specifically linked to one gene. A major drawback of this method is that it is difficult to identify specific mutations responsible for the induced mutant phenotype. To overcome this challenge, several groups have introduced replication-deficient retroviruses or transposons as mutagens, which can insert into the genome to cause mutations. In this method, the mutagenic agent is inserted into the 1-cell stage if it is a transposon, or the 1000-cell stage embryos if it is a retrovirus. However, the overall mutagenic frequency from retroviruses and transposons has been estimated to be much lower than ENU, thus requiring a significantly more bountiful library of mutagenized fish. Both random mutagenesis and insertional mutagenesis methods have led to the generation of mutant collections with defects in kidney development [53,77,90,110,111,112,113]. Within the zebrafish pronephros, examples of aberrant morphological defects that could be attributed to reduced kidney function include pericardial edema, kidney cysts, hydrocephalus of the brain or body curvature [53,77].
Since the early live screens, scientists have expanded the methods of identifying mutant features. One simple way of phenotypic screening is by using a molecular marker(s) to visualize a particular cell or tissue type, using techniques such as whole mount in situ hybridization (WISH) or immunostaining [111]. The use of a tissue-specific transgenic line marked by fluorescent protein expression can alleviate the hands-on effort and sample fixation issue caused by in situ hybridization or immunostaining. In addition, restriction enzymes could offer a simple and useful tool to detect mutation. If the mutation affects a particular site on the DNA that a restriction enzyme has been known to affect, such as the removal of a restriction enzyme site from WT embryos, a restriction enzyme digest assay could be used to distinguish between WT and mutant embryos. Lastly, DNA extracted from embryos from mutant lines could be used for PCR and sent for sequencing, allowing for robust and direct identification of the genetic lesion.
3.3. Reverse Genetics: Loss-of-Function Methods Using Morpholinos and Genome Editing
Loss-of-function studies have been performed using various approaches, including morpholino oligos, CRISPR/Cas9 system, TALENs and ZFNs [46,108]. In comparison, techniques with RNA interference (RNAi) have proven to be problematic in zebrafish [114]. Morpholinos are a short antisense oligonucleotide, up to 25 basepairs (bps) long, designed to target the processed mRNA or pre-mRNA transcript of the animal [115]. Morpholino oligos that target the ATG start site block translation, while morpholinos that target the splice donor or acceptor site of a given exon–intron boundary can disrupt the spliceosome [115]. This can lead to numerous outcomes, such as introns being present after RNA processing. In such a case, a splice site morpholino might cause a frameshift mutation, resulting in a premature stop codon during translation, causing a truncated protein that will later be degraded. A benefit of splice-site morpholino over ATG-site morpholino is that RNA transcripts can be reverse transcribed into DNA, and the presence of the mis-spliced transcripts can be detected using RT-PCR and sequencing [115,116]. In the zebrafish model, morpholinos are typically delivered to the 1-cell-stage embryo via microinjection, and effects last for up to several days (1–3, or more), allowing for an easy model to study development (Figure 2A) [115].
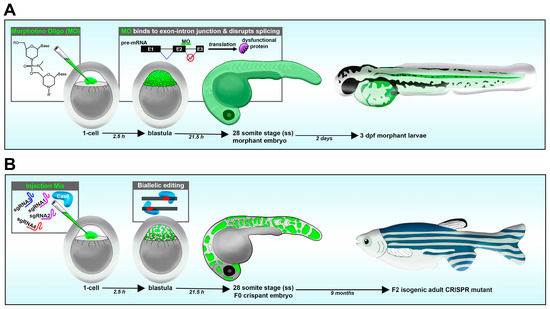
Figure 2.
Reverse genetic loss-of-function strategies in the zebrafish embryo. (A) Morpholino knockdown. (B) CRISPR-Cas9.
Other loss-of-function methods which have been used in the zebrafish include zinc finger nucleases (ZFNs) [117,118,119,120] and transcription-activator-like (TAL) effector nucleases (TALENs) [121,122,123,124]. ZFNs were the earliest tool of genome-editing with the use of endonucleases [117,118,119,120]. They contain chimeric enzymes with a zinc finger (ZF) domain fused with the FokI endonuclease domain. The ZF domain mediates the binding to DNA, while the FokI endonuclease domain performs the nucleic acid cleavage. ZF domains can be engineered to bind to a 9–18 bp DNA sequence, and once that binding happens, the FokI domain causes a double-stranded break in the DNA, which is then repaired by either homologous cell repair or non-homologous end joining. Similarly to ZFNs, TALENs contain chimeric proteins with a structure containing a modular DNA-binding domain that is fused together with a FokI nuclease, causing a double-stranded break at a specific site in the gene of interest [121,122,123,124]. TALENs originate from virulence factors in Xanthomonas bacteria. The difference between TALENs and ZFNs are that DNA-binding modules of TALENs are naturally occurring, termed TALs. TALs include a series of conserved domains across 34 amino acids, with the only difference being between Positions 12 and 13, where DNA contact sites happen, termed repeat variable di-residues.
In recent years, the CRISPR/Cas9 system has become the most popular tool to study gene knockout in the zebrafish [123,124]. CRISPR stands for “clustered regularly interspaced short palindromic repeats”, which are segments of prokaryotic DNA that bacteria use to defend against foreign DNA elements by binding to these sequences and recruiting the CRISPR-associated protein 9 (Cas9) to destroy such a DNA sequence [125]. Interestingly, researchers can utilize this ancient self-defense mechanism to edit the genome of animal models such as the zebrafish [126,127,128,129]. In this design, a guide RNA (gRNA) can be designed to bind specifically to a gene of interest which will recruit a Cas9 endonuclease, which will make a double-strand cut in the DNA sequence. This cut will thus result in the generation of a random insertion/deletion (indel) mutation in the genome as a result of non-homologous end joining. The introduction of specific mutations can also be performed by including single strand oligos with the guide RNA and Cas9 mixture [130]. CRISPR/Cas9 engineered embryos can later be extracted for DNA for sequencing to detect the genetic alterations. Additionally, the T7 endonuclease assay can be used to detect mutations caused by CRISPR/Cas9. In this assay, T7 endonuclease will recognize deformities in heteroduplex DNA and make a cleavage, as compared to WT samples. The results could be easily detected via an agarose gel, making the T7 endonuclease assay a cost-effective and simple way of verifying the CRISPR reaction.
Similar to morpholino oligos, in CRISPR/Cas9, both the gRNA and Cas9 enzyme are delivered into zebrafish embryos in the 1-cell stage via microinjection. Further, a cocktail of multiple gRNAs can be simultaneously injected with the Cas9 to increase the probability of mutation, and to introduce biallelic disruptions and thus permit analysis in the F0 embryos for a gene of interest (Figure 2B). CRISPR/Cas9 differs from morpholino oligos in that the target of disruption is DNA instead of RNA. Therefore, CRISPR/Cas9 can serve as a powerful method to generate knockout or knock-in alleles depending on the materials which are injected. Resulting crispants (F0) can be raised and heterozygotes can be outcrossed with WT to generate F1 generation, consisting of ~50% WT and ~50% heterozygotes. Selected F1 heterozygotes can then be incrossed with each other to generate F2 progeny with 25% homozygous for mutation. One caveat is that gene targeting during the initial 1-cell microinjection can lead to genome modifications in later cleavage cycles, such as in the 2- or 4-cell stages, and thus individual embryos can be mosaics of cells with multiple different mutations. Thus, when raising an F1 generation, each individual may have varying mutations and detailed analysis is necessary when finding stable lines. There have been continuing advances that reduce off-target modifications to the genome when employing CRISPR/Cas9, such as the development of high-fidelity Cas9 derivatives [131].
