Sculpting an Embryo: The Interplay between Mechanical Force and Cell Division
Abstract
:1. Introduction
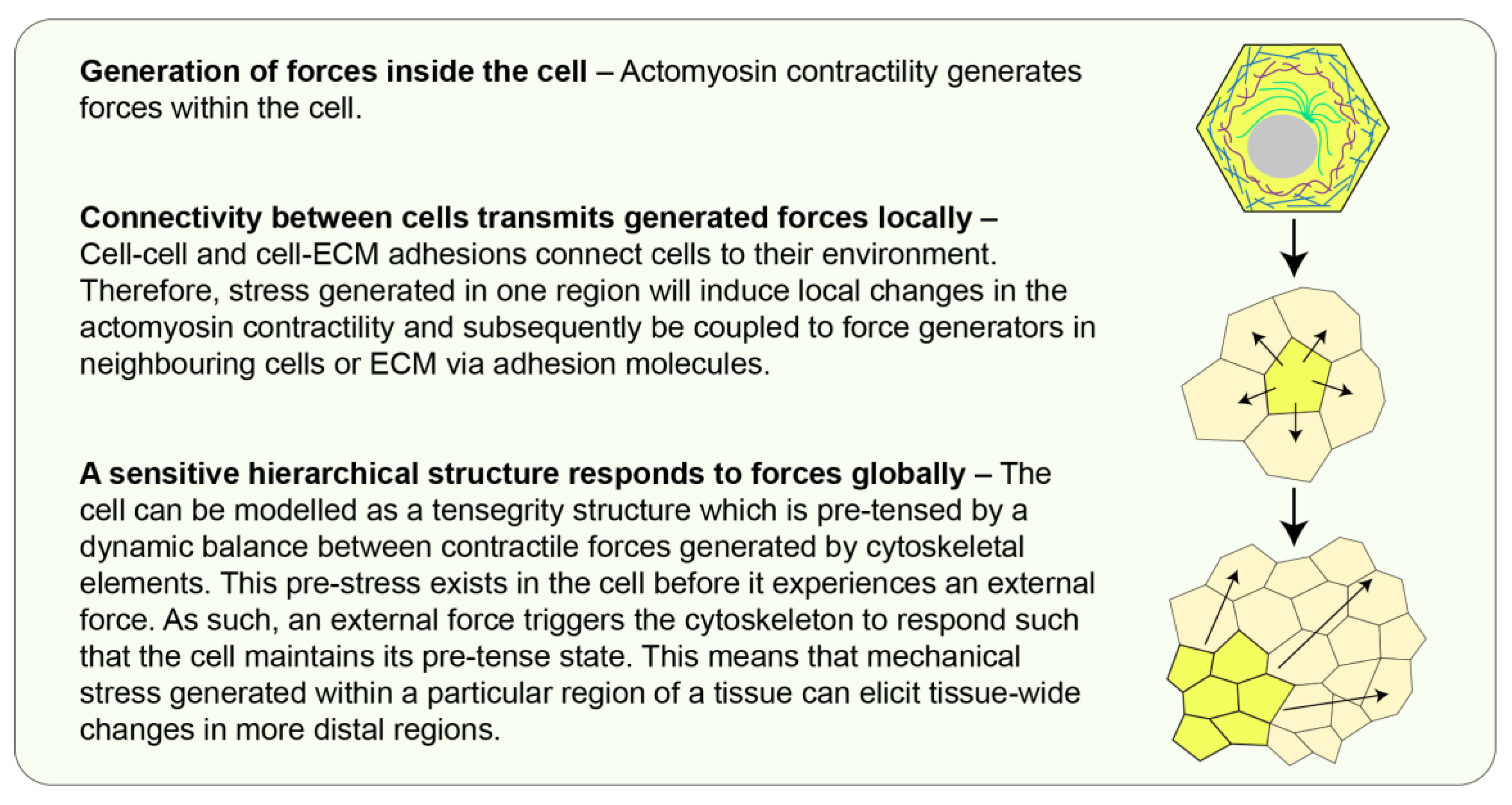
2. Mechanical Force Generation and Transmission in Tissues
3. Cell Division as a Foundation of Tissue Architecture
4. Forces and Cell Division Rate
5. Forces and Cell Division Orientation
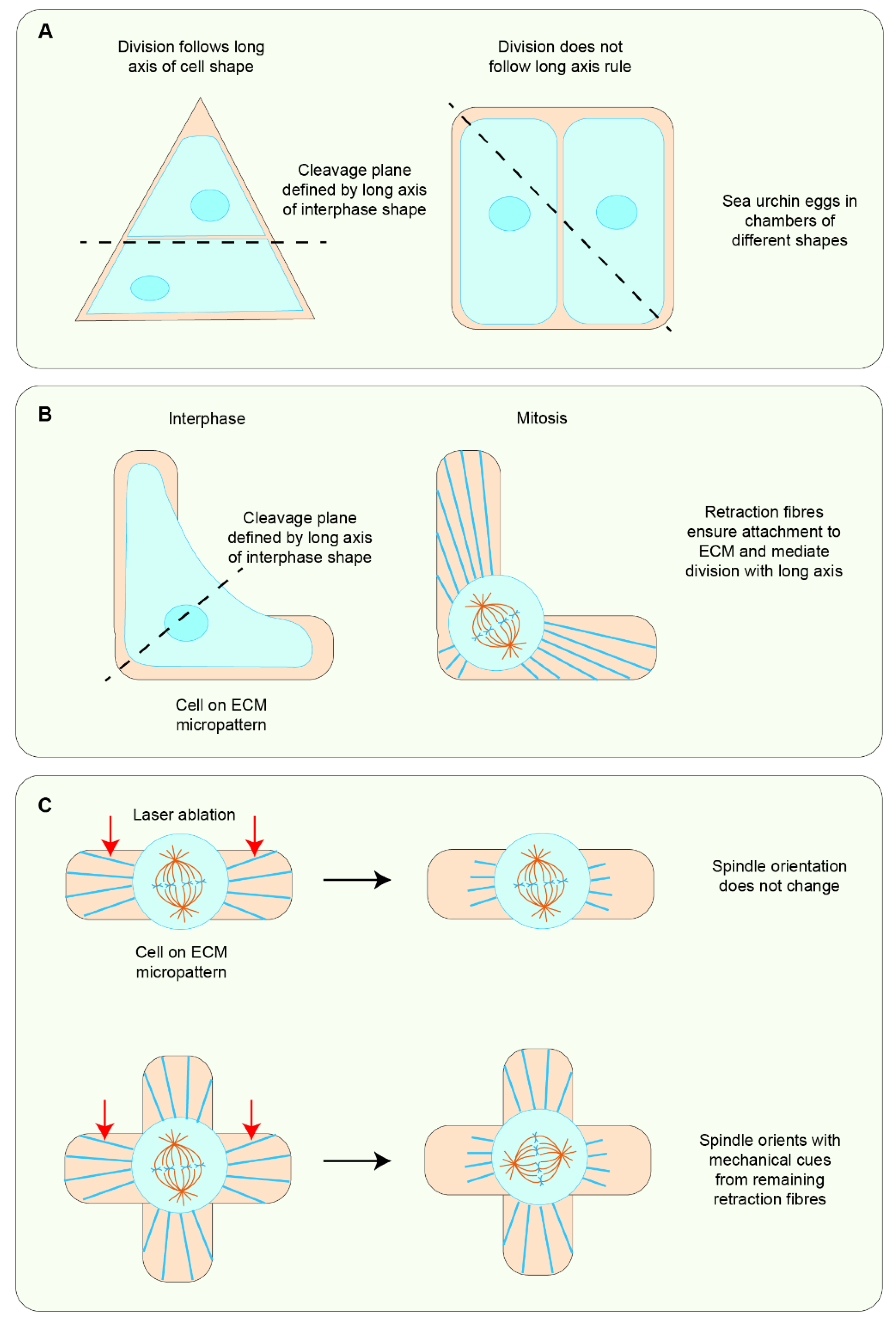
5.1. Cell Shape Change as the Primary Cue in Mechanosensitive Cell Division Orientation
5.2. Force Can Be Sensed Independently of Cell Shape to Orient Cell Divisions
5.3. Force and Cell Shape Function Synergistically to Orient Global Cell Divisions in Tissues
6. Mitotic Rounding as an Additional Cue for Morphogenesis
7. Potential Mechanotransducers Linking Forces to Mitosis
7.1. Mechanosensitive Channels
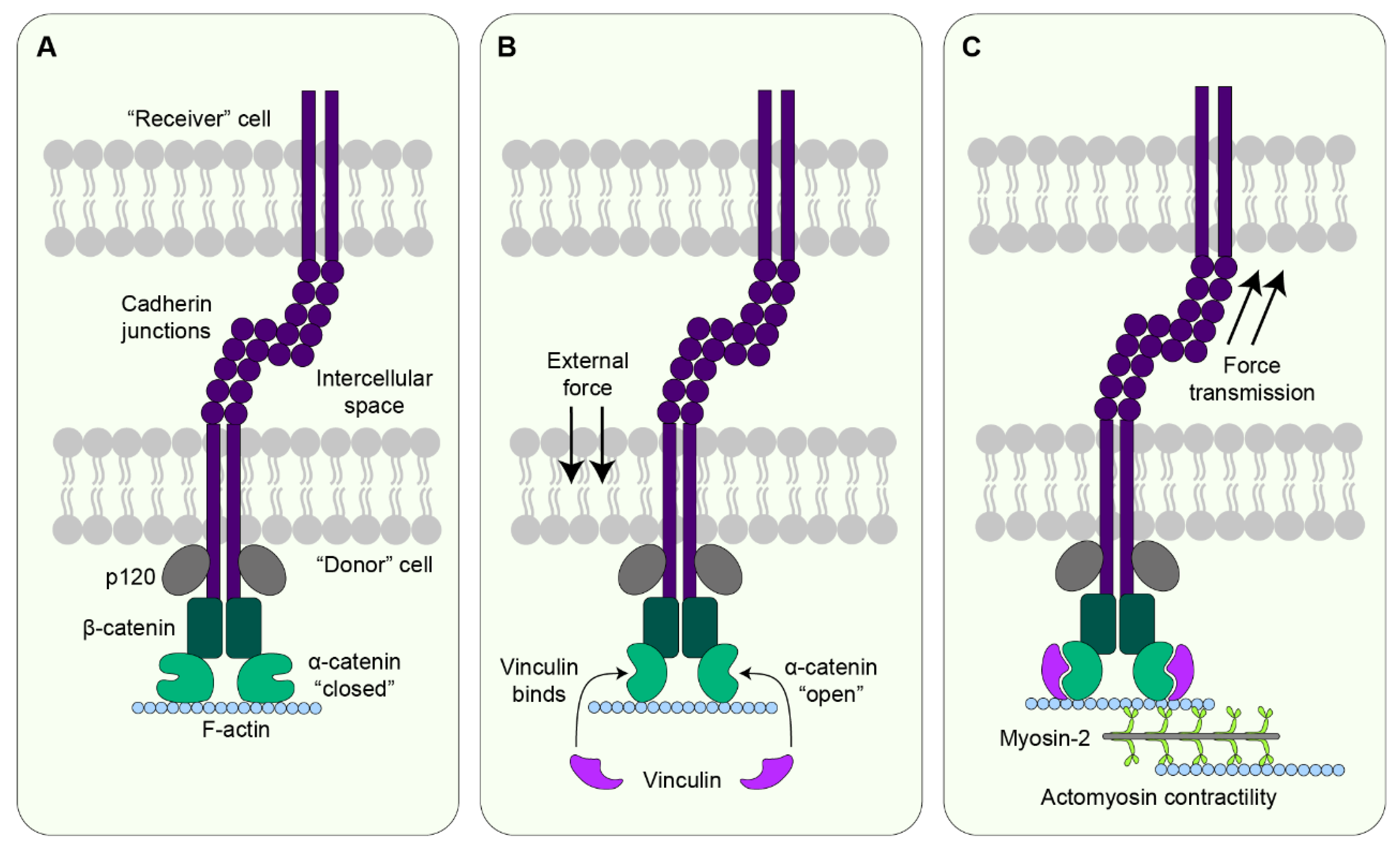
7.2. Cadherin
7.3. LGN/NuMA
7.4. Actin-Binding Proteins
7.5. Myosin
8. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campinho, P.; Behrndt, M.; Ranft, J.; Risler, T.; Minc, N.; Heisenberg, C.P. Tension-oriented cell divisions limit anisotropic tissue tension in epithelial spreading during zebrafish epiboly. Nat. Cell Biol. 2013, 15, 1405–1414. [Google Scholar] [CrossRef]
- Fletcher, G.C.; Diaz-de-la-Loza, M.D.; Borreguero-Muñoz, N.; Holder, M.; Aguilar-Aragon, M.; Thompson, B.J. Mechanical strain regulates the Hippo pathway in Drosophila. Development 2018, 145, dev159467. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Mahadevan, L.; Tabin, C.J. BMP signaling controls buckling forces to modulate looping morphogenesis of the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.; Finet, C.; Blanchard, G.B.; Sanson, B. Actomyosin-Driven Tension at Compartmental Boundaries Orients Cell Division Independently of Cell Geometry In Vivo. Dev. Cell 2018, 47, 727–740.e726. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Hu, Y.; Wang, Z.; Jiang, K.; Zhan, C.; Marshall, W.F.; Tang, N. Mechanical Forces Program the Orientation of Cell Division during Airway Tube Morphogenesis. Dev. Cell 2018, 44, 313–325.e315. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.F.; Hunter, M.V.; Wang, G.; McFaul, C.; Yip, C.M.; Fernandez-Gonzalez, R. Automated cell tracking identifies mechanically oriented cell divisions during Drosophila axis elongation. Development 2017, 144, 1350–1361. [Google Scholar] [CrossRef]
- Nestor-Bergmann, A.; Goddard, G.; Woolner, S. Force and the spindle: Mechanical cues in mitotic spindle orientation. Semin. Cell Dev. Biol. 2014, 34, 133–139. [Google Scholar] [CrossRef]
