Methyl Protodioscin Promotes Ferroptosis of Prostate Cancer Cells by Facilitating Dissociation of RB1CC1 from the Detergent-Resistant Membranes and Its Nuclear Translocation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents
2.3. Cell Viability Assay
2.4. Cell Scratch Damage Repair and Cell Invasion Assays
2.5. Separation and Extraction of DRMs
2.6. Immunoblot Analysis
2.7. Immunofluorescence
2.8. Measurements of Glutathione, Malondialdehyde, and ROS Levels
2.9. Determination of Iron Content and Cholesterol Content
2.10. RNA Extraction and Real-Time Quantitative Reverse Transcription (RT) PCR
2.11. In Vivo Experiments
2.12. Prediction of Palmitoylation Modification Sites
2.13. Statistical Analysis
3. Results
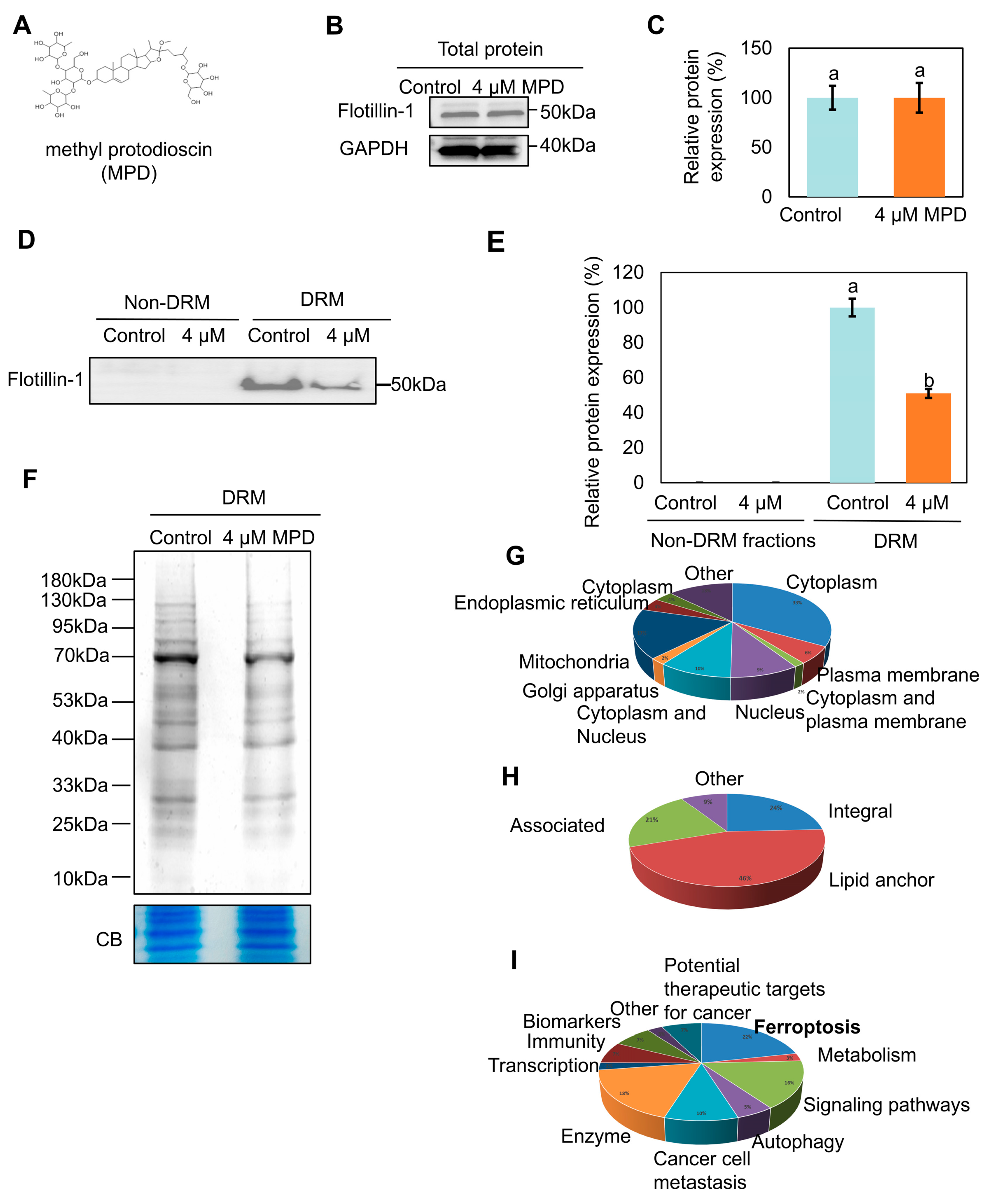
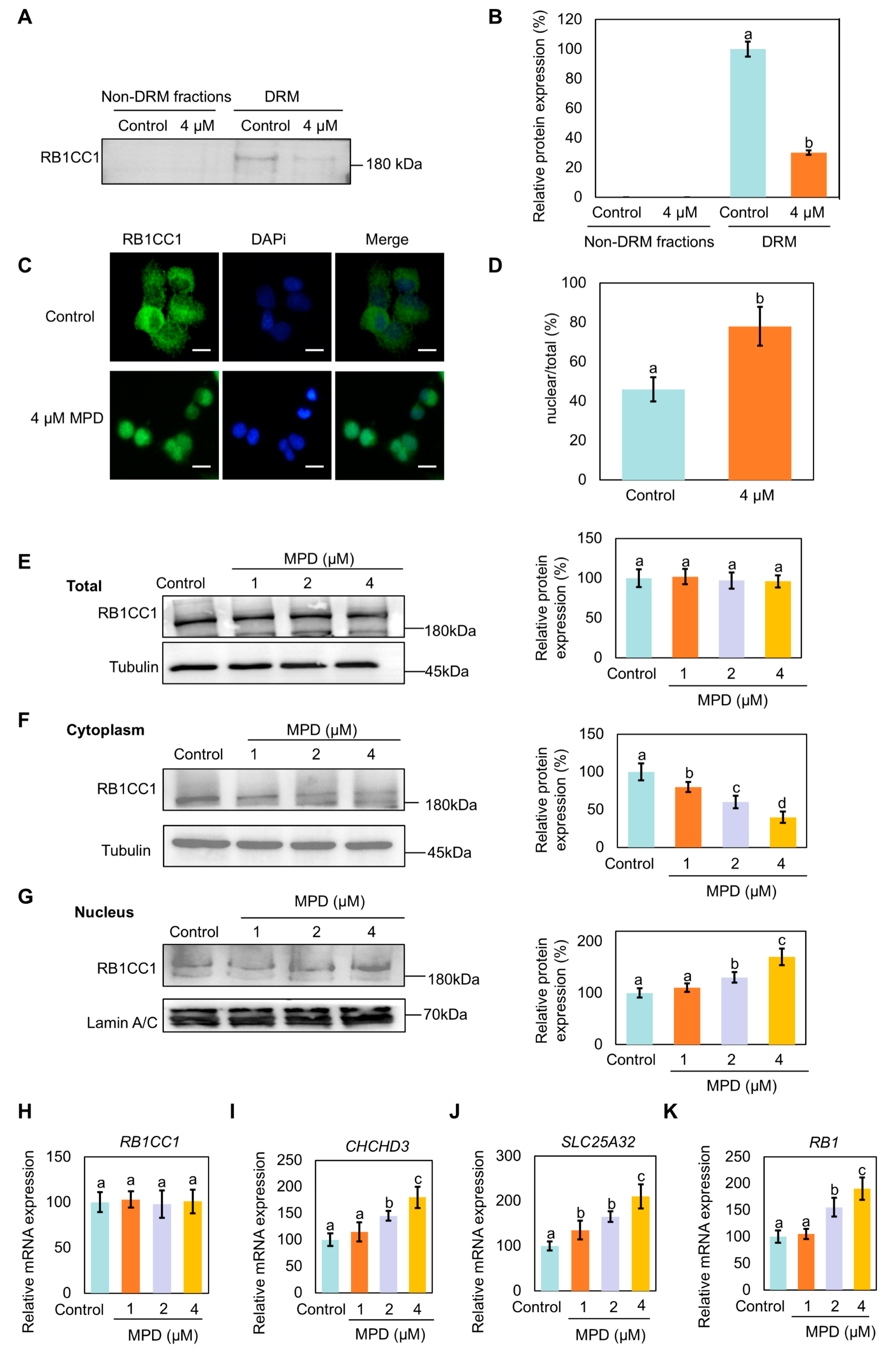
3.1. MPD Induces Protein Rearrangements in the DRMs
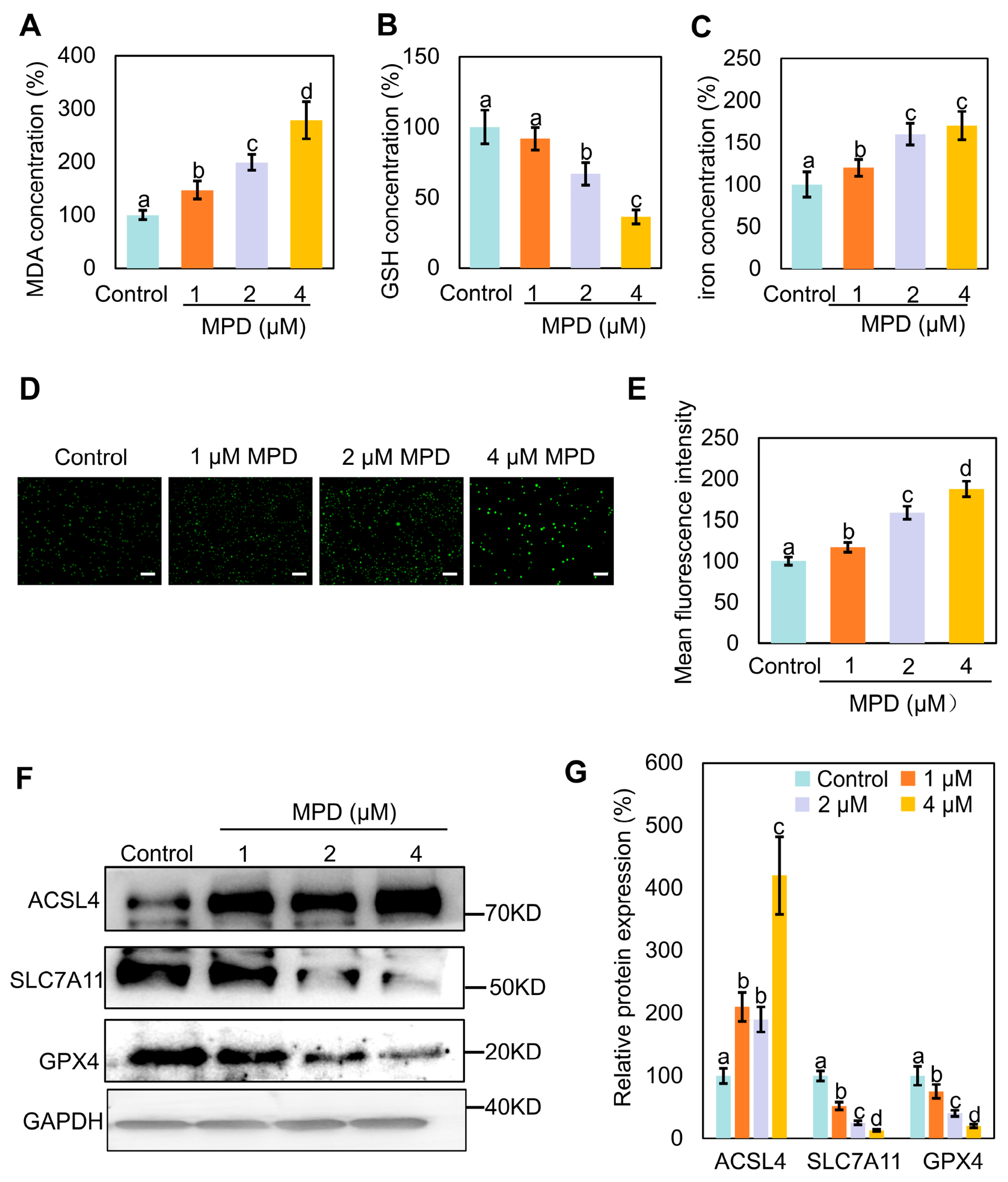
3.2. MPD Appears to Promote Ferroptosis in Prostate Cancer Cells
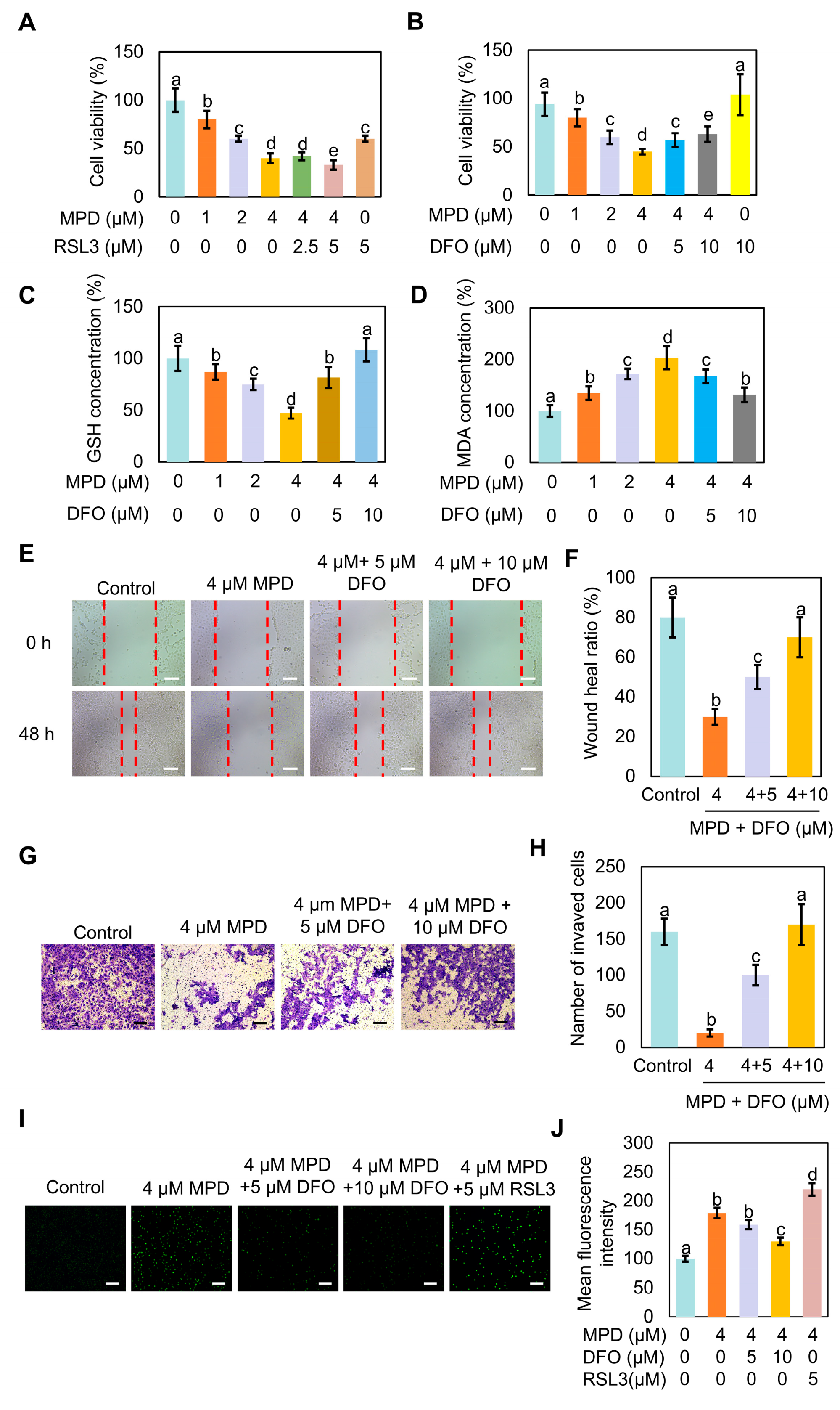
3.3. Ferroptosis Inhibitor DFO Reverses MPD-Associated Ferroptosis in DU145 Cells
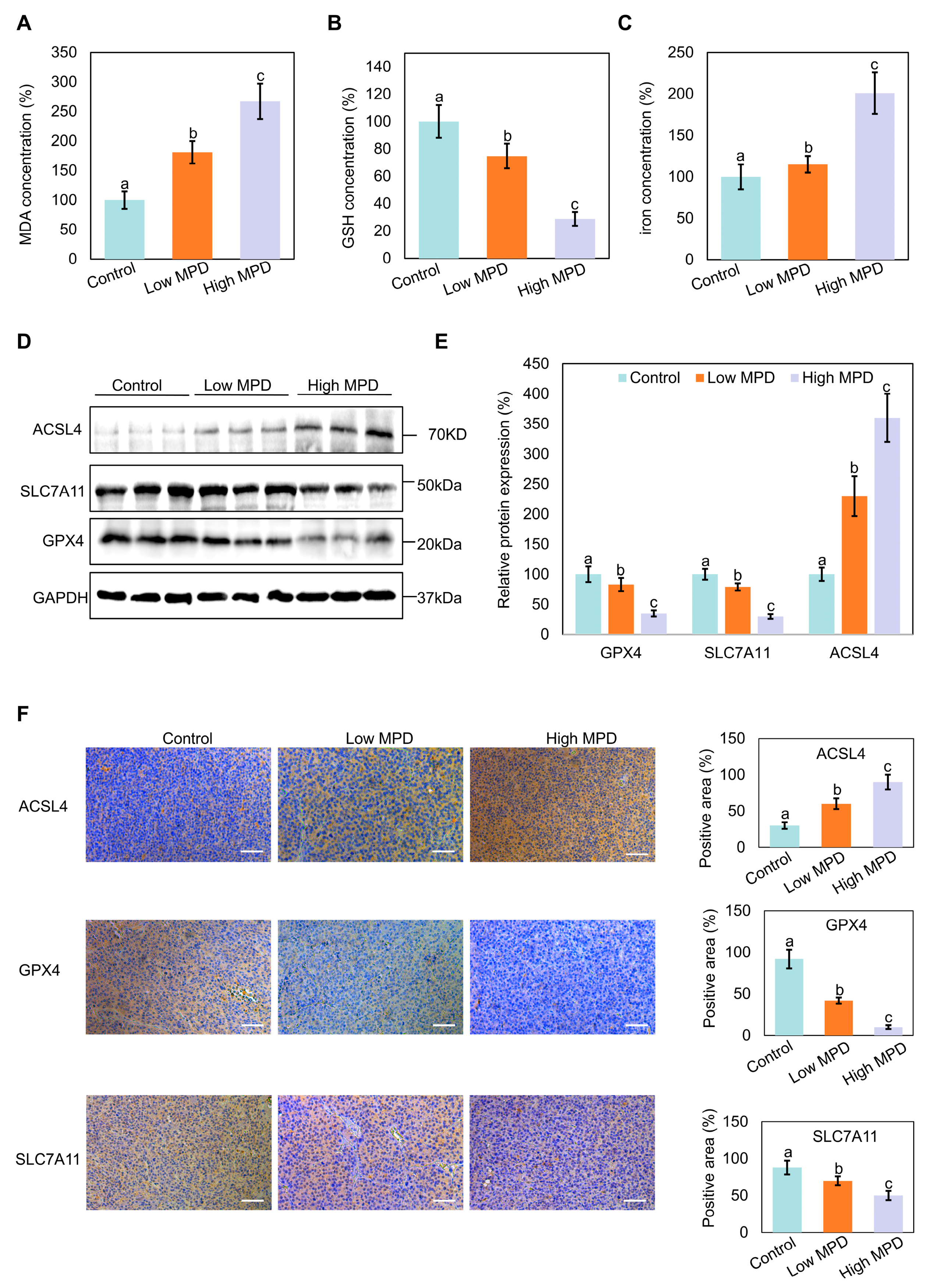
3.4. MPD Appears to Induce Ferroptosis in Prostate Cancer In Vivo
3.5. Nuclear Translocation of RB1CC1 Following Its Dissociation from DRMs
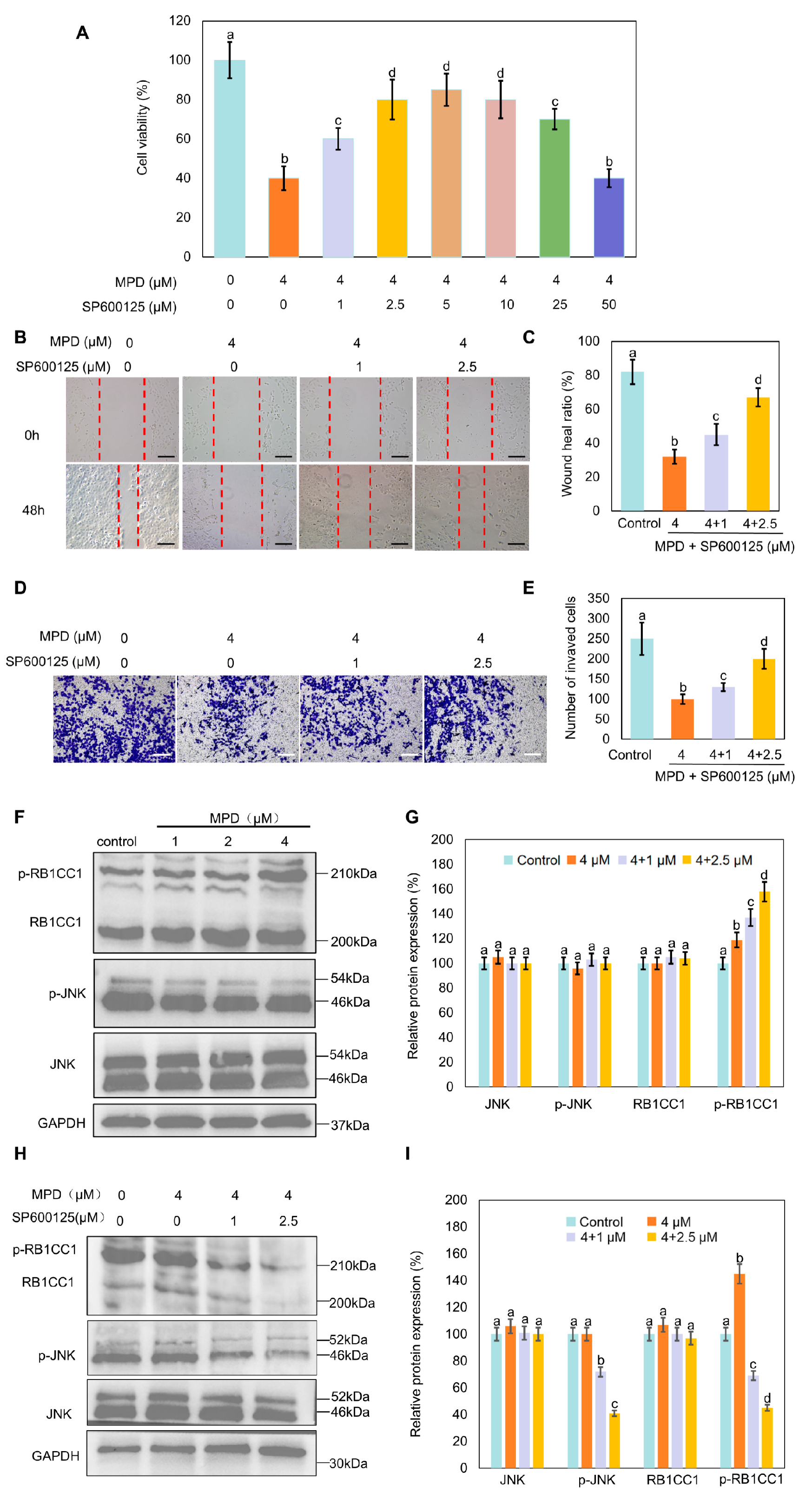
3.6. JNK Inhibitor SP600125 Increases MPD-Reduced Cell Proliferation, Migration, and Invasion, and Decreases MPD-Induced RB1CC1 Phosphorylation
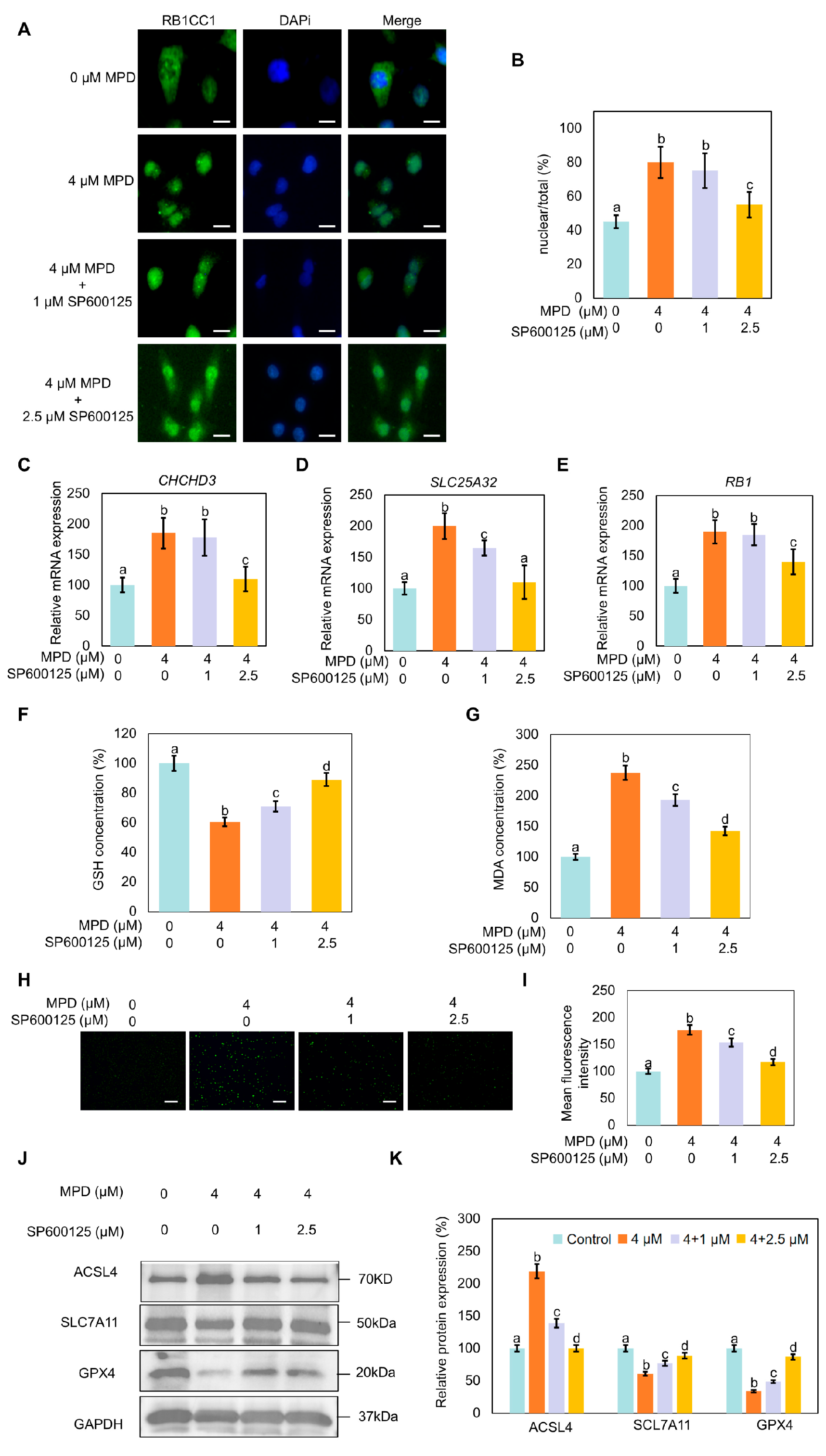
