Tissue Rotation of the Xenopus Anterior–Posterior Neural Axis Reveals Profound but Transient Plasticity at the Mid-Gastrula Stage
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Embryo Collection
2.2. Neural Ectoderm Transplantation
2.3. In Situ Hybridization, FlDx Detection, and Whole Mount Imaging
2.4. Histology and Imaging of Slides
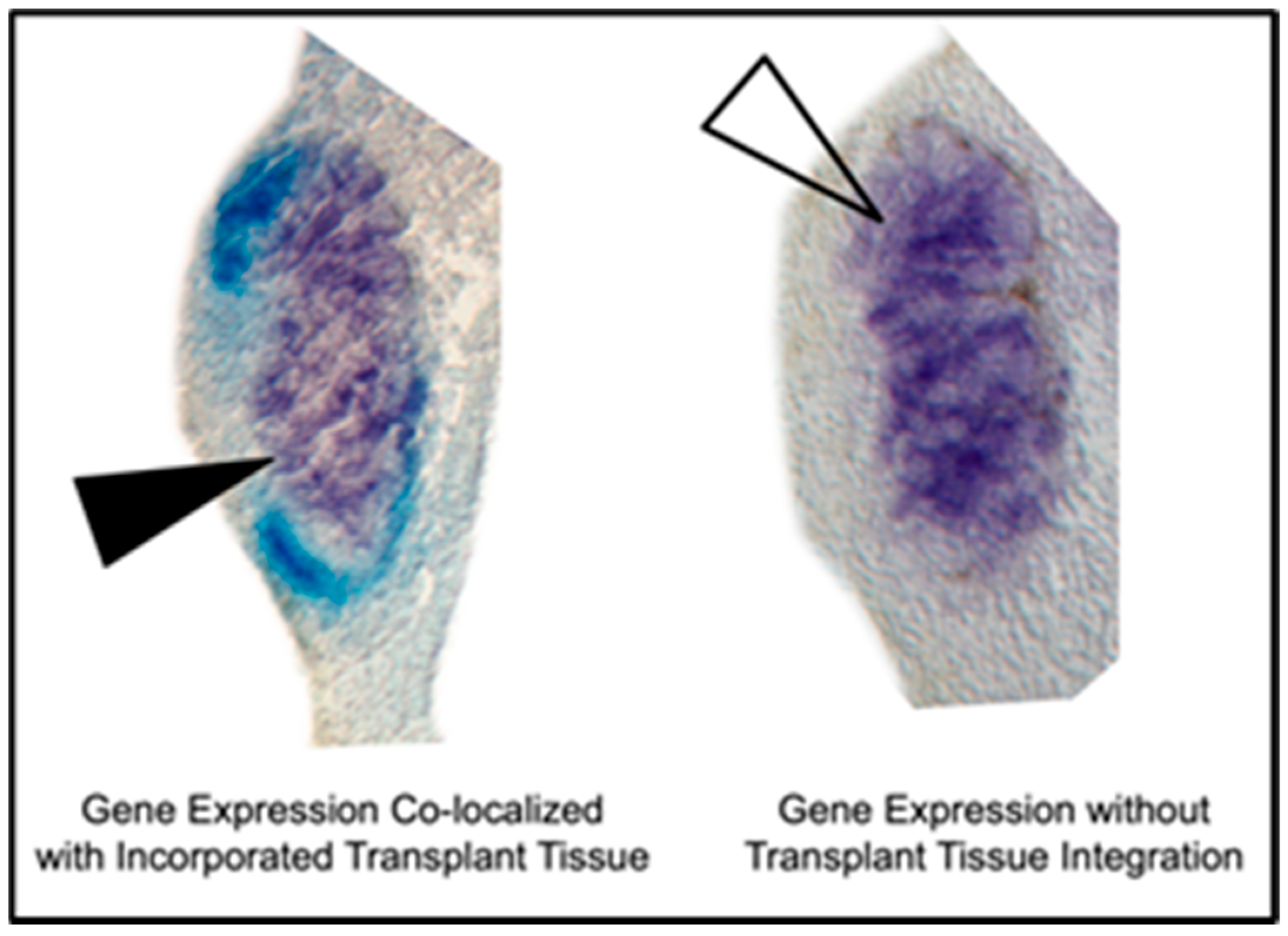
2.5. Histological Gene Expression Analysis
3. Results
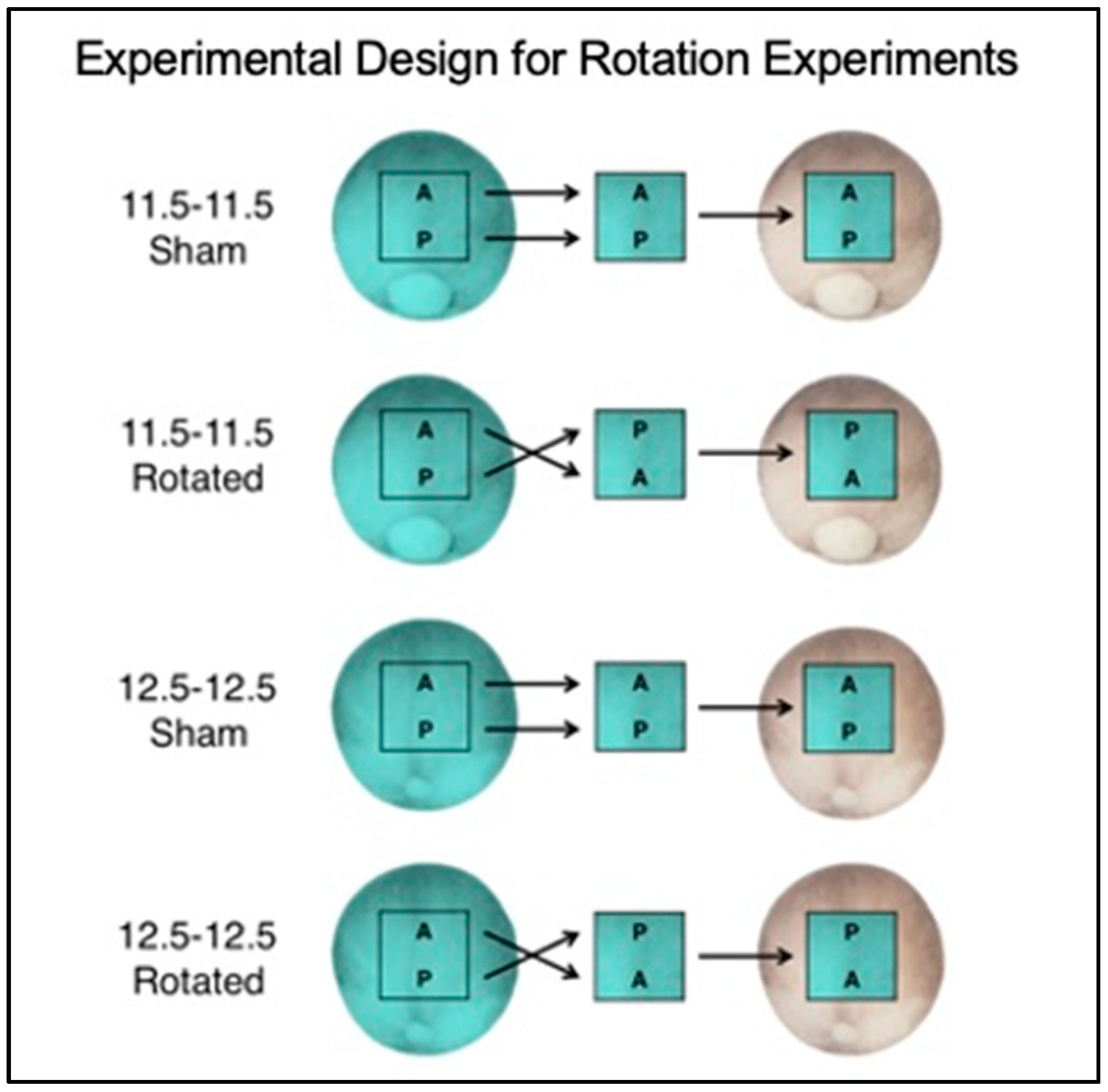
3.1. Experimental Overview
3.2. Mid-Gastrula Rotated Embryos Display Relatively Normal Morphology while Late Gastrula Rotated Embryos Show Significant Abnormality
3.3. Mid-Gastrula Rotated Embryos Display Comparatively Normal Regional Marker Gene Expression Compared to Aberrant, Ectopic Gene Expression in Late Gastrula Rotated Embryos
3.3.1. Overview
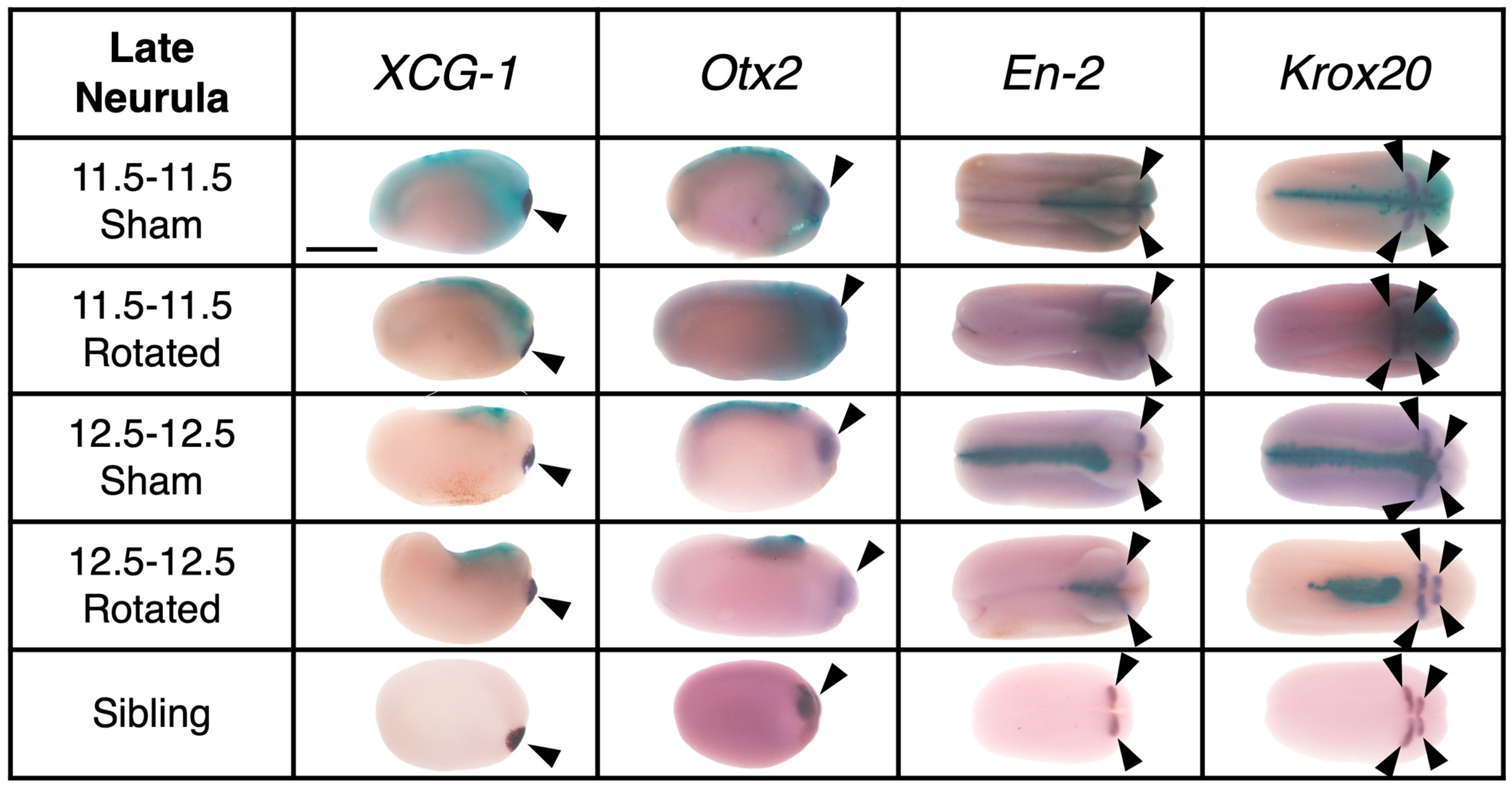
3.3.2. Transplants Assayed at Late Neurula Stages
3.3.3. Transplants Assayed at Hatching Stages
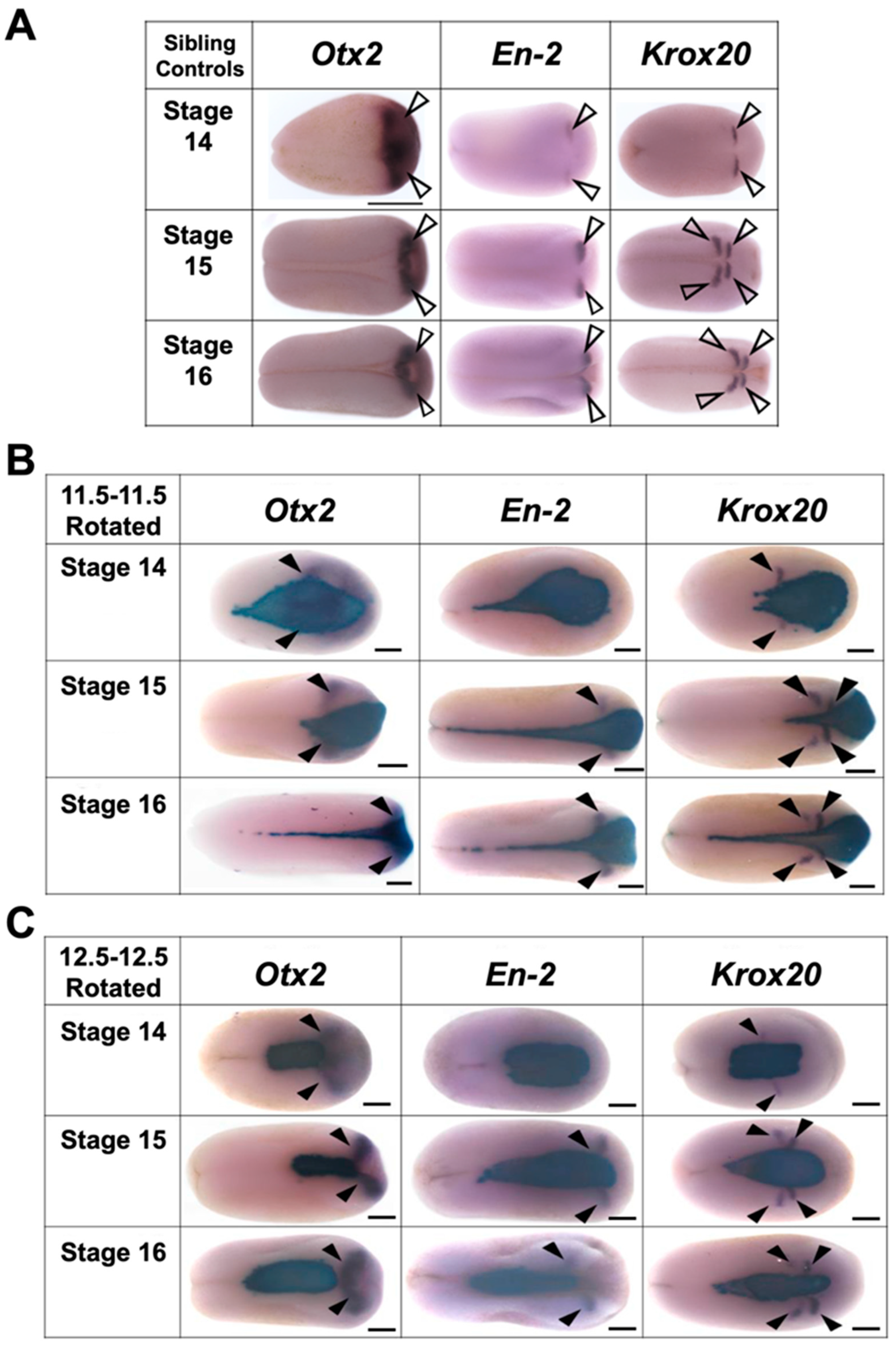
3.4. Mid-Gastrula Rotation Embryos Recover and Display Appropriate Marker Gene Expression by the Mid-Neurula Stage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, S.F. Continuity and change: Paradigm shifts in neural induction. Int. J. Dev. Biol. 2001, 45, 155–164. [Google Scholar] [PubMed]
- Stern, C.D. Neural induction: Old problem, new findings, yet more questions. Development 2005, 132, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Sasai, N.; Kadoya, M.; Ong Lee Chen, A. Neural induction: Historical views and application to pluripotent stem cells. Dev. Growth Differ. 2021, 63, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.D.; Moody, S.A.; Casey, E.S. Neural induction and factors that stabilize a neural fate. Birth Defects Res. C Embryo Today 2009, 87, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Kimelman, D.; Martin, B.L. Anterior–posterior patterning in early development: Three strategies. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 253–266. [Google Scholar] [CrossRef]
- Tuazon, F.B.; Mullins, M.C. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin. Cell Dev. Biol. 2015, 42, 118–133. [Google Scholar] [CrossRef]
- Martinez Arias, A.; Steventon, B. On the nature and function of organizers. Development 2018, 145, dev159525. [Google Scholar] [CrossRef]
- Metzis, V.; Steinhauser, S.; Pakanavicius, E.; Gouti, M.; Stamataki, D.; Ivanovitch, K.; Watson, T.; Rayon, T.; Mousavy Gharavy, S.N.; Lovell-Badge, R.; et al. Nervous System Regionalization Entails Axial Allocation before Neural Differentiation. Cell 2018, 175, 1105–1118.e17. [Google Scholar] [CrossRef]