- Finegan, T.M.; Bergstralh, D.T. Division orientation: Disentangling shape and mechanical forces. Cell Cycle 2019, 18, 1187–1198. [Google Scholar] [CrossRef]
- Charras, G.; Yap, A.S. Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr. Biol. 2018, 28, R445–R457. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; Uchida, N.; Imamura, Y.; Nagafuchi, A.; Fujimoto, K.; Uemura, T.; Vermeulen, S.; van Roy, F.; Adamson, E.D.; Takeichi, M. Alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 1998, 142, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Weiss, E.E.; Kroemker, M.; Rüdiger, A.H.; Jockusch, B.M.; Rüdiger, M. Vinculin is part of the cadherin-catenin junctional complex: Complex formation between alpha-catenin and vinculin. J. Cell Biol. 1998, 141, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Shewan, A.M.; Maddugoda, M.; Kraemer, A.; Stehbens, S.J.; Verma, S.; Kovacs, E.M.; Yap, A.S. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell 2005, 16, 4531–4542. [Google Scholar] [CrossRef] [PubMed]
- Smutny, M.; Cox, H.L.; Leerberg, J.M.; Kovacs, E.M.; Conti, M.A.; Ferguson, C.; Hamilton, N.A.; Parton, R.G.; Adelstein, R.S.; Yap, A.S. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat. Cell Biol. 2010, 12, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Ingber, D.E.; Wang, N.; Stamenovic, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef]
- StamenoviĆ, D.; Wang, N.; Ingber, D.E. 4—Cellular tensegrity models and cell-substrate interactions. In Principles of Cellular Engineering; King, M.R., Ed.; Academic Press: Burlington, NJ, USA, 2006; pp. 81–101. [Google Scholar]
- Taubenberger, A.V.; Baum, B.; Matthews, H.K. The Mechanics of Mitotic Cell Rounding. Front. Cell Dev. Biol. 2020, 8, 687. [Google Scholar] [CrossRef]
- Monster, J.L.; Donker, L.; Vliem, M.J.; Win, Z.; Matthews, H.K.; Cheah, J.S.; Yamada, S.; de Rooij, J.; Baum, B.; Gloerich, M. An asymmetric junctional mechanoresponse coordinates mitotic rounding with epithelial integrity. J. Cell Biol. 2021, 220, e202001042. [Google Scholar] [CrossRef]
- Sorce, B.; Escobedo, C.; Toyoda, Y.; Stewart, M.P.; Cattin, C.J.; Newton, R.; Banerjee, I.; Stettler, A.; Roska, B.; Eaton, S.; et al. Mitotic cells contract actomyosin cortex and generate pressure to round against or escape epithelial confinement. Nat. Commun. 2015, 6, 8872. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nam, S.; Yim, D.; Camuglia, J.; Martin, J.L.; Sanders, E.N.; O’Brien, L.E.; Martin, A.C.; Kim, T.; Chaudhuri, O. The nature of cell division forces in epithelial monolayers. J. Cell Biol. 2021, 220, e202011106. [Google Scholar] [CrossRef]