3.7. JNK Inhibitor SP600125 Inhibits MPD-Induced RB1CC1 Nuclear Translocation and Ferroptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPD | Methyl Protodioscin |
| DRMs | Detergent-Resistant Membranes |
| RB1CC1 | RB1-Inducible Coiled-Coil 1 |
| CRPC | Castration-Resistant Prostate Cancer |
| GSH | Glutathione |
| GPX4 | Glutathione Peroxidase 4 |
| GSSG | Glutathione Disulfide |
| TFR1 | Transferrin Receptor Protein 1 |
| Fpn | Ferroportin |
| CHCHD3 | Coiled-Coil-Helix-Coiled-Coil-Helix Domain Containing 3 |
| ROS | Reactive oxygen species |
| ACSL4 | Acyl-CoA synthetase long chain family member 4 |
| SLC7A11 | Solute carrier family 7 member 11 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.H.A.; Dominic, A.; Lujan, F.E.; Senthilkumar, S.; Bhattacharya, P.K.; Frigo, D.E.; Subramani, E. Unlocking ferroptosis in prostate cancer—The road to novel therapies and imaging markers. Nat. Rev. Urol. 2024, 21, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 1–16. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, Y.; Ding, J.-H.; Jin, X.; Ma, D.; Li, D.-Q.; Shi, J.-X.; Huang, W.; Wang, Y.-P.; Jiang, Y.-Z. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023, 35, 84.e108–100.e108. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Hu, H.; Zhang, X.; Zeng, S.; Li, J.; Dong, X.; Deng, X.; Zhang, J.; Zhang, Y. METTL17 coordinates ferroptosis and tumorigenesis by regulating mitochondrial translation in colorectal cancer. Redox Biol. 2024, 71, 103087. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, T.; Qian, W.; Ji, J.; Cai, Q.; Jin, Y.; Jiang, J.; Zhang, J. HNF4A-BAP31-VDAC1 axis synchronously regulates cell proliferation and ferroptosis in gastric cancer. Cell Death Dis. 2023, 14, 356. [Google Scholar] [CrossRef]
- Guo, K.; Lu, M.; Bi, J.; Yao, T.; Gao, J.; Ren, F.; Zhu, L. Ferroptosis: Mechanism, immunotherapy and role in ovarian cancer. Front. Immunol. 2024, 15, 1410018. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Ke, A.; Guo, K. Ferroptosis and its interaction with tumor immune microenvironment in liver cancer. Biochim. Biophys. Acta-Rev. Cancer 2023, 1878, 188848. [Google Scholar] [CrossRef]
- Longo, A.; Manganelli, V.; Misasi, R.; Riitano, G.; Caglar, T.R.; Fasciolo, E.; Recalchi, S.; Sorice, M.; Garofalo, T. Extracellular Vesicles in the Crosstalk of Autophagy and Apoptosis: A Role for Lipid Rafts. Cells 2025, 14, 749. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhang, P.; Xiao, W.; Feng, B.; Chen, L.Y.; Li, S.; Li, P.; Zhao, W.Z.; Qi, X.T.; Yin, L.P. TMD1 domain and CRAC motif determine the association and disassociation of MxIRT1 with detergent--resistant membranes. Traffic 2018, 19, 122–137. [Google Scholar] [CrossRef]
- Auriac, A.; Willemetz, A.; Canonne-Hergaux, F. Lipid raft-dependent endocytosis: A new route for hepcidin-mediated regulation of ferroportin in macrophages. Haematologica 2010, 95, 1269. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Polavarapu, R.; Karmali, V.; Guo, L.; Van Dam, R.; Cheng, Q.; Akahori, H.; Saeed, O.; Nakano, M.; Pachura, K. Hepcidin-ferroportin axis controls toll-like receptor 4 dependent macrophage inflammatory responses in human atherosclerotic plaques. Atherosclerosis 2015, 241, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, P.; Ma, W.; Pan, H.; Hong, W.; Chen, G.; Ding, H.; Tang, W.; Lin, G.; Zhang, Z. Protective effects of methyl protodioscin against lipid disorders and liver injury in hyperlipidemic gerbils. Heliyon 2023, 9, e22785. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Zhang, Y.-S.; Zhang, F.; Zhang, Y.-Y.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Methyl protodioscin from Polygonatum sibiricum inhibits cervical cancer through cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2019, 132, 110655. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, C.-s.; Gao, H.; Zhan, L.; Wang, F.; Qu, C.; Li, Y.; Wang, P.; Chen, H.; Meng, Z. Natural compound methyl protodioscin suppresses proliferation and inhibits glycolysis in pancreatic cancer. Evid.-Based Complement. Altern. Med. 2018, 2018, 7343090. [Google Scholar] [CrossRef]
- Xu, Z.; Song, T.; Yang, X.; Cong, L.; Yin, L.; Xu, Y.; Han, X.; Gao, M.; Xu, L. TMT-based proteomics reveals methylprotodioscin alleviates oxidative stress and inflammation via COX6C in myocardial infraction. Biomed. Pharmacother. 2024, 180, 117489. [Google Scholar] [CrossRef]