- Al Anber, A.; Martin, B.L. Transformation of a neural activation and patterning model. EMBO Rep. 2019, 20, e48060. [Google Scholar] [CrossRef]
- Exner, C.R.T.; Willsey, H.R. Xenopus leads the way: Frogs as a pioneering model to understand the human brain. Genesis 2021, 59, e23405. [Google Scholar] [CrossRef]
- Trevers, K.E.; Prajapati, R.S.; Hintze, M.; Stower, M.J.; Strobl, A.C.; Tambalo, M.; Ranganathan, R.; Moncaut, N.; Khan, M.A.F.; Stern, C.D.; et al. Neural induction by the node and placode induction by head mesoderm share an initial state resembling neural plate border and ES cells. Proc. Natl. Acad. Sci. USA 2018, 115, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.A.; Klein, S.L.; Karpinski, B.A.; Maynard, T.M.; Lamantia, A.S. On becoming neural: What the embryo can tell us about differentiating neural stem cells. Am. J. Stem Cells 2013, 2, 74–94. [Google Scholar] [PubMed]
- Gaur, S.; Mandelbaum, M.; Herold, M.; Majumdar, H.D.; Neilson, K.M.; Maynard, T.M.; Mood, K.; Daar, I.O.; Moody, S.A. Neural transcription factors bias cleavage stage blastomeres to give rise to neural ectoderm. Genesis 2016, 54, 334–349. [Google Scholar] [CrossRef]
- Dubey, A.; Saint-Jeannet, J.P. Anterior patterning genes induced by Zic1 are sensitive to retinoic acid and its metabolite, 4-oxo-RA. Dev. Dyn 2022, 251, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Park, S.; Lee, U.; Kim, J. The Organizer and Its Signaling in Embryonic Development. J. Dev. Biol. 2021, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Bedzhov, I.; Graham, S.J.; Leung, C.Y.; Zernicka-Goetz, M. Developmental plasticity, cell fate specification and morphogenesis in the early mouse embryo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130538. [Google Scholar] [CrossRef]
- Lafuente, E.; Beldade, P. Genomics of Developmental Plasticity in Animals. Front. Genet. 2019, 10, 720. [Google Scholar] [CrossRef]
- Sztal, T.E.; Stainier, D.Y.R. Transcriptional adaptation: A mechanism underlying genetic robustness. Development 2020, 147, dev186452. [Google Scholar] [CrossRef]
- Skipper, M.; Weiss, U.; Gray, N. Plasticity. Nature 2010, 465, 703. [Google Scholar] [CrossRef]
- Martinez Arias, A.; Nichols, J.; Schroter, C. A molecular basis for developmental plasticity in early mammalian embryos. Development 2013, 140, 3499–3510. [Google Scholar] [CrossRef]
- Wymeersch, F.J.; Huang, Y.; Blin, G.; Cambray, N.; Wilkie, R.; Wong, F.C.; Wilson, V. Position-dependent plasticity of distinct progenitor types in the primitive streak. Elife 2016, 5, e10042. [Google Scholar] [CrossRef] [PubMed]
