Abstract
Medical robotics nowadays can prevent, treat, or alleviate numerous severe conditions, including the dire consequences of stroke. Our objective was to determine the effect of employing a robotic soft exoskeleton in therapy on the development of the early mobilization, gait, and coordination in stroke patients. The ReStore™ Soft Exo-Suit, a wearable exosuit developed by a leading company with exoskeleton technology, was utilized. It is a powered, lightweight device intended for use in stroke rehabilitation for people with lower limb disability. We performed a randomized clinical intervention, using a before–after trial design in a university hospital setting. A total of 48 patients with a history of stroke were included, of whom 39 were randomized and 30 completed the study. Interventions: Barthel Index and modified Rankin scale (mRS) patients were randomly assigned to a non-physical intervention control (n = 9 of 39 completed, 30 withdrew before baseline testing), or to a high-intensity agility program (15 sessions, 5 weeks, n = 30 completed). The main focus of assessment was on the Modified Rankin Scale. Additionally, we evaluated secondary factors including daily life functionality, five dimensions of health-related quality of life, the Beck depression inventory, the 6 min walk test (6MWT), the Berg Balance Scale (BBS), and static balance (center of pressure). The Robot-Assisted Gait Therapy (ROB/RAGT) program led to significant improvements across various measures, including a 37% improvement in Barthel Index scores, a 56% increase in 10 m walking speed, and a 68% improvement in 6 min walking distance, as well as notable enhancements in balance and stability. Additionally, the intervention group demonstrated significant gains in all these aspects compared to the control group. In conclusion, the use of robotic therapy can be beneficial in stroke rehabilitation. These devices support the restoration and improvement of movement in various ways and contribute to restoring balance and stability.
1. Introduction
In recent years, the use of technology-based neuro-rehabilitation approaches has increased to meet high demand resulting from the rising number of stroke victims [1,2]. Medical robotics nowadays plays a crucial role in preventing, treating, or alleviating numerous severe conditions, assisting in surgeries, hospitals, or rehabilitation [3,4,5,6]. Stroke, one of the most frequently occurring diseases worldwide, often leads to permanent disability [7]. The condition is caused by the death of brain cells due to the blockage of a blood vessel supplying the brain (ischemic stroke), or bleeding into or around the brain (hemorrhagic stroke) [8,9]. This interruption results in not receiving fresh oxygenated blood in certain parts of the brain, leading to the death of neurons in that region, which is a primary contributor to long-term disabilities. Following a stroke, the effects are almost immediate and vary depending on the extent of damage within the brain. Common symptoms include sudden numbness, weakness, or paralysis on one side of the body, or more severely, on both sides. These symptoms may manifest as a weakened arm, leg, or eyelid; challenges in speaking or understanding speech; sudden blurred or lost vision; particularly in one eye; dizziness; confusion; instability; and/or severe headaches [7,10]. More than half of stroke survivors experience some degree of lasting hemiparesis or hemiplegia due to damage of the neural tissues, rendering them unable to perform daily activities without assistance [8,10,11].
A large number of lower limb exoskeletons have been developed over the years, usually with the purpose of assisting or augmenting human walking [1,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. Some exoskeletons are stationary, mounted above treadmills and used to provide gait retraining or rehabilitation for persons with disabilities or injuries. Other exoskeletons are mobile, and those can be used either to support the full body weight of an individual or to provide partial assistive forces [29]. Devices in the former category are intended to be used by paralyzed individuals, enabling them to walk in the case where they were previously unable to do so. In the latter category, exoskeletons providing partial gait assistance can be used either by disabled individuals for rehabilitation or gait augmentation, or by healthy individuals to improve strength or endurance [30].
The aim of this research was to assess the importance of robotic technology by comparing Robot-Assisted Gait Therapy (ROB) with standard therapy treatment (STT) in stroke rehabilitation. In the ROB case, we employed robotic devices to aid patients’ motor rehabilitation targeting the limbs (Figure 1). In the STT group, we helped rehabilitation with traditional techniques. This research is expected to illuminate the efficacy of robotic treatment in stroke rehabilitation. The results can contribute to faster, more intensive rehabilitation of patients and improve the quality of their life after a stroke.
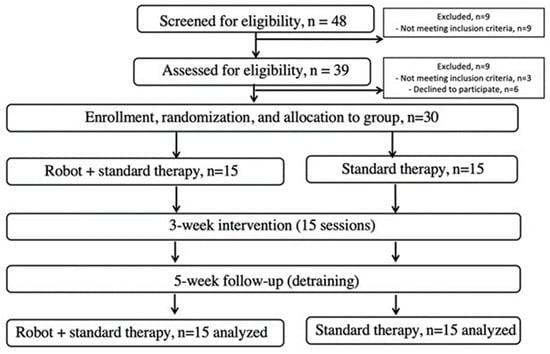
Figure 1.
