Requirements and Solutions for Motion Limb Assistance of COVID-19 Patients
Abstract
:1. Introduction
2. Effects of COVID-19 in Limb Motion Capabilities
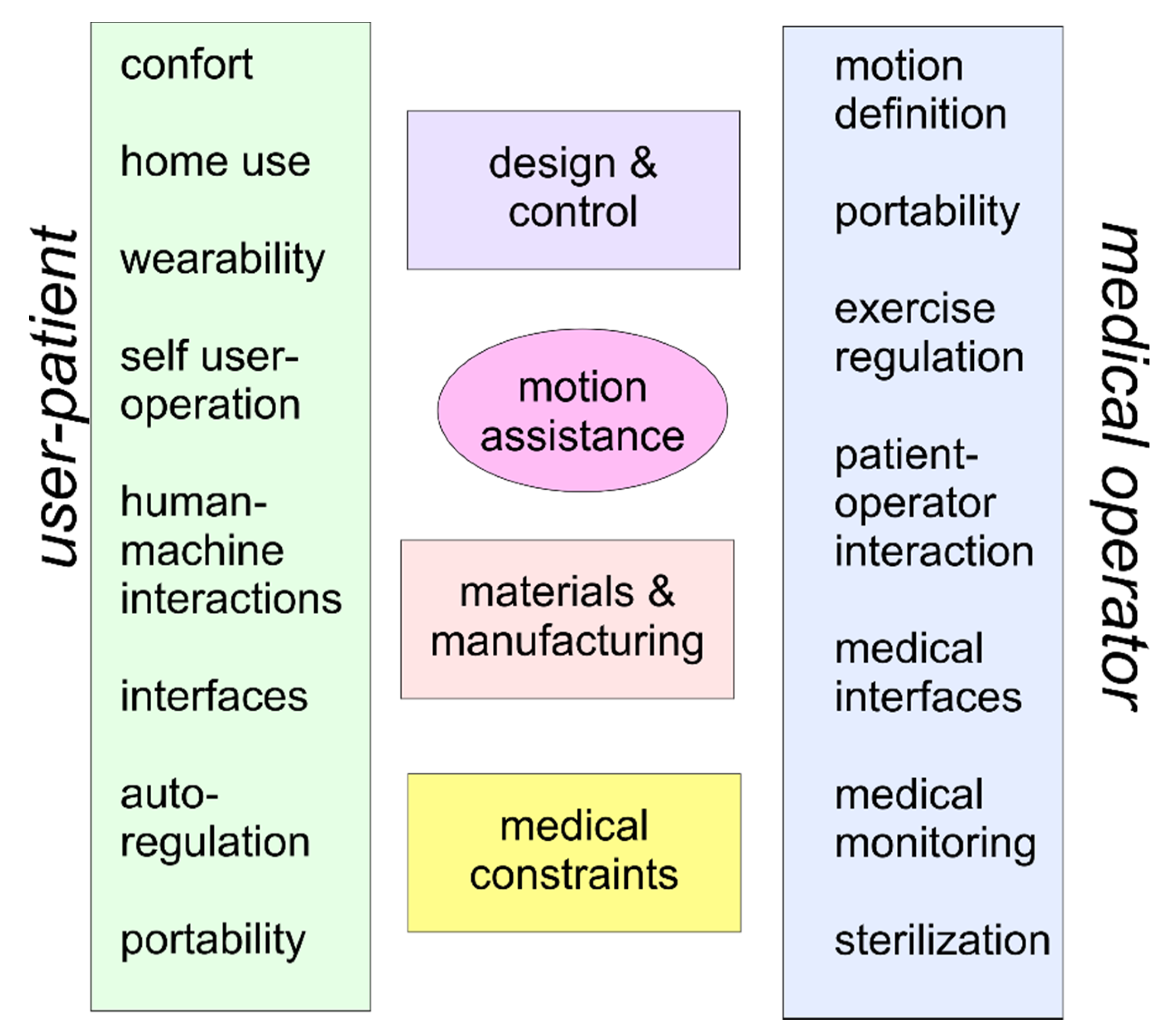
3. Problems and Requirements
- Comfort: User comfort can be considered a requirement with constraints for the functional medical purposes of a motion assistance device, although sometimes it is considered of minor importance. The comfort in weak COVID patients requires specific considerations in terms of the wearing and usage of the device not only for design and functional problems, but even for man–machine interactions and psychological effects on a user.