- Nam, S.; Chaudhuri, O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nat. Phys. 2018, 14, 621–628. [Google Scholar] [CrossRef]
- Nam, S.; Lin, Y.-H.; Kim, T.; Chaudhuri, O. Cellular Pushing Forces during Mitosis Drive Mitotic Elongation in Collagen Gels. Adv. Sci. 2021, 8, 2000403. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A. Cancer: An unknown territory; rethinking before going ahead. Genes Dis. 2021, 8, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.M.; Vincent, J.-P. Oriented cell divisions in the extending germband of Drosophila. Development 2007, 134, 3049–3054. [Google Scholar] [CrossRef]
- Leise, W.F., 3rd; Mueller, P.R. Inhibition of the cell cycle is required for convergent extension of the paraxial mesoderm during Xenopus neurulation. Development 2004, 131, 1703–1715. [Google Scholar] [CrossRef]
- Bénazéraf, B.; Francois, P.; Baker, R.E.; Denans, N.; Little, C.D.; Pourquié, O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature 2010, 466, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Mo, C.; Fraser, S.E. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 2004, 430, 689–693. [Google Scholar] [CrossRef]
- Ciruna, B.; Jenny, A.; Lee, D.; Mlodzik, M.; Schier, A.F. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 2006, 439, 220–224. [Google Scholar] [CrossRef]
- Quesada-Hernández, E.; Caneparo, L.; Schneider, S.; Winkler, S.; Liebling, M.; Fraser, S.E.; Heisenberg, C.P. Stereotypical cell division orientation controls neural rod midline formation in zebrafish. Curr. Biol. 2010, 20, 1966–1972. [Google Scholar] [CrossRef]
- Baena-López, L.A.; Baonza, A.; García-Bellido, A. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 2005, 15, 1640–1644. [Google Scholar] [CrossRef]
- Lisica, A.; Fouchard, J.; Kelkar, M.; Wyatt, T.P.J.; Duque, J.; Ndiaye, A.-B.; Bonfanti, A.; Baum, B.; Kabla, A.J.; Charras, G.T. Tension at intercellular junctions is necessary for accurate orientation of cell division in the epithelium plane. bioRxiv 2022. [Google Scholar] [CrossRef]
- Box, K.; Joyce, B.W.; Devenport, D. Epithelial geometry regulates spindle orientation and progenitor fate during formation of the mammalian epidermis. eLife 2019, 8, e47102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, H.; Wen, W. Phase Separation and Mechanical Forces in Regulating Asymmetric Cell Division of Neural Stem Cells. Int. J. Mol. Sci. 2021, 22, 267. [Google Scholar] [CrossRef] [PubMed]