- Solini, G.E.; Dong, C.; Saha, M. Embryonic transplantation experiments: Past, present, and future. Trends Dev. Biol. 2017, 10, 13–30. [Google Scholar] [PubMed]
- Spemann, H. Uber eine neue Methode der embryonal Transplantaion. Verh. Der Dtsch. Zool. Ges. 1906, 16, 195–202. [Google Scholar]
- Spemann, H. Uber die Entwicklung umgedrehter Hirnteile bie Amphibien embryonen. In Zoologische Jahrbücher; 1912; Volume 3, pp. 1–48. Available online: https://books.google.com.hk/books?hl=en&lr=&id=Iv5JAQAAMAAJ&oi=fnd&pg=PA1&dq=Uber+die+Entwicklung+umgedrehter+Hirnteile+bie+Amphibien+embryonen.&ots=Vve-qL8zUT&sig=8gmpTTLlm0fNIYgCYhcw2tMGL6Y&redir_esc=y#v=onepage&q=Uber%20die%20Entwicklung%20umgedrehter%20Hirnteile%20bie%20Amphibien%20embryonen.&f=false (accessed on 5 September 2022).
- Roach, F.C. Differentiation of the central nervous system after axial reversals of the medulaary plate of Amblystoma. J. Exp. Zool. 1945, 99, 53–77. [Google Scholar] [CrossRef]
- Nicholas, J.S. Results of Inversion of Neural Plate Material. Proc. Natl. Acad. Sci. USA 1957, 43, 542–545. [Google Scholar] [CrossRef]
- Sladecek, F. Regulative tendencies of the central nervous system during embryogenesis of the axolotl (Amyblystoma mexicanum Cope). Acta Soc. Zool. Bohemoslov. 1955, 19, 138–149. [Google Scholar]
- Jacobson, A.G. The Determination and Positioning of the Nose, Lens and Ear. Iii. Effects of Reversing the Antero-Posterior Axis of Epidermis, Neural Plate and Neural Fold. J. Exp. Zool 1963, 154, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Szaro, B.; Ide, C.; Kaye, C.; Tompkins, R. Regulation in the neural plate of Xenopus laevis demonstrated by genetic markers. J. Exp. Zool. 1985, 234, 117–129. [Google Scholar] [CrossRef]
- Waddington, C.H.; Yao, T. Studies on regional specificity within the organization centre of urodeles. J. Exp. Biol. 1950, 27, 126–144. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D.; Albert, B. The role of competence in the cranio-caudal segregation of the nervous system. Dev. Growth Differ. 1990, 32, 23–31. [Google Scholar] [CrossRef]
- Guthrie, S.; Muchamore, I.; Kuroiwa, A.; Marshall, H.; Krumlauf, R.; Lumsden, A. Neuroectodermal autonomy of Hox-2.9 expression revealed by rhombomere transpositions. Nature 1992, 356, 157–159. [Google Scholar] [CrossRef]
