Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors
Abstract
1. Introduction
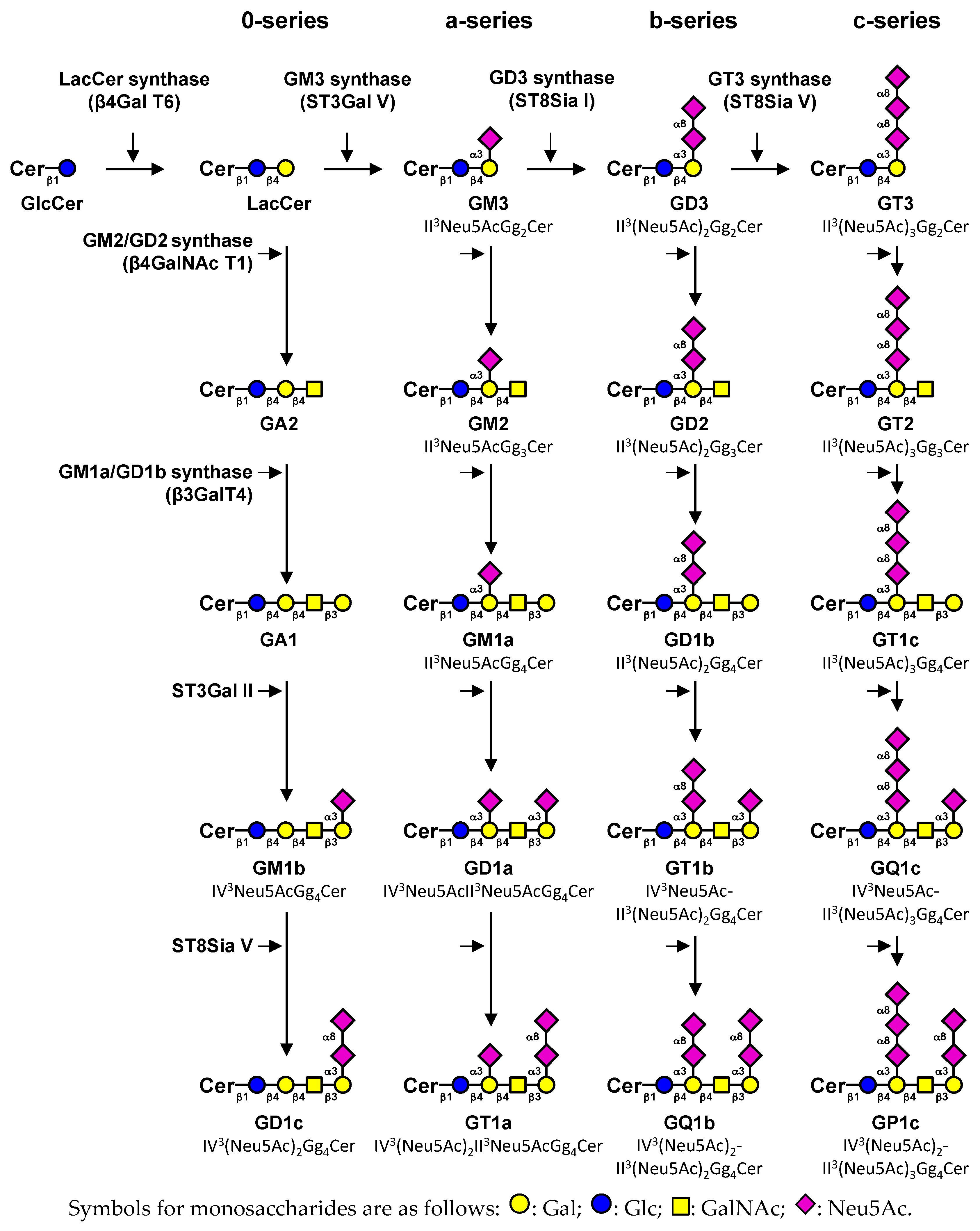
2. Expression of Gangliosides in Human Tissues
2.1. Monosialogangliosides Expression
2.2. Disialogangliosides Expression
3. Gangliosides Involved in Cell Fate
3.1. Gangliosides as Essential Components for The Maintenance of Cell Signaling
3.2. The Anti-Tumoral Role of Monosialogangliosides in ND Tumors
3.3. Monosialogangliosides as Enhancers of Tumorigenesis
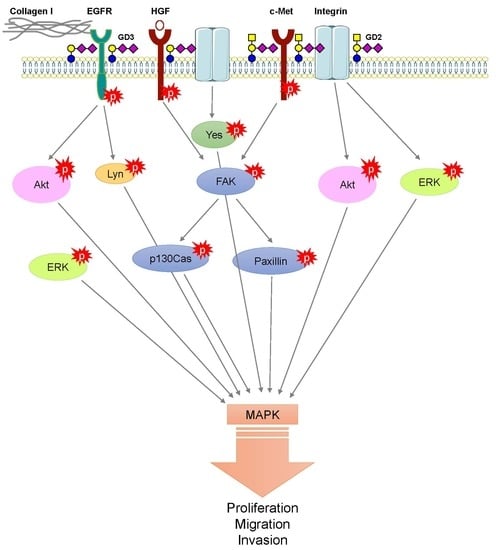
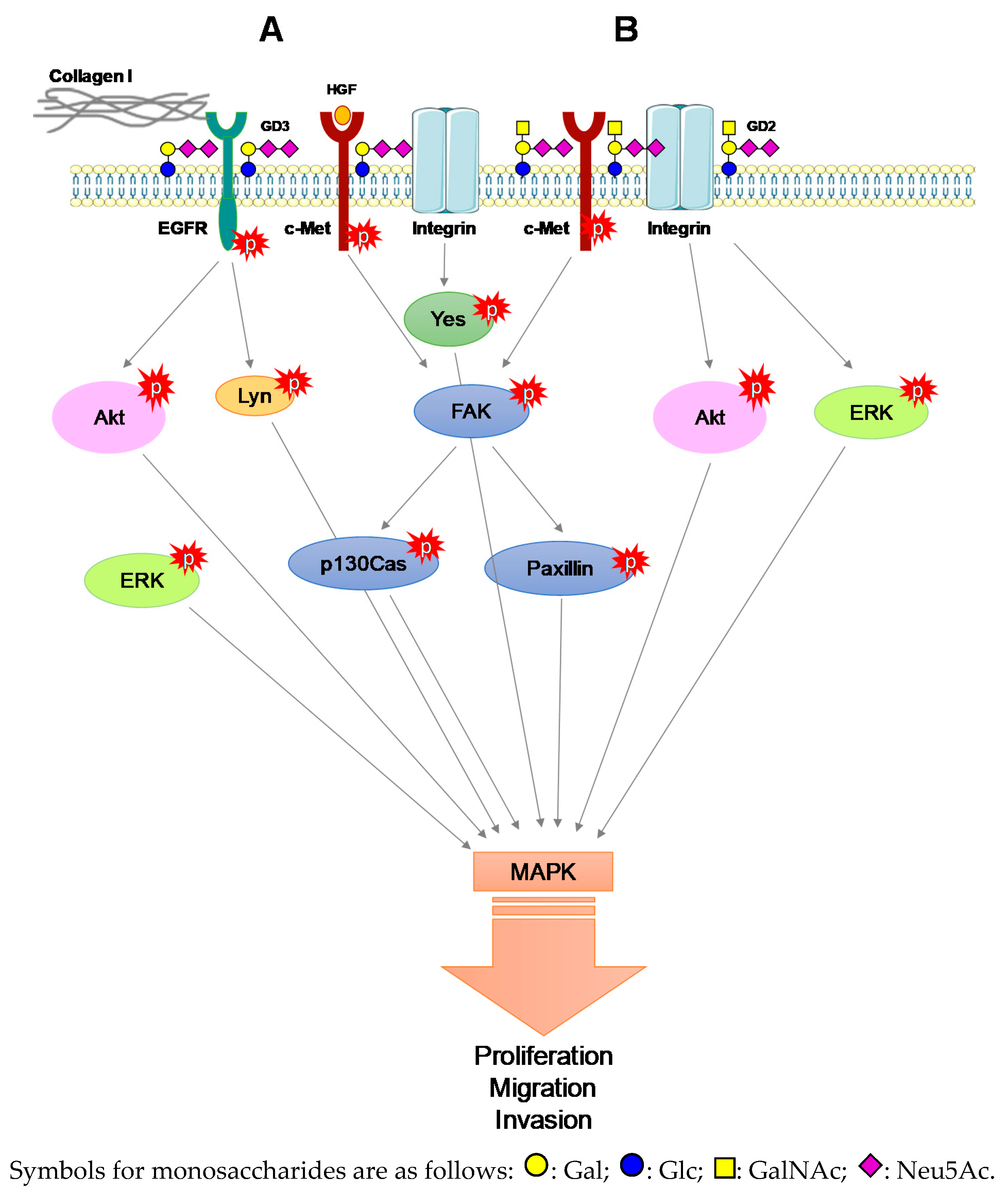
3.4. The Pro-Tumoral Role of Cell Membrane GD2 and GD3
3.5. Alternative Roles of O-acetylated Derivatives of GD3 and GD2 in ND Tumors
3.6. Gangliosides Are EMT Modulators
4. Immune System Reactivity against Ganglioside Expression
4.1. Chemoresistance Supported by Gangliosides
4.2. Gangliosides as Therapeutic Targets for Cancer
4.3. Anti-Ganglioside Immunotherapy
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. Ganglioside designation. Adv. Exp. Med. Biol. 1980, 125, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.G.; Balmaña, M.; Macedo, J.A.; Poças, J.; Fernandes, Â.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhães, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell Immunol. 2018, 333, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Groux-Degroote, S.; Rodríguez-Walker, M.; Dewald, J.H.; Daniotti, J.L.; Delannoy, P. Gangliosides in Cancer Cell Signaling. Prog. Mol. Biol. Transl. Sci. 2018, 156, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, X.; Pang, X.; Li, L.; Wang, J.; Yang, C.; Du, G. Ganglioside GD3 synthase (GD3S), a novel cancer drug target. Acta. Pharm. Sin. B 2018, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Yu, R.K. Gangliosides: Structure, isolation, and analysis. Methods Enzymol. 1982, 83, 139–191. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem. Sci. 2015, 40, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ledeen, R.W.; Wu, G. Gangliosides of the nervous system. In Gangliosides, Methods and Protocals Protocols; Sonnino, S., Prinetti, A., Eds.; Humana Press: New York, NY, USA, 2018; pp. 19–56. [Google Scholar]