- Seldin, L.; Poulson, N.D.; Foote, H.P.; Lechler, T. NuMA localization, stability, and function in spindle orientation involve 4.1 and Cdk1 interactions. Mol. Biol. Cell 2013, 24, 3651–3662. [Google Scholar] [CrossRef] [PubMed]
- Benham-Pyle, B.W.; Pruitt, B.L.; Nelson, W.J. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 2015, 348, 1024–1027. [Google Scholar] [CrossRef]
- Finegan, T.M.; Na, D.; Cammarota, C.; Skeeters, A.V.; Nádasi, T.J.; Dawney, N.S.; Fletcher, A.G.; Oakes, P.W.; Bergstralh, D.T. Tissue tension and not interphase cell shape determines cell division orientation in the Drosophila follicular epithelium. EMBO J. 2019, 38, e100072. [Google Scholar] [CrossRef]
- Fink, J.; Carpi, N.; Betz, T.; Bétard, A.; Chebah, M.; Azioune, A.; Bornens, M.; Sykes, C.; Fetler, L.; Cuvelier, D.; et al. External forces control mitotic spindle positioning. Nat. Cell Biol. 2011, 13, 771–778. [Google Scholar] [CrossRef]
- Godard, B.G.; Dumollard, R.; Munro, E.; Chenevert, J.; Hebras, C.; McDougall, A.; Heisenberg, C.P. Apical Relaxation during Mitotic Rounding Promotes Tension-Oriented Cell Division. Dev. Cell 2020, 55, 695–706.e694. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef]
- Hart, K.C.; Tan, J.; Siemers, K.A.; Sim, J.Y.; Pruitt, B.L.; Nelson, W.J.; Gloerich, M. E-cadherin and LGN align epithelial cell divisions with tissue tension independently of cell shape. Proc. Natl. Acad. Sci. USA 2017, 114, E5845–E5853. [Google Scholar] [CrossRef]
- Mao, Y.; Tournier, A.L.; Hoppe, A.; Kester, L.; Thompson, B.J.; Tapon, N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. EMBO J. 2013, 32, 2790–2803. [Google Scholar] [CrossRef] [Green Version]
- Minc, N.; Burgess, D.; Chang, F. Influence of cell geometry on division-plane positioning. Cell 2011, 144, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Nestor-Bergmann, A.; Stooke-Vaughan, G.A.; Goddard, G.K.; Starborg, T.; Jensen, O.E.; Woolner, S. Decoupling the Roles of Cell Shape and Mechanical Stress in Orienting and Cueing Epithelial Mitosis. Cell Rep. 2019, 26, 2088–2100.e2084. [Google Scholar] [CrossRef]
- Théry, M.; Jiménez-Dalmaroni, A.; Racine, V.; Bornens, M.; Jülicher, F. Experimental and theoretical study of mitotic spindle orientation. Nature 2007, 447, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Théry, M.; Racine, V.; Pépin, A.; Piel, M.; Chen, Y.; Sibarita, J.B.; Bornens, M. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 2005, 7, 947–953. [Google Scholar] [CrossRef]