- Kuratani, S.C.; Eichele, G. Rhombomere transplantation repatterns the segmental organization of cranial nerves and reveals cell-autonomous expression of a homeodomain protein. Development 1993, 117, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, S.; Prince, V.; Lumsden, A. Selective dispersal of avian rhombomere cells in orthotopic and heterotopic grafts. Development 1993, 118, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Grapin-Botton, A.; Bonnin, M.A.; McNaughton, L.A.; Krumlauf, R.; Le Douarin, N.M. Plasticity of transposed rhombomeres: Hox gene induction is correlated with phenotypic modifications. Development 1995, 121, 2707–2721. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.; Hornbruch, A.; Lumsden, A. Independent assignment of antero-posterior and dorso-ventral positional values in the developing chick hindbrain. Curr. Biol. 1995, 5, 205–214. [Google Scholar] [CrossRef]
- Itasaki, N.; Sharpe, J.; Morrison, A.; Krumlauf, R. Reprogramming Hox expression in the vertebrate hindbrain: Influence of paraxial mesoderm and rhombomere transposition. Neuron 1996, 16, 487–500. [Google Scholar] [CrossRef]
- Saldivar, J.R.; Krull, C.E.; Krumlauf, R.; Ariza-McNaughton, L.; Bronner-Fraser, M. Rhombomere of origin determines autonomous versus environmentally regulated expression of Hoxa-3 in the avian embryo. Development 1996, 122, 895–904. [Google Scholar] [CrossRef]
- Schilling, T.F.; Prince, V.; Ingham, P.W. Plasticity in zebrafish hox expression in the hindbrain and cranial neural crest. Dev. Biol. 2001, 231, 201–216. [Google Scholar] [CrossRef]
- Staudt, N.; Houart, C. The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol. 2007, 5, e69. [Google Scholar] [CrossRef]
- Buitrago-Delgado, E.; Nordin, K.; Rao, A.; Geary, L.; LaBonne, C. NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science 2015, 348, 1332–1335. [Google Scholar] [CrossRef]
- Roellig, D.; Tan-Cabugao, J.; Esaian, S.; Bronner, M.E. Dynamic transcriptional signature and cell fate analysis reveals plasticity of individual neural plate border cells. Elife 2017, 6, e21620. [Google Scholar] [CrossRef] [PubMed]
- Krumlauf, R.; Wilkinson, D.G. Segmentation and patterning of the vertebrate hindbrain. Development 2021, 148, dev186460. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.W.; Izpisua Belmonte, J.C.; Christen, B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev. Dyn. 2009, 238, 1226–1248. [Google Scholar] [CrossRef] [PubMed]
- Sater, A.K.; Moody, S.A. Using Xenopus to understand human disease and developmental disorders. Genesis 2017, 55, e22997. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Ott, T. Xenopus: An Undervalued Model Organism to Study and Model Human Genetic Disease. Cells Tissues Organs 2018, 205, 303–313. [Google Scholar] [CrossRef]
