The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ice Recrystallization Inhibition (IRI) Assay
2.3. Statistical Analysis
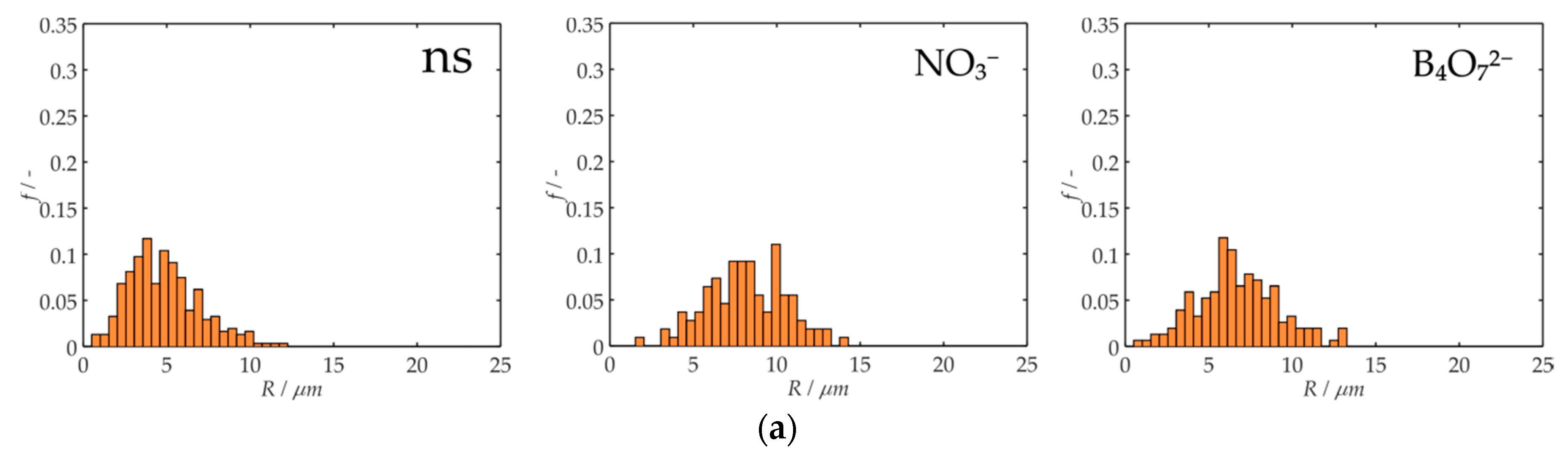
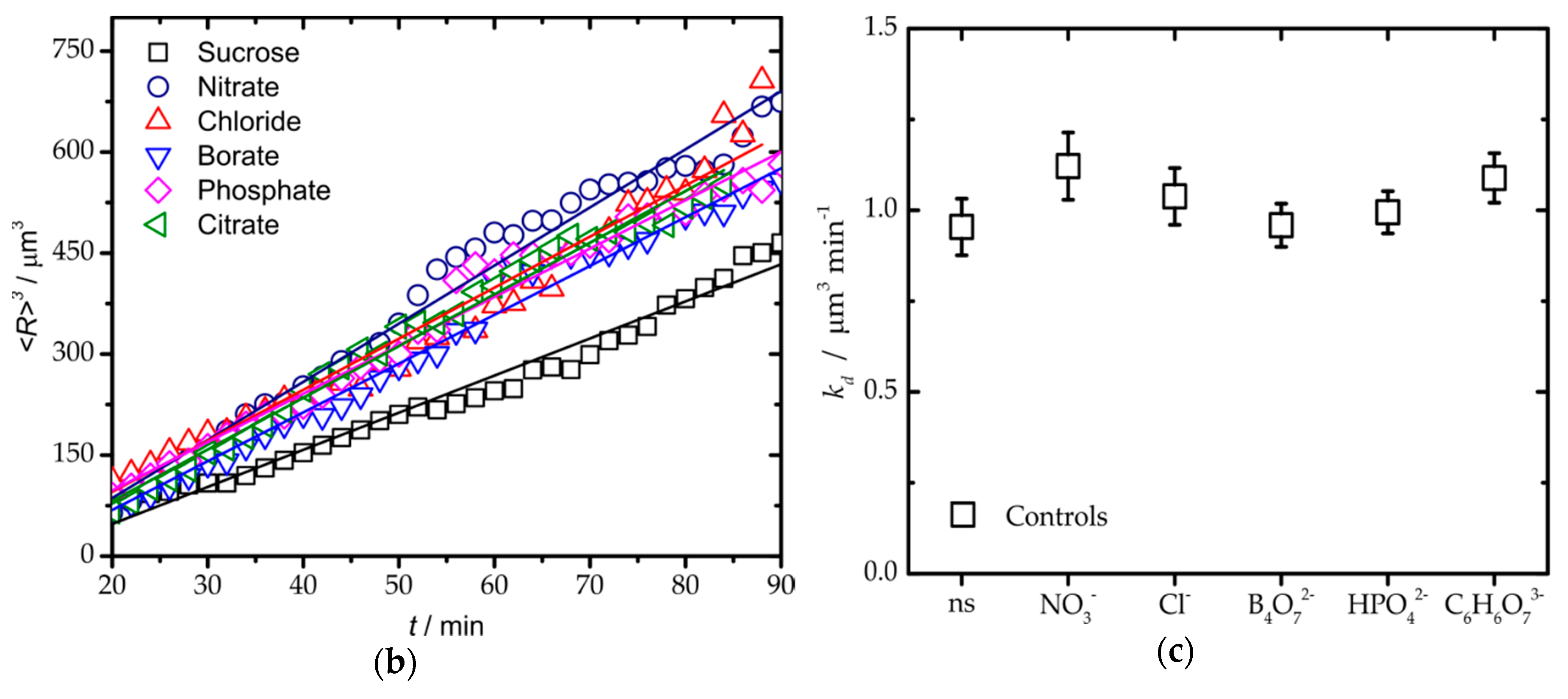
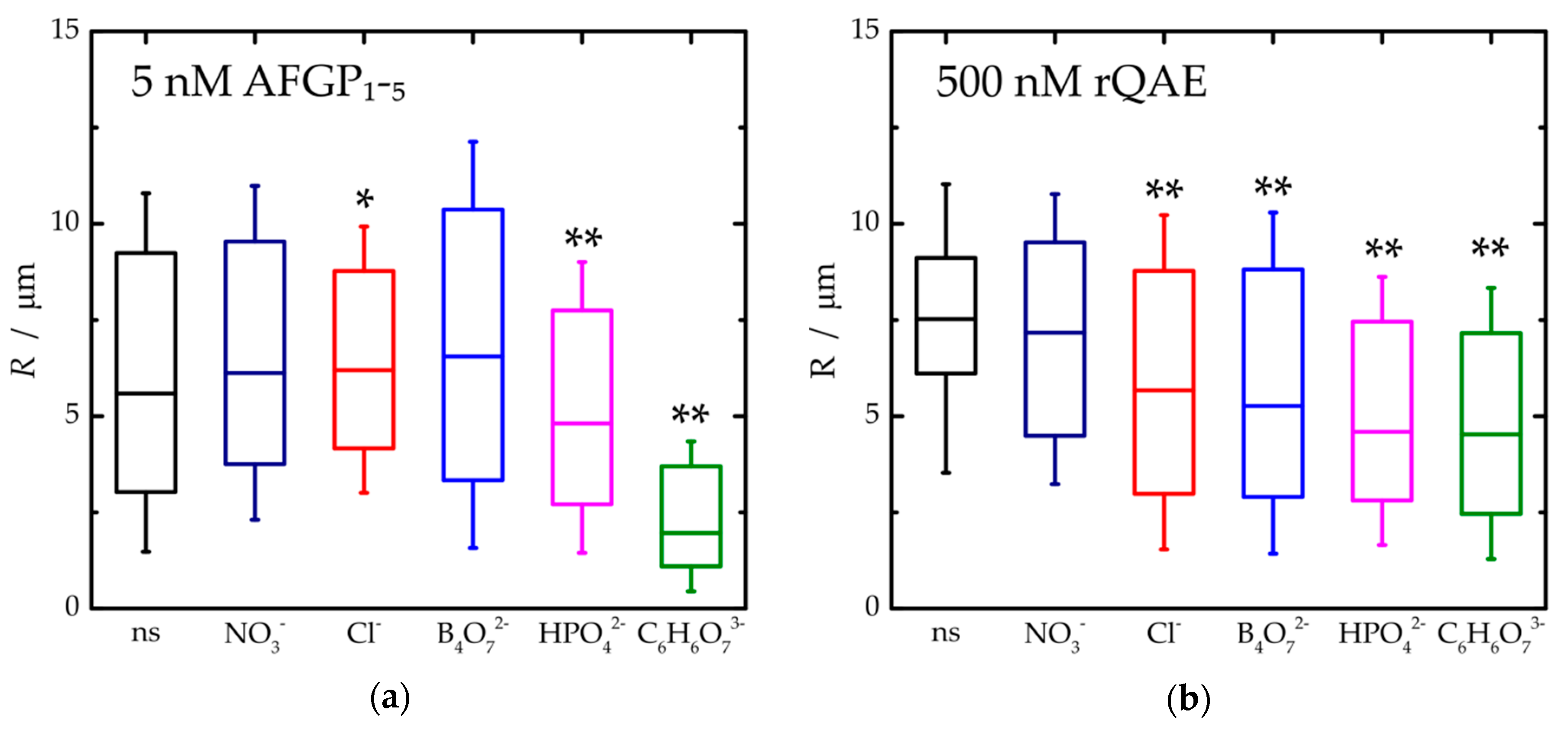
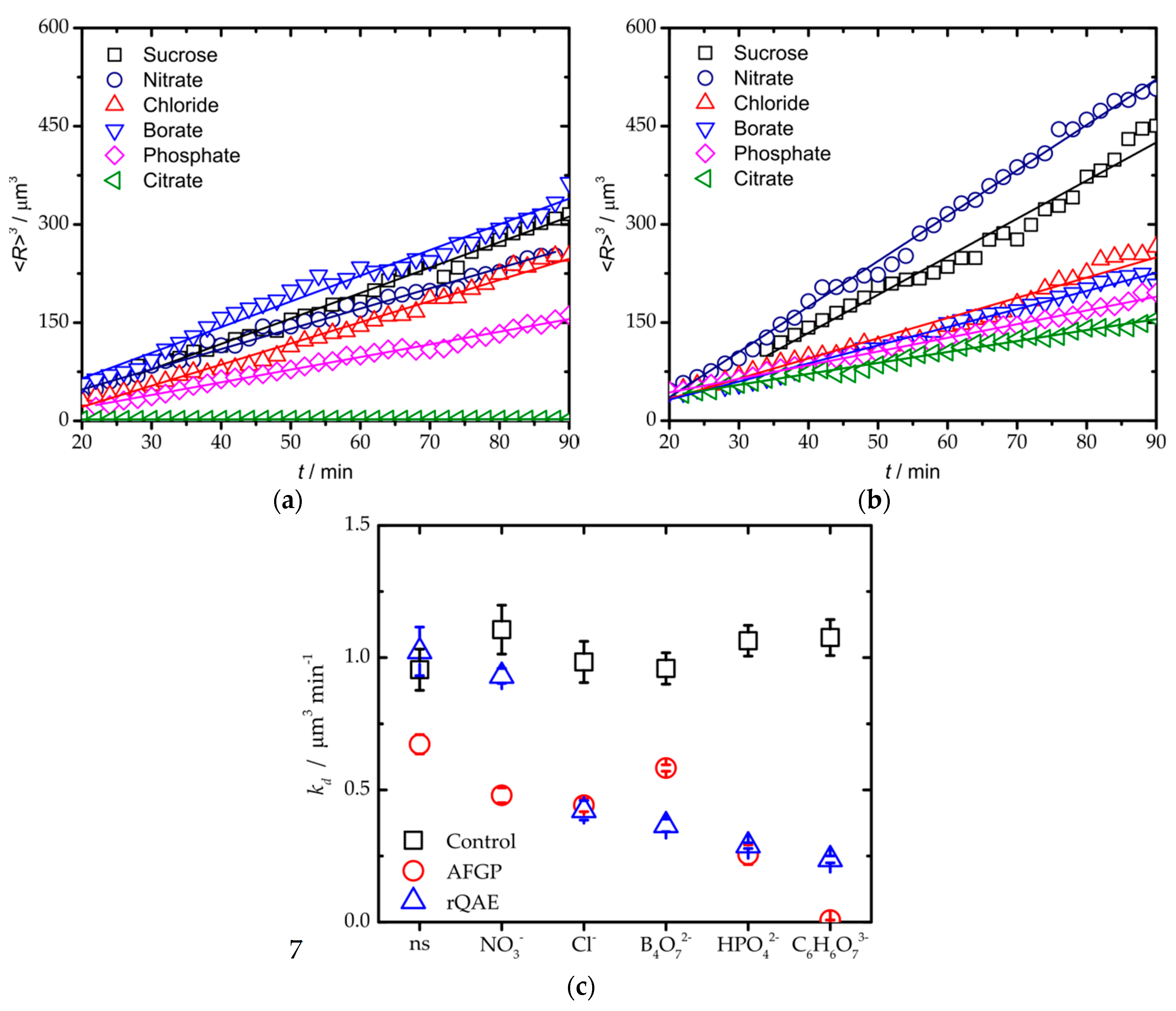
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazur, P. Freezing of living cells: Mechanisms and implications. Am. J. Physiol. 1984, 247, C125–142. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.L.; Lecak, J.; Acker, J.P. Biopreservation of Red Blood Cells: Past, Present, and Future. Transfus. Med. Rev. 2005, 19, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar] [CrossRef]
- Olijve, L.L.; Meister, K.; DeVries, A.L.; Duman, J.G.; Guo, S.; Bakker, H.J.; Voets, I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H.; Koo, B.W. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Hansen, T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Proc. Natl. Acad. Sci. USA 1992, 89, 8953–8957. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.K.; Youm, H.W.; Kim, H.J.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effects of three different types of antifreeze proteins on mouse ovarian tissue cryopreservation and transplantation. PLoS ONE 2015, 10, e0126252. [Google Scholar] [CrossRef]