- Chen, J.; Qin, P.; Tao, Z.; Ding, W.; Yao, Y.; Xu, W.; Yin, D.; Tan, S. Anticancer activity of methyl protodioscin against prostate cancer by modulation of cholesterol-associated MAPK signaling pathway via FOXO1 induction. Biol. Pharm. Bull. 2023, 46, 574–585. [Google Scholar] [CrossRef]
- Ning, W.; Jiang, P.; Guo, Y.; Wang, C.; Tan, X.; Zhang, W.; Peng, D.; Xue, Y. GPS-Palm: A deep learning-based graphic presentation system for the prediction of S-palmitoylation sites in proteins. Brief. Bioinform. 2020, 22, 1836–1847. [Google Scholar] [CrossRef]
- Wang, R.; Huang, W.; Cai, K.; Xiao, S.; Zhang, W.; Hu, X.; Guo, J.; Mao, L.; Yuan, W.; Xu, Y. FLOT1 promotes gastric cancer progression and metastasis through BCAR1/ERK signaling. Int. J. Biol. Sci. 2023, 19, 5104. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liu, C.; Li, L.; Yang, M.; Jiang, N.; Luo, S.; Sun, L. Acyl-CoA synthase ACSL4: An essential target in ferroptosis and fatty acid metabolism. Chin. Med. J. 2023, 136, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Talty, R.; Johnson, C.H. Targeting ferroptosis to treat colorectal cancer. Trends Cell Biol. 2023, 33, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cui, C.; Jiao, D.; Zhu, X. JAK/STAT signaling as a key regulator of ferroptosis: Mechanisms and therapeutic potentials in cancer and diseases. Cancer Cell Int. 2025, 25, 83. [Google Scholar] [CrossRef]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remião, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef]