It is highly desirable to study genetic lesions induced by CRISPR/Cas9 or other modes of gene editing, as studies have suggested that there are wider off-target effects of agents such as morpholinos that contribute to morphant phenotypes [132]. However, one downside of crispants or stable mutant lines generated via CRISPR/Cas9 is that milder phenotypes in many cases have been found to be the result of compensatory mechanisms where other genes are upregulated in response to the loss-of-function [133]. Thus, careful controls such as rescue studies and gene expression analyses are necessary to best validate each model and understand its potential research limitations [134].
3.4. Reverse Genetics: Gain-of-Function Approaches with mRNA and Transgenic Models
In addition to loss-of-function studies, gain-of-function studies also allow researchers to properly study how certain genes influence development [46,108]. Gain-of-function can be achieved by transient overexpression via mRNA or transgenic heat shock [114]. Gain-of-function studies are helpful to study how the overexpression of a gene of interest affects nephron development, or to perform rescue studies in loss-of-function models and test the specificity. The microinjection of synthetic 5′ capped mRNA (cRNA) (as opposed to the delivery of plasmid DNA) leads to a relatively homogenous distribution of the mRNA and resultant protein throughout the developing embryo when performed in the zebrafish embryo in the 1-cell stage (Figure 3A) [114]. cRNA can also be co-injected with other molecules such as morpholino oligos to rescue nephron segment-specific phenotypes caused by morpholino oligos. Embryos could then be fixed at the time point of interest along with WT embryos or morphant embryos and utilized for further analysis of nephron segments such as whole mount in situ hybridization (WISH). While cRNA injection into embryos has shown success in overexpression or rescue from loss-of-function studies, as a transient method, cRNA injection might not successfully rescue nephron segment defects due to dosage, or may lead to disruptions in other developmental events [114].
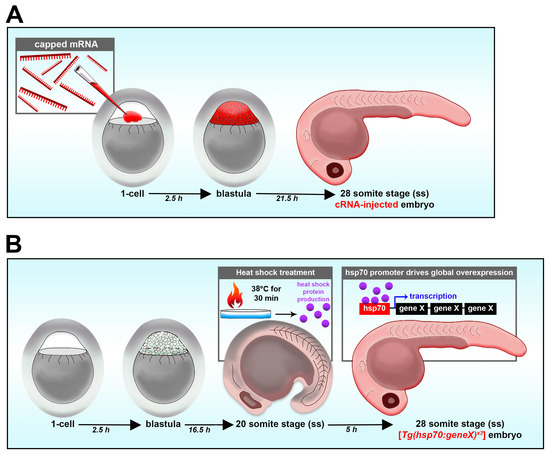
Figure 3.
Reverse genetic gain-of-function strategies in the zebrafish embryo. (A) Overexpression through mRNA delivery. (B) Transgenic mediated overexpression.
In such cases, the use of a heat shock transgenic line is an alternative conditional strategy to achieve gain-of-function [114,135]. This could be achieved by creating a transgenic line with the Hsp70 promoter tagged to the gene of interest, called [Tg(hsp70:geneX)]. The transgenic line could be generated via the use of the Tol2 transposase system, which has been regarded as a powerful method to generate transgenic lines. This is achieved by injecting a construct including the Tol2 vector Hsp70 coupled with the gene of interest together with the Tol2 transposase mRNA into the one-cell-stage embryos. The Tol2 transposase will help to insert the construct into the genome of the embryos. Once the transgenic line is established, their embryos could be used for gain-of-function studies. To activate the transgene, heat shock treatment would be applied to the embryo at the desired time point of interest, such as 37 °C for 30 min in the 20 somite stage (Figure 3B). The heat shock treatment would promote the overexpression of the gene of interest for gain-of-function or rescue studies [135].
4. Advances in Understanding Distal Segment Development
Through a combination of forward genetic screens and reverse genetic approaches, zebrafish researchers have uncovered many key principles of pronephros development over the past 25 years. Seminal forward screens identified crucial transcription factors such as pax2a and hnf1ba [53,110]. The conducting of large-scale gene expression analysis screens and subsequent deposition of these data into public databases such as the Zebrafish Information Network (ZFIN, https://zfin.org/, accessed on 1 January 2023) [136,137] provided a wealth of information regarding the putative candidate genes involved in organogenesis, including the kidney. Screens for morphological signs of renal dysfunction such as edema have also been performed and used to identify the relevant kidney genes [77,90]. Subsequent targeted screens that directly assessed the presence of nephron segment populations with molecular markers have also successfully uncovered relevant genetic factors and signaling pathways. The mechanisms that regulate specification and early pattern formation of renal progenitors have been nicely reviewed elsewhere [138]. In the following sections, we will explore several recently published studies that used the study of selected candidates and/or the characterization and cloning of mutants from nephron segment screens to add new insights into our understanding about the development of distal segments, with a focus on the DE, DL and CS cell lineages.
4.1. The Mecom/Tbx2a/2b/Emx1 Network in DL Pronephros Development
Genetic studies from several reports have revealed a set of transcription factors that are necessary for promoting the DL lineage. The mds1/evi1 complex (mecom) transcription factor was the earliest known marker of distal tubule progenitors [73,77]. Interestingly, in 2014, Li et al. reported that the knockdown of mecom leads to a reduced DL segment length [139]. In conjunction, they found that the proximal tubule (including both the PCT and PST segments) expands caudally [139]. Several years later, Drummond et al. identified roles for the t-box2a (tbx2a) and t-box2b (tbx2b) transcription factors in DL development as well [140]. The researchers found that the single knockdown of tbx2a or tbx2b led to the formation of a small DL segment, similar to the tbx2b genetic mutant, from beyond, or fbyc144, which encodes a point mutation that introduces a premature stop codon within the T-box DNA-binding domain [140,141]. Embryos that were doubly deficient for tbx2a/b displayed a similar phenotype to each of the single gene knockdowns, suggesting the possibility that they act within the same pathway to influence the DL’s fate [140]. Subsequent research by Morales et al. in 2019 determined that mecom acts upstream of tbx2a/b, finding that tbx2b overexpression was sufficient to rescue mecom-deficient embryos [142]. Furthermore, they identified that the transcription factor empty spiracles homeobox gene 1 (emx1) was highly expressed in distal tubule progenitors [142]. The domain of emx1+ distal tubule progenitors was reduced in both tbx2a- and tbx2b-deficient embryos, and emx1 was sufficient to rescue DL formation in the latter [142]. These and other studies enabled the authors to propose a working model for DL fate in which Mecom promotes tbx2a/b expression, and Tbx2a/b in turn promotes emx1 expression. Future studies with genetic mutants will be a useful way to test this model further. Additionally, transcriptional profiling of renal progenitors in these various genetic mutants will likely reveal important, currently unappreciated insights about how the population is altered in the absence of these factors. Another interesting facet to this pathway is the evidence that the transcriptional coactivator Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Ppargc1a) is necessary for the development of tbx2+ DL progenitors, and that the short DL segment in ppargc1asa131186 mutants can be rescued via tbx2b overexpression [143].