- Home use: A home device presents problems for both the design and operation when a device is conceived to be handled directly by a user in an environment, such as home frames, which are not accommodated for medical devices. Thus, particular attention can be necessary in terms of the sizes, functionalities, and interactivity with a user when considering additional requirements to address medical hygiene and environmental safety.
- Wearability: Wearability depends on comfort and the possibility of user autonomy in using a device. It is also a fundamental aspect for user acceptance, and it affects interactions with the patient’s arm, the ease of application, and the use of the device with specific other sensors.
- Self-user operation: Autonomy in operating a motion assistance device is an important characteristic for acceptance and practical usage. This aspect includes learning, training, and handling the operation of the device, including regulation and monitoring during motion exercise.
- Human–machine interactions: The interactions among limbs with their parts and the device determine specific characteristics for motion assistance in terms of requirements and constraints, but they also present specifications for the design and functionality of a device with proper levels for movements and actions of the device connected to the user’s limb.
- Interfaces: Interfaces are necessary in terms of device operation for movement adjustments in the user’s exercise, and they are also required for motion and clinical monitoring with supervision by medical operators, e.g., via smartphone.
- Auto-regulation: A device can be conveniently designed with intelligent self-regulation in order to operate with proper levels of autonomy for the user’s personal safety. The regulation and control of the device operation during motion assistance can be designed with control algorithms that should be developed with functions depending on the user’s reactions.
- Safety: User safety is a fundamental aspect for any motion assisting device, such as a machine design interacting with a human body. High-reliability levels and risk awareness are expected both by users and medical operators, following a full understanding of the design and operation of a device.
- Portability: The ease of transportation and setting up of an assisting device can be fundamental for the device to be properly located in a hospital or home frame, as well as for avoiding interference with other assisting equipment.
- As per the medical operator side, attention should be paid to:
- Motion definition: The motion characteristics in the exercise of motion assistance require clinical medical diagnosis of the patient conditions by a medical operator and the possibility of performing motion exercises properly with the device available. The determination of assisted motion requires synergy and integration of the device’s motion capabilities with the physiotherapy therapy that is indicated by a medical operator for a specific user-patient.
- Portability: Portability is a fundamental characteristic of motion assisting devices to satisfy the needs of patients and therapy plans established by physiotherapists, as well as to adapt the motion assistance to specific patient constraints and conditions, whether the location of the assistance is in hospital or home frames. The portability feature can also be expressed in terms of light design, easy operation, and easy adaptability to patient anatomy.
- Exercise regulation: Exercise regulation is a key aspect of motion assistance both for users and medical operators according to a prescribed medical therapy. Thus, the regulation and control of the motion exercises will be assisted by suitable sensors that are useful also for monitoring and remote control by a medical supervisor.
- Patient–operator interaction: Interaction between a patient and medical operators during a motion-assisted exercise session can be expected in such a way that a real-time reaction is ensured to give outputs and instructions for necessary adjustments in the exercise, as well as to share data and problems on the exercise results.
- Medical interfaces: Medical interfaces are fundamental equipment components in a motion assisting device with the aim of monitoring and checking clinical and medical parameters during motion exercise sessions. Those medical interfaces, such as sensors and vision systems, can be considered necessary complements when integrated into a device for an interactive operation by users and medical operators.
- Medical monitoring: Medical monitoring and supervision are indispensable for evaluating the effects of motion exercises as well as for permitting the timely indications of medical operators to a user-patient during and in anticipation of an exercise session. Such medical monitoring equipment can include sensors and medical interfaces as well as special equipment useful for monitoring activity with video transmissions and even remote modes.