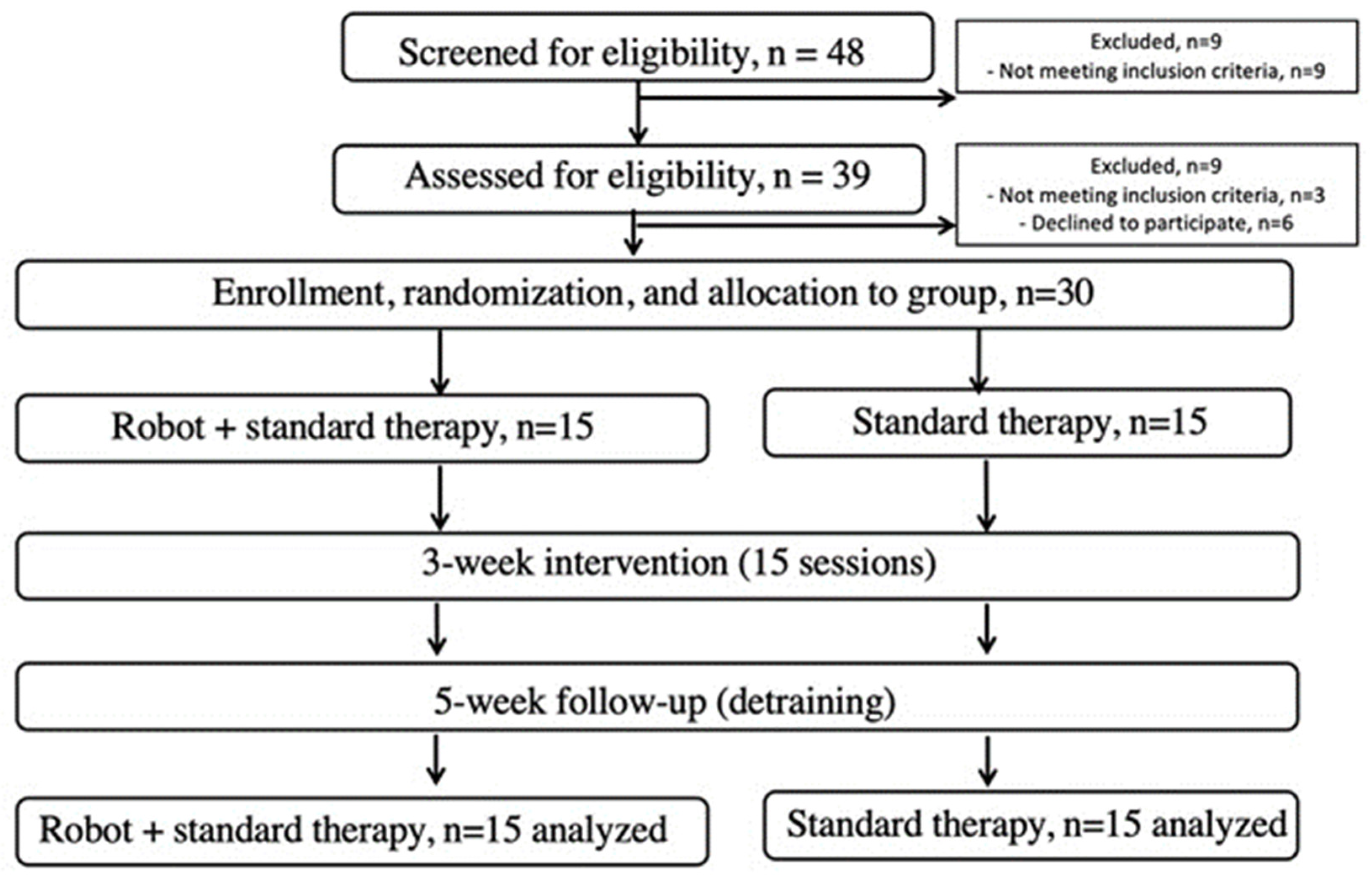
Prisma flow chart of the study conducted to assess the efficacy of a robotic rehabilitation system.
2. Materials and Methods
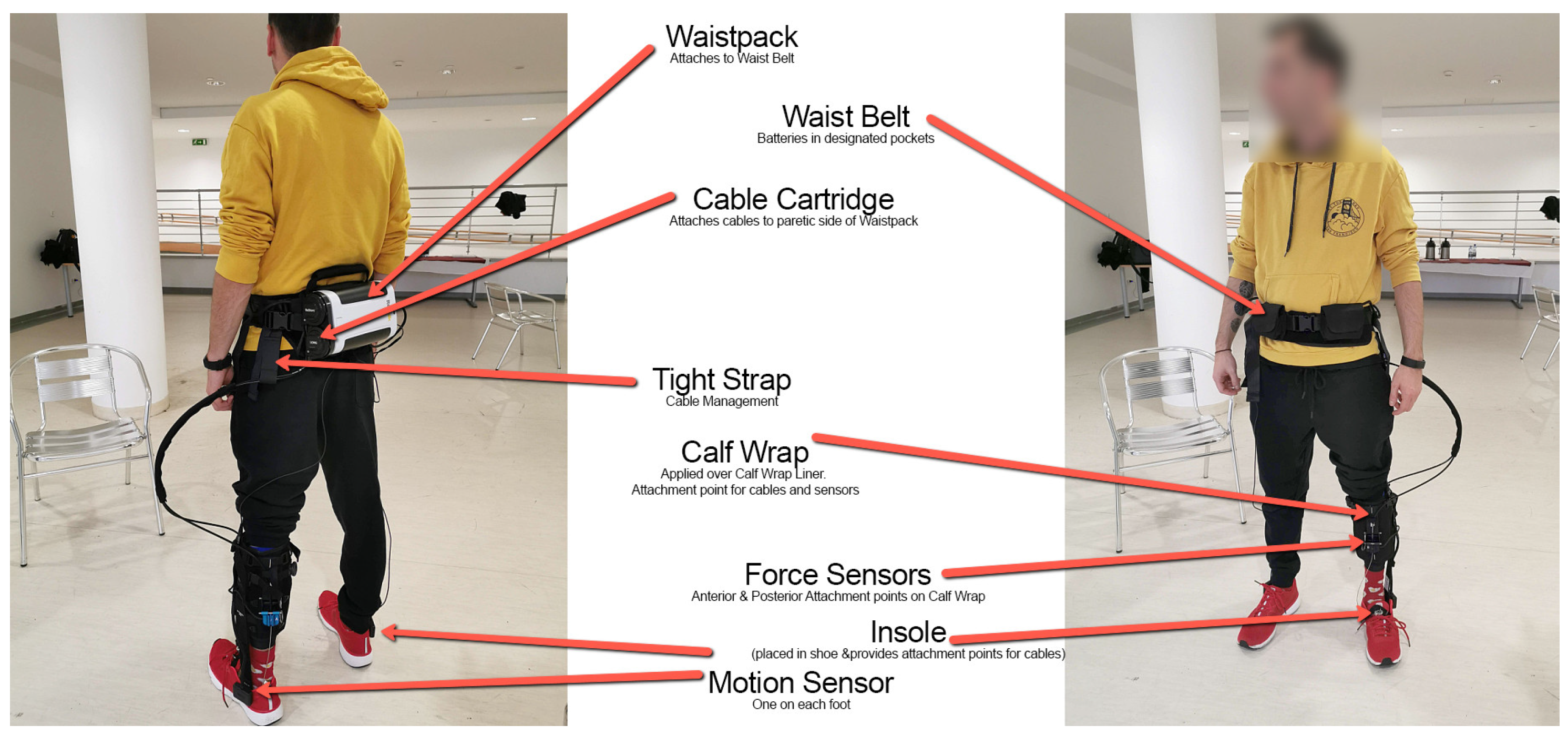
We performed a multisite, interventional, non-comparative study to evaluate the efficiency of a lower limb robotic device in subjects with hemiplegia/hemiparesis due to ischemic or hemorrhagic stroke. We used the ReStore Soft Exo Suit (Figure 2), an exoskeleton robot designed for the post-stroke rehabilitation of gait (ReWalk, Fototronic Ltd., Budapest, Hungary) [31,32]. The ReStore system has been cleared (CE marked in the EU as a medical device) for functional training, supported by its sensory data-driven force/torque control.
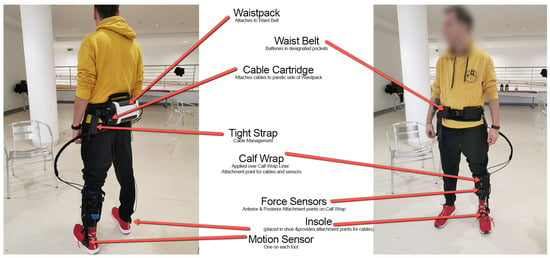
Figure 2.