- Borodinsky, L.N. Xenopus laevis as a Model Organism for the Study of Spinal Cord Formation, Development, Function and Regeneration. Front. Neural Circuits 2017, 11, 90. [Google Scholar] [CrossRef]
- Fortriede, J.D.; Pells, T.J.; Chu, S.; Chaturvedi, P.; Wang, D.; Fisher, M.E.; James-Zorn, C.; Wang, Y.; Nenni, M.J.; Burns, K.A.; et al. Xenbase: Deep integration of GEO & SRA RNA-seq and ChIP-seq data in a model organism database. Nucleic Acids Res. 2020, 48, D776–D782. [Google Scholar] [CrossRef]
- Fainsod, A.; Moody, S.A. Xenopus From Basic Biology to Disease Models in the Genomic Era; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Microinjection of RNA and preparation of secreted proteins from Xenopus oocytes. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5538. [Google Scholar] [CrossRef]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Calibration of the injection volume for microinjection of Xenopus oocytes and embryos. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5537. [Google Scholar] [CrossRef]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Microinjection of Xenopus embryos. Cold Spring Harb. Protoc. 2010, 2010, pdb.ip81. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus laevis (Daudin); Garland Publishing Inc.: New York, NY, USA, 1994; ISBN 0-8153-1896-0. [Google Scholar]
- Moody, S.A. Lineage Tracing and Fate Mapping in Xenopus Embryos. Cold Spring Harb. Protoc. 2018, 2018, pdb-rot097253. [Google Scholar] [CrossRef] [PubMed]
- Sive, H.; Grainger, R.; Harland, R. Early Development of Xenopus laevis: A Laboratory Manual; Cold Spring Harbor Press: Long Island, NY, USA, 2000. [Google Scholar]
- Solini, G.E.; Pownall, M.E.; Hillenbrand, M.J.; Tocheny, C.E.; Paudel, S.; Halleran, A.D.; Bianchi, C.H.; Huyck, R.W.; Saha, M.S. Xenopus embryos show a compensatory response following perturbation of the Notch signaling pathway. Dev. Biol. 2020, 460, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sive, H.L.; Hattori, K.; Weintraub, H. Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell 1989, 58, 171–180. [Google Scholar] [CrossRef]
- Saha, M.S.; Grainger, R.M. A labile period in the determination of the anterior–posterior axis during early neural development in Xenopus. Neuron 1992, 8, 1003–1014. [Google Scholar] [CrossRef]
- Plouhinec, J.L.; Medina-Ruiz, S.; Borday, C.; Bernard, E.; Vert, J.P.; Eisen, M.B.; Harland, R.M.; Monsoro-Burq, A.H. A molecular atlas of the developing ectoderm defines neural, neural crest, placode, and nonneural progenitor identity in vertebrates. PLoS Biol. 2017, 15, e2004045. [Google Scholar] [CrossRef]
