Life-Saver or Undertaker: The Relationship between Primary Cilia and Cell Death in Vertebrate Embryonic Development
Abstract
1. Introduction
2. IFT Proteins and Cell Death
3. Ciliary Base Proteins and Cell Death
4. Does the Number of Primary Cilia Correlate with the Apoptosis Rate?
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, L.; Scholey, J. Intraflagellar transport at a glance. J. Cell Sci. 2009, 122, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Leroux, M. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Craige, B.; Tsao, C.; Diener, D.; Hou, Y.; Lechtreck, K.; Rosenbaum, J.; Witman, G. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 2010, 190, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Li, C.; Kida, K.; Inglis, P.; Mohan, S.; Semenec, L.; Bialas, N.; Stupay, R.; Chen, N.; Blacque, O.; et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 2011, 192, 1023–1041. [Google Scholar] [CrossRef]
- Warburton-Pitt, S.; Jauregui, A.; Li, C.; Wang, J.; Leroux, M.; Barr, M. Ciliogenesis in Caenorhabditis elegans requires genetic interactions between ciliary middle segment localized NPHP-2 (inversin) and transition zone-associated proteins. J. Cell Sci. 2012, 125, 2592–2603. [Google Scholar]
- Awata, J.; Takada, S.; Standley, C.; Lechtreck, K.; Bellvé, K.; Pazour, G.; Fogarty, K.; Witman, G. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J. Cell Sci. 2014, 127, 4714–4727. [Google Scholar]
- Li, C.; Jensen, V.; Park, K.; Kennedy, J.; Garcia-Gonzalo, F.; Romani, M.; De Mori, R.; Bruel, A.; Gaillard, D.; Doray, B.; et al. MKS5 and CEP290 Dependent Assembly Pathway of the Ciliary Transition Zone. PLoS Biol. 2016, 14, e1002416. [Google Scholar] [CrossRef]
- Lin, H.; Guo, S.; Dutcher, S. RPGRIP1L helps to establish the ciliary gate for entry of proteins. J. Cell Sci. 2018, 131, jcs220905. [Google Scholar] [CrossRef]
- Wiegering, A.; Dildrop, R.; Vesque, C.; Khanna, H.; Schneider-Maunoury, S.; Gerhardt, C. Rpgrip1l controls ciliary gating by ensuring the proper amount of Cep290 at the vertebrate transition zone. Mol. Biol. Cell. 2021, mbcE20030190. [Google Scholar] [CrossRef]
- Sang, L.; Miller, J.; Corbit, K.; Giles, R.; Brauer, M.; Otto, E.; Baye, L.; Wen, X.; Scales, S.; Kwong, M.; et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 2011, 145, 513–528. [Google Scholar] [CrossRef]
- Czarnecki, P.G.; Shah, J.V. The ciliary transition zone: From morphology and molecules to medicine. Trends in Cell Biol. 2012, 22, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Wiegering, A.; Dildrop, R.; Kalfhues, L.; Spychala, A.; Kuschel, S.; Lier, J.; Zobel, T.; Dahmen, S.; Leu, T.; Struchtrup, A.; et al. Cell type-specific regulation of ciliary transition zone assembly in vertebrates. EMBO J. 2018, 37, e97791. [Google Scholar] [CrossRef] [PubMed]