- Wyatt, T.P.; Harris, A.R.; Lam, M.; Cheng, Q.; Bellis, J.; Dimitracopoulos, A.; Kabla, A.J.; Charras, G.T.; Baum, B. Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc. Natl. Acad. Sci. USA 2015, 112, 5726–5731. [Google Scholar] [CrossRef] [PubMed]
- Donker, L.; Vliem, M.J.; Canever, H.; Gómez-González, M.; Bosch-Padrós, M.; Pannekoek, W.-J.; Trepat, X.; Borghi, N.; Gloerich, M. A mechanical G2 checkpoint controls epithelial cell division through E-cadherin-mediated regulation of Wee1-Cdk1. bioRxiv 2021. [Google Scholar] [CrossRef]
- Streichan, S.J.; Hoerner, C.R.; Schneidt, T.; Holzer, D.; Hufnagel, L. Spatial constraints control cell proliferation in tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 5586–5591. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.-B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef]
- Nam, S.; Gupta, V.K.; Lee, H.P.; Lee, J.Y.; Wisdom, K.M.; Varma, S.; Flaum, E.M.; Davis, C.; West, R.B.; Chaudhuri, O. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27(Kip1) signaling axis. Sci. Adv. 2019, 5, eaaw6171. [Google Scholar] [CrossRef]
- Hayakawa, K.; Hirata, H.; Samsonov, M.; Sokabe, M. Planar compression of extracellular substrates induces S phase arrest via ATM-independent CHK2 activation. Biochem. Biophys. Res. Commun. 2018, 506, 983–989. [Google Scholar] [CrossRef]
- Pan, Y.; Heemskerk, I.; Ibar, C.; Shraiman Boris, I.; Irvine Kenneth, D. Differential growth triggers mechanical feedback that elevates Hippo signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E6974–E6983. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.; Schappell, L.E.; Mayer, C.R.; Duke, A.A.; Armiger, T.J.; Arsenovic, P.T.; Mohan, A.; Dahl, K.N.; Gleghorn, J.P.; Conway, D.E. Osmotic Gradients in Epithelial Acini Increase Mechanical Tension across E-cadherin, Drive Morphogenesis, and Maintain Homeostasis. Curr. Biol. 2020, 30, 624–633.e624. [Google Scholar] [CrossRef] [PubMed]
- Aureille, J.; Buffière-Ribot, V.; Harvey, B.E.; Boyault, C.; Pernet, L.; Andersen, T.; Bacola, G.; Balland, M.; Fraboulet, S.; Van Landeghem, L.; et al. Nuclear envelope deformation controls cell cycle progression in response to mechanical force. EMBO Rep. 2019, 20, e48084. [Google Scholar] [CrossRef] [PubMed]