4.2. Role of Irx3b and Irx1a in DE Pronephros Development
There have been several key insights into DE segment formation in the vertebrate pronephros. Seminal work in the frog pronephros first associated several Iroquois transcription factors, including Irx1 and Irx3, with nephron segment development [144]. When assessed in the zebrafish, irx3b was found to be necessary and sufficient for the DE fate [77]. The knockdown of irx3b led to a near absence of cells expressing DE-specific markers [77]. Instead, the irx3b-deficient embryos had other lineages such as the CS exhibiting a population increase [77]. Furthermore, when Morales et al. examined emx1-deficient embryos, they observed expanded expression domains of both irx3b and irx1a in the distal tubule progenitors, suggesting that they might be a target of Emx1 and might explain the expansions in DE and CS populations in emx1-deficient embryos [142]. In subsequent work, Naylor et al. demonstrated that the CS lineage emerges from the DE population, thus providing a context to understand these phenotypes [145]. Additionally, prostaglandin signaling negatively regulates the irx3b expression domain and likely explains why interruptions in this signaling are associated with an expanded DE and reduced DL segment identity [146,147].
4.3. Tfap2a/b Control DE Differentiation/Maturation
Crucial insights into the control of DE lineage differentiation came from studies on the transcription factor AP-2 alpha and AP-2 beta genes [148]. In this work, Chambers et al. isolated the tfap2a mutant terminus (trm) through a forward haploid genetic screen strategy [111] to find renal regulators by assessing the formation of alternating pronephros segments [148]. Compared to wild-type embryos, trm mutants showed diminished expression of the DE-specific segment marker slc12a1 (Figure 4A). Whole genome sequencing revealed that the trm lesion corresponded to a G > A substitution at a conserved splice donor site in tfap2a. Transcript analysis revealed that mutants expressed abnormal tfap2a isoforms compared to wild-type embryos, including three that encoded premature stop codons. A complementation test confirmed that this novel tfap2a lesion causes the abnormal renal phenotype in trm mutant embryos, and tfap2a morpholino knockdown led to a similar effect (Figure 4A). Furthermore, the trm mutants exhibited similar abnormalities as previously reported tfap2a mutants in the developing neural crest, such as in the craniofacial cartilage, and these defects were rescued in overexpression studies with wild-type tfap2a transcripts [148]. Interestingly, both tfap2a and its closely related family member tfap2b were found to be expressed in renal progenitors [148], as noted in previous gene expression studies [136,137]. tfap2b loss-of-function did not alter distal segment development (Figure 4A). Interestingly, embryos that were doubly deficient for tfap2a/2b had the most dramatic defects in distal segment development, which requires future study. However, a loss of tfap2a led to diminished tfap2b expression, suggesting partial redundancy between the factors with tfap2a acting upstream of tfap2b.
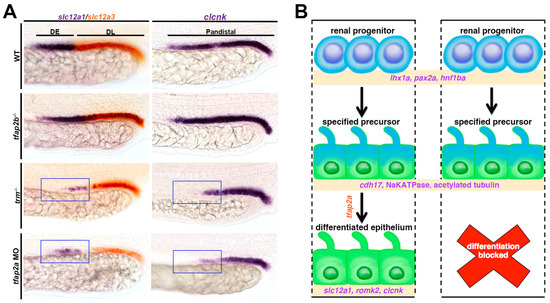
Figure 4.
Role of Tfap2 transcription factors in DE segment differentiation in the zebrafish pronephros. (A) trm mutants exhibit reductions (indicated by blue boxes) in the expression of transcripts that encode DE segment solute transporters, such as slc12a1. (B) Tfap2a is a requisite for discrete aspects of DE differentiation, but is not required for features such as polarity establishment and ciliogenesis. Adapted from Ref. [148].
The severely reduced expression of DE markers in trm mutants but normal pattern of segment domains suggested that tfap2a was necessary for the terminal differential of distal nephrons (Figure 4B). trm mutant DE cells exhibited some differentiated features such as clearly demarcated apical-based polarity, as well as a discernible nephron lumen, together suggesting normal tubulogenesis. The cilia arrangement and morphology in trm mutants were also determined to be comparable with wild-type embryos, suggesting that trm mutant DE cells are able to undergo ciliogenesis. However, the trm mutant DE cells were found to have a diminished expression of specific solute transporters, leading researchers to surmise that Tfap2a was explicitly requisite for the DE to express a cadre of distal solute transporter proteins (Figure 4A). Additional genetic studies placed tfap2a downstream of irx3b, but upstream of irx1a in controlling DE differentiation. Taken together, these data suggest a model where trm mutant renal progenitor cells develop normally until a final stage of differentiation, undergoing typical specification and some aspects of differentiation such as epithelialization and organelle development (Figure 4B). However, trm mutant cells then fail to activate the necessary distal solute transporters in the absence of appropriate Tfap2a expression (Figure 4B). In this model, tfap2a is upstream of tfap2b. However, there is a level of redundant function that is speculated to promote the expression of the various distal solute transporter gene targets. The study does not determine whether tfap2a and tfap2b interact directly or not, which is a potential area for future investigation.
4.4. Tfap2a Autoregulation through the Kctd15a/b Repressors
Two candidate components of the Tfap2a nephrogenesis gene regulatory network (GRN), kctd15a and kctd15b, were hypothesized [149] because previous studies have established conserved roles for the Kctd15 potassium channel tetramerization domain genes as Tfap2 repressors [150,151]. For example, both Kctd15a/b inhibit neural crest development in zebrafish through the direct repression of Tfap2a [150,151]. Both kctd15a and kctd15b exhibited similar expressions in the distal nephron throughout pronephros development, in an overlapping pattern with tfap2a [149,152,153]. Loss-of-function experiments revealed that kctd15a, kctd15b and kctd15a/b morphants and crispants had expanded expression domains of several DE-specific differentiation markers compared to wild-type embryos [149]. The largest domains were observed in the kctd15a/b doubly deficient embryos, which formed nearly double the number of cells that expressed a DE signature (e.g., slc12a1+, kcnj1a.1+) as their wild-type counterparts [149]. Interestingly, these expansions were attributed to the co-expression of the DE signature in the adjacent proximal and distal tubule segments [149]. Furthermore, kctd15-deficient embryos developed a significantly expanded Corpuscles of Stannius (CS) lineage, which was derived from DE precursors [145]. These data indicate that kctd15a/b are required to suppress the expression of DE features, and to repress CS fate, in renal progenitors during nephrogenesis [149].
Interestingly, kctd15a/b-deficient embryos showed a significant expansion of the Tfap2a protein, especially in the proximal nephron [149]. These results suggest that when kctd15a and kctd15b are negated, the number of Tfap2a-expressing cells increases [149]. Indeed, the tfap2a expression domain was greatly increased in kctd15a/b-deficient embryos [149]. Furthermore, trm mutants exhibited significant reductions in kctd15a/b expression [149]. Finally, triple tfap2a/kctd15a/b loss-of-function studies also suggest that kctd15a and kctd15b are only able to function through tfap2a [149]. In summary, these findings support a model whereby an autoregulatory feedback loop driven by tfap2a expression regulates the transcription of its repressors, kctd15a and kctd15b (Figure 5).
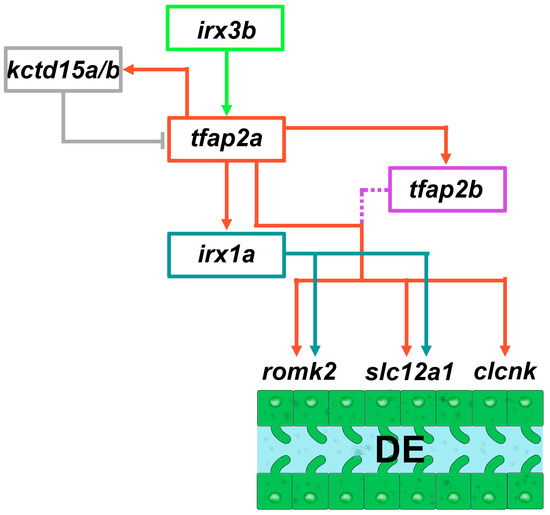
Figure 5.