- Akella, J.; Silva, M.; Morsci, N.; Nguyen, K.; Rice, W.; Hall, D.; Barr, M. Cell type-specific structural plasticity of the ciliary transition zone in C. elegans. Biol. Cell 2019, 111, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Fisch, C.; Dupuis-Williams, P. Ultrastructure of cilia and flagella - back to the future! Biol. Cell 2011, 103, 249–270. [Google Scholar] [CrossRef]
- Quinlan, R.; Tobin, J.; Beales, P. Modeling ciliopathies: Primary cilia in development and disease. Curr. Top. Dev. Biol. 2008, 84, 249–310. [Google Scholar] [CrossRef]
- Wheway, G.; Lord, J.; Baralle, D. Splicing in the pathogenesis, diagnosis and treatment of ciliopathies. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194433. [Google Scholar] [CrossRef]
- Castresana, J. Cancer as a Ciliopathy: The Primary Cilium as a New Therapeutic Target. J. Carcinog. Mutagene 2015, 6, e119. [Google Scholar] [CrossRef]
- Gerhardt, C.; Leu, T.; Lier, J.; Rüther, U. The cilia-regulated proteasome and its role in the development of ciliopathies and cancer. Cilia 2016, 5, 14. [Google Scholar] [CrossRef]
- Higgins, M.; Obaidi, I.; McMorrow, T. Primary cilia and their role in cancer. Oncol. Lett. 2019, 17, 3041–3047. [Google Scholar] [CrossRef]
- Ki, S.; Jeong, H.; Lee, J. Primary Cilia in Glial Cells: An Oasis in the Journey to Overcoming Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 736888. [Google Scholar] [CrossRef]
- Liu, H.; Kiseleva, A.; Golemis, E. Ciliary signalling in cancer. Nat. Rev. Cancer 2018, 18, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Kutchy, N.; Chen, L.; Meigs, D.; Hu, G. Primary cilia and ciliary signaling pathways in aging and age-related brain disorders. Neurobiol. Dis. 2022, 163, 105607. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Seol, A.; So, P.; Ermilov, A.; Bichakjian, C.; Epstein, E.J.; Dlugosz, A.; Reiter, J. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat. Med. 2009, 15, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Basten, S.; Willekers, S.; Vermaat, J.; Slaats, G.; Voest, E.; van Diest, P.; Giles, R. Reduced cilia frequencies in human renal cell carcinomas versus neighboring parenchymal tissue. Cilia 2013, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Arjumand, W.; Sultana, S. Role of VHL gene mutation in human renal cell carcinoma. Tumour Biol. 2012, 33, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.; Harten, S.; Tran, M.; Maxwell, P. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J. Am. Soc. Nephrol. 2006, 17, 1801–1806. [Google Scholar] [CrossRef]
- Guadiana, S.; Semple-Rowland, S.; Daroszewski, D.; Madorsky, I.; Breunig, J.; Mykytyn, K.; Sarkisian, M. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J. Neurosci. 2013, 33, 2626–2638. [Google Scholar] [CrossRef]
- Wheway, G.; Nazlamova, L.; Hancock, J. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.; Christensen, S. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef]
