Automated Remote Insect Surveillance at a Global Scale and the Internet of Things
Abstract
1. Introduction
- (a)
- photo-interruption of either entering or falling insects in several types of traps (e.g., Red-palm weevil traps, pitfall traps, funnel traps). Photo-interruption is also used in electronic gates installed in beehives. A low power emitter of infrared light and a coupled photodiode form a sheet of light covering the entrance of the trap. The flow of light is interrupted from an insect entering and thus it is counted,
- (b)
- (c)
2. Materials and Methods
2.1. Trap Type #1: The Picusan Trap
2.2. Trap Type #2: The Stored-Grain Pitfall Trap
2.3. Trap Type #3: The Lindgren Trap
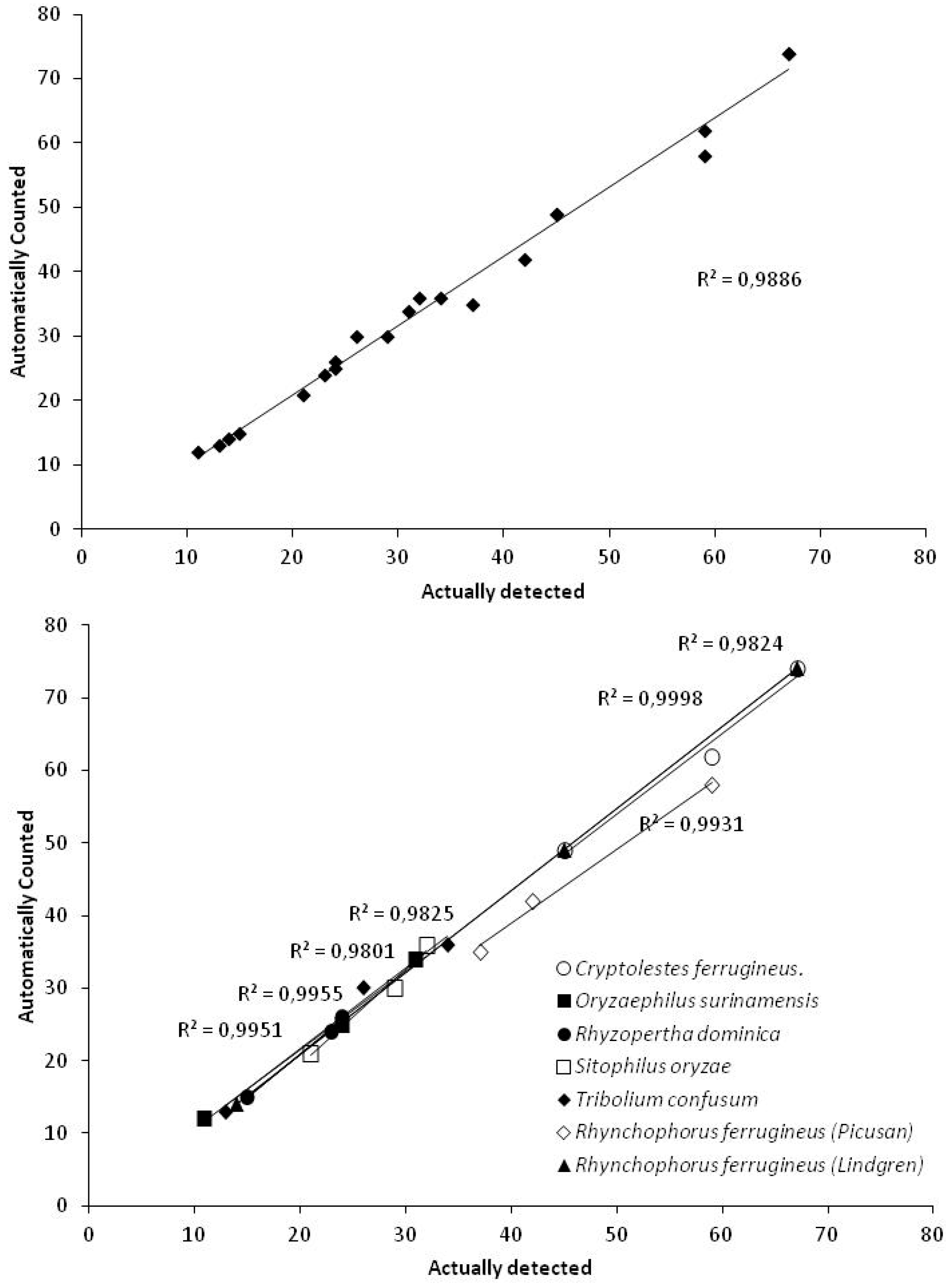
3. Results & Discussion
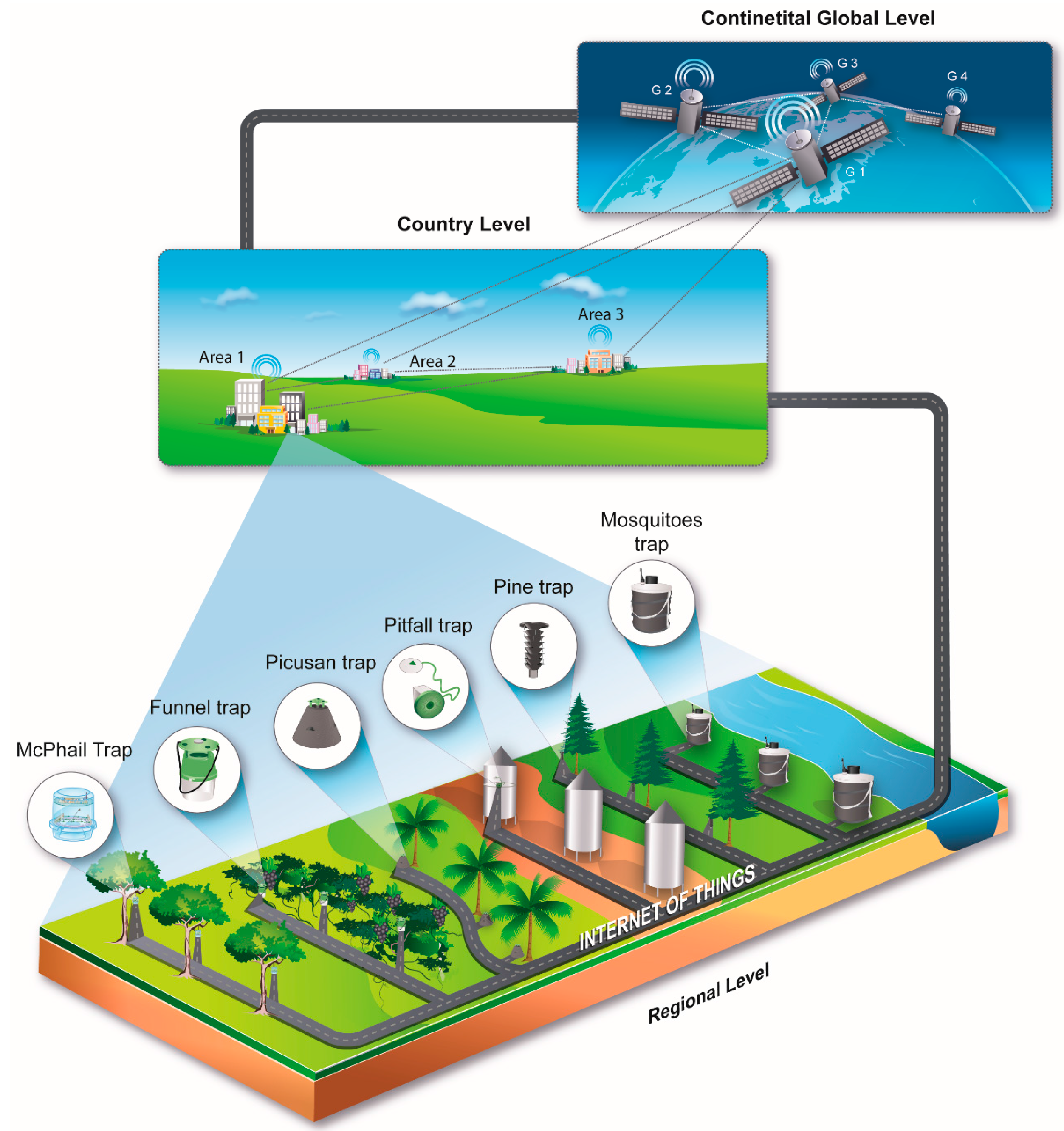
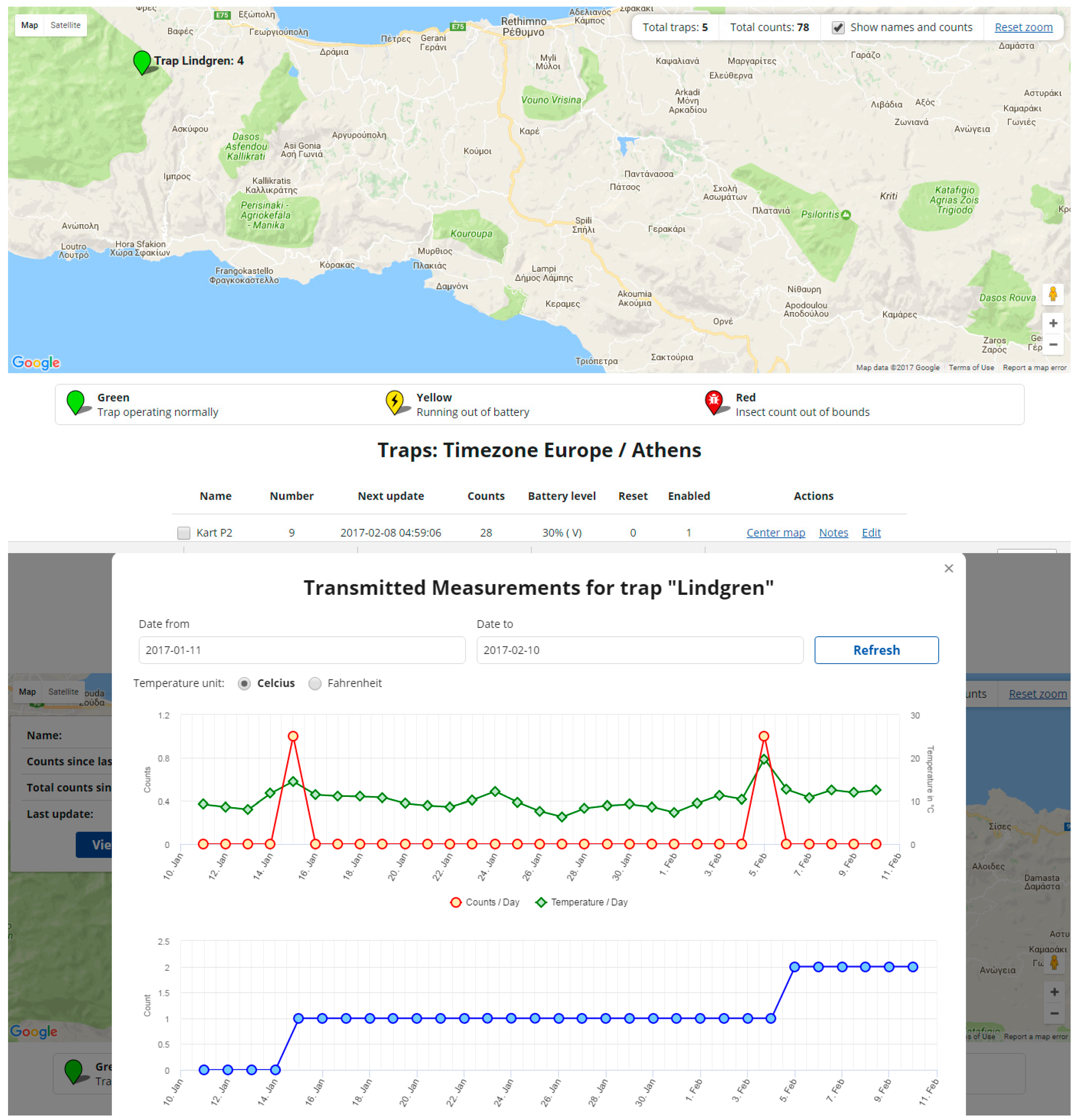
4. Data Processing and the IoT
- (a)
- Counts delivered on a pre-scheduled basis along with the time-stamps of each insect entrance to the traps.