Working model of components in the Tfap2 gene regulatory network that controls distal segment differentiation during zebrafish pronephros development. tfap2a is positioned as an intermediary factor within a cascade of the irx3-irx1a transcription factors that promote DE formation. Furthermore, tfap2a positively regulates the expression of tfap2b, and auto-regulates itself by modulating the expression of the kctd15a/b repressors. Adapted from Refs. [148,149].
5. Conclusions
Here, we have discussed several critical advances in delineating the genetic factors which dictate lineage fate choice and differentiation of nephron segments in the zebrafish embryonic kidney. Ongoing work continues to identify genes of interest [154,155,156,157]. Continued investigations to elucidate the interrelationships and functions of these identified factors are an exciting future area of investigation. Additionally, data from various single cell sequencing approaches [158,159,160,161,162] are expanding our knowledge of the nephron and will be pivotal to advancing knowledge about nephrogenesis, which can be used in future applications such as regenerative medicine [163].
Author Contributions
Conceptualization, T.K.N., M.P., B.E.C. and R.A.W.; writing—original draft preparation, T.K.N., M.P. and R.A.W.; writing—review and editing, T.K.N., M.P., B.E.C. and R.A.W.; visualization, B.E.C.; supervision, R.A.W.; project administration, R.A.W.; funding acquisition, M.P. and R.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by funds from the University of Notre Dame College of Science to R.A.W., University of Notre Dame Graduate School Teaching Assistant funding to T.K.N., a University of Notre Dame College of Science Summer Undergraduate Research Fellowship from the Glynn Family Honors Program awarded to M.P., and Elizabeth and Michael Gallagher by their generous gift to the University of Notre Dame to support stem cell research. The funders had no role in the study design, data collection and analysis, decision to publish or manuscript preparation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the University of Notre Dame Department of Biological Sciences for their support. We have deep gratitude to the Freimann Life Science Center (FLSC) and the Center for Zebrafish Research at the University of Notre Dame for the overseeing and care of our zebrafish aquarium. T.K.N. thanks R.A.W, R.R. and P.B for their incredible mentorship, and the Integrated Biomedical Sciences program for their support. R.A.W. thanks G.R.W. for unwavering support and encouragement, as well as B.C., C.T.G.M., K.P.E. and M.R.M. for their support and advice. Finally, we thank all of the past and current members of our lab for their outstanding discussions about nephron ontogeny.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Preuss, H.G. Basics of renal anatomy and physiology. Clin. Lab. Med. 1993, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, M.P.; Zeidel, M.L. Homeostasis, the milieu intérieur, and the wisdom of the nephron. Clin. J. Am. Soc. Nephrol. 2014, 9, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.S.; Rohacs, T.; Susztak, K. How many cell types are in the kidney and what do they do? Annu. Rev. Physiol. 2022, 84, 507–531. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M.R.; Quaggin, S.E.; Hoenig, M.P.; Dworkin, L.D. The glomerulus: The sphere of influence. Clin. J. Am. Soc. Nephrol. 2014, 9, 1461–1469. [Google Scholar] [CrossRef]
- Garg, P. A review of podocyte biology. Am. J. Nephrol. 2018, 47, 3–13. [Google Scholar] [CrossRef]
- Zhuo, J.L.; Li, X.C. Proximal nephron. Compr. Physiol. 2013, 3, 1079–1123. [Google Scholar] [PubMed]
- Curthoys, N.P.; Moe, O.W. Proximal tubule function and response to acidosis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1627–1638. [Google Scholar] [CrossRef]
- Dantzler, W.H.; Layton, A.T.; Layton, H.E.; Pannabecker, T.L. Urine-concentrating mechanism in the inner medulla: Function of the thin limbs of the loops of Henle. Clin. J. Am. Soc. Nephrol. 2014, 9, 1781–1789. [Google Scholar] [CrossRef]
- Mount, D.B. Thick ascending limb of the loop of Henle. Clin. J. Am. Soc. Nephrol. 2014, 9, 1974–1986. [Google Scholar] [CrossRef]
- Subramanya, A.R.; Ellison, D.H. Distal convoluted tubule. Clin. J. Am. Soc. Nephrol. 2014, 9, 2147–2163. [Google Scholar] [CrossRef]
- Pearce, D.; Soundararajan, R.; Trimpert, C.; Kashlan, O.B.; Deen, P.M.T.; Kohan, D.E. Collecting duct principal cell transport processes and their regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 135–146. [Google Scholar] [CrossRef]
- Roy, A.; Al-bataineh, M.M.; Pastor-Soler, N.M. Collecting duct intercalated cell function and regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Bertram, J.F.; Douglas-Denton, R.N.; Diouf, B.; Hughson, M.D.; Hoy, W.E. Human nephron number: Implications for health and disease. Pediatr. Nephrol. 2011, 26, 1529–1533. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Shukha, K.; Brenner, B.M. Low nephron number and its clinical consequences. Rambam Maimonides Med. J. 2011, 2, e0061. [Google Scholar] [CrossRef] [PubMed]
- Black, M.J.; Sutherland, M.R.; Gubhaju, L.; Kent, A.L.; Dahlstrom, J.E.; Moore, L. When birth comes early: Effects on nephrogenesis. Nephrology (Carlton) 2013, 18, 180–182. [Google Scholar] [CrossRef]
- McCampbell, K.K.; Wingert, R.A. Renal stem cells: Fact or science fiction? Biochem. J. 2012, 444, 153–168. [Google Scholar] [CrossRef]
- Schedl, A. Renal abnormalities and their developmental origin. Nat. Rev. Genet. 2007, 8, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Dursun, H.; Bayazit, A.K.; Büyükçelik, M.; Soran, M.; Noyan, A.; Anarat, A. Associated anomalies in children with congenital solitary functioning kidney. Pediatr. Surg. Int. 2005, 21, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Westland, R.; Schreuder, M.F.; Ket, J.C.F.; van Wijk, J.A.E. Unilateral renal agenesis: A systematic review on associated anomalies and renal injury. Nephrol. Dial. Transplant. 2013, 28, 1844–1855. [Google Scholar] [CrossRef]
- Vivante, A.; Kohl, S.; Hwang, D.; Dworschak, G.C.; Hildebrandt, F. Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr. Nephrol. 2014, 29, 695–704. [Google Scholar] [CrossRef]
- Rodriguez, M.M. Congenital anomalies of the kidney and the urinary tract (CAKUT). Fetal Pediatr. Pathol. 2014, 33, 293–320. [Google Scholar] [CrossRef]
- Nicolaou, N.; Renkema, K.Y.; Bongers, E.M.; Giles, R.H.; Knoers, N.V. Genetic, environmental, and epigenetic factors involved in CAKUT. Nat. Rev. Nephrol. 2015, 11, 720–731. [Google Scholar] [CrossRef]