- Wang, J.H. A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology 2000, 41, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Q.; Yang, X.; Layne, J.R., Jr.; Devries, A.L. Antifreeze glycoproteins from antarctic notothenioid fishes fail to protect the rat cardiac explant during hypothermic and freezing preservation. Cryobiology 1994, 31, 185–192. [Google Scholar] [CrossRef]
- Kunz, W.; Henle, J.; Ninham, B.W. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid Interface Sci. 2004, 9, 19–37. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. Hofmeister effects: An explanation for the impact of ionic liquids on biocatalysis. J. Biotechnol. 2009, 144, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cremer, P.S. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Amornwittawat, N.; Wang, S.; Duman, J.G.; Wen, X. Polycarboxylates enhance beetle antifreeze protein activity. Proteins Proteom. 2008, 1784, 1942–1948. [Google Scholar] [CrossRef][Green Version]
- Duman, J.G. The inhibition of ice nucleators by insect antifreeze proteins is enhanced by glycerol and citrate. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2002, 172, 163–168. [Google Scholar] [CrossRef]
- Kristiansen, E.; Pedersen, S.A.; Zachariassen, K.E. Salt-induced enhancement of antifreeze protein activity: A salting-out effect. Cryobiology 2008, 57, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Amornwittawat, N.; Banatlao, J.; Chung, M.; Kao, Y.; Wen, X. Hofmeister Effects of Common Monovalent Salts on the Beetle Antifreeze Protein Activity. J. Phys. Chem. B 2009, 113, 13891–13894. [Google Scholar] [CrossRef][Green Version]
- Oude Vrielink, A.S.; Aloi, A.; Olijve, L.L.; Voets, I.K. Interaction of ice binding proteins with ice, water and ions. Biointerphases 2016, 11, 018906. [Google Scholar] [CrossRef]
- Budke, C.; Dreyer, A.; Jaeger, J.; Gimpel, K.; Berkemeier, T.; Bonin, A.S.; Nagel, L.; Plattner, C.; DeVries, A.L.; Sewald, N.; et al. Quantitative Efficacy Classification of Ice Recrystallization Inhibition Agents. Crystal Growth Design 2014, 14, 4285–4294. [Google Scholar] [CrossRef]
- Budke, C.; Heggemann, C.; Koch, M.; Sewald, N.; Koop, T. Ice recrystallization kinetics in the presence of synthetic antifreeze glycoprotein analogues using the framework of LSW theory. J. Phys. Chem. B 2009, 113, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Olijve, L.L.C.; Oude Vrielink, A.S.; Voets, I.K. A Simple and Quantitative Method to Evaluate Ice Recrystallization Kinetics Using the Circle Hough Transform Algorithm. Cryst. Growth Design 2016, 16, 4190–4195. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Yeh, Y.; Osuga, D.T.; Feeney, R.E. Antifreeze Glycoproteins from Antarctic Fish: Inactivation by borate. J. Biol. Chem. 1976, 251, 3033–3036. [Google Scholar] [PubMed]