- (b)
- Environmental data (mainly humidity, temperature and GPS tag).
- (c)
- Wingbeat recordings uploaded to a server (in the case of McPhail and mosquito traps).
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Oerke, E.C.; Dehne, H.W.; Schönbeck, F.; Weber, A. Crop Production and Crop Protection: Estimated Losses in Major Food and Cash Crops; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Porter, J.H.; Nagy, E.; Kratz, T.K.; Hanson, P. New eyes on the world: Advanced sensors for ecology. BioScience 2009, 59, 385–397. [Google Scholar] [CrossRef]
- Chen, C.-P.; Chuang, C.-L.; Jiang, J.-A. Ecological Monitoring Using Wireless Sensor Networks—Overview, Challenges, and Opportunities. In Smart Sensors, Measurement and Instrumentation; Book Section in Advancement in Sensing Technology, V. 1; Mukhopadhyay, S.C., Jayasundera, K.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–21. [Google Scholar]
- Aide, T.M.; Corrada-Bravo, C.; Campos-Cerqueira, M.; Milan, C.; Vega, G.; Alvarez, R. Real-time bioacoustics monitoring and automated species identification. PeerJ 2013, 1, e103. [Google Scholar] [CrossRef] [PubMed]
- Chesmore, D. Automated bioacoustic identification of species. An. Acad. Bras. Ciênc. 2004, 76, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Potamitis, I. Automatic Classification of a Taxon-Rich Community Recorded in the Wild. PLoS ONE 2014, 9, e96936. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.L.; Freeman, R.; Collen, A.; Dietz, C.; Fenton, M.B.; Jones, G.; Obrist, M.K.; Puechmaille, S.J.; Sattler, T.; Siemers, B.M.; et al. A continental-scale tool for acoustic identification of European bats. J. Appl. Ecol. 2012, 49, 1064–1074. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Tatlas, N.-A. Automated Surveillance of Fruit Flies. Sensors 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Potamitis, I.; Ganchev, T.; Kontodimas, D. On Automatic Bioacoustic Detection of Pests: The Cases of Rhynchophorus ferrugineus and Sitophilus oryzae. J. Econ. Entomol. 2009, 102, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, D.K.; Clark, C.W. Recognizing transient low-frequency whale sounds by spectrogram correlation. J. Acoust. Soc. Am. 2000, 107, 3518–3529. [Google Scholar] [CrossRef] [PubMed]
- Ospina, O.E.; Villanueva-Rivera, L.J.; Corrada-Bravo, C.J.; Mitchell, A.T. Variable response of anuran calling activity to daily precipitation and temperature: Implications for climate change. Ecosphere 2013. [Google Scholar] [CrossRef]