- Romagnani, P.; Lasagni, L.; Remuzzi, G. Renal progenitors: An evolutionary conserved strategy for kidney regeneration. Nat. Rev. Nephrol. 2013, 9, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Peired, A.J.; Melica, M.E.; Molli, A.; Nardi, C.; Romagnani, P.; Lasagni, L. Molecular mechanisms of renal progenitor regulation: How many pieces in the puzzle? Cells 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Naved, B.A.; Bonventre, J.V.; Hubbell, J.A.; Hukriede, N.A.; Humphreys, B.D.; Kesselman, C.; Valerius, M.T.; McMahon, A.P.; Shankland, S.J.; Wertheim, J.A.; et al. Kidney repair and regeneration: Perspectives of the NIDDK (Re)Building a Kidney consortium. Kidney Int. 2022, 101, 845–853. [Google Scholar] [CrossRef]
- Chambers, J.M.; Wingert, R.A. Advances in understanding vertebrate nephrogenesis. Tissue Barriers 2020, 8, e1832844. [Google Scholar] [CrossRef] [PubMed]
- Little, M.H. Returning to kidney development to deliver synthetic kidneys. Dev. Biol. 2021, 474, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W. Kidneys sans glomeruli. Am. J. Physiol. Renal Physiol. 2004, 286, F811–F827. [Google Scholar] [CrossRef]
- Holz, P.H. Anatomy and physiology of the reptile renal system. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Orosz, S.E.; Echols, M.S. The urinary and osmoregulatory systems of birds. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Keogh, L.; Kilroy, D.; Bhattacharjee, S. The struggle to equilibrate outer and inner milieus: Renal evolution revisited. Ann. Anat. 2021, 233, 151610. [Google Scholar] [CrossRef]
- Schnell, J.; Achieng, M.; Lindström, N.O. Principles of human and mouse nephron development. Nat. Rev. Nephrol. 2022, 18, 628–642. [Google Scholar] [CrossRef]
- Senarat, S.; Kettratad, J.; Pairohakul, S.; Ampawong, S.; Huggins, B.P.; Coleman, M.M.; Kaneko, G. An update on the evolutionary origin of aglomerular kidney with structural and ultrastructural descriptions of the kidney in three fish species. J. Fish Biol. 2022, 100, 1283–1298. [Google Scholar] [CrossRef]
- Evans, R.G. Evolution of the glomerulus in a marine environment and its implications for renal function in terrestrial vertebrates. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R143–R151. [Google Scholar] [CrossRef]
- Wingert, R.A.; Davidson, A.J. The zebrafish pronephros: A model to study nephron segmentation. Kidney Int. 2008, 73, 1120–1127. [Google Scholar] [CrossRef]
- Ebarasi, L.; Oddsson, A.; Hultenby, K.; Betsholtz, C.; Tryggvason, K. Zebrafish: A model system for the study of vertebrate renal development, function, and pathophysiology. Curr. Opin. Nephrol. Hypertens. 2011, 20, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, G.F.; Wingert, R.A. Kidney organogenesis in the zebrafish: Insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 559–585. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, P.T.; Wingert, R.A. Using zebrafish to study podocyte genesis during kidney development and regeneration. Genesis 2014, 52, 771–792. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J. Kidney regeneration in fish. Nephron Exp. Nephrol. 2014, 126, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Desgrange, A.; Cereghini, S. Nephron patterning: Lessons from Xenopus, zebrafish and mouse studies. Cells 2015, 4, 483–499. [Google Scholar] [CrossRef]
- McKee, R.A.; Wingert, R.A. Zebrafish renal pathology: Emerging models of acute kidney injury. Curr. Pathobiol. Rep. 2015, 3, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Drummond, B.E.; Wingert, R.A. Insights into kidney stem cell development and regeneration using zebrafish. World J. Stem Cells 2016, 8, 22–31. [Google Scholar] [CrossRef]
- Marra, A.N.; Li, Y.; Wingert, R.A. Antennas of organ morphogenesis: The roles of cilia in vertebrate kidney development. Genesis 2016, 54, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.A.; Davidson, A.J. Zebrafish kidney development. Methods Cell Biol. 2016, 134, 391–429. [Google Scholar] [PubMed]
- Poureetezadi, S.J.; Wingert, R.A. Little fish, big catch: Zebrafish as a model for kidney disease. Kidney Int. 2016, 89, 1204–1210. [Google Scholar] [CrossRef]
- Morales, E.E.; Wingert, R.A. Zebrafish as a model of kidney disease. Results Probl. Cell Differ. 2017, 60, 55–75. [Google Scholar]
- Elmonem, M.A.; Berlingerio, S.P.; van den Heuvel, L.P.; de Witte, P.A.; Lowe, M.; Levtchenko, E.N. Genetic renal diseases: The emerging role of zebrafish models. Cells 2018, 7, 130. [Google Scholar] [CrossRef]
- Chambers, B.E.; Wingert, R.A. Renal progenitors: Roles in kidney disease and regeneration. World J. Stem Cells 2016, 8, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Fatma, S.; Nayak, U.; Swain, R.K. Methods to generate and evaluate zebrafish models of human kidney diseases. Int. J. Dev. Biol. 2021, 65, 475–485. [Google Scholar] [CrossRef]
- Wesselman, H.M.; Nguyen, T.K.; Chambers, J.M.; Drummond, B.E.; Wingert, R.A. Advances in understanding the genetic mechanisms of zebrafish renal multiciliated cell development. J. Dev. Biol. 2022, 11, 1. [Google Scholar] [CrossRef]
- Bolten, J.S.; Pratsinis, A.; Alter, C.L.; Fricker, G.; Huwyler, J. Zebrafish (Danio rerio) larva as an in vivo vertebrate model to study renal function. Am. J. Physiol. Renal Physiol. 2022, 322, F280–F294. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.A.; Majumdar, A.; Hentschel, H.; Elger, M.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Stemple, D.L.; Zwartkruis, F.; Rangini, Z.; et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 1998, 125, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Drummond, I.A. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev. Genet. 1999, 24, 220–229. [Google Scholar] [CrossRef]
- Majumdar, A.; Drummond, I.A. The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev. Biol. 2000, 222, 147–157. [Google Scholar] [CrossRef]
- Serluca, F.C.; Fishman, M.C. Pre-pattern in the pronephric kidney field of zebrafish. Development 2001, 128, 2233–2241. [Google Scholar] [CrossRef]
- Hsu, H.; Lin, G.; Chung, B. Parallel early development of zebrafish interrenal glands and pronephros: Differential control by Wt1 and Ff1b. Development 2003, 130, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Drummond, B.E.; Ercanbrack, W.S.; Wingert, R.A. Modeling podocyte ontogeny and podocytopathies with the zebrafish. J. Dev. Biol. 2023, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Zucker, A.G.; Wiessner, S.; Jensen, A.M.; Drummond, I.A. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev. Biol. 2005, 285, 316–329. [Google Scholar] [CrossRef]
- Kramer-Zucker, A.G.; Olale, F.; Haycraft, C.J.; Yoder, B.K.; Schier, A.F.; Drummond, I.A. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development 2005, 132, 1907–1921. [Google Scholar] [CrossRef]
- Bollig, F.; Mehringer, R.; Perner, B.; Hartung, C.; Schäfer, M.; Schartl, M.; Volff, J.; Winkler, C.; Englert, C. Identification and comparative expression analysis of a second Wt1 gene in zebrafish. Dev. Dyn. 2006, 235, 554–561. [Google Scholar] [CrossRef]
- Perner, B.; Englert, C.; Bollig, F. The Wilms tumor genes Wt1a and Wt1b control different steps during formation of the zebrafish pronephros. Dev. Biol. 2007, 309, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jiang, Y.J. Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 2007, 3, e18. [Google Scholar] [CrossRef]