- Safety: Safety issues and medical constraints must be taken into account as a first requirement in the usage of motion assisting devices, and even if medical issues cannot be represented with clear technical constraints, they may identify new problems and new solutions in terms of the technical design and medical operation of those devices.
- Sterilization: Sterilization is an essential aspect in dealing with the COVID patients and therefore requires suitable care in terms of design and operation in order to ensure full cleanness with a fairly simple procedure after each use of a device, either by the user-patient alone or by an assistant to the user.
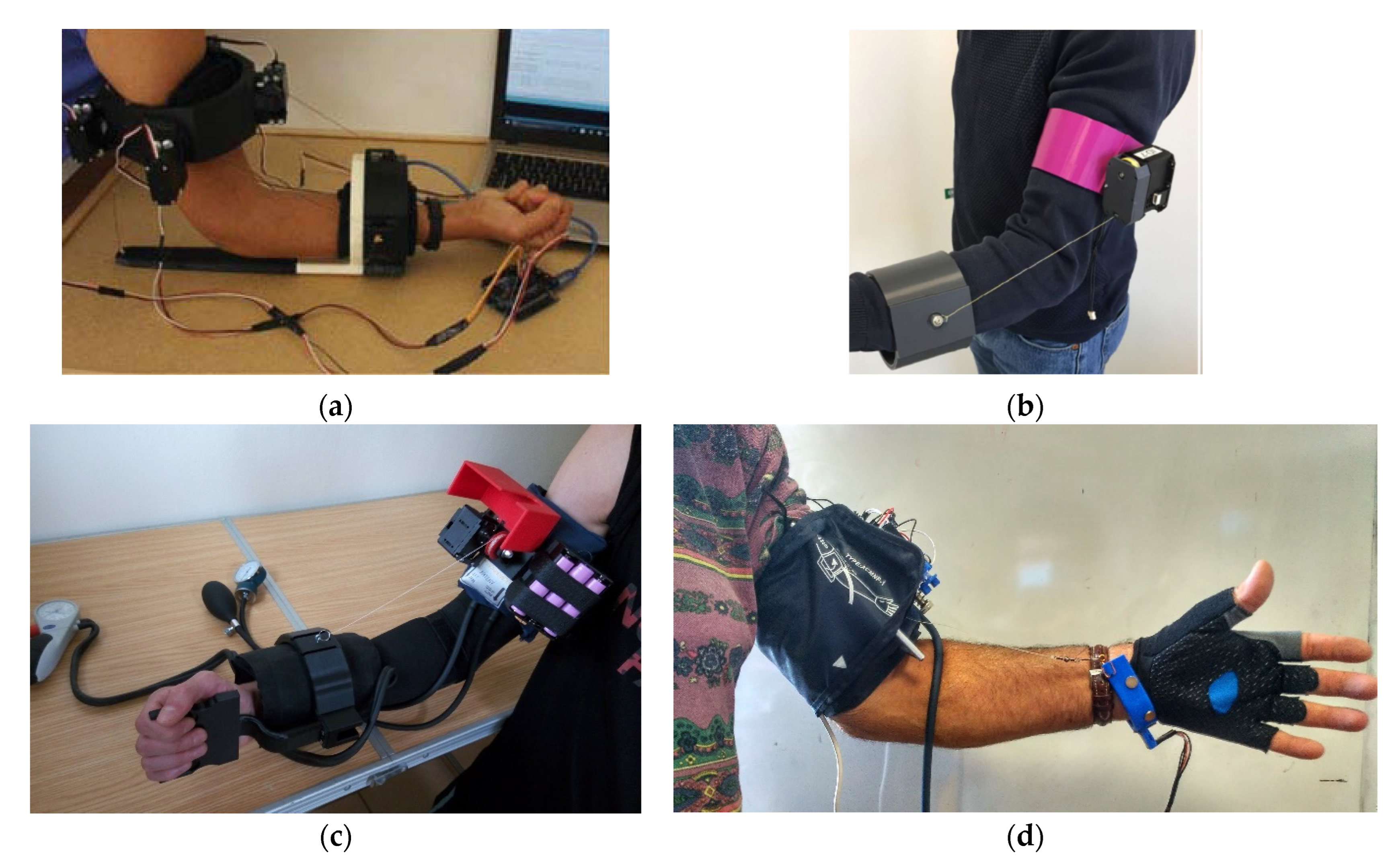
4. A Survey on Existing Medical Devices
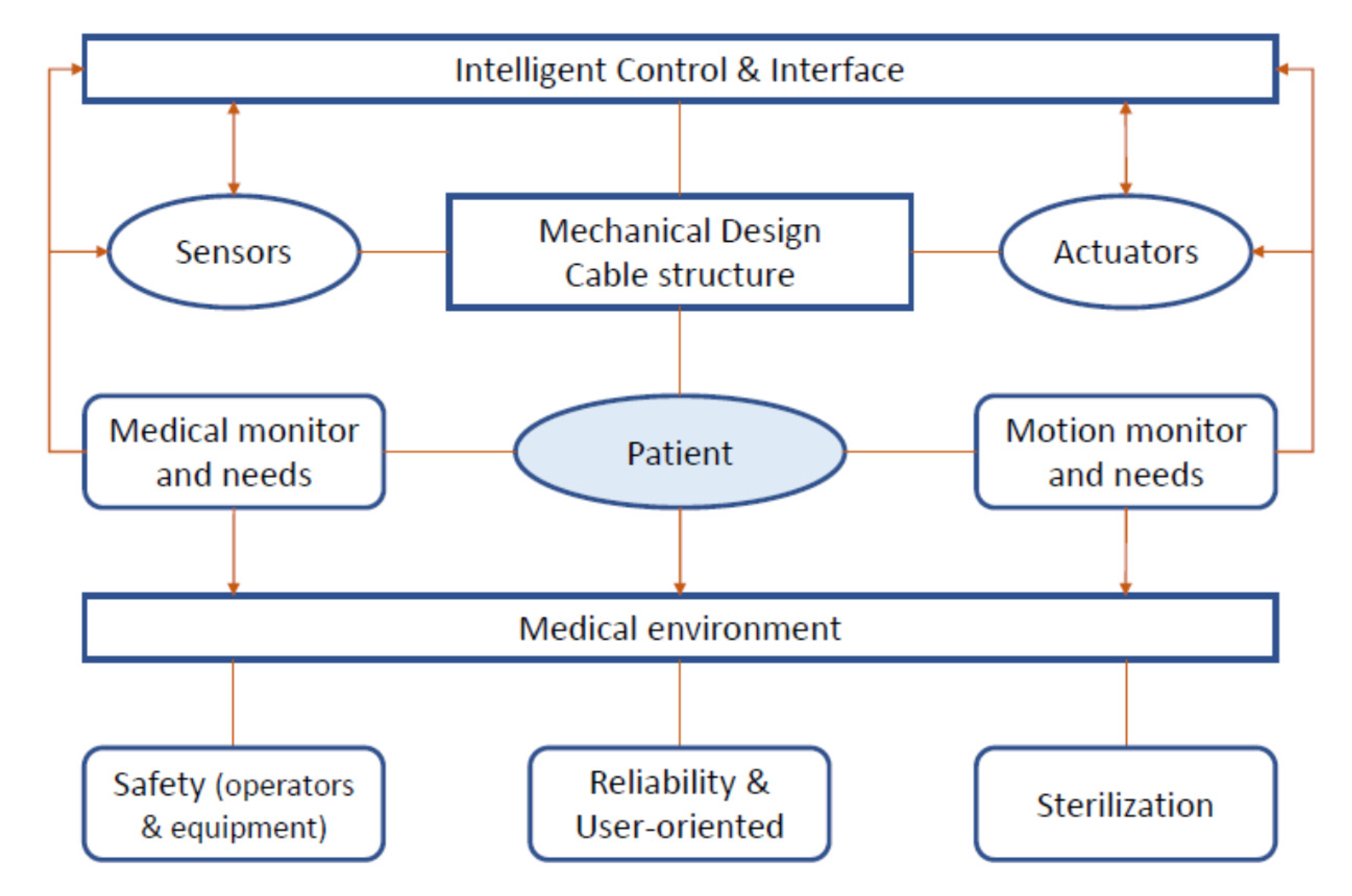
5. Requirements for Updated or New Solutions
- Cost-oriented: more and more people require rehabilitation and exercising after COVID-19 recovery, so it is important to reduce both the production (and sale) and operation cost to better reach patients at an affordable budget.