- Hertwig, O. Ueber den Werth der ersten Furchungszellen für die Organbildung des Embryo Experimentelle Studien am Frosch-und Tritonei. Arch. Für Mikrosk. Anat. 1893, 42, 662–807. [Google Scholar] [CrossRef]
- Legoff, L.; Rouault, H.; Lecuit, T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development 2013, 140, 4051–4059. [Google Scholar] [CrossRef]
- Cattin Cedric, J.; Düggelin, M.; Martinez-Martin, D.; Gerber, C.; Müller Daniel, J.; Stewart Martin, P. Mechanical control of mitotic progression in single animal cells. Proc. Natl. Acad. Sci. USA 2015, 112, 11258–11263. [Google Scholar] [CrossRef]
- Bosveld, F.; Markova, O.; Guirao, B.; Martin, C.; Wang, Z.; Pierre, A.; Balakireva, M.; Gaugue, I.; Ainslie, A.; Christophorou, N.; et al. Epithelial tricellular junctions act as interphase cell shape sensors to orient mitosis. Nature 2016, 530, 495–498. [Google Scholar] [CrossRef]
- Camuglia, J.; Chanet, S.; Martin, A.C. Morphogenetic forces planar polarize LGN/Pins in the embryonic head during Drosophila gastrulation. Elife 2022, 11, e78779. [Google Scholar] [CrossRef]
- Kelkar, M.; Bohec, P.; Smith Matthew, B.; Sreenivasan, V.; Lisica, A.; Valon, L.; Ferber, E.; Baum, B.; Salbreux, G.; Charras, G. Spindle reorientation in response to mechanical stress is an emergent property of the spindle positioning mechanisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2121868119. [Google Scholar] [CrossRef]
- Hoijman, E.; Rubbini, D.; Colombelli, J.; Alsina, B. Mitotic cell rounding and epithelial thinning regulate lumen growth and shape. Nat. Commun. 2015, 6, 7355. [Google Scholar] [CrossRef] [Green Version]
- Freddo, A.M.; Shoffner, S.K.; Shao, Y.; Taniguchi, K.; Grosse, A.S.; Guysinger, M.N.; Wang, S.; Rudraraju, S.; Margolis, B.; Garikipati, K.; et al. Coordination of signaling and tissue mechanics during morphogenesis of murine intestinal villi: A role for mitotic cell rounding. Integr. Biol. 2016, 8, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hayashi, S. Mitotic cell rounding accelerates epithelial invagination. Nature 2013, 494, 125–129. [Google Scholar] [CrossRef]
- Petridou, N.I.; Grigolon, S.; Salbreux, G.; Hannezo, E.; Heisenberg, C.-P. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat. Cell Biol. 2019, 21, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Mathiah, N.; Despin-Guitard, E.; Stower, M.; Nahaboo, W.; Eski, E.S.; Singh, S.P.; Srinivas, S.; Migeotte, I. Asymmetry in the frequency and position of mitosis in the mouse embryo epiblast at gastrulation. EMBO Rep. 2020, 21, e50944. [Google Scholar] [CrossRef] [PubMed]