- Mongera, A.; Michaut, A.; Guillot, C.; Xiong, F.; Pourquie, O. Mechanics of Anteroposterior Axis Formation in Vertebrates. Annu. Rev. Cell Dev. Biol. 2019, 35, 259–283. [Google Scholar] [CrossRef]
- Monsoro-Burq, A.H. Xenopus Embryo: Neural Induction. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2020. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Mullins, M.C. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development 2013, 140, 1970–1980. [Google Scholar] [CrossRef]
- Pera, E.M.; Acosta, H.; Gouignard, N.; Climent, M.; Arregi, I. Active signals, gradient formation and regional specificity in neural induction. Exp. Cell Res. 2014, 321, 25–31. [Google Scholar] [CrossRef]
- Carron, C.; Shi, D.L. Specification of anteroposterior axis by combinatorial signaling during Xenopus development. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 150–168. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D. Activation and organization of the central nervous system in amphibians. Part III. Synthesis of a new working hypothesis. J. Exp. Zool. 1952, 108, 83–108. [Google Scholar] [CrossRef]
- Slack, J.M.; Tannahill, D. Mechanism of anteroposterior axis specification in vertebrates. Lessons from the amphibians. Development 1992, 114, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Gamse, J.; Sive, H. Vertebrate anteroposterior patterning: The Xenopus neurectoderm as a paradigm. Bioessays 2000, 22, 976–986. [Google Scholar] [CrossRef]
- Niehrs, C. Regionally specific induction by the Spemann-Mangold organizer. Nat. Rev. Genet. 2004, 5, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The role of Xenopus developmental biology in unraveling Wnt signalling and antero-posterior axis formation. Dev. Biol. 2022, 482, 1–6. [Google Scholar] [CrossRef]
- Polevoy, H.; Gutkovich, Y.E.; Michaelov, A.; Volovik, Y.; Elkouby, Y.M.; Frank, D. New roles for Wnt and BMP signaling in neural anteroposterior patterning. EMBO Rep. 2019, 20, e45842. [Google Scholar] [CrossRef]
- Britton, G.; Heemskerk, I.; Hodge, R.; Qutub, A.A.; Warmflash, A. A novel self-organizing embryonic stem cell system reveals signaling logic underlying the patterning of human ectoderm. Development 2019, 146, dev179093. [Google Scholar] [CrossRef]
- Kjolby, R.A.S.; Truchado-Garcia, M.; Iruvanti, S.; Harland, R.M. Integration of Wnt and FGF signaling in the Xenopus gastrula at TCF and Ets binding sites shows the importance of short-range repression by TCF in patterning the marginal zone. Development 2019, 146, dev179580. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Konishi, H.; Miyamoto, T.; Nagata, T.; Uchida, M.; Suzuki, A. Coordinated regulation of the dorsal-ventral and anterior–posterior patterning of Xenopus embryos by the BTB/POZ zinc finger protein Zbtb14. Dev. Growth Differ. 2018, 60, 158–173. [Google Scholar] [CrossRef]