- Lightweight and compact: the device should be easy to carry around the house and should be compact to improve home delivery.
- Easy to use: Even if telerehabilitation is carried out, a patient should be requested to perform some exercises on its own. In this scenario, the device should be easy to use by a patient to ensure good rehabilitation and exercising results.
- Durable: Different patients may provide different care to the equipment. To reduce broken equipment (thus increased costs), the device should be durable, even after several cycles of treatment (and different patients).
- Easy to disinfect: If the rehabilitation phase is limited, the same device could be cleaned for reuse and even be given to another patient. The number of parts and gaps should be reduced, and the materials used should be compatible with the disinfection processes.
- Safe: Apart from the rehabilitation process, the device should not harm the patient in other ways. Safety measures must be implemented in the control design and operation, and with proper control by using proper sensors.
6. An Example: CADEL Solution
- Wear the equipment.
- Adjust the ring platforms.
- Connect the cables from the arm platform to the forearm platform.
- Start the exercise.
7. Conclusions
8. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Di Lallo, A.; Murphy, R.; Krieger, A.; Zhu, J.; Taylor, R.H.; Su, H. Medical Robots for Infectious Diseases: Lessons and Challenges from the COVID-19 Pandemic. IEEE Robot. Autom. Mag. 2021, 28, 18–27. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Argenzian, M.G.; Bruc, S.L.; Slate, C.L.; Tia, J.R.; Baldwi, M.R.; Barr, R.G.; Chan, B.P.; Cha, K.H.; Cho, J.J.; Gavin, N.; et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020, 369, m1996. [Google Scholar] [CrossRef]
- Stam, H.J.; Stucki, G.; Bickenbach, J. COVID-19 and post intensive care syndrome: A call for action. J. Rehabil. Med. 2020, 52, jrm00044. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, M.J.; Haddadi, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Hasanzadeh, S.; Jamshidi, P.; Murthi, M.; Mirsaeidi, M. COVID-19 Clinical Characteristics, and Sex-Specific Risk of Mortality: Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Thomas, P.; Baldwin, C.; Bissett, B.; Boden, I.; Gosselink, R.; Granger, C.L.; Hodgson, C.; Jones, A.Y.; Kho, M.E.; Moses, R.; et al. Physiotherapy management for COVID-19 in the acute hospital setting: Clinical practice recommendations. J. Physiother. 2020, 66, 73–82. [Google Scholar] [CrossRef]
- Kress, J.P.; Hall, J.B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014, 370, 1626–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, G.; Barrett, H.; Patel, P.; Soni, S. One hundred eighteen days on a ventilator: A COVID-19 success story against all odds. BMJ Case Rep. 2021, 14, e239631. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.; Seiler, B.; Tomonaga, Y.; Schwenkglenks, M.; Puhan, M.A.; Yebyo, H.G. Systematic early versus late mobilization or standard early mobilization in mechanically ventilated adult ICU patients: Systematic review and meta-analysis. Crit. Care 2021, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Skeletalmuscle damage in COVID-19: A call for action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef]