- Bao, X.; Luo, X.; Bai, X.; Lv, Y.; Weng, X.; Zhang, S.; Leng, Y.; Huang, J.; Dai, X.; Wang, Y.; et al. Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic. Biol. Med. 2023, 201, 76–88. [Google Scholar] [CrossRef]
- Xue, X.; Ma, L.; Zhang, X.; Xu, X.; Guo, S.; Wang, Y.; Qiu, S.; Cui, J.; Guo, W.; Yu, Y. Tumour cells are sensitised to ferroptosis via RB1CC1-mediated transcriptional reprogramming. Clin. Transl. Med. 2022, 12, e747. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, J.; Wang, Z.; Liu, J.; Zhao, J.; Liu, X.; Zhang, Y.; Liu, G.; Zhao, Z.; Li, W. SLC25A32 promotes malignant progression of glioblastoma by activating PI3K-AKT signaling pathway. BMC Cancer 2023, 23, 589. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Dyson, N.J. RB1: A prototype tumor suppressor and an enigma. Genes Dev. 2016, 30, 1492–1502. [Google Scholar] [CrossRef]
- Wang, H.-H.; Fan, S.-Q.; Zhan, Y.-T.; Peng, S.-P.; Wang, W.-Y. Suppression of the SLC7A11/glutathione axis causes ferroptosis and apoptosis and alters the mitogen-activated protein kinase pathway in nasopharyngeal carcinoma. Int. J. Biol. Macromol. 2024, 254, 127976. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X.; et al. LPCAT3 is transcriptionally regulated by YAP/ZEB/EP300 and collaborates with ACSL4 and YAP to determine ferroptosis sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.J.; Song, Y.; Van Horn, W.D.; Hustedt, E.J.; Schafer, J.M.; Hadziselimovic, A.; Beel, A.J.; Sanders, C.R. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science 2012, 336, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Hao, Q.; Liang, Y.; Kong, E. Protein palmitoylation in cancer: Molecular functions and therapeutic potential. Mol. Oncol. 2023, 17, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, S.F.; Abrami, L.; Linder, M.E.; Bamji, S.X.; Dickinson, B.C.; van der Goot, F.G. Mechanisms and functions of protein S-acylation. Nat. Rev. Mol. Cell Biol. 2024, 25, 488–509. [Google Scholar] [CrossRef]
- Lorent, J.H.; Levental, I. Structural determinants of protein partitioning into ordered membrane domains and lipid rafts. Chem. Phys. Lipids 2015, 192, 23–32. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Clusters of apoptotic signaling molecule-enriched rafts, CASMERs: Membrane platforms for protein assembly in Fas/CD95 signaling and targets in cancer therapy. Biochem. Soc. Trans. 2022, 50, 1105–1118. [Google Scholar] [CrossRef]
- Gyoten, M.; Luo, Y.; Fujiwara-Tani, R.; Mori, S.; Ogata, R.; Kishi, S.; Kuniyasu, H. Lovastatin treatment inducing apoptosis in human pancreatic cancer cells by inhibiting cholesterol rafts in plasma membrane and mitochondria. Int. J. Mol. Sci. 2023, 24, 16814. [Google Scholar] [CrossRef]
- Lee, M.-H.; Kim, H.L.; Seo, H.; Jung, S.; Kim, B.-J. A secreted form of chorismate mutase (Rv1885c) in Mycobacterium bovis BCG contributes to pathogenesis by inhibiting mitochondria-mediated apoptotic cell death of macrophages. J. Biomed. Sci. 2023, 30, 95. [Google Scholar] [CrossRef]