- Kotani, M.; Kawashima, I.; Ozawa, H.; Terashima, T.; Tai, T. Differential distribution of major gangliosides in rat central nervous system detected by specific monoclonal antibodies. Glycobiology 1993, 3, 137–146. [Google Scholar] [CrossRef]
- Bolot, G.; David, M.J.; Taki, T.; Handa, S.; Kasama, T.; Richard, M.; Pignat, J.C.; Thomas, L.; Portoukalian, J. Analysis of glycosphingolipids of human head and neck carcinomas with comparison to normal tissue. Biochem. Mol. Biol. Int. 1998, 46, 125–135. [Google Scholar] [CrossRef]
- Dewald, J.H.; Cavdarli, S.; Steenackers, A.; Delannoy, C.P.; Mortuaire, M.; Spriet, C.; Noël, M.; Groux-Degroote, S.; Delannoy, P. TNF differentially regulates ganglioside biosynthesis and expression in breast cancer cell lines. PloS ONE 2018, 13, e0196369. [Google Scholar] [CrossRef] [PubMed]
- Noll, E.N.; Lin, J.; Nakatsuji, Y.; Miller, R.H.; Black, P.M. GM3 as a novel growth regulator for human gliomas. Exp. Neurol. 2001, 168, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, Y.; Miller, R.H. Selective cell-cycle arrest and induction of apoptosis in proliferating neural cells by ganglioside GM3. Exp. Neurol. 2001, 168, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Izumoto, S.; Suzuki, T.; Kinoshita, M.; Kagawa, N.; Wada, K.; Hashimoto, N.; Maruno, M.; Nakatsuji, Y.; Yoshimine, T. Ganglioside GM3 inhibits proliferation and invasion of glioma. J. Neurooncol. 2005, 71, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Jamal, O. Expression of the gangliosides GD3 and GD2 on lymphocytes in tissue sections of melanoma. Pathology 1989, 21, 51–58. [Google Scholar] [CrossRef]
- Shibuya, H.; Hamamura, K.; Hotta, H.; Matsumoto, Y.; Nishida, Y.; Hattori, H.; Furukawa, K.; Ueda, M.; Furukawa, K. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012, 103, 16561664. [Google Scholar] [CrossRef]
- Roth, M.; Linkowski, M.; Tarim, J.; Piperdi, S.; Sowers, R.; Geller, D.; Gill, J.; Gorlick, R. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 2014, 120, 548–554. [Google Scholar] [CrossRef]