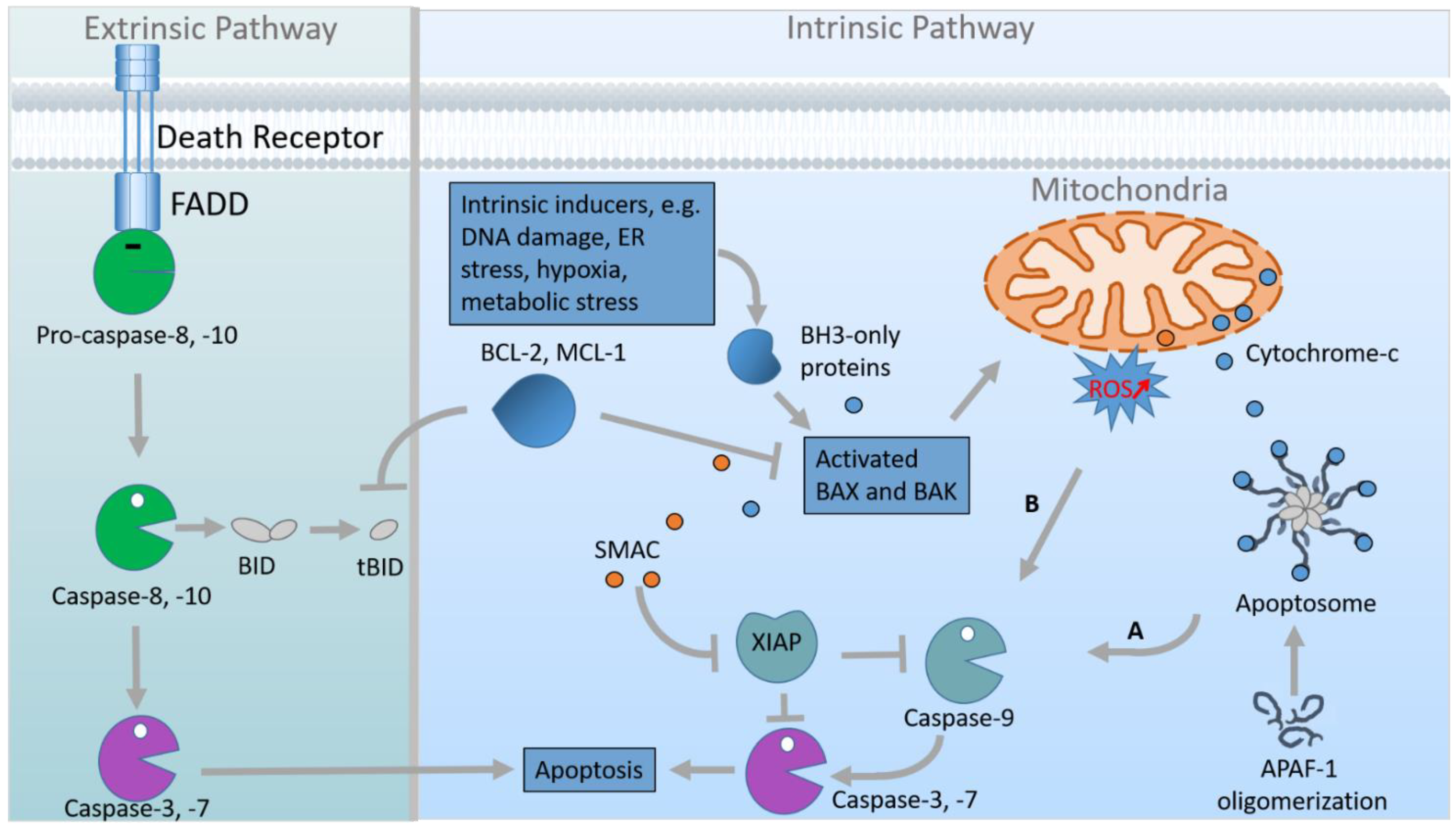
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.; Abrams, J.; Adam, D.; Agostinis, P.; Alnemri, E.; Altucci, L.; Amelio, I.; Andrews, D.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Conradt, B. Genetic control of programmed cell death during animal development. Annu. Rev. Genet. 2009, 43, 493–523. [Google Scholar] [CrossRef] [PubMed]
- Ghose, P.; Shaham, S. Cell death in animal development. Development 2020, 147, dev191882. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Voss, A.; Strasser, A. The essentials of developmental apoptosis. F1000Res. 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sui, X.; Ren, H. From procaspase-8 to caspase-8: Revisiting structural functions of caspase-8. J. Cell. Physiol. 2010, 225, 316–320. [Google Scholar] [CrossRef]
- Green, D.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Bratton, S.; Salvesen, G. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 2010, 123, 3209–3214. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.; Ahmad, M.; Alnemri, E.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Indrieri, A.; Conte, I.; Chesi, G.; Romano, A.; Quartararo, J.; Tatè, R.; Ghezzi, D.; Zeviani, M.; Goffrini, P.; Ferrero, I.; et al. The impairment of HCCS leads to MLS syndrome by activating a non-canonical cell death pathway in the brain and eyes. EMBO Mol. Med. 2013, 5, 280–293. [Google Scholar] [CrossRef]
- Bazzi, H.; Anderson, K. Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo. Proc. Natl. Acad. Sci. USA 2014, 111, E1491–E1500. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.; Perkins, B. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 Intraflagellar Transport mutants. Vision Res. 2009, 49, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, P.; Davison, C.; Casanova, G.; Badano, J.; Zolessi, F. Characterization of primary cilia during the differentiation of retinal ganglion cells in the zebrafish. Neural Dev. 2016, 11, 10. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Malicki, J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 2004, 42, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Vion, A.; Alt, S.; Klaus-Bergmann, A.; Szymborska, A.; Zheng, T.; Perovic, T.; Hammoutene, A.; Oliveira, M.; Bartels-Klein, E.; Hollfinger, I.; et al. Primary cilia sensitize endothelial cells to BMP and prevent excessive vascular regression. J. Cell Biol. 2018, 217, 1651–1665. [Google Scholar] [CrossRef]
- Gorivodsky, M.; Mukhopadhyay, M.; Wilsch-Braeuninger, M.; Phillips, M.; Teufel, A.; Kim, C.; Malik, N.; Huttner, W.; Westphal, H. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev. Biol. 2009, 325, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, K.; Sul, H.; Hong, H.; Kim, K.; Kero, J.; Shong, M. Loss of primary cilia promotes mitochondria-dependent apoptosis in thyroid cancer. Sci. Rep. 2021, 11, 4181. [Google Scholar] [CrossRef]