The overview of the ReStore Soft Exo Suit, ReWalk system (Image credit: ReWalk Robotics).
2.1. Participants and Design
Patient inclusion criteria were as follows: first occasion of ischemic stroke diagnosed by a neurologist based on CT or MRI scans; elapsed time after stroke of 2 to 4 weeks; mobility and postural limitation determined by neurologic examination; and a Modified Rankin Scale (mRS) score of 2 or higher. Exclusion criteria included a history of multiple strokes; systolic resting blood pressure (sRBP) less than 120 or greater than 160 mmHg; orthostatic hypotension; carotid artery stenosis; severe heart disease; hemophilia; traumatic brain injury; seizure disorder; uncontrolled diabetes; abnormal electroencephalography; Mini Mental State Examination score less than 22; abnormal blood panel; use of sedatives; irregular medication schedule; serious aphasia (Western Aphasia Battery, 25); serious visual or hearing impairments; serious sensory dysfunction; serious orthopedic problems; neurologic conditions affecting motor function (PD, multiple sclerosis, multiple system atrophy, Guillain–Barré syndrome); alcoholism; recreational drug use; smoking after stroke diagnosis; inability to walk a minimum of 100 m with or without a walking aid in 6 min; Berg Balance Scale (BBS) score of 32 or less; Barthel Index (BI) score of 70 or less; inability to understand verbal instructions or prompts from a television screen; or current participation in a self-directed or formal group exercise program other than standard physical therapy. The 30 participants who had previously experienced a stroke were divided into two groups: a Robot-Assisted Gait Therapy (ROB, n = 15) group and a Standard Therapy (STT, n = 15) group. There were no significant differences in demographic and baseline data of the patients. The mean age of the participants was approx. 65 years. Most strokes occurred in the left hemisphere. Participants signed a written informed consent. The Institutional Research Ethics Committee approved the study protocol (IKEB2021/10), which was registered as a clinical trial (NCT05300867). All patients were cared for in the acute part of the neurological department.
We started patient treatment in the subacute state under hospital conditions. All patients remained in the rehabilitation ward until the end of the treatment. This study followed a pre–post–follow-up design. The participants were assessed at three different time points: pre-intervention (baseline), post-intervention (immediately after the intervention), and follow-up (a certain period after the intervention). In the Robot-Assisted Gait Therapy (ROB), patients received standard therapy with robotic treatment. In the Standard Therapy Treatment (STT), we used only standard therapy. The research was carried out in three stages. The first assessment occurred sub-acutely on the 5th day after the initial treatment. The second examination was scheduled for 15 working days later, followed by a third follow-up evaluation five weeks after the initial treatment.
Notably, all assessments were conducted without the use of the robotic device. For the 6 min walk test, both groups were evaluated, encompassing patients who received robotic treatment as well as those who underwent physiotherapy. The robotic treatment protocol was as follows: before the fitting of the robotic device, there was a 5 min warm-up session that included joint mobilization and passive movements as needed. Following the fitting, there was a 5 min warm-up walk with robotic assistance, designed to encourage patient cooperation with the robot for seamless operation. The main session consisted of a 10 min dimensional walk (along a line), during which the patients maintained a pulse rate between 110 and 130, monitored with a Polar watch. This took place along a 100-meter-long corridor. Subsequently, there were five sets of 2 min obstacle avoidance/slalom walks on a 20-meter-long course. Here, there was one-meter space between each pair of obstacles, and for every 2 min of walking, there was one minute of rest. The aim was to complete the slalom walk as swiftly and accurately as possible.
Finally, there was a 6 min period of weight-bearing exercise on a 100-meter-long track. This entailed 50 m of brisk walking followed by 50 m of relaxed walking. During the rest periods, the robot was dismounted for 5 min, followed by a 10 min stretching session. The physiotherapy treatment protocol mirrored that of the robotic treatment, with the same warm-up and fitting procedures. The main session, too, followed the same structure, including the dimensional walk, obstacle avoidance/slalom walks, and weight-bearing exercise.
2.2. Outcomes
2.2.1. Primary Outcomes
The mRS and Barthel Index were employed as the primary outcome measures to assess functional status and activities of daily living in stroke survivors. The mRS demonstrated significant improvement across all intervention groups. Specifically, the Robot-Assisted Gait Therapy (ROB) group exhibited a substantial decrease in mRS scores, indicating enhanced functional independence post-treatment. The Standard Therapy Treatment (STT) group also exhibited notable improvement in mRS scores post-intervention, with a trend towards further amelioration at follow-up. While the effect size was comparatively smaller than in the ROB case, the improvement remained clinically significant. In concurrence with mRS results, the Barthel Index demonstrated substantial improvement across all intervention groups. The ROB group displayed a remarkable increase in Barthel Index scores at post-treatment, indicative of enhanced activities of daily living. This improvement was sustained at follow-up.
2.2.2. Secondary Outcomes
The Berg Balance Scale was employed to assess postural control and balance. The ROB group demonstrated a substantial improvement in Berg Balance Scale scores post-intervention, surpassing the improvements observed in the STT group. These improvements remained stable at follow-up, indicating sustained enhancement in postural control. The 10 m maximal walking speed test provided objective measures of mobility and gait. The ROB group exhibited a substantial improvement in walking speed post-intervention, with a further enhancement observed at follow-up. The ROB group exhibited remarkable improvements in distance walked during the 6 MWT, indicating enhanced endurance levels. These improvements were sustained at follow-up. The STT group exhibited a modest improvement in 6 MWT distance post-intervention, with a trend towards further enhancement at follow-up. The secondary outcome measures corroborated the positive effects observed in the primary outcomes, underscoring the efficacy of the respective interventions in enhancing functional recovery and mobility in stroke survivors.
2.3. Intervention
Patients assessed using the Barthel Index and mRS Scale were randomly assigned to either a non-physical intervention control group (n = 9 out of 39 completed, 30 withdrew before baseline testing), or a high-intensity agility program (15 sessions over 5 weeks, n = 30 completed). Both groups began with joint warm-up exercises and passive stretching as necessary (5 min), followed by slow walking to prepare the muscles and body for the subsequent activity (5 min). Each session concluded with 10 min of stretching for both groups (Figure 1).