- Ziebarth, A.J.; Felder, M.A.; Harter, J.; Connor, J.P. Uterine leiomyosarcoma diffusely express disialoganglioside GD2 and bind the therapeutic immunocytokine 14.18-IL2: Implications for immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1149–1153. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Klier, F.G. Disialoganglioside GD2 distributes preferentially into substrate-associated microprocesses on human melanoma cells during their attachment to fibronectin. J. Cell Biol. 1986, 102, 1887–1897. [Google Scholar] [CrossRef]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar]
- Schulz, G.; Cheresh, D.A.; Varki, N.M.; Yu, A.; Staffileno, L.K.; Reisfeld, R.A. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984, 44, 5914–5920. [Google Scholar] [PubMed]
- Marquina, G.; Waki, H.; Fernandez, L.E.; Kon, K.; Carr, A.; Valiente, O.; Perez, R.; Ando, S. Gangliosides expressed in human breast cancer. Cancer Res. 1996, 56, 5165–5171. [Google Scholar] [PubMed]
- Dobrenkov, K.; Ostrovnaya, I.; Gu, J.; Cheung, I.Y.; Cheung, N.K. Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatr. Blood Cancer 2016, 63, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Saraf, A.J.; Dickman, P.S.; Hingorani, P. Disialoganglioside GD2 expression in pediatric rhabdomyosarcoma: A case series and review of the literature. J Pediatr. Hematol. Oncol. 2019, 41, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.; Barbolini, M.; Ficarra, G.; Tazzioli, G.; Manni, P.; Petrachi, T.; Mastrolia, I.; Orvieto, E.; Spano, C.; Prapa, M.; et al. GD2 expression in breast cancer. Oncotarget 2017, 8, 31592–31600. [Google Scholar] [CrossRef] [PubMed]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated With Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362. [Google Scholar] [CrossRef]
- Sinha, D.; Mandal, C.; Bhattacharya, D.K. Identification of 9-O Acetyl sialoglycoconjugates (9-OAcSGs) as biomarkers in childhood acute lymphoblastic leukemia using a lectin, achatininh, as a probe. Leukemia 1999, 13, 119–125. [Google Scholar] [CrossRef]