- Virgirinia, R.P.; Jahan, N.; Okada, M.; Takebayashi-Suzuki, K.; Yoshida, H.; Nakamura, M.; Akao, H.; Yoshimoto, Y.; Fatchiyah, F.; Ueno, N.; et al. Cdc2-like kinase 2 (Clk2) promotes early neural development in Xenopus embryos. Dev. Growth Differ. 2019, 61, 365–377. [Google Scholar] [CrossRef]
- Parihar, M.; Bendelac-Kapon, L.; Gur, M.; Abbou, T.; Belorkar, A.; Achanta, S.; Kinberg, K.; Vadigepalli, R.; Fainsod, A. Retinoic Acid Fluctuation Activates an Uneven, Direction-Dependent Network-Wide Robustness Response in Early Embryogenesis. Front. Cell Dev. Biol. 2021, 9, 747969. [Google Scholar] [CrossRef]
- Girgin, M.U.; Broguiere, N.; Mattolini, L.; Lutolf, M.P. Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. Stem Cell Rep. 2021, 16, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, S.S.; Henry, J.J.; Krebs, J.E. Neural and eye-specific defects associated with loss of the imitation switch (ISWI) chromatin remodeler in Xenopus laevis. Mech Dev. 2005, 122, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Richardson, G.A.; Kroll, K.L. The SWI/SNF chromatin remodeling protein Brg1 is required for vertebrate neurogenesis and mediates transactivation of Ngn and NeuroD. Development 2005, 132, 105–115. [Google Scholar] [CrossRef]
- Yoo, A.S.; Crabtree, G.R. ATP-dependent chromatin remodeling in neural development. Curr. Opin. Neurobiol. 2009, 19, 120–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, B.; Christian, K.M.; He, C.; Jin, P.; Ming, G.L.; Song, H. Epigenetic mechanisms in neurogenesis. Nat. Rev. Neurosci. 2016, 17, 537–549. [Google Scholar] [CrossRef]
- Wullimann, M.F.; Rink, E.; Vernier, P.; Schlosser, G. Secondary neurogenesis in the brain of the African clawed frog, Xenopus laevis, as revealed by PCNA, Delta-1, Neurogenin-related-1, and NeuroD expression. J. Comp. Neurol. 2005, 489, 387–402. [Google Scholar] [CrossRef]
- Poznanski, A.; Keller, R. The role of planar and early vertical signaling in patterning the expression of Hoxb-1 in Xenopus. Dev. Biol. 1997, 184, 351–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albers, B. Competence as the Main Factor Determining the Size of the Neural Plate. Dev. Growth Differ. 1987, 29, 535–545. [Google Scholar] [CrossRef]
- Servetnick, M.; Grainger, R.M. Changes in neural and lens competence in Xenopus ectoderm: Evidence for an autonomous developmental timer. Development 1991, 112, 177–188. [Google Scholar] [CrossRef]