- Davis, H.B.; Mott, C.J.B. Interaction of boric acid and borates with carbohydrates and related substances. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1980, 76. [Google Scholar] [CrossRef]
- Meister, K.; DeVries, A.L.; Bakker, H.J.; Drori, R. Antifreeze Glycoproteins Bind Irreversibly to Ice. J. Am. Chem. Soc. 2018, 140, 9365–9368. [Google Scholar] [CrossRef] [PubMed]
- Meister, K.; Ebbinghaus, S.; Xu, Y.; Duman, J.G.; DeVries, A.; Gruebele, M.; Leitner, D.M.; Havenith, M. Long-range protein-water dynamics in hyperactive insect antifreeze proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surís-Valls, R.; Voets, I.K. The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins. Biomolecules 2019, 9, 347. https://doi.org/10.3390/biom9080347
Surís-Valls R, Voets IK. The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins. Biomolecules. 2019; 9(8):347. https://doi.org/10.3390/biom9080347
Chicago/Turabian StyleSurís-Valls, Romà, and Ilja K. Voets. 2019. "The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins" Biomolecules 9, no. 8: 347. https://doi.org/10.3390/biom9080347
APA StyleSurís-Valls, R., & Voets, I. K. (2019). The Impact of Salts on the Ice Recrystallization Inhibition Activity of Antifreeze (Glyco)Proteins. Biomolecules, 9(8), 347. https://doi.org/10.3390/biom9080347