- Ribeiro-Resende, V.T.; Oliveira-Silva, A.; Ouverney-Brandão, S.; Santiago, M.F.; Hedin-Pereira, C.; Mendez-Otero, R. Ganglioside 9-O-acetyl GD3 expression is upregulated in the regenerating peripheral nerve. Neuroscience 2007, 147, 97–105. [Google Scholar] [CrossRef]
- Gocht, A.; Rutter, G.; Kniep, B. Changed expression of 9-O-acetyl GD3 (CDw60) in benign and atypical proliferative lesions and carcinomas of the human breast. Histochem. Cell Biol. 1998, 110, 217–229. [Google Scholar] [CrossRef]
- Parameswaran, R.; Lim, M.; Arutyunyan, A.; Abdel-Azim, H.; Hurtz, C.; Lau, K.; Müschen, M.; Yu, R.K.; von Itzstein, M.; Heisterkamp, N.; et al. O-acetylated N-acetylneuraminic acid as a novel target for therapy in human pre-B acute lymphoblastic leukemia. J. Exp. Med. 2013, 210, 805–819. [Google Scholar] [CrossRef]
- Merritt, W.D.; Sztein, M.B.; Reaman, G.H. Detection of GD3 ganglioside in childhood acute lymphoblastic leukemia with monoclonal antibody to GD3: Restriction to immunophenotypically defined T-cell disease. J. Cell. Biochem. 1988, 37, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.; Allman, R.; Mason, M.D. Ganglioside expression in lung cancer cell lines. Lung Cancer 1997, 18, 21–33. [Google Scholar] [CrossRef]
- Birks, S.M.; Danquah, J.O.; King, L.; Vlasak, R.; Gorecki, D.C.; Pilkington, G.J. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro. Oncol. 2011, 13, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rueda, N.; Desselle, A.; Cochonneau, D.; Chaumette, T.; Clemenceau, B.; Leprieur, S.; Bougras, G.; Supiot, S.; Mussini, J.M.; Barbet, J.; et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PLoS ONE 2011, 6, e25220. [Google Scholar] [CrossRef] [PubMed]
- Cochonneau, D.; Terme, M.; Michaud, A.; Dorvillius, M.; Gautier, N.; Frikeche, J.; Alvarez-Rueda, N.; Bougras, G.; Aubry, J.; Paris, F.; et al. Cell cycle arrest and apoptosis induced by O-acetyl-GD2-specific monoclonal antibody 8B6 inhibits tumor growth in vitro and in vivo. Cancer Lett. 2013, 333, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J.; Cochonneau, D.; Fougeray, S.; Oliver, L.; Geraldo, F.; Terme, M.; Dorvillius, M.; Loussouarn, D.; Vallette, F.; Paris, F.; et al. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget 2016, 7, 41172–41185. [Google Scholar] [CrossRef] [PubMed]