- Chen, Y.; Why, A.; Batista, G.; Mafra-Neto, A.; Keogh, E. Flying insect classification with inexpensive sensors. J. Insect Behav. 2014, 27, 657–677. [Google Scholar] [CrossRef]
- Eliopoulos, P.A.; Potamitis, I.; Kontodimas, D.C.; Givropoulou, E.G. Detection of Adult Beetles Inside the Stored Wheat Mass Based on Their Acoustic Emissions. J. Econ. Entomol. 2015, 108, 2808–2814. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, P.A.; Potamitis, I.; Kontodimas, D.C. Estimation of population density of stored grain pests via bioacoustic detection. Crop Prot. 2016, 85, 71–78. [Google Scholar] [CrossRef]
- Aldryhim, Y.N.; Ayedh, H.Y.A. Diel flight activity patterns of the red palm weevil (Coleoptera: Curculionidae) as monitored by smart traps. Fla. Entomol. 2015, 98, 1019–1024. [Google Scholar] [CrossRef]
- Fanini, L.; Longo, S.; Cervo, R.; Roversi, P.F.; Mazza, G. Daily activity and non-random occurrence of captures in the Asian palm weevils. Ethol. Ecol. Evolut. 2014, 26, 95–203. [Google Scholar] [CrossRef]
- Murchie, A.K.; Burn, D.J.; Kirk, W.D.J.; Williams, I.H. A novel mechanism for time-sorting insect catches, and its use to derive the diel flight periodicity of brassica pod midge Dasineura brassicae (Diptera: Cecidomyiidae). Bull. Entomol. Res. 2001, 91, 199–204. [Google Scholar] [PubMed]
- Batiste, W.C.; Olson, W.H.; Berlowitz, A. Codling moth: Influence of temperature and daylight intensity on periodicity of daily flight in the field. J. Econ. Entomol. 1973, 66, 883–892. [Google Scholar] [CrossRef]
- Hendricks, D.E. Portable electronic detector system used with inverted-cone sex pheromone traps to determine periodicity and moth captures. Environ. Entomol. 1985, 14, 199–204. [Google Scholar] [CrossRef]
- Kondo, A.; Sano, T.; Tanaka, F. Automatic record using camera of diel periodicity of pheromone trap catches. Jpn. J. Appl. Entomol. Zool. 1994, 38, 197–199. [Google Scholar] [CrossRef]
- Engelmann, F. The Physiology of Insect Reproduction; International Series of Monographs in Pure and Applied Biology Zoology; Pergamon Press: Oxford, UK, 1970; Volume 44. [Google Scholar]
- Saunders, D.S. Insect Clocks; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Hendricks, D.E. Electronic system for detecting trapped boll weevils in the field and transferring incident information to a computer. Southwest. Entomol. 1990, 15, 39–48. [Google Scholar]