2.3.1. Robot-Assisted Gait Therapy (ROB) Group
In the ROB group, the donning/mounting of the robotic device took 5 min. The system recorded the movement pattern of the non-involved (non-paretic) side. Two servo motors generated plantarflexion and dorsiflexion of the involved ankle through connector cables. Patients walked with the wireless control unit affixed to their waist with a belt, which housed two batteries to power the robot. The actuators with motion sensors were strapped to the shank of each leg. Ankle plantarflexion and dorsiflexion would be online (real time with Bluetooth)- configured and the state of walking balance was displayed on the control unit’s screen. Based on the patient’s gait pattern, the therapist could adjust dorsiflexion and plantarflexion assistances independently. After the proper mounting of the device, the next step was a 5 min warm-up walk with robotic assistance—the goal was to teach the patient to work together with the robot and to be able to work smoothly. Patients, escorted by a therapist, walked for 10 min at a heart rate of 110–130 b × min−1 in a 100-meter-long hallway. They also performed 10 min of slalom walking around obstacles placed ~1 m apart over a 20-meter-long course, with 1 min of rest after each 2 min. The aim was to walk as rapidly and safely as possible. Finally, patients walked for 6 min alternating high- vs. low-intensity walking for 50 m. At the end of the session, patients sat down and the robot was taken off in about 5 min.
2.3.2. Standard Therapy Treatment (STT) Group
In the STT group, participants received standard therapy treatment, which consisted of traditional rehabilitation techniques. This therapy encompassed a range of exercises targeting various aspects of movement recovery, functional ability, and mobility. The objective of the treatment was to enhance the participants’ condition and functional abilities to mitigate the effects of the illness or injury. The control (CON) group underwent standard care as prescribed by the government, which entailed 30 min of daily group exercises while seated, and 30 min of individual physical therapy involving walking and balance exercises at local clinics. Seated exercises aimed to strengthen upper extremity and trunk muscles through movements such as lifting, lowering, and rotating medicine balls and weighted sticks. Standing exercises targeted lower extremity function, encompassing various stepping variations (forward, backward, and diagonally while standing on one leg); weight shifting; coordination exercises with arm movements while walking with or without sensory aids; and squatting movements with arm support on a backed chair to reinforce the lower extremity extensor mechanism. Following the exercise sessions, each participant in both groups received a 20 min medical massage of the lower extremities. Participants were instructed to log their symptoms, which were reviewed by therapists daily, and were advised not to modify their diet, medication, or physical activity habits throughout the study period.
2.4. Statistical Analyses
Data were reported as mean ± SD or median and interquartile ranges. An a priori power analysis using G*Power with the following input parameters was conducted to obtain medium-sized Group × Time interaction for distance walked over six minutes: effect size of f = 0.25; type I error of 0.05; type II error of 0.80; two groups; three measurements moments; and a correlation of r = 0.50 among groups [1]. These analyses were based on prior data [8,9]. Our analyses revealed a total sample size of ~33, i.e., 16–17 participants per group before an expected dropout of 20%.
The two groups’ baseline characteristics were compared with an independent t-test. The main analysis was a Group (ROB, STT) by Time (pre-intervention, post-intervention, and follow-up) analysis of variance with repeated measures on Time, followed by Tukey post hoc contrasts. These changes were further characterized via Cohen’s effect size (Cohen’s d values ≤0.49 indicate small, 0.50 ≤ d ≤ 0.79 medium, and ≥0.80 large effect sizes) [10]. Ordinal data or data not normally distributed were analyzed with non-parametric analyses. For these variables, we performed Friedman tests to determine the effects of the interventions on outcome over time. Kendall’s W quantified the effect size. A significant Friedman test was followed by a Wilcoxon test as a post hoc analysis to determine whether changes from pre-intervention to post-intervention, and post-intervention to follow-up were significant. The effect size for these within-group changes were computed through the test of probability of superiority for dependent samples (PSdep; a value of 0 or 1 denotes maximal, a value of 0.5 denotes an effect size of zero) [11]. Between-group differences at pre-intervention, post-intervention, and follow-up were assessed using the Mann–Whitney Test and the effect size characterized by η2 (η2 = 0.01: small effects; η2 = 0.06: medium effects; η2 = 0.14: large effects. To determine whether the robotic intervention affected the distance walked with and without wearing the robot in ROB, we performed a Condition of walking (robot, no robot) by Time (pre-intervention, post-intervention, follow-up) analysis of variance with repeated measures on both factors. The level of significance was set for all analyses at p < 0.05.
3. Results
3.1. Baseline Characteristics
Table 1 shows the descriptive data for the two groups. There were no differences between the groups in descriptive characteristics at baseline (all p > 0.05). Each group had 11 males of the 15 PwST enrolled. Over 70% of strokes occurred in the left hemisphere in each group. Nearly 70% of PwST smoked and 25% reported high levels of alcohol consumption in each group. Hypertension (9/30 PwST), ischemic heart disease (5/30), and diabetes (4/30) were the most frequent co-morbidities.

Table 1.
Descriptive characteristics of the two groups of patients.
3.2. Intervention Effects
Table 2 shows the intervention effects. There were no differences between the two groups in any of the variables at baseline (all p > 0.05).

Table 2.
Intervention effects on primary and secondary outcomes.
3.3. Primary Outcome
The Friedman test revealed that ROB improved the MRS median scores (Table 2, Friedman χ2 = 27.8, Kendall’s W effect size = 0.93). Post hoc analyses showed that the 2-unit pre- to post-intervention improvement by ROB was significant (Wilcoxon post hoc test, p = 0.001, PSdep effect size = 0.99), and the additional 1-unit improvement at follow-up was significant (p = 0.008, PSdep effect size = 0.79). The Friedman test revealed that STT improved the MRS median scores (Table 2, Friedman χ2 = 25.2, Kendall’s W effect size = 0.84). Post hoc analyses revealed that the 1-unit pre- to post-intervention improvement by STT was significant (p = 0.001, PSdep effect size = 0.78), but did not change further by follow-up (p = 0.180, PSdep effect size = 0.50). The Mann–Whitney test did not show between-group differences at post-intervention (U = 67.5, p = 0.061, η2 = 0.38). The 1-unit between-group difference at follow-up was significant (U = 39.0, p = 0.002, η2 = 0.93).
3.4. Secondary Outcomes
The Friedman test revealed that ROB improved Barthel Index median scores (Table 2, Friedman χ2 =27.4, Kendall’s W effect size = 0.912). Post hoc analyses showed that the 20-point pre- to post-intervention improvement by ROB was significant (Wilcoxon post hoc test, p = 0.001, PSdep effect size = 0.97), and this improved score did not change further at follow-up (p = 0.157, PSdep effect size = 0.48). The Friedman test revealed that STT improved the Barthel Index median scores (Table 2, Friedman χ2 = 27.1, Kendall’s W effect size = 0.90). Post hoc analyses revealed that the 10-point pre- to post-intervention improvement by STT was significant (p = 0.001, PSdep effect size = 0.851), but did not change further during follow-up (p = 0.059, PSdep effect size = 0.44). The Mann–Whitney Test showed that the two groups differed by 10 points at post-intervention (U = 37.0, p = 0.001, effect size η2 = 0.78), and ROB was still 5 points higher (improved) than STT at follow-up (U = 22.5, p = 0.001, effect size η2 = 0.1.06).