- Cavdarli, S.; Dewald, J.H.; Yamakawa, N.; Guérardel, Y.; Terme, M.; Le Doussal, J.M.; Delannoy, P.; Groux-Degroote, S. Identification of 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2) as main O-acetylated sialic acid species of GD2 in breast cancer cells. Glycoconj. J. 2019, 36, 79–90. [Google Scholar] [CrossRef]
- Terme, M.; Dorvillius, M.; Cochonneau, D.; Chaumette, T.; Xiao, W.; Diccianni, M.B.; Barbet, J.; Yu, A.L.; Paris, F.; Sorkin, L.S.; et al. Chimeric antibody c.8B6 to O-acetyl-GD2 mediates the same efficient anti-neuroblastoma effects as therapeutic ch14.18 antibody to GD2 without antibody induced allodynia. PLoS ONE 2014, 9, e87210. [Google Scholar] [CrossRef]
- Yamashita, T.; Wu, Y.P.; Sandhoff, R.; Werth, N.; Mizukami, H.; Ellis, J.M.; Dupree, J.L.; Geyer, R.; Sandhoff, K.; Proia, R.L. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 2725–2730. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, T.W.; Kang, S.K.; Lee, Y.C.; Ko, J.H.; Kim, J.G.; Kim, C.H. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology 2006, 16, 573–583. [Google Scholar] [CrossRef]
- Hamamura, K.; Furukawa, K.; Hayashi, T.; Hattori, T.; Nakano, J.; Nakashima, H.; Okuda, T.; Mizutani, H.; Hattori, H.; Ueda, M.; et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Aixinjueluo, W.; Furukawa, K.; Zhang, Q.; Hamamura, K.; Tokuda, N.; Yoshida, S.; Ueda, R.; Furukawa, K. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: Roles of anoikis. J. Biol. Chem. 2005, 280, 29828–29836. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bremer, E.G.; Schlessinger, J.; Hakomori, S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 1986, 261, 2434–2440. [Google Scholar] [PubMed]
- Mirkin, B.L.; Clark, S.H.; Zhang, C. Inhibition of human neuroblastoma cell proliferation and EGF receptor phosphorylation by gangliosides GM1, GM3, GD1a and GT1b. Cell Prolif. 2002, 35, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Nakayama, K.; Hikita, T.; Handa, K.; Hakomori, S.I. Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 18987–18991. [Google Scholar] [CrossRef]