- Liu, Y.; Pathak, N.; Kramer-Zucker, A.; Drummond, I.A. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development 2007, 134, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, T.; Urban, J.M.; Gebhart, N.; Hatta, K.; Kawakami, K.; Ono, F. Formation of the spinal network in zebrafish determined by domain-specific pax genes. J. Comp. Neurol. 2011, 519, 1562–1579. [Google Scholar] [CrossRef]
- Tena, J.J.; Neto, A.; de la Calle-Mustienes, E.; Bras-Pereira, C.; Casares, F.; Gómez-Skarmeta, J.L. Odd-Skipped genes encode repressors that control kidney development. Dev. Biol. 2007, 301, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Mudumana, S.P.; Hentschel, D.; Liu, Y.; Vasilyev, A.; Drummond, I.A. odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development 2008, 135, 3355–3367. [Google Scholar] [CrossRef]
- Neto, A.; Mercader, N.; Gómez-Skarmeta, J.L. The Osr1 and Osr2 genes act in the pronephric anlage downstream of retinoic acid signaling and upstream of Wnt2b to maintain pectoral fin development. Development 2012, 139, 301–311. [Google Scholar] [CrossRef]
- Tomar, R.; Mudumana, S.P.; Pathak, N.; Hukriede, N.A.; Drummond, I.A. Osr1 is required for podocyte development downstream of Wt1a. J. Am. Soc. Nephrol. 2014, 25, 2539–2545. [Google Scholar] [CrossRef]
- Perens, E.A.; Garavito-Aguilar, Z.V.; Guio-Vega, G.P.; Peña, K.T.; Schindler, Y.L.; Yelon, D. Hand2 inhibits kidney specification while promoting vein formation within the posterior mesoderm. eLife 2016, 5, e19941. [Google Scholar] [CrossRef]
- Perens, E.A.; Diaz, J.T.; Quesnel, A.; Crump, J.G.; Yelon, D. osr1 couples intermediate mesoderm cell fate with temporal dynamics of vessel progenitor cell differentiation. Development 2021, 148, dev198408. [Google Scholar] [CrossRef] [PubMed]
- Drummond, B.E.; Chambers, B.E.; Wesselman, H.M.; Gibson, S.; Arceri, L.; Ulrich, M.N.; Gerlach, G.F.; Kroeger, P.T.; Leshchiner, I.; Goessling, W.; et al. osr1 maintains renal progenitors and regulates podocyte development by promoting wnt2ba via the antagonism of hand2. Biomedicines 2022, 10, 2868. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.A.; Selleck, R.; Yu, J.; Song, H.; Chen, Z.; Song, A.; Zhou, Y.; Thisse, B.; Thisse, C.; McMahon, A.P.; et al. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007, 3, e189. [Google Scholar] [CrossRef]
- Ebarasi, L.; He, L.; Hultenby, K.; Takemoto, M.; Betsholtz, C.; Tryggvason, K.; Majumdar, A. A reverse genetic screen in the zebrafish identifies Crb2b as a regulator of the glomerular filtration barrier. Dev. Biol. 2009, 334, 1–9. [Google Scholar] [CrossRef]
- Lyons, J.P.; Miller, R.K.; Zhou, X.; Weidinger, G.; Deroo, T.; Denayer, T.; Park, J.; Ji, H.; Hong, J.Y.; Li, A.; et al. Requirement of Wnt/Β-Catenin signaling in pronephric kidney development. Mech. Dev. 2009, 126, 142–159. [Google Scholar] [CrossRef] [PubMed]
- de Groh, E.D.; Swanhart, L.M.; Cosentino, C.C.; Jackson, R.L.; Dai, W.; Kitchens, C.A.; Day, B.W.; Smithgall, T.E.; Hukriede, N.A. Inhibition of histone deacetylase expands the renal progenitor cell population. J. Am. Soc. Nephrol. 2010, 21, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Wingert, R.A.; Davidson, A.J. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev. Dyn. 2011, 240, 2011–2027. [Google Scholar] [CrossRef]
- O’Brien, L.L.; Grimaldi, M.; Kostun, Z.; Wingert, R.A.; Selleck, R.; Davidson, A.J. Wt1a, Foxc1a, and the Notch Mediator Rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev. Biol. 2011, 358, 318–330. [Google Scholar] [CrossRef]
- Wang, H.; Lehtonen, S.; Chen, Y.C.; Heikkila, E.; Panula, P.; Holthofer, H. Neph3 associates with regulation of glomerular and neural development in zebrafish. Differentiation 2012, 83, 38–46. [Google Scholar] [CrossRef]
- Ichimura, K.; Powell, R.; Nakamura, T.; Kurihara, H.; Sakai, T.; Obara, T. Podocalyxin regulates pronephric glomerular development in zebrafish. Physiol. Rep. 2013, 1, e00074. [Google Scholar] [CrossRef]
- Naylor, R.W.; Przepiorski, A.; Ren, Q. HNF1B is essential for nephron segmentation during nephrogenesis. J. Am. Soc. Nephrol. 2013, 24, 77–87. [Google Scholar] [CrossRef]
- Gerlach, G.F.; Wingert, R.A. Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev. Biol. 2014, 396, 183–200. [Google Scholar] [CrossRef] [PubMed]
- McKee, R.; Gerlach, G.F.; Jou, J.; Cheng, C.N.; Wingert, R.A. Temporal and spatial expression of tight junction genes during zebrafish pronephros development. Gene Expr. Patterns 2014, 16, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Fukuyo, Y.; Nakamura, T.; Bubenshchikova, E.; Powell, R.; Tsuji, T.; Janknecht, R.; Obara, T. Nephrin and Podocin functions are highly conserved between the zebrafish pronephros and mammalian metanephros. Mol. Med. Rep. 2014, 9, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.N.; Li, Y.; Marra, A.N.; Verdun, V.; Wingert, R.A. Flat mount preparation for observation and analysis of zebrafish embryo specimens stained by whole mount in situ hybridization. J. Vis. Exp. 2014, 89, 51604. [Google Scholar]
- Cheng, C.N.; Wingert, R.A. Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in zebrafish. Dev. Biol. 2015, 399, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Pietsch, S.; Tan, Z.; Perner, B.; Sierig, R.; Kruspe, D.; Groth, M.; Witzgall, R.; Gröne, H.; Platzer, M.; et al. Integration of cistromic and transcriptomic analyses identifies Nphs2, Mafb, and Magi2 as Wilms’ Tumor 1 target genes in podocyte differentiation and maintenance. J. Am. Soc. Nephrol. 2015, 26, 2118–2128. [Google Scholar] [CrossRef]
- Marra, A.N.; Wingert, R.A. Epithelial cell fate in the nephron tubule is mediated by the ETS transcription factors etv5a and etv4 during zebrafish kidney development. Dev. Biol. 2016, 411, 231–245. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Zeng, C.; Wang, L.; Xu, F.; Hou, Q.; Liu, Z. Ultrastructural characterization of the pronephric glomerulus development in zebrafish. J. Morphol. 2016, 277, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, P.T.; Drummond, B.E.; Miceli, R.; McKernan, M.; Gerlach, G.F.; Marra, A.N.; Fox, A.; McCampbell, K.K.; Leshchiner, I.; Rodriguez-Mari, A.; et al. The zebrafish kidney mutant zeppelin reveals that brca2/fancd1 is essential for pronephros development. Dev. Biol. 2017, 428, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Boucher, R.C.; Bollig, F.; Englert, C.; Hildebrandt, F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am. J. Physiol. Renal Physiol. 2010, 299, F1040–F1047. [Google Scholar] [CrossRef]
- Diep, C.Q.; Peng, Z.; Ukah, T.K.; Kelly, P.M.; Daigle, R.V.; Davidson, A.J. Development of the zebrafish mesonephros. Genesis 2015, 53, 257–269. [Google Scholar] [CrossRef]