- Benham-Pyle, B.W.; Sim, J.Y.; Hart, K.C.; Pruitt, B.L.; Nelson, W.J. Increasing β-catenin/Wnt3A activity levels drive mechanical strain-induced cell cycle progression through mitosis. Elife 2016, 5, e19799. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, Y.; Miyazaki, N.; Matsumoto, A.; Nagae, S.; Yonemura, S.; Tanoue, T.; Iwasaki, K.; Takeichi, M. Giant cadherins Fat and Dachsous self-bend to organize properly spaced intercellular junctions. Proc. Natl. Acad. Sci. USA 2014, 111, 16011–16016. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Liang, X.; Chen, J.; Hong, J.; Li, L.; He, Q.; Cai, X. History and progression of Fat cadherins in health and disease. OncoTargets Ther. 2016, 9, 7337–7343. [Google Scholar] [CrossRef]
- Silva, E.; Tsatskis, Y.; Gardano, L.; Tapon, N.; McNeill, H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr. Biol. 2006, 16, 2081–2089. [Google Scholar] [CrossRef]
- Willecke, M.; Hamaratoglu, F.; Kango-Singh, M.; Udan, R.; Chen, C.L.; Tao, C.; Zhang, X.; Halder, G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 2006, 16, 2090–2100. [Google Scholar] [CrossRef]
- Kiyomitsu, T.; Boerner, S. The Nuclear Mitotic Apparatus (NuMA) Protein: A Key Player for Nuclear Formation, Spindle Assembly, and Spindle Positioning. Front. Cell Dev. Biol. 2021, 9, 653801. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, H.; Wan, Q.; Liu, J.; Xiao, Z.; Siderovski, D.P.; Du, Q. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 2010, 189, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Gloerich, M.; Bianchini, J.M.; Siemers, K.A.; Cohen, D.J.; Nelson, W.J. Cell division orientation is coupled to cell-cell adhesion by the E-cadherin/LGN complex. Nat. Commun. 2017, 8, 13996. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, A.E.; Cleveland, D.W. NuMA after 30 years: The matrix revisited. Trends Cell Biol. 2010, 20, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Ohta, N.; Hisata, K.; Raabe, T.; Matsuzaki, F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nat. Cell Biol. 2006, 8, 586–593. [Google Scholar] [CrossRef]
- Siller, K.H.; Cabernard, C.; Doe, C.Q. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nat. Cell Biol. 2006, 8, 594–600. [Google Scholar] [CrossRef]
- Sawyer, J.K.; Harris, N.J.; Slep, K.C.; Gaul, U.; Peifer, M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J. Cell Biol. 2009, 186, 57–73. [Google Scholar] [CrossRef]