- Jiang, J.A.; Tseng, C.L.; Lu, F.M.; Yang, E.C.; Wu, Z.S.; Chen, C.P.; Lin, S.H.; Lin, K.C.; Liao, C.S. A GSM-based remote wireless automatic monitoring system for field information: A case study for ecological monitoring of the oriental fruit fly, Bactrocera dorsalis (Hendel). Comput. Electron. Agric. 2008, 62, 243–259. [Google Scholar] [CrossRef]
- Okuyama, T.; Yang, E.; Chen, C.; Lin, T.; Chuang, C.; Jiang, J. Using automated monitoring systems to uncover pest population dynamics in agricultural fields. Agric. Syst. 2011, 104, 666–670. [Google Scholar] [CrossRef]
- Faleiro, J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Vacas, S.; Primo, J.; Navarro-Llopis, V. Advances in the use of trapping systems for Rhynchophorus ferrugineus (Coleoptera: Curculionidae): Traps and attractants. J. Econ. Entomol. 2013, 106, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Montiel, C.; García-Coapio, G.; Rojas, J.C.; Malo, E.A.; Cruz-López, L.; Del Real, I.; González-Hernández, H. Aggregation pheromone of the agave weevil, Scyphophorus acupunctatus. Entomol. Exp. Appl. 2008, 127, 207–217. [Google Scholar] [CrossRef]
- Aguilar, J.F.S.; Hernández, H.G.; Vázquez, J.L.L.; Martínez, A.E.; Mendoza, F.J.F.; Garza, Á.M. Scyphophorus Acupunctatus Gyllenhal, Plaga del Agave Tequilero en Jalisco, México; Colegio de Postgraduados: Córdoba, México, 2001. [Google Scholar]
- Reed, C.R.; Wright, V.F.; Mize, T.W.; Pedersen, J.R.; Evans, B.J. Pitfall traps and grain samples as indicators of insects in farm-stored wheat. J. Econ. Entomol. 1991, 84, 1381–1387. [Google Scholar] [CrossRef]
- Toews, M.D.; Nansen, C. 21 Trapping and Interpreting Captures of Stored Grain Insects. Stored Prod. Prot. 2012, 21, 243. [Google Scholar]
- White, N.D.G.; Arbogast, R.T.; Fields, P.G.; Hillmann, R.C.; Loschiavo, S.R.; Subramanyam, B.; Throne, J.E.; Wright, V.F. The development and use of pitfall and probe traps for capturing insects in stored grain. J. Kansas Entomol. Soc. 1990, 63, 506–525. [Google Scholar]
- Neethirajan, S.; Karunakaran, C.; Jayas, D.S.; White, N.D.G. Detection techniques for stored-product insects in grain. Food Control 2007, 18, 157–162. [Google Scholar] [CrossRef]
- Aulicky, R.; Stejskal, V.; Kucerova, Z.; Trematerra, P. Trapping of internal and external feeding stored grain beetle pests with two types of pitfall traps: A two-year field study. Plant Prot. Sci. 2016, 52, 45–53. [Google Scholar] [CrossRef]