- Mancini, P.; Ossipova, O.; Sokol, S.Y. The dorsal blastopore lip is a source of signals inducing planar cell polarity in the Xenopus neural plate. Biol. Open 2021, 10, bio058761. [Google Scholar] [CrossRef]
- Leung, H.C.; Leclerc, C.; Moreau, M.; Shipley, A.M.; Miller, A.L.; Webb, S.E. Transmembrane H(+) fluxes and the regulation of neural induction in Xenopus laevis. Zygote 2022, 30, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Bischof, J.; Levin, M. Morphological Coordination: A Common Ancestral Function Unifying Neural and Non-Neural Signaling. Physiology 2020, 35, 16–30. [Google Scholar] [CrossRef]
- Xue, X.; Sun, Y.; Resto-Irizarry, A.M.; Yuan, Y.; Aw Yong, K.M.; Zheng, Y.; Weng, S.; Shao, Y.; Chai, Y.; Studer, L.; et al. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 2018, 17, 633–641. [Google Scholar] [CrossRef]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; Garcia Arcos, J.M.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817.e22. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.H.; Schattinger, P.A.; Takayoshi, E.E.; Wills, A.E. Hif1alpha and Wnt are required for posterior gene expression during Xenopus tropicalis tail regeneration. Dev. Biol. 2022, 483, 157–168. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolkhovitinov, L.; Weselman, B.T.; Shaw, G.A.; Dong, C.; Giribhattanavar, J.; Saha, M.S. Tissue Rotation of the Xenopus Anterior–Posterior Neural Axis Reveals Profound but Transient Plasticity at the Mid-Gastrula Stage. J. Dev. Biol. 2022, 10, 38. https://doi.org/10.3390/jdb10030038
Bolkhovitinov L, Weselman BT, Shaw GA, Dong C, Giribhattanavar J, Saha MS. Tissue Rotation of the Xenopus Anterior–Posterior Neural Axis Reveals Profound but Transient Plasticity at the Mid-Gastrula Stage. Journal of Developmental Biology. 2022; 10(3):38. https://doi.org/10.3390/jdb10030038
Chicago/Turabian StyleBolkhovitinov, Lyuba, Bryan T. Weselman, Gladys A. Shaw, Chen Dong, Janhavi Giribhattanavar, and Margaret S. Saha. 2022. "Tissue Rotation of the Xenopus Anterior–Posterior Neural Axis Reveals Profound but Transient Plasticity at the Mid-Gastrula Stage" Journal of Developmental Biology 10, no. 3: 38. https://doi.org/10.3390/jdb10030038
APA StyleBolkhovitinov, L., Weselman, B. T., Shaw, G. A., Dong, C., Giribhattanavar, J., & Saha, M. S. (2022). Tissue Rotation of the Xenopus Anterior–Posterior Neural Axis Reveals Profound but Transient Plasticity at the Mid-Gastrula Stage. Journal of Developmental Biology, 10(3), 38. https://doi.org/10.3390/jdb10030038