- Robert, A.; Margall-Ducos, G.; Guidotti, J.; Brégerie, O.; Celati, C.; Bréchot, C.; Desdouets, C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell Sci. 2007, 120, 628–637. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, K.; Oh, H.; Ji-Ae, K.; Kim, H.; Cho, H.; Lee, J.; Yeong Ko, J.; Choi, J.; Jeong, Y.; et al. IFT46 plays an essential role in cilia development. Dev. Biol. 2015, 400, 248–257. [Google Scholar] [CrossRef]
- Schock, E.; Chang, C.; Struve, J.; Chang, Y.; Chang, J.; Delany, M.; Brugmann, S. Using the avian mutant talpid2 as a disease model for understanding the oral-facial phenotypes of oral-facial-digital syndrome. Dis. Model. Mech. 2015, 8, 855–866. [Google Scholar] [CrossRef]
- Dvorak, L.; Fallon, J. Talpid2 mutant chick limb has anteroposterior polarity and altered patterns of programmed cell death. Anat. Rec. 1991, 231, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Schock, E.; O’Hare, E.; Dodgson, J.; Cheng, H.; Muir, W.; Edelmann, R.; Delany, M.; Brugmann, S. The cellular and molecular etiology of the craniofacial defects in the avian ciliopathic mutant talpid2. Development 2014, 141, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.r.; Reiter, J. A primer on the mouse basal body. Cilia 2016, 5, 17. [Google Scholar] [CrossRef]
- Ye, X.; Zeng, H.; Ning, G.; Reiter, J.; Liu, A. C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.; Bonatto Paese, C.; Carroll, A.; Struve, J.; Nagy, N.; Brugmann, S. Mutation in the Ciliary Protein C2CD3 Reveals Organ-Specific Mechanisms of Hedgehog Signal Transduction in Avian Embryos. J. Dev. Biol. 2021, 9, 12. [Google Scholar] [CrossRef]
- Hoover, A.; Wynkoop, A.; Zeng, H.; Jia, J.; Niswander, L.; Liu, A. C2cd3 is required for cilia formation and Hedgehog signaling in mouse. Development 2008, 135, 4049–4058. [Google Scholar] [CrossRef]
- Thauvin-Robinet, C.; Lee, J.; Lopez, E.; Herranz-Pérez, V.; Shida, T.; Franco, B.; Jego, L.; Ye, F.; Pasquier, L.; Loget, P.; et al. The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nat. Genet. 2014, 46, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.; Reiter, J. Ciliary Hedgehog signaling regulates cell survival to build the facial midline. Elife 2021, 10, e68558. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Dong, H.; Gong, S.; Wei, B.; Luo, M.; Wang, H.; Wu, X.; Liu, W.; Xu, X.; et al. Loss of Tctn3 causes neuronal apoptosis and neural tube defects in mice. Cell Death Dis. 2018, 9, 520. [Google Scholar] [CrossRef]
- Tiwari, S.; Hudson, S.; Gattone, V.n.; Miller, C.; Chernoff, E.; Belecky-Adams, T. Meckelin 3 is necessary for photoreceptor outer segment development in rat Meckel syndrome. PLoS ONE 2013, 8, e59306. [Google Scholar] [CrossRef]