- Lindgren, B.S. A multiple funnel trap for scolytid beetles (Coleoptera). Can. Entomol. 1983, 115, 299–302. [Google Scholar] [CrossRef]
- Bentz, B.J. Mountain pine beetle population sampling: Inferences from Lindgren pheromone traps and tree emergence cages. Can. J. For. Res. 2006, 36, 351–360. [Google Scholar] [CrossRef]
- Shieh, J.C.; Wang, J.Y.; Lin, T.S.; Lin, C.H.; Yang, E.C.; Tsai, Y.J.; Jiang, J.A. A GSM-based field monitoring system for Spodoptera litura (Fabricius). Eng. Agric. Environ. Food 2011, 4, 77–82. [Google Scholar] [CrossRef]
- Liao, M.S.; Chuang, C.L.; Lin, T.S.; Chen, C.P.; Zheng, X.Y.; Chen, P.T.; Jiang, J.A. Development of an autonomous early warning system for Bactrocera dorsalis (Hendel) outbreaks in remote fruit orchards. Comput. Electron. Agric. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Deqin, X.; Qiumei, Y.; Junqian, F.; Xiaohui, D.; Jianzhao, F.; Yaowen, Y.; Yongyue, L. A multi-target trapping and tracking algorithm for Bactrocera Dorsalis based on cost model. Comput. Electron. Agric. 2016, 123, 224–231. [Google Scholar] [CrossRef]
- Xia, C.; Lee, J.M.; Li, Y.; Chung, B.K.; Chon, T.S. In situ detection of small-size insect pests sampled on traps using multifractal analysis. Opt. Eng. 2012, 51, 027001-1–027001-12. [Google Scholar] [CrossRef]
- Boissard, P.; Martin, V.; Moisan, S. A cognitive vision approach to early pest detection in greenhouse crops. Comput. Electron. Agric. 2008, 62, 81–93. [Google Scholar] [CrossRef]
- Ding, W.; Taylor, G. Automatic moth detection from trap images for pest management. Comput. Electron. Agric. 2016, 123, 17–28. [Google Scholar] [CrossRef]
- López, O.; Rach, M.M.; Migallon, H.; Malumbres, M.P.; Bonastre, A.; Serrano, J.J. Monitoring pest insect traps by means of low-power image sensor technologies. Sensors 2012, 12, 15801–15819. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Maini, S.; Molari, G.; Rondelli, V. Automatic trap for moth detection in integrated pest management. Bull. Insectol. 2011, 64, 247–251. [Google Scholar]
- Douglas, E.N. The Premonition Trap: First Field Trials of a Robotic Smart Trap for Mosquitoes with Species Recognition. In Proceedings of the 47th Annual Conference of Society for Vector Ecology, Anchorage, Alaska, 11–15 September 2016. [Google Scholar]