Berg Balance Scale scores revealed a Group by Time interaction (F = 10.5, p < 0.001, η2 = 0.27, Table 2). ROB improved by 7.2 (±3.86, 37% ±20, d = 2.21) vs. STT which improved by 2.7 points (±2.31, 14% ±13, d = 0.91, both p < 0.001), and this 4.5 points (23%) improvement difference was significant (d = 1.16, Tukey post hoc, p < 0.05). These improvements did not further change at follow-up (p > 0.05), and the 4.2 points between-group difference at follow-up was significant (p < 0.05, d = 1.38). The 10-m maximal walking speed data revealed a Group by Time interaction (F = 6.7, p < 0.002, η2 = 0.20, Table 2). ROB improved 10 m walking speed by 0.57 m/s (±0.30, 56% ±31, d = 2.36). STT improved 10 m walking speed by 0.28 m/s (±0.22, 27% ±22, d = 1.40, both < 0.001). This 0.28 m/s improvement difference was significant (p < 0.001, d = 1.12). The 0.11 m/s (d = 0.53) faster gait in ROB vs. STT was still significant at follow-up (p < 0.05).
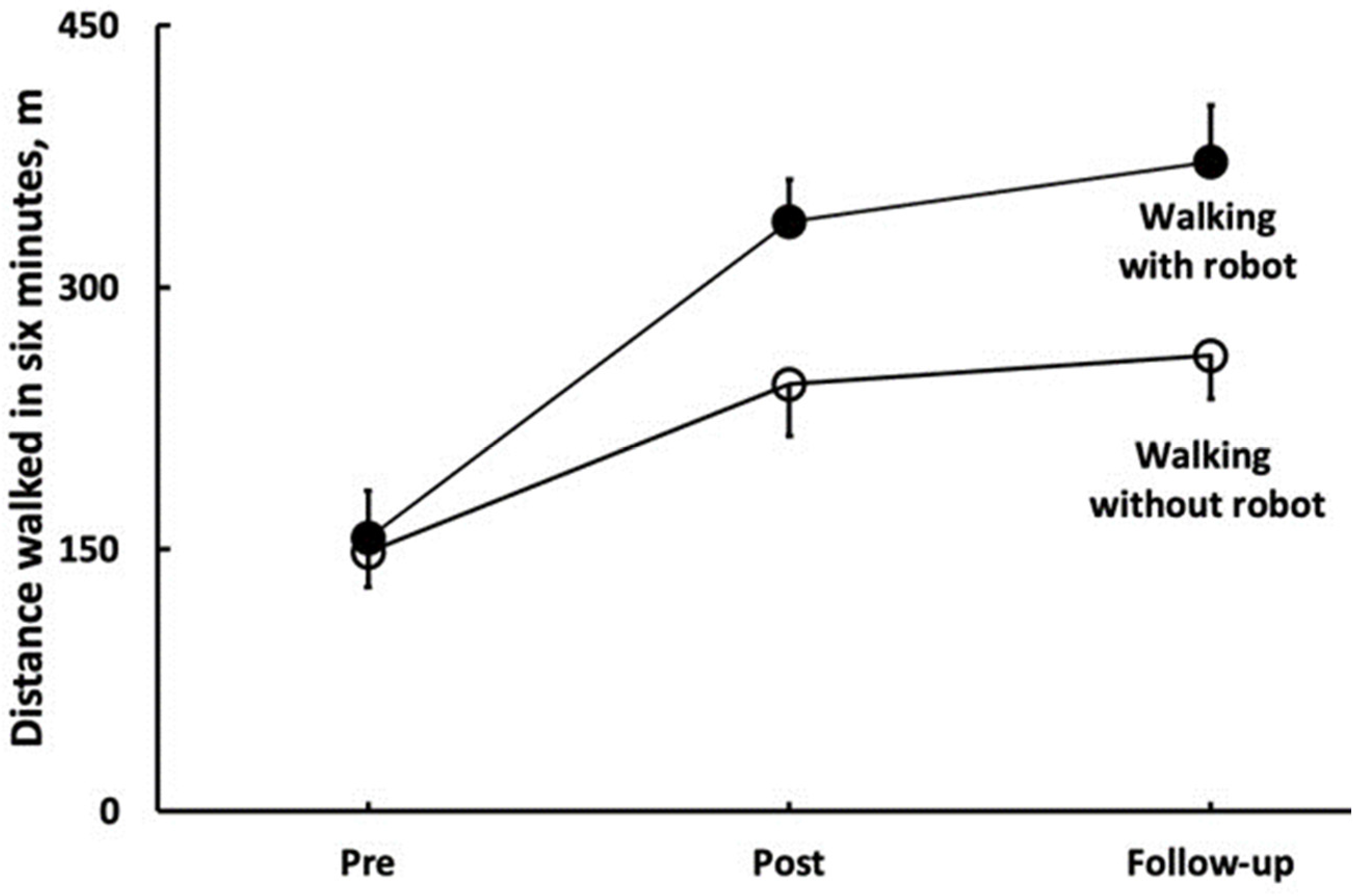
The 6MWT data revealed a Group by Time interaction (F = 34.9, p < 0.001, η2 = 0.56, Table 2). In the 6MWT, ROB improved by 96 m (±37, 68% ±32, d = 3.69), and STT improved by 32 m (±26, 23% ±20, d = 1.35, both p < 0.001); these improvements did not change significantly during follow-up (ROB: 8% ±11; STT: 3% ±10, both p > 0.05). Figure 2 shows that the 6MWT distance walked with, vs. without, wearing the robot was 93 m longer at post and 111 m longer at follow-up in ROB (both p < 0.001). Sway while standing with a wide stance and eyes open improved similarly in the two groups at post (3.6 cm ±3.99, 28% ±36, Time main effect, F = 24.6, p < 0.001, η2 = 0.47, Table 2), and these improvements were maintained at follow-up. Sway while standing with a wide stance and eyes closed improved similarly in the two groups at post-intervention (3.0 cm ±5.33, 18% ±45, Time main effect, F = 7.9, p < 0.001, η2 = 0.22, Table 2), and these improvements did not change at follow-up. Sway while standing with a narrow stance and eyes open improved similarly in the two groups at post-intervention (1.9 cm ±5.12, 9% ±42, Time main effect, F = 4.6, p < 0.014, η2 = 0.14, Table 2), and these improvements did not change at follow-up. Sway while standing with a narrow stance and eyes closed did not change in any group, neither at post-intervention, nor at follow-up (all p > 0.05, Table 2). Figure 2 depicts the effects of standard rehabilitation coupled with robotic training on the distance walked during six minutes with (filled symbols) and without robot (open symbols). The two conditions of walking (robot, no robot) interacted with time (F = 50.4, p = 0.001, η2 = 0.79). In the condition of walking with the robot, PwST (n = 15) walked 181 m (±42) or 123% (±50) further (p < 0.001) after the robotic intervention (post, d = 7.1). After a 5-week no-intervention follow-up, they walked 35 m (±22) or 10% (±7) further (d = 1.2, p = 0.011). When the same PwST were tested for the distance walked without the robot, they also walked 96 m (±37) or 68% (±32) further (post, d = 3.7, p = 0.001), and they maintained this gain but did not further increase it at follow-up (d = 0.6, p = 0.281). At post-intervention, the difference in distance walked between the two conditions was 93 m (d = 3.4, p = 0.001). At follow-up, the between-condition difference in distance walked was 111 m (d = 3.9, p = 0.001), as presented in Figure 3.