- Warda, M.; Tekin, S.; Gamal, M.; Khafaga, N.; Çelebi, F.; Tarantino, G. Lipid rafts: Novel therapeutic targets for metabolic, neurodegenerative, oncological, and cardiovascular diseases. Lipids Health Dis. 2025, 24, 147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, R.; Hu, C.; Zhao, Y.; Wu, S.; Cao, S.; Xu, L.; Yin, D.; Tan, S. Methyl Protodioscin Promotes Ferroptosis of Prostate Cancer Cells by Facilitating Dissociation of RB1CC1 from the Detergent-Resistant Membranes and Its Nuclear Translocation. Biomolecules 2026, 16, 38. https://doi.org/10.3390/biom16010038
Wang R, Hu C, Zhao Y, Wu S, Cao S, Xu L, Yin D, Tan S. Methyl Protodioscin Promotes Ferroptosis of Prostate Cancer Cells by Facilitating Dissociation of RB1CC1 from the Detergent-Resistant Membranes and Its Nuclear Translocation. Biomolecules. 2026; 16(1):38. https://doi.org/10.3390/biom16010038
Chicago/Turabian StyleWang, Ruonan, Chaoyu Hu, Yi Zhao, Shuhan Wu, Shujuan Cao, Leiming Xu, Dengke Yin, and Song Tan. 2026. "Methyl Protodioscin Promotes Ferroptosis of Prostate Cancer Cells by Facilitating Dissociation of RB1CC1 from the Detergent-Resistant Membranes and Its Nuclear Translocation" Biomolecules 16, no. 1: 38. https://doi.org/10.3390/biom16010038
APA StyleWang, R., Hu, C., Zhao, Y., Wu, S., Cao, S., Xu, L., Yin, D., & Tan, S. (2026). Methyl Protodioscin Promotes Ferroptosis of Prostate Cancer Cells by Facilitating Dissociation of RB1CC1 from the Detergent-Resistant Membranes and Its Nuclear Translocation. Biomolecules, 16(1), 38. https://doi.org/10.3390/biom16010038