- Weng, R.; Yang, T.; Huang, C.; Chang, C.; Wang, W.; Liao, J. Super-Resolution Imaging Reveals TCTN2 Depletion-Induced IFT88 Lumen Leakage and Ciliary Weakening. Biophys. J. 2018, 115, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalo, F.; Corbit, K.; Sirerol-Piquer, M.; Ramaswami, G.; Otto, E.; Noriega, T.; Seol, A.; Robinson, J.; Bennett, C.; Josifova, D.; et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 2011, 43, 776–784. [Google Scholar] [CrossRef]
- Aoto, K.; Trainor, P. Co-ordinated brain and craniofacial development depend upon Patched1/XIAP regulation of cell survival. Hum. Mol. Genet. 2015, 24, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Nishizaki, Y.; Hui, C.; Nakafuku, M.; Kondoh, H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: Implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 1999, 126, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Aoto, K.; Nishimura, T.; Eto, K.; Motoyama, J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev. Biol. 2002, 251, 320–332. [Google Scholar] [CrossRef]
- Bastida, M.; Delgado, M.; Wang, B.; Fallon, J.; Fernandez-Teran, M.; Ros, M. Levels of Gli3 repressor correlate with Bmp4 expression and apoptosis during limb development. Dev. Dyn. 2004, 231, 148–160. [Google Scholar] [CrossRef]
- Matissek, S.; Elsawa, S. GLI3: A mediator of genetic diseases, development and cancer. Cell Commun. Signal. 2020, 18, 54. [Google Scholar] [CrossRef]
- Gerhardt, C.; Lier, J.; Burmühl, S.; Struchtrup, A.; Deutschmann, K.; Vetter, M.; Leu, T.; Reeg, S.; Grune, T.; Rüther, U. The transition zone protein Rpgrip1l regulates proteasomal activity at the primary cilium. J. Cell Biol. 2015, 210, 115–133. [Google Scholar] [CrossRef]
- Gerhardt, C.; Lier, J.; Kuschel, S.; Rüther, U. The ciliary protein Ftm is required for ventricular wall and septal development. PLoS ONE 2013, 8, e57545. [Google Scholar] [CrossRef]
- Wang, L.; De Solis, A.; Goffer, Y.; Birkenbach, K.; Engle, S.; Tanis, R.; Levenson, J.; Li, X.; Rausch, R.; Purohit, M.; et al. Ciliary gene RPGRIP1L is required for hypothalamic arcuate neuron development. JCI Insight 2019, 4, e123337. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, M.; Peng, W.; Li, Q.; Feng, Z.; Li, Z.; Wang, M.; Feng, X.; Liu, F.; Huang, J. Sonic hedgehog signalling pathway regulates apoptosis through Smo protein in human umbilical vein endothelial cells. Rheumatology 2015, 54, 1093–1102. [Google Scholar] [CrossRef]
- De-Castro, A.; Rodrigues, D.; De-Castro, M.; Vieira, N.; Vieira, C.; Carvalho, A.; Gassmann, R.; Abreu, C.; Dantas, T. WDR60-mediated dynein-2 loading into cilia powers retrograde IFT and transition zone crossing. J. Cell Biol. 2022, 221, e202010178. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Dong, M.; Yu, H.; Xie, Y.; Yu, Y.; Cao, Y.; Kong, Z.; Zhou, B.; Xu, Y.; Yang, T.; et al. Knockdown of TCTN1 Strongly Decreases Growth of Human Colon Cancer Cells. Med. Sci. Monit. 2017, 23, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xia, X.; Yang, Z.; Tian, Y.; Di, J.; Guo, M. Silencing of TCTN1 inhibits proliferation, induces cell cycle arrest and apoptosis in human thyroid cancer. Exp. Ther. Med. 2017, 14, 3720–3726. [Google Scholar] [CrossRef] [PubMed]