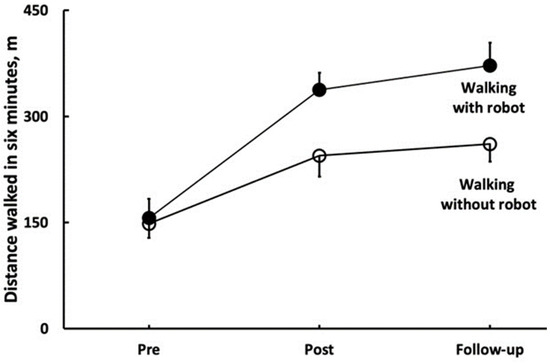
Figure 3.
Key outcome of robotic rehabilitation support—the distance patients were able to walk with robot assistance.
4. Discussion
This study compares the effects of incorporating soft exoskeletons in stroke rehabilitation using robotic-assisted therapy (ROB) and standard therapy treatment (STT). The results demonstrate significant improvements in mobility, balance, and overall functional abilities of those stroke patients who underwent ROB. Both ROB and STT led to improvements in the primary outcome measure, as indicated by the significant increase in MRS median scores. However, at follow-up, the improvement in the ROB case was advancing, while STT’s improvement remained stagnant.
In addition to the primary outcome, both groups experienced enhancements in secondary outcome measures, including Barthel Index median scores, Berg Balance Scale scores, the 10 m maximal walking speed, 6 MWT distance, and sway. Interestingly, ROB outperformed STT at follow-up, with a sustained 5-point higher improvement in Berg Balance score. Moreover, the 10 m maximal walking speed improvement difference remained significant in favor of ROB both at pre- and post-assessment, and follow-up. Notably, the 6 MWT distance walked with the soft exoskeleton was much greater at both post-assessment and follow-up, showing the potential long-term benefits of incorporating exoskeletons. Sway measures exhibited consistent improvements in both groups at post-assessment, which persisted during the follow-up period. However, Sway NEC showed no change in either group at post-assessment or follow-up. These findings emphasize the value of integrating soft exoskeletons like ROB into comprehensive stroke rehabilitation programs, offering lasting improvements in motor skills and daily activities. Rehabilitation with exoskeletons aimed to improve gait in patients with neurological diseases is a hot topic. Previous research has already proven that robotic exoskeletons are very useful in rehabilitation after stroke [8,9,10,14]. Contrary to the findings of another study analysis [27], in our study, there was a significant positive outcome using robotic exoskeletons, highlighting the benefits on the 6 MWT.
Based on our research, it can be said that exoskeletons physically strengthen the force and stability of patients, support their joints, and can contribute to restoring or improving their ability to move. Exoskeletons enable intensive and more effective therapy for patients after stroke. The devices allow patients to experience a sense of movement and independence, which can improve their self-confidence. As a result, they can be more motivated in the rehabilitation process and put more effort into relearning and recovery [33,34,35]. Current research is focused on improving functional ambulation using exoskeletons to improve safe community ambulation [27]. As an aim, our study focused on the use of exoskeletons in neuro-rehabilitation departments. Robotic therapy treatment in these departments is useful, because we can follow the patients’ progress better and there is more time to get used to the exoskeleton. Some other studies focused on exoskeletons’ long-term effects [36]. We tried to explore exoskeletons in the early rehabilitation after stroke; we obtained similar results as in another study [37], the post-intervention results showed improvements with both standard therapy and robotic therapy, but the latter had a higher impact on the mentioned results. However, considering follow-up results, only the robotic therapy showed a progressive tendency. Similar incremental benefits have been reported in other studies as well [38,39,40].
These research outcomes are considered significant from the regulatory point of view as well, since the new Medical Device Regulation (MDR) in the EU explicitly makes post-market surveillance and outcome-monitoring compulsory for medical device manufacturers [41,42,43], and there are similar challenges posed by the U.S. Food and Drug Administration [44].
When using robot treatments in everyday practice, there are many things which need attention. For example, the robot device must fit the patient’s body properly to be effective. Because body sizes and types are each unique, there can be challenges in achieving optimal fixation and fit. If the device is not properly fitted or fixed, it cannot adequately support and guide the patient’s movement. Robotic devices may sometimes require complex settings or ongoing maintenance to function properly. Device management and maintenance may require additional time and resources from therapists and patients. The aim of this research was to investigate the effectiveness of exoskeletons in the gait rehabilitation of patients after stroke. Nowadays, many new therapies are used in stroke rehabilitations. In the future, it will be an interesting research topic to examine the beneficial effects of these therapies and to compare them.
Moreover, as has been seen with other robotic therapeutic or interventional robotic devices [3,45,46], the overall cost and complexity of maintenance has been an inhibiting factor regarding clinical application. Supporting evidence for clinical outcome significance remains a main goal of treatment solution providers.
5. Limitations
The main limitation of this research is that it was conducted with a relatively small number of patients. With larger groups the level of significance increases, which leads to fewer errors in terms of quantity and quality. For example, it could be stated with greater certainty that the more effective improvement in the robotic rehabilitation therapy group is not just coincidental.
The other limitation is that we examined only a short follow-up period. In addition, we do not have knowledge on the long-term outcomes yet, and do not know the duration of the benefits.
6. Conclusions
The use of robotic therapy treatment can be useful in the rehabilitation of post-stroke patients. Stroke can often cause paralysis or weakness on one side of the body. Robotic treatment can help restore movement in affected limbs. These devices allow patients to begin a range of motion exercises earlier, even if they lack sufficient muscle strength or stability. Robotic therapy contributes to rapid, intensive recovery, while increasing the patients’ self-confidence and improving the quality of their everyday life.