- Reimschuessel, R. A fish model of renal regeneration and development. ILAR J. 2001, 42, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.Q.; Ma, D.; Deo, R.C.; Holm, T.M.; Naylor, R.W.; Arora, N.; Wingert, R.A.; Bollig, F.; Djordjevic, G.; Lichman, B.; et al. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 2011, 470, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J. Uncharted waters: Nephrogenesis and renal regeneration in fish and mammals. Pediatr. Nephrol. 2011, 26, 1435–1443. [Google Scholar] [CrossRef]
- McCampbell, K.K.; Wingert, R.A. New tides: Using zebrafish to study renal regeneration. Transl. Res. 2012, 163, 109–122. [Google Scholar] [CrossRef]
- McCampbell, K.K.; Springer, K.N.; Wingert, R.A. Analysis of nephron composition and function in the adult zebrafish kidney. J. Vis. Exp. 2014, 90, e51644. [Google Scholar]
- McCampbell, K.K.; Springer, K.N.; Wingert, R.A. Atlas of cellular dynamics during zebrafish adult kidney regeneration. Stem Cells Int. 2015, 2015, 547636. [Google Scholar] [CrossRef]
- Kamei, C.N.; Gallegos, T.F.; Liu, Y.; Hukriede, N.; Drummond, I.A. Wnt signaling mediates new nephron formation during zebrafish kidney regeneration. Development 2019, 146, dev168294. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, T.; He, X.; Fu, Y.; Dai, L.; Wang, B.; Wu, Y.; He, J.; Li, Y.; Zhang, F.; et al. Dual roles of hydrogen peroxide in promoting zebrafish renal repair and regeneration. Biochem. Biophys. Res. Commun. 2019, 516, 680–685. [Google Scholar] [CrossRef]
- Gallegos, T.F.; Kamei, C.N.; Rohly, M.; Drummond, I.A. Fibroblast growth factor signaling mediates progenitor cell aggregation and nephron regeneration in the adult zebrafish kidney. Dev. Biol. 2019, 454, 44–51. [Google Scholar] [CrossRef]
- Liu, X.; Yu, T.; Tan, X.; Jin, D.; Yang, W.; Zhang, J.; Dai, L.; He, Z.; Li, D.; Zhang, Y.; et al. Renal interstitial cells promote nephron regeneration by secreting prostaglandin E2. eLife 2023, 12, e81438. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.B.; Westerfield, M. Zebrafish models in translational research: Tipping the scales toward advancements in human health. Dis. Model Mech. 2014, 7, 739–743. [Google Scholar] [CrossRef]
- Irion, U.; Nüsslein-Volhard, C. Developmental genetics with model organisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2122148119. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Lawson, N.D.; Wolfe, S.A. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev. Cell 2011, 21, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Molinari, E.; Sayer, J.A. Disease modeling to understand the pathomechanisms of human genetic kidney disorders. Clin. J. Am. Soc. Nephrol. 2020, 15, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Amsterdam, A.; Pazour, G.J.; Cole, D.G.; Miller, M.S.; Hopkins, N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 2004, 131, 4085–4093. [Google Scholar] [CrossRef]
- Kroeger, P.T.; Poureetezadi, S.J.; McKee, R.; Jou, J.; Miceli, R.; Wingert, R.A. Production of haploid zebrafish embryos by in vitro fertilization. J. Vis. Exp. 2014, 89, 51708. [Google Scholar]
- Ebarasi, L.; Ashraf, S.; Bierzynska, A.; Gee, H.Y.; McCarthy, H.J.; Lovric, S.; Sadowski, C.E.; Pabst, W.; Vega-Warner, V.; Fang, H.; et al. Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am. J. Hum. Genet. 2015, 96, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, J.; Pandey, G.; Westhoff, J.H. Zebrafish as a model for drug screening in genetic kidney diseases. Front. Pediatr. 2018, 6, 183. [Google Scholar] [CrossRef]
- Skromne, I.; Prince, V.E. Current perspectives in zebrafish reverse genetics: Moving forward. Dev. Dyn. 2008, 237, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Nasevicius, A.; Ekker, S.C. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000, 26, 216–220. [Google Scholar] [CrossRef]
- Draper, B.W.; Morcos, P.A.; Kimmel, C.B. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: A quantifiable method for gene knockdown. Genesis 2001, 30, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Doyon, Y.; McCammon, J.M.; Miller, J.C.; Faraji, F.; Ngo, C.; Katibah, G.E.; Amora, R.; Hocking, T.D.; Zhang, L.; Rebar, E.J.; et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 702–708. [Google Scholar] [CrossRef]
- Meng, X.; Noyes, M.B.; Zhu, L.J.; Lawson, N.D.; Wolfe, S.A. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat. Biotechnol. 2008, 26, 695–701. [Google Scholar] [CrossRef]
- Gupta, A.; Meng, X.; Zhu, L.J.; Lawson, N.D.; Wolfe, S.A. Zinc finger protein-dependent and -independent contributions to the in vivo off-target activity of zinc finger nucleases. Nucleic Acids Res. 2011, 39, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Smith, T.; McNulty, J.; Rayla, A.L.; Lakshmanan, A.; Siekmann, A.F.; Buffardi, M.; Meng, X.; Shin, J.; Padmanabhan, A.; et al. Evaluation and application of modularly assembled zinc-finger nucleases in zebrafish. Development 2011, 138, 4555–4564. [Google Scholar] [CrossRef]
- Miller, J.C.; Tan, S.; Qiao, G.; Barlow, K.A.; Wang, J.; Xia, D.F.; Meng, X.; Paschon, D.E.; Leung, E.; Hinkley, S.J.; et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011, 29, 143–148. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161. [Google Scholar] [CrossRef]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef]
- Auer, T.O.; Duroure, K.; De Cian, A.; Concordet, J.-P.; Del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2014, 24, 142–153. [Google Scholar] [CrossRef]
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407. [Google Scholar] [CrossRef]
- Tsai, S.Q.; Zheng, Z.; Nguyen, N.T.; Liebers, M.; Topkar, V.V.; Thapar, V.; Wyvekens, N.; Khayter, C.; Iafrate, A.J.; Le, L.P.; et al. GUIDE-Seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 2015, 33, 187–197. [Google Scholar] [CrossRef]
- Hoffmann, S.; Mullins, L.; Rider, S.; Brown, C.; Buckley, C.B.; Assmus, A.; Li, Z.; Sierra Beltran, M.; Henderson, N.; Del Pozo, J.; et al. Comparative studies of renin-null zebrafish and mice provide new functional insights. Hypertension 2022, 79, e56–e66. [Google Scholar] [CrossRef]
- Prykhozhij, S.V.; Cordeiro-Santanach, A.; Caceres, L.; Berman, J.N. Genome editing in zebrafish using high-fidelity Cas9 nucleases: Choosing the right nuclease for the task. Methods Mol. Biol. 2020, 2115, 385–405. [Google Scholar]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Holper, S.; Kruger, M.; Stainier, D.Y.R. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef]
- Outtandy, P.; Russell, C.; Kleta, R.; Bockenhauer, D. Zebrafish as a model for kidney function and disease. Pediatr. Nephrol. 2019, 34, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Scheer, N.; Campos-Ortega, J.A. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 1999, 80, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Thisse, C.; Thisse, B. Fast Release Clones: A High Throughput Expression Analysis. ZFIN Direct Data Submission. 2004. Available online: http://zfin.org/ZDB-PUB-040907-1 (accessed on 1 January 2023).