- Lam, M.S.Y.; Lisica, A.; Ramkumar, N.; Hunter, G.; Mao, Y.; Charras, G.; Baum, B. Isotropic myosin-generated tissue tension is required for the dynamic orientation of the mitotic spindle. Mol. Biol. Cell 2020, 31, 1370–1379. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Cramer, L.P.; Baum, B.; McGee, K.M. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 2004, 117, 361–372. [Google Scholar] [CrossRef]
- Christodoulou, N.; Skourides, P.A. Cell-Autonomous Ca(2+) Flashes Elicit Pulsed Contractions of an Apical Actin Network to Drive Apical Constriction during Neural Tube Closure. Cell Rep. 2015, 13, 2189–2202. [Google Scholar] [CrossRef]
- Streichan, S.J.; Lefebvre, M.F.; Noll, N.; Wieschaus, E.F.; Shraiman, B.I. Global morphogenetic flow is accurately predicted by the spatial distribution of myosin motors. Elife 2018, 7, e27454. [Google Scholar] [CrossRef]
- Coravos, J.S.; Mason, F.M.; Martin, A.C. Actomyosin Pulsing in Tissue Integrity Maintenance during Morphogenesis. Trends Cell Biol. 2017, 27, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Davidson, L.A. Punctuated actin contractions during convergent extension and their permissive regulation by the non-canonical Wnt-signaling pathway. J. Cell Sci. 2011, 124, 635–646. [Google Scholar] [CrossRef]
- Miao, H.; Blankenship, J.T. The pulse of morphogenesis: Actomyosin dynamics and regulation in epithelia. Development 2020, 147, dev186502. [Google Scholar] [CrossRef] [PubMed]
- Sedzinski, J.; Biro, M.; Oswald, A.; Tinevez, J.-Y.; Salbreux, G.; Paluch, E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 2011, 476, 462–466. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarannum, N.; Singh, R.; Woolner, S. Sculpting an Embryo: The Interplay between Mechanical Force and Cell Division. J. Dev. Biol. 2022, 10, 37. https://doi.org/10.3390/jdb10030037
Tarannum N, Singh R, Woolner S. Sculpting an Embryo: The Interplay between Mechanical Force and Cell Division. Journal of Developmental Biology. 2022; 10(3):37. https://doi.org/10.3390/jdb10030037
Chicago/Turabian StyleTarannum, Nawseen, Rohan Singh, and Sarah Woolner. 2022. "Sculpting an Embryo: The Interplay between Mechanical Force and Cell Division" Journal of Developmental Biology 10, no. 3: 37. https://doi.org/10.3390/jdb10030037
APA StyleTarannum, N., Singh, R., & Woolner, S. (2022). Sculpting an Embryo: The Interplay between Mechanical Force and Cell Division. Journal of Developmental Biology, 10(3), 37. https://doi.org/10.3390/jdb10030037