- Medrinal, C.; Prieur, G.; Bonnevie, T.; Gravier, F.E.; Mayard, D.; Desmalles, E.; Smondack, P.; Lamia, B.; Combret, Y.; Fossat, G. Muscle weakness, functional capacities and recovery for COVID-19 ICU survivors. BMC Anesthesiol. 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, H.; Prieske, O.; Herz, M.; Moran, J.; Hohne, J.; Kliegl, R.; Ramirez-Campillo, R.; Behm, D.G.; Hortobagyi, T.; Granacher, U. Home-based exercise programmes improve physical fitness of healthy older adults: A PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Res. Rev. 2021, 67, 101265. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: Results of the ECLB-COVID19 international online survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Clinical Management: Living Guidance, 25 January 2021; Technical Report; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Salvitti, S.; Repossini, E. Perception, experience and knowledge of early physiotherapy in intensive care units of Rome: A survey. Monaldi Arch. Chest Dis. 2021, 90, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Quigley, A.; Johnson, H.; McArthur, C. Transforming the provision of physiotherapy in the time of COVID-19: A call to action for telerehabilitation. Physiother. Can. 2021, 73, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, M.A.; Galea, O.A.; O’Leary, S.P.; Hill, A.J.; Russell, T.G. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: A systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fernandez, A.; Lobo-Prat, J.; Font-Llagunes, J.M. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J. NeuroEng. Rehabil. 2021, 18, 22. [Google Scholar] [CrossRef]
- Kozisek, A.; Ceccarelli, M.; Laribi, M.A.; Ferrara, L. Experimental characterization of a cable-driven device for elbow motion assistance. In New Trends in Medical and Service Robotics; Springer: Cham, Switzerland, 2021; pp. 71–78. [Google Scholar] [CrossRef]
- Esquenazi, A.; Talaty, M.; Packel, A.; Saulino, M. The Rewalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am. J. Phys. Med. Rehabil. 2012, 91, 911–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanabe, S.; Hirano, S.; Saitoh, E. Wearable Power-Assist Locomotor (WPAL) for supporting upright walking in persons with paraplegia. NeuroRehabilitation 2013, 33, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Mao, H.F.; Hu, J.S.; Wang, T.Y.; Tsai, Y.J.; Hsu, W.L. The effects of gait training using powered lower limb exoskeleton robot on individuals with complete spinal cord injury. J. NeuroEng. Rehabil. 2018, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- Prange, G.B.; Jannink, M.J.; Groothuis-Oudshoorn, C.G.; Hermens, H.J.; Ijzerman, M.J. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J. Rehabil. Res. Dev. 2006, 43, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejasz, P.; Eschweiler, J.; Gerlach-Hahn, K.; Jansen-Troy, A.; Leonhardt, S. A survey on robotic devices for upper limb rehabilitation. J. NeuroEng. Rehabil. 2014, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiero, S.; Celia, A.; Rosati, G.; Armani, M. Robotic-Assisted Rehabilitation of the Upper Limb After Acute Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, M. Low-Cost Robots for Research and Teaching Activity. IEEE Autom. Robot. Mag. 2003, 10, 37–45. [Google Scholar] [CrossRef]
- Patton, J.; Jayaraman, A. (Eds.) Proceedings of 2019 International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 4–28 June 2019; IEEE Explorer. Available online: https://ieeexplore.ieee.org/xpl/conhome/8765245/proceeding (accessed on 8 March 2022).