- Thisse, C.; Thisse, B. Expression from: Unexpected Novel Relational Links Uncovered by Extensive Developmental Profiling of Nuclear Receptor Expression. ZFIN Direct Data Submission. 2008. Available online: http://zfin.org/ZDB-PUB-080220-1 (accessed on 1 January 2023).
- Naylor, R.W.; Qubisi, S.S.; Davidson, A.J. Zebrafish pronephros development. Results Probl. Cell Differ. 2017, 60, 27–53. [Google Scholar] [PubMed]
- Li, Y.; Cheng, C.N.; Verdun, V.A.; Wingert, R.A. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev. Biol. 2014, 386, 111–122. [Google Scholar] [CrossRef]
- Drummond, B.E.; Li, Y.; Marra, A.N.; Cheng, C.N.; Wingert, R.A. The tbx2a/b transcription factors direct pronephros segmentation and corpuscle of Stannius formation in zebrafish. Dev. Biol. 2017, 421, 52–66. [Google Scholar] [CrossRef]
- Snelson, C.D.; Santhakumar, K.; Halpern, M.E.; Gamse, J.T. tbx2b is required for the development of the parapineal organ. Development 2008, 135, 1693–1702. [Google Scholar] [CrossRef][Green Version]
- Morales, E.E.; Handa, N.; Drummond, B.E.; Chambers, J.M.; Marra, A.N.; Addiego, A.; Wingert, R.A. Homeogene emx1 is required for nephron distal segment development in zebrafish. Sci. Rep. 2018, 8, 18038. [Google Scholar] [CrossRef]
- Chambers, J.M.; Poureetezadi, S.J.; Addiego, A.; Lahne, M.; Wingert, R.A. ppargc1a controls nephron segmentation during zebrafish embryonic kidney ontogeny. eLife 2018, 7, e40266. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, L.; Raciti, D.; Airik, R.; Kispert, A.; Brandli, A.W. The prepattern transcription factor Irx3 directs nephron segment identity. Genes. Dev. 2007, 21, 2358–2370. [Google Scholar] [CrossRef]
- Naylor, R.W.; Chang, H.G.; Qubisi, S.; Davidson, A.J. A novel mechanism of gland formation in zebrafish involving transdifferentiation of renal epithelial cells and live cell extrusion. eLife 2018, 7, e38911. [Google Scholar] [CrossRef]
- Poureetezadi, S.J.; Cheng, C.N.; Chambers, J.M.; Drummond, B.E.; Wingert, R.A. Prostaglandin signaling regulates nephron segment patterning of renal progenitors during zebrafish kidney development. eLife 2016, 5, e17551. [Google Scholar] [CrossRef]
- Marra, A.N.; Adeeb, B.D.; Chambers, B.E.; Drummond, B.E.; Ulrich, M.; Addiego, A.; Springer, M.; Poureetezadi, S.J.; Chambers, J.M.; Ronshaugen, M.; et al. Prostaglandin signaling regulates renal multiciliated cell specification and maturation. Proc. Natl. Acad. Sci. USA 2019, 116, 8409–8418. [Google Scholar] [CrossRef]
- Chambers, B.E.; Gerlach, G.F.; Clark, E.G.; Chen, K.H.; Levesque, A.E.; Leshchiner, I.; Goessling, W.; Wingert, R.A. Tfap2a is a novel gatekeeper of nephron differentiation during kidney development. Development 2019, 146, dev172387. [Google Scholar] [CrossRef]
- Chambers, B.E.; Clark, E.G.; Gatz, A.E.; Wingert, R.A. Kctd15 regulates nephron segment development by repressing Tfap2a activity. Development 2020, 147, dev191973. [Google Scholar] [CrossRef]
- Zarelli, V.E.; Dawid, I.B. Inhibition of neural crest formation by Kctd15 involves regulation of transcription factor AP-2. Proc. Natl. Acad. Sci. USA 2013, 110, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.; Rebbert, M.; Wang, C.; Chen, X.; Heffer, A.; Zarelli, V.E.; Dawid, I.B.; Zhao, H. Genes regulated by potassium channel tetramerization domain containing 15 (Kctd15) in the developing neural crest. Int. J. Dev. Biol. 2016, 60, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Suzuki, T.; Nishida, E.; Kusakabe, M. Identification and characterization of Xenopus kctd15, an ectodermal gene repressed by the FGF pathway. Int. J. Dev. Biol. 2012, 56, 393–402. [Google Scholar] [CrossRef]
- Dutta, S.; Dawid, I.B. Kctd15 inhibits neural crest formation by attenuating Wnt/β-catenin signaling output. Development 2010, 137, 3013–3018. [Google Scholar] [CrossRef]
- Chambers, J.M.; Addiego, A.; Flores-Mireles, A.L.; Wingert, R.A. Ppargc1a controls ciliated cell development by regulating prostaglandin biosynthesis. Cell Rep. 2020, 33, 108370. [Google Scholar] [CrossRef]
- Marra, A.N.; Cheng, C.N.; Adeeb, B.; Addiego, A.; Wesselman, H.M.; Chambers, B.E.; Chambers, J.M.; Wingert, R.A. Iroquois transcription factor irx2a is required for multiciliated and transporter cell fate decisions during zebrafish pronephros development. Sci. Rep. 2019, 9, 6454. [Google Scholar] [CrossRef]
- Weaver, N.E.; Healy, A.; Wingert, R.A. gldc is essential for renal progenitor patterning during kidney development. Biomedicines 2022, 10, 3220. [Google Scholar] [CrossRef] [PubMed]
- Wesselman, H.M.; Gatz, A.E.; Pfaff, M.R.; Arceri, L.; Wingert, R.A. Estrogen signaling influences nephron segmentation of the zebrafish embryonic kidney. Cells 2023, 12, 666. [Google Scholar] [CrossRef]
- Tang, Q.; Iyer, S.; Lobbardi, R.; Moore, J.C.; Chen, H.; Lareau, C.; Hebert, C.; Shaw, M.L.; Neftel, C.; Suva, M.L.; et al. Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J. Exp. Med. 2017, 214, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Cianciolo Cosentino, C.; Loffing-Cueni, D.; Neuhauss, S.C.F.; Loffing, J. Comparative transcriptomic analysis identifies evolutionarily conserved gene products in the vertebrate renal distal convoluted tubule. Pflügers Arch.-Eur. J. Physiol. 2017, 469, 859–867. [Google Scholar] [CrossRef]
- Sander, V.; Salleh, L.; Naylor, R.W.; Schierding, W.; Sontam, D.; O’Sullivan, J.M.; Davidson, A.J. Transcriptional profiling of the zebrafish proximal tubule. Am. J. Physiol. Renal Physiol. 2019, 317, F478–F488. [Google Scholar] [CrossRef]
- Brown, C.; Mullins, L.J.; Wesencraft, K.; McConnell, G.; Beltran, M.; Henderson, N.C.; Conway, B.; Hoffmann, S.; Rider, S.; Mullins, J.J. scRNA transcription profile of adult zebrafish podocytes using a novel reporter strain. Cell Physiol. Biochem. 2021, 55, 35–47. [Google Scholar] [PubMed]
- Corkins, M.E.; Achieng, M.; DeLay, B.D.; Krneta-Stankic, V.; Cain, M.P.; Walker, B.L.; Chen, J.; Lindström, N.O.; Miller, R.K. A comparative study of cellular diversity between the Xenopus pronephric and mouse metanephric nephron. Kidney Int. 2023, 103, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Chambers, B.E.; Weaver, N.E.; Wingert, R.A. The “3Ds” of Growing Kidney Organoids: Advances in Nephron Development, Disease Modeling, and Drug Screening. Cells 2023, 12, 549. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).