7. Suppliers
- MediTECH Electronic GmbH, 95
- ReWalk system: Restore, soft exoskeleton
- Statistical Package for the Social Sciences, SPSS, version 22; IBM.
Author Contributions
Authors contributed equally to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the Department of Neurology, Somogy County Kaposi Móricz Teaching University Hospital, and by a “Regional Health Development” award from the Doctoral School of the Faculty of Health Sciences, University of Pécs. The research was supported by the NKFIH from the project ‘Research on the health application of artificial intelligence, digital imaging, employment and material technology developments by linking the scientific results of Széchenyi István University and Semmelweis University’, under grant number TKP2021-EGA-21.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Kaposi Mór Oktató Kórház (IKEB2021/10).
Data Availability Statement
Authors are committed to making the data available upon request.
Acknowledgments
Authors express their sincere gratitude to Zoltán Vadászi for his contributions and engineering support throughout the project. J.T. was supported in part by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, Hungary. T. Haidegger is a Consolidator Researcher, supported by the Distinguished Researcher program of Óbuda University.
Conflicts of Interest
The authors declare no conflicts of interest. The authors state that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Abbreviations
| ROB | Robot-Assisted Gait Therapy |
| STT | Standard Therapy Treatment |
| MRS | Modified Rankin Scale |
| BI | Barthel Index |
| BBS | Berg Balance Scale |
| 6MWT | 6 Minute Walk Test |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| rSBP | Resting Systolic Blood Pressure |
| IT | Inertial Technology |
| PwST | Patients with Stroke |
| ReStore Soft Exo Suit | Robotic Exoskeleton Device |
| ReWalk | Manufacturer of Robotic Exoskeleton |
| cm | Centimeters |
| m | Meters |
| m/s | Meters per Second |
| min | Minutes |
| SD | Standard Deviation |
| G*Power | Statistical Power Analysis Software |
| η2 | Eta-squared (Effect Size) |
| PSdep | Probability of Superiority for Dependent Samples |
| NEC | Eyes Closed |
| STT | Soft Exoskeleton |
References
- Esquenazi, A.; Talaty, M.; Packel, A.; Saulino, M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 2013, 92, 617–624. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, W.; Zhang, W.; Ding, X. A Review on Lower Limb Rehabilitation Exoskeleton Robots. Chin. J. Mech. Eng. 2019, 32, 74. [Google Scholar] [CrossRef]
- Fichtinger, G.; Troccaz, J.; Haidegger, T. Image-Guided Interventional Robotics: Lost in Translation? Proc. IEEE 2022, 110, 932–950. [Google Scholar] [CrossRef]
- Haidegger, T.; Speidel, S.; Stoyanov, D.; Satava, R.M. Robot-assisted minimally invasive surgery—Surgical robotics in the data age. Proc. IEEE 2022, 110, 835–846. [Google Scholar] [CrossRef]
- Haidegger, T.; Mai, V.; Mörch, C.; Boesl, D.; Jacobs, A.; Rao, R.B.; Khamis, A.; Lach, L.; Vanderborght, B. Robotics: Enabler and inhibitor of the Sustainable Development Goals. Sustain. Prod. Consum. 2023, 43, 422–434. [Google Scholar] [CrossRef]
- Han, S.; Wang, H.; Yu, H. Human–Robot Interaction Evaluation-Based AAN Control for Upper Limb Rehabilitation Robots Driven by Series Elastic Actuators. IEEE Trans. Robot. 2023, 39, 3437–3451. [Google Scholar] [CrossRef]
- Tollár, J.; Vetrovsky, T.; Széphelyi, K.; Csutorás, B.; Prontvai, N.; Ács, P.; Hortobágyi, T. Effects of 2-year-long Maintenance Training and Detraining on 558 Subacute Ischemic Stroke Patients’; Clinical-Motor Symptoms. Med. Sci. Sports Exerc. 2022, 55, 607–613. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.; Yoon, B. Effects of wearable robot-assisted gait training on walking ability and quality in individuals with stroke: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1315–1328. [Google Scholar]
- Cai, L.; Chen, C.; Chen, Y.; Wu, J. Exoskeleton rehabilitation robot training methods for stroke rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2018, 15, 1–15. [Google Scholar]
- Loureiro, R.C.; Collin, C.A.; Harwin, W.S. Upper limb robot mediated stroke therapy—GENTLE/s approach. Auton. Robot. 2019, 43, 733–746. [Google Scholar]
- Huo, W.; Xia, C.; Wang, J.; Ma, X. A review of wearable lower limb exoskeletons for gait training. Disabil. Rehabil. Assist. Technol. 2020, 1–16. [Google Scholar]
- Da Silva Cameirão, M.; Bermúdez IBadia, S.; Duarte, E.; Verschure, P.F. Virtual reality-based rehabilitation speeds up functional recovery of the upper extremities after stroke: A randomized controlled pilot study in the acute phase of stroke using the Rehabilitation Gaming System. Restor. Neurol. Neurosci. 2011, 29, 287–298. [Google Scholar] [CrossRef]
- Kazerooni, H.; Steger, R. (Eds.) Exoskeletons in Rehabilitation Robotics: Tremor Suppression; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Gorgey, A.S. Robotic exoskeletons: The current pros and cons. World J. Orthop. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, R.C.; Harwin, W.S. Exoskeletons and active orthoses: Literature review, design examples and future directions. Med. Eng. Phys. 2016, 38, 1159–1173. [Google Scholar]
- Veneman, J.F.; Kruidhof, R.; Hekman, E.E.; Ekkelenkamp, R.; Van Asseldonk, E.H.; Van der Kooij, H. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Castermans, T.; Hoellinger, T.; Maciejasz, P.; Dupeyron, A. The CYBERLEGs exoskeleton: An autonomous wearable robot for supporting people with mobility impairments. IEEE Robot. Autom. Mag. 2011, 18, 37–45. [Google Scholar]
- Esquenazi, A.; Talaty, M.; Jayaraman, A. Powered exoskeletons for walking assistance in persons with central nervous system injuries: A narrative review. PMR 2016, 9, 46–62. [Google Scholar] [CrossRef]
- Asbeck, A.T.; De Rossi, S.; Holt, K.G.; Walsh, C.J. A biologically inspired soft exosuit for walking assistance. Int. J. Robot. Res. 2015, 34, 744–762. [Google Scholar] [CrossRef]
- Polygerinos, P.; Galloway, K.C.; Savage, E.; Herman, M.; Walsh, C.J. EMG controlled soft robotic glove for assistance during activities of daily living. Robot. Auton. Syst. 2015, 73, 171–178. [Google Scholar]