- Ma, J.; Zhou, X.; Li, S.; Li, Z. Connecting Agriculture to the Internet of Things through Sensor Networks. In Proceedings of the 2011 International Conference on Internet of Things and 4th International Conference on Cyber, Physical and Social Computing, Dalian, China, 19–22 October 2011; pp. 184–187. [Google Scholar]
- Shi, Y.; Wang, Z.; Wang, X.; Zhang, S. Internet of Things Application to Monitoring Plant Disease and Insect Pests. In Proceedings of the China International Conference on Applied Science and Engineering Innovation (ASEI 2015), Xi’an, China, 30–31 August 2015. [Google Scholar]
- Shahzadi, R. Internet of Things based Expert System for Smart Agriculture, (IJACSA). Int. J. Adv. Comput. Sci. Appl. 2016, 7. [Google Scholar] [CrossRef]
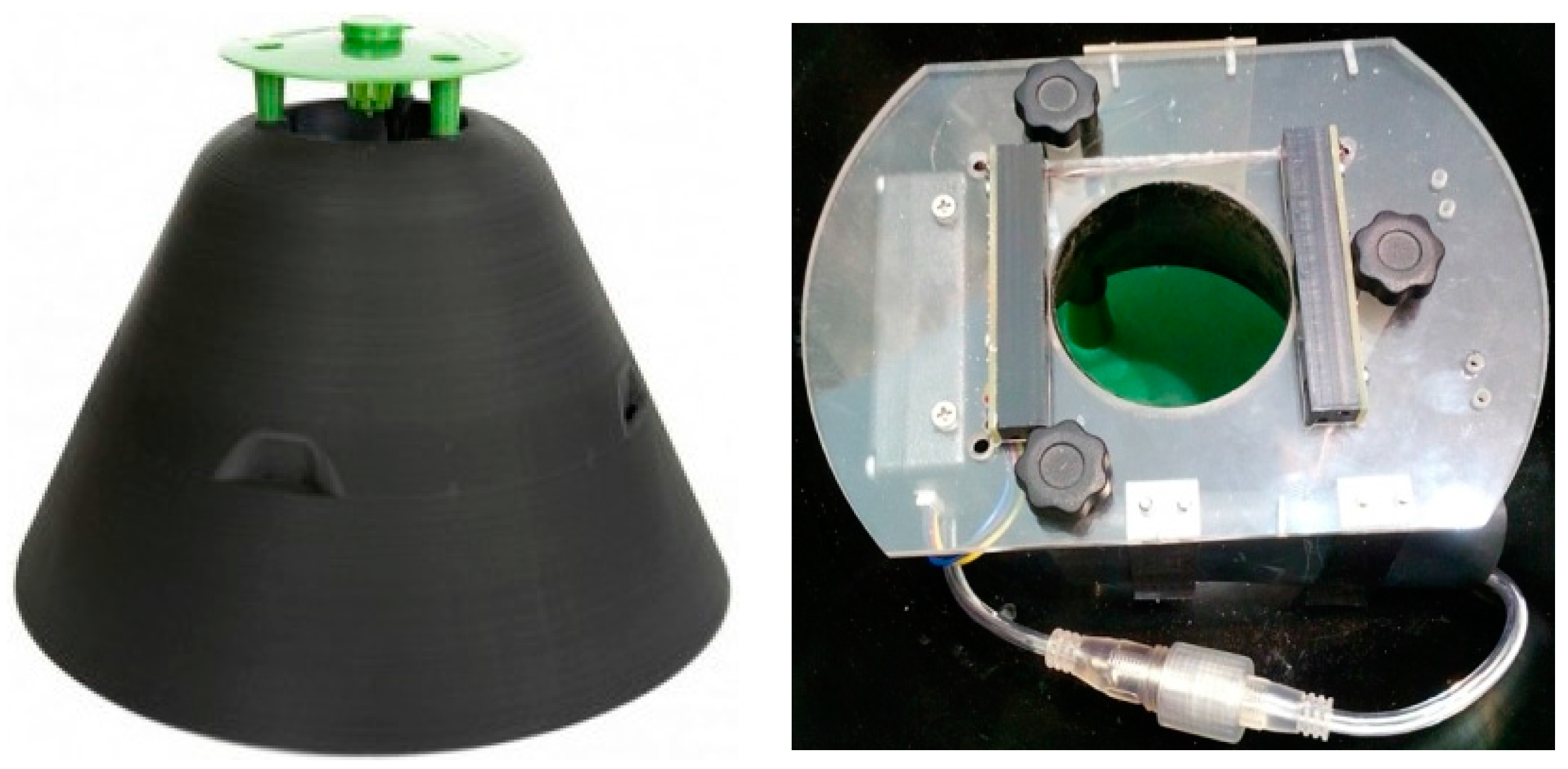


| Trap Type | LEDs/Photodiodes | Diffuser |
|---|---|---|
| Picusan | 5 | NO |
| Stored-grain pitfall | 16 | YES |
| Lindgren | 5 | NO |
| Trap Type | Species | Manually Verified | Automatically Counted | Correlation Coefficient (r) |
|---|---|---|---|---|
| Picusan 1 | R. ferrugineus | 37 | 35 | 0.9966 |
| 42 | 42 | |||
| 59 | 58 | |||
| Pitfall 2 | C. ferrugineus. | 59 | 62 | 0.9912 |
| 45 | 49 | |||
| 67 | 74 | |||
| O. surinamensis | 31 | 34 | 0.9978 | |
| 11 | 12 | |||
| 24 | 25 | |||
| R. dominica | 15 | 15 | 0.9976 | |
| 23 | 24 | |||
| 24 | 26 | |||
| S. oryzae | 21 | 21 | 0.9900 | |
| 32 | 36 | |||
| 29 | 30 | |||
| T. confusum | 13 | 13 | 0.9912 | |
| 26 | 30 | |||
| 34 | 36 | |||
| Lindgren 3 | R. ferrugineus. | 14 | 14 | 0.9999 |
| 45 | 49 | |||
| 67 | 74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potamitis, I.; Eliopoulos, P.; Rigakis, I. Automated Remote Insect Surveillance at a Global Scale and the Internet of Things. Robotics 2017, 6, 19. https://doi.org/10.3390/robotics6030019
Potamitis I, Eliopoulos P, Rigakis I. Automated Remote Insect Surveillance at a Global Scale and the Internet of Things. Robotics. 2017; 6(3):19. https://doi.org/10.3390/robotics6030019
Chicago/Turabian StylePotamitis, Ilyas, Panagiotis Eliopoulos, and Iraklis Rigakis. 2017. "Automated Remote Insect Surveillance at a Global Scale and the Internet of Things" Robotics 6, no. 3: 19. https://doi.org/10.3390/robotics6030019
APA StylePotamitis, I., Eliopoulos, P., & Rigakis, I. (2017). Automated Remote Insect Surveillance at a Global Scale and the Internet of Things. Robotics, 6(3), 19. https://doi.org/10.3390/robotics6030019