- Wodarczyk, C.; Distefano, G.; Rowe, I.; Gaetani, M.; Bricoli, B.; Muorah, M.; Spitaleri, A.; Mannella, V.; Ricchiuto, P.; Pema, M.; et al. Nephrocystin-1 forms a complex with polycystin-1 via a polyproline motif/SH3 domain interaction and regulates the apoptotic response in mammals. PLoS ONE 2010, 5, e12719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, X. Apoptosis in Polycystic Kidney Disease: From Pathogenesis to Treatment. In Polycystic Kidney Disease; Li, X., Ed.; Codon Publications: Brisbane, Australia, 2015. [Google Scholar]
- Choi, S.; Chacon-Heszele, M.; Huang, L.; McKenna, S.; Wilson, F.; Zuo, X.; Lipschutz, J. Cdc42 deficiency causes ciliary abnormalities and cystic kidneys. J. Am. Soc. Nephrol. 2013, 24, 1435–1450. [Google Scholar] [CrossRef]
- Hafsia, N.; Forien, M.; Renaudin, F.; Delacour, D.; Reboul, P.; Van Lent, P.; Cohen-Solal, M.; Lioté, F.; Poirier, F.; Ea, H. Galectin 3 Deficiency Alters Chondrocyte Primary Cilium Formation and Exacerbates Cartilage Destruction via Mitochondrial Apoptosis. Int. J. Mol. Sci. 2020, 21, 1486. [Google Scholar] [CrossRef]
- Farooq, M.; Lindbæk, L.; Krogh, N.; Doganli, C.; Keller, C.; Mönnich, M.; Gonçalves, A.; Sakthivel, S.; Mang, Y.; Fatima, A.; et al. RRP7A links primary microcephaly to dysfunction of ribosome biogenesis, resorption of primary cilia, and neurogenesis. Nat. Commun. 2020, 11, 5816. [Google Scholar] [CrossRef]
- Ding, W.; Wu, Q.; Sun, L.; Pan, N.; Wang, X. Cenpj Regulates Cilia Disassembly and Neurogenesis in the Developing Mouse Cortex. J. Neurosci. 2019, 39, 1994–2010. [Google Scholar] [CrossRef]
- Forschbach, V.; Goppelt-Struebe, M.; Kunzelmann, K.; Schreiber, R.; Piedagnel, R.; Kraus, A.; Eckardt, K.; Buchholz, B. Anoctamin 6 is localized in the primary cilium of renal tubular cells and is involved in apoptosis-dependent cyst lumen formation. Cell Death Dis. 2015, 6, e1899. [Google Scholar] [CrossRef]
- Porter, A.; Jänicke, R. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Grasl-Kraupp, B.; Ruttkay-Nedecky, B.; Koudelka, H.; Bukowska, K.; Bursch, W.; Schulte-Hermann, R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: A cautionary note. Hepatology 1995, 21, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
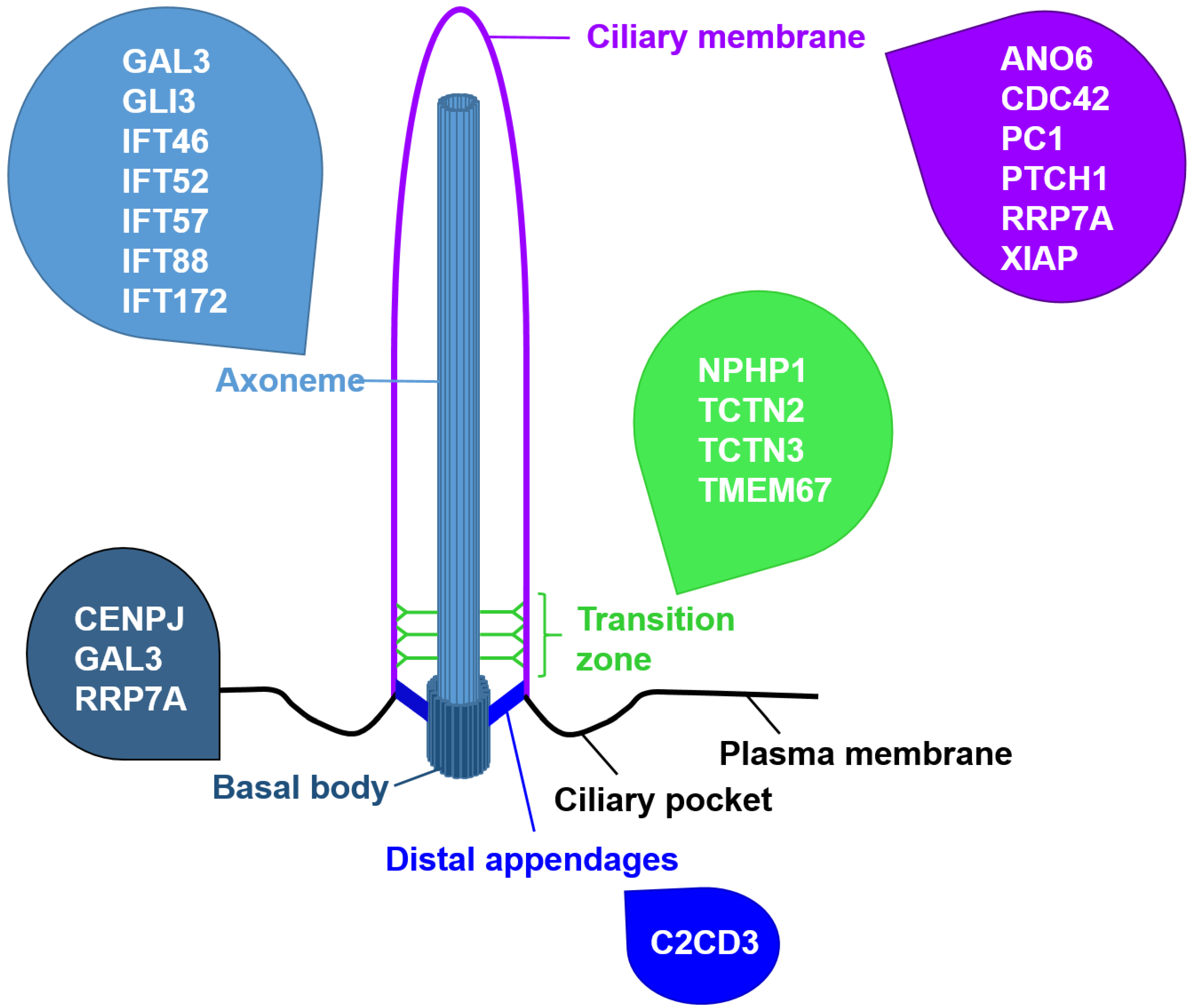
| Name of the Protein | Impact on Apoptosis |
|---|---|
| ANO6 | Ano6-deficient renal epithelial cells display reduced apoptosis [81] |
| C2CD3 | Limb buds and limbs of talpid2 (ta2) mutant avian embryos present reduced apoptosis [51] |
| CDC42 | Cdc42−/− mouse embryonic kidneys exhibit elevated apoptosis [77] |
| CENPJ | CENPJ-deficient developing mouse cerebrum cortices display increased apoptosis [80] |