- Park, S.; Ha, J.W.; Kim, J. Soft exosuit for hip assistance with a soft posterior module. Soft Robot. 2018, 5, 700–711. [Google Scholar]
- Panizzolo, F.A.; Galiana, I.; Asbeck, A.T.; Siviy, C.; Schmidt, K.; Holt, K.G.; Walsh, C.J. A biologically inspired multi-joint soft exosuit that can reduce the energy cost of loaded walking. J. Neuroeng. Rehabil. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, B.T.; Lee, S.; Malcolm, P.; Rossi, D.M.; Grimmer, M.; Siviy, C.; Karavas, N.; Wagner, D.; Asbeck, A.; Galiana, I.; et al. Assistance magnitude versus metabolic cost reductions for a tethered multiarticular soft exosuit. Sci. Robot. 2017, 2, eaah4416. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Panizzolo, F.A.; Siviy, C.; Malcolm, P.; Galiana, I.; Walsh, C.J. Effect of timing of hip extension assistance during loaded walking with a soft exosuit. J. Neuroeng. Rehabil. 2018, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and bio- medical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power for the Behavioral Sciences; Erblaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Karunakaran, K.K.; Gute, S.; Ames, G.R.; Chervin, K.; Dandola, C.M.; Nolan, J.K. Effect of robotic exoskeleton gait training during acute stroke on functional ambulation. NeuroRehabilitation 2021, 48, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.O.; Vizi, M.B.; Galambos, P.; Szalay, T. Direct drive hand exoskeleton for robot-assisted post stroke rehabilitation. Acta Polytech. Hung. 2021, 18, 37–54. [Google Scholar] [CrossRef]
- Di Tommaso, F.; Tamburella, F.; Lorusso, M.; Gastaldi, L.; Molinari, M.; Tagliamonte, N.L. Biomechanics of Exoskeleton-Assisted Treadmill Walking. In Proceedings of the 2023 International Conference on Rehabilitation Robotics (ICORR), Singapore, 24–28 September 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Chisholm, A.E.; Alamro, R.A.; Williams, A.M.M.; Lam, T. Overground vs. treadmill-based robotic gait training to improve seated balance in people with motor-complete spinal cord injury: A case report. J. Neuroeng. Rehabil. 2017, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Awad, L.N.; Esquenazi, A.; Francisco, G.E.; Nolan, K.J.; Jayaraman, A. The ReWalk ReStore™ soft robotic exosuit: A multi-site clinical trial of the safety, reliability, and feasibility of exosuit-augmented post-stroke gait rehabilitation. J. Neuroeng. Rehabil. 2020, 17, 80. [Google Scholar] [CrossRef]
- Awad, L.N.; Bae, J.; O’donnell, K.; De Rossi, S.M.; Hendron, K.; Sloot, L.H.; Kudzia, P.; Allen, S.; Holt, K.G.; Ellis, T.D.; et al. A soft robotic exosuit improves walking in patients after stroke. Sci. Transl. Med. 2017, 9, eaai9084. [Google Scholar] [CrossRef]
- Grissom, R.J.; Kim, J.J. Effect Sizes for Research: Univariate and Multivariate Applications, 2nd ed.; Routledge: London, UK, 2012. [Google Scholar]
- Tollar, J.; Nagy, F.; Csutoras, B.; Prontvai, N.; Nagy, Z.; Török, K.; Blényesi, E.; Vajda, Z.; Farkas, D.; Tóth, B.E.; et al. High Frequency and Intensity Rehabilitation in 641 Subacute Ischemic Stroke Patients. Arch. Phys. Med. Rehabil. 2021, 102, 9–18. [Google Scholar] [CrossRef]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; de Sire, A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: A systematic review. Eur. J. Phys. Rehabil. Med. 2021, 58, 1–8. [Google Scholar] [CrossRef]
- Postol, N.; Grissel, J.; McHugh, C.; Bivard, A.; Spratt, N.J.; Marquez, J. Effects of therapy with a free-standing robotic exoskeleton on motor function and other health indicators in people with severe mobility impairment due to chronic stroke: A quasi-controlled study. J. Rehabil. Assist. Technol. Eng. 2021, 8, 3–4. [Google Scholar] [CrossRef]
- Awad, L.N.; Kudzia, P.; Revi, D.A.; Ellis, T.D.; Walsh, C.J. Walking faster and farther with a soft robotic exosuit: Implications for post-stroke gait assistance and rehabilitation. IEEE Open J. Eng. Med. Biol. 2020, 1, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, S.; Xue, Q. Lower limb rehabilitation exoskeleton robot: A review. Adv. Mech. Eng. 2021, 13, 1–17. [Google Scholar] [CrossRef]
- Sarajchi, M.; Sirlantzis, K. Pediatric Robotic Lower-Limb Exoskeleton: An Innovative Design and Kinematic Analysis. IEEE Access 2023, 11, 115219–115230. [Google Scholar] [CrossRef]
- Vassallo, C.; Zinni, G.; Maludrottu, S.; Laffranchi, M.; De Michieli, L. Stairs and ramps ascent and descent: How to design feasible gait patterns for a powered lower-limb exoskeleton. In Proceedings of the 2022 9th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), Seoul, Republic of Korea, 21–24 August 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Pentek, M.; Zrubka, Z.; Gulacsi, L.; Weszl, M.; Czere, J.T.; Tamás, P. 10 pragmatic points to consider when performing a systematic literature review of clinical evidence on digital medical devices. Acta Polytech. Hung. 2023, 20, 110–128. [Google Scholar]
- Zah, V.; Burrell, A.; Asche, C.; Zrubka, Z. Paying for Digital Health Interventions-What Evidence is Needed? Acta Polytech. Hung. 2022, 19, 179–199. [Google Scholar] [CrossRef]
- Hölgyesi, Á.; Zrubka, Z.; Gulácsi, L.; Baji, P.; Tamás, H.; Kozlovszky, M.; Weszl, M.; Kovács, L.; Péntek, M. Robot-assisted surgery and artificial intelligence-based tumour diag-nostics: Social preferences with a representative cross-sectional survey. BMC Med. Inform. Decis. Mak. 2024, 24, 1–12, article in press. [Google Scholar]
- Eguren, D.; Contreras-Vidal, J.L. Navigating the FDA Medical Device Regulatory Pathways for Pediatric Lower Limb Exoskeleton Devices. IEEE Syst. J. 2021, 15, 2361–2368. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Khan, A.A.; Muzammil, M. Lower limb rehabilitation robotics: The current understanding and technology. Work 2021, 69, 775–793. [Google Scholar] [CrossRef]
- Pană, C.F.; Popescu, D.; Rădulescu, V.M. Patent Review of Lower Limb Rehabilitation Robotic Systems by Sensors and Actuation Systems Used. Sensors 2023, 23, 6237. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).