- Molaei, A.; Foomany, N.A.; Parsapour, M.; Dargahi, J. A portable low-cost 3D-printed wrist rehabilitation robot: Design and development. Mech. Mach. Theory 2022, 171, 104719. [Google Scholar] [CrossRef]
- Carbone, G.; Gerding, E.C.; Corves, B.; Cafolla, D.; Russo, M.; Ceccarelli, M. Design of a Two-DOFs driving mechanism for a motion-assisted finger exoskeleton. Appl. Sci. 2020, 10, 2619. [Google Scholar] [CrossRef] [Green Version]
- Washabaugh, E.P.; Guo, J.; Chang, C.K.; Remy, C.D.; Krishnan, C. A portable passive rehabilitation robot for upper-extremity functional resistance training. IEEE Trans. Biomed. Eng. 2018, 66, 496–508. [Google Scholar] [CrossRef]
- Chaparro-Rico, B.D.; Cafolla, D.; Ceccarelli, M.; Castillo-Castaneda, E. NURSE-2 DoF device for arm motion guidance: Kinematic, dynamic, and FEM analysis. Appl. Sci. 2020, 10, 2139. [Google Scholar] [CrossRef] [Green Version]
- Berezny, N.; Dowlatshahi, D.; Ahmadi, M. Feasibility of a One Degree-of-Freedom Linear Robot for Bed-Bound Stroke Rehabilitation. J. Med. Devices 2021, 15, 034501. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Romdhane, L. Design issues for human-machine platform interface in cable-based parallel manipulators for physiotherapy applications. J. Zhejiang Univ. Sci. A 2010, 11, 231–239. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Ferrara, L.; Petuya, V. Design of a cable-driven device for elbow rehabilitation and exercise. In Interdisciplinary Applications of Kinematics; Springer: Cham, Switzerland, 2019; pp. 61–68. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Bottin, M.; Rosati, G. Portable Cable-Driven Exoskeleton for Elbow Motion Assistance. Italy Patent pending n.102020000004885, 9 March 2020. [Google Scholar]
- Ceccarelli, M.; Ferrara, L.; Petuya, V. Device for Elbow Rehabilitation. Italy Patent pending no. IT. 102017000083887, 29 October 2019. [Google Scholar]
- Ceccarelli, M.; Russo, M.; Urizar Arana, M.; Riabtsev, M.; Fort, A.; Amine Laribi, M. Portable Device for Motion Assistance of the Elbow. Italy Patent pending n.102021000013229, 20 May 2021. [Google Scholar]
- Zuccon, G.; Bottin, M.; Ceccarelli, M.; Rosati, G. Design and performance of an elbow assisting mechanism. Machines 2020, 8, 68. [Google Scholar] [CrossRef]
- Laribi, M.A.; Ceccarelli, M.; Sandoval, J.; Bottin, M.; Rosati, G. Experimental Validation of Light Cable-Driven Elbow-Assisting Device L-CADEL Design. J. Bionic Eng. 2022, 19, 416–428. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Riabtsev, M.; Fort, A.; Russo, M.; Laribi, M.A.; Urizar, M. Design and experimental characterization of l-CADEL v2, an assistive device for elbow motion. Sensors 2021, 21, 5149. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Ceccarelli, M. Analysis of a wearable robotic system for ankle rehabilitation. Machines 2020, 8, 48. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, M.; Bottin, M.; Russo, M.; Rosati, G.; Laribi, M.A.; Petuya, V. Requirements and Solutions for Motion Limb Assistance of COVID-19 Patients. Robotics 2022, 11, 45. https://doi.org/10.3390/robotics11020045
Ceccarelli M, Bottin M, Russo M, Rosati G, Laribi MA, Petuya V. Requirements and Solutions for Motion Limb Assistance of COVID-19 Patients. Robotics. 2022; 11(2):45. https://doi.org/10.3390/robotics11020045
Chicago/Turabian StyleCeccarelli, Marco, Matteo Bottin, Matteo Russo, Giulio Rosati, Med Amine Laribi, and Victor Petuya. 2022. "Requirements and Solutions for Motion Limb Assistance of COVID-19 Patients" Robotics 11, no. 2: 45. https://doi.org/10.3390/robotics11020045
APA StyleCeccarelli, M., Bottin, M., Russo, M., Rosati, G., Laribi, M. A., & Petuya, V. (2022). Requirements and Solutions for Motion Limb Assistance of COVID-19 Patients. Robotics, 11(2), 45. https://doi.org/10.3390/robotics11020045