| GAL3 | Loss of GAL3 leads to enhanced chondrocyte apoptosis [78] |
| GLI3 | GLI3 regulates apoptosis in the developing neural tube, face, and limb buds of mice [65,66,67] |
| IFT46 | Overexpression of IFT46 in zebrafish embryos induces excessive apoptosis in the central nervous system [49] |
| IFT52 | Ift52 mutant zebrafish show an increased apoptosis of photoreceptors in the eyes [44] |
| IFT57 | Ift57 mutant zebrafish show an increased apoptosis of photoreceptors in the eyes [42] |
| IFT88 | Ift88-deficient zebrafish retinas exhibit increased apoptosis [43]; Ift88−/− zebrafish display photoreceptor apoptosis [42]; Ift88-deficient zebrafish ears show increased cell death [44]; Tg-Cre;Ift88flox/flox mice suffering from thyrocyte-specific loss of primary cilia display increased apoptosis of thyrocytes [47]; overexpression of IFT88 in HeLa cells caused apoptosis in 40% of the overexpressing cells [48] |
| IFT172 | Ift172 mutant zebrafish show an increased apoptosis of photoreceptors in the eyes [42] |
| NPHP1 | Depletion of NPHP1 in MDCK cells led to elevated apoptosis and cystic kidneys of patients with mutations in NPHP1 exhibit elevated apoptosis [75] |
| PC1 | Overexpression of PC1 protects against apoptosis stimulation [76] |
| PTCH1 | PTCH1 promotes apoptosis in a cell type-specific manner by regulating CASP9 activity [63] |
| RRP7A | Loss of RRP7A in zebrafish results in enhanced apoptosis [79] |
| SMO | SMO negatively regulates apoptosis in endothelial cells [71] |
| TCTN2 | Tctn2-negative mouse embryos display increased apoptosis in the ventral neuroectoderm and facial ectoderm [58] |
| TCTN3 | Loss of TCTN3 results in increased apoptosis in the brain of murine embryos [59] |
| TMEM67 | Tmem67 mutant rats show elevated apoptosis in the eyes [60] |
| XIAP | By binding to the C terminus of PTCH1, XIAP mediates its apoptosis-promoting function [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfirrmann, T.; Gerhardt, C. Life-Saver or Undertaker: The Relationship between Primary Cilia and Cell Death in Vertebrate Embryonic Development. J. Dev. Biol. 2022, 10, 52. https://doi.org/10.3390/jdb10040052
Pfirrmann T, Gerhardt C. Life-Saver or Undertaker: The Relationship between Primary Cilia and Cell Death in Vertebrate Embryonic Development. Journal of Developmental Biology. 2022; 10(4):52. https://doi.org/10.3390/jdb10040052
Chicago/Turabian StylePfirrmann, Thorsten, and Christoph Gerhardt. 2022. "Life-Saver or Undertaker: The Relationship between Primary Cilia and Cell Death in Vertebrate Embryonic Development" Journal of Developmental Biology 10, no. 4: 52. https://doi.org/10.3390/jdb10040052
APA StylePfirrmann, T., & Gerhardt, C. (2022). Life-Saver or Undertaker: The Relationship between Primary Cilia and Cell Death in Vertebrate Embryonic Development. Journal of Developmental Biology, 10(4), 52. https://doi.org/10.3390/jdb10040052