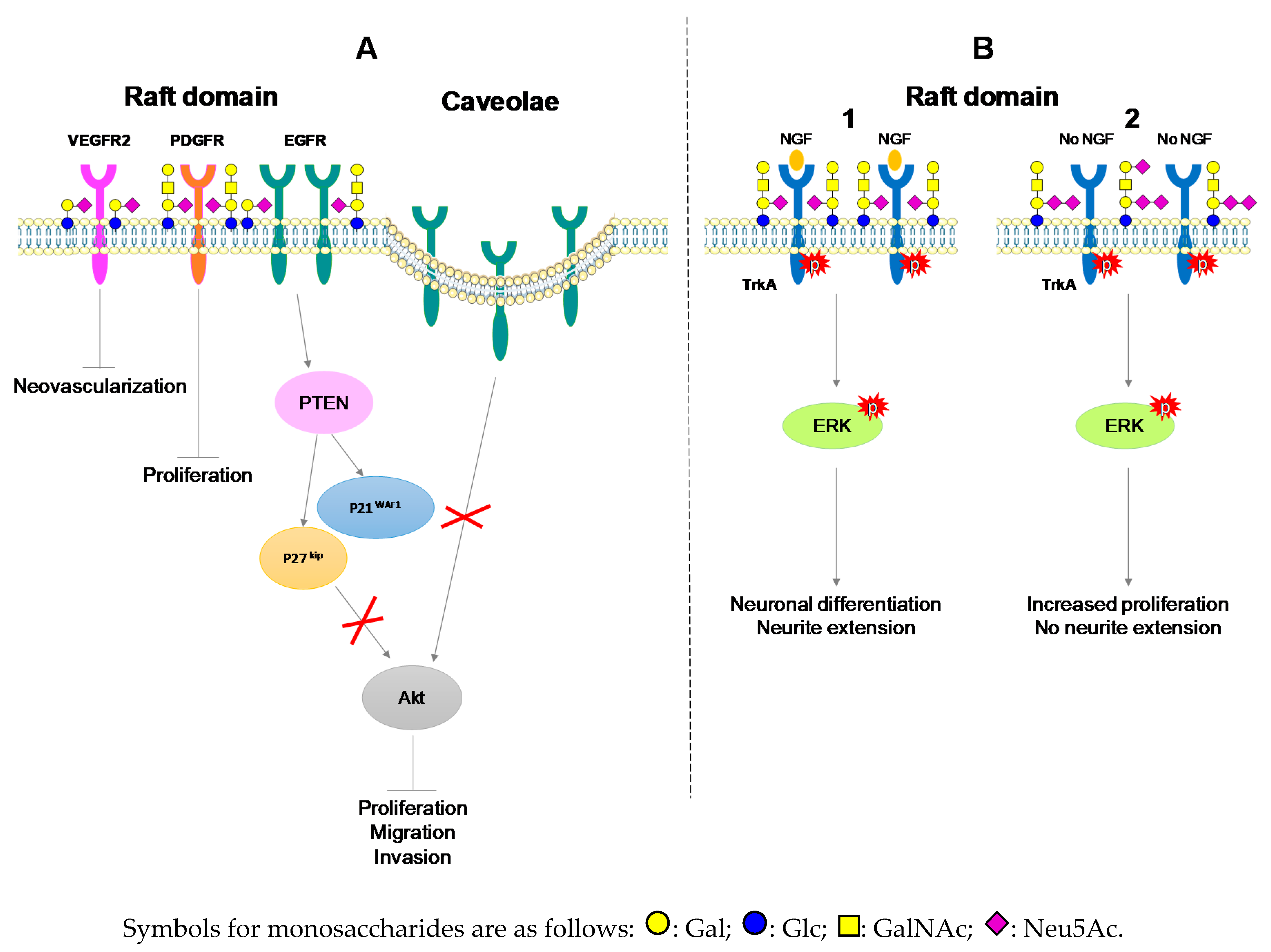
- Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Caveolae and signalling in cancer. Nat. Rev. Cancer 2015, 15, 225–237. [Google Scholar] [CrossRef]
- Zhuo, D.; Guan, F. Ganglioside GM1 promotes contact inhibition of growth by regulating the localization of epidermal growth factor receptor from glycosphingolipid-enriched microdomain to caveolae. Cell Prolif. 2019, e12639. [Google Scholar] [CrossRef]
- Mitsuda, T.; Furukawa, K.; Fukumoto, S.; Miyazaki, H.; Urano, T.; Furukawa, K. Overexpression of ganglioside GM1 results in the dispersion of platelet-derived growth factor receptor from glycolipid-enriched microdomains and in the suppression of cell growth signals. J. Biol. Chem. 2002, 277, 11239–11246. [Google Scholar] [CrossRef]
- Chung, T.W.; Kim, S.J.; Choi, H.J.; Kim, K.J.; Kim, M.J.; Kim, S.H.; Lee, H.J.; Ko, J.H.; Lee, Y.C.; Suzuki, A.; et al. Ganglioside GM3 inhibits VEGF/VEGFR-2-mediated angiogenesis: Direct interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef]
- Mutoh, T.; Tokuda, A.; Miyadai, T.; Hamaguchi, M.; Fujiki, N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA 1995, 92, 5087–5091. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Mutoh, T.; Hasegawa, T.; Miyazaki, H.; Okada, M.; Goto, G.; Furukawa, K.; Urano, T. GD3 synthase gene expression in PC12 cells results in the continuous activation of TrkA and ERK1/2 and enhanced proliferation. J. Biol. Chem. 2000, 275, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, J.; Mi, W.; Yang, J.; Han, F.; Lu, X.; Yu, W. Silencing of GM3 synthase suppresses lung metastasis of murine breast cancer cells. Breast Cancer Res. 2008, 10, R1. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, R.K. Interaction of ganglioside GD3 with an EGF receptor sustains the self-renewal ability of mouse neural stem cells in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 19137–19142. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef]
- Liang, Y.J.; Wang, C.Y.; Wang, I.A.; Chen, Y.W.; Li, L.T.; Lin, C.Y.; Ho, M.Y.; Chou, T.L.; Wang, Y.H.; Chiou, S.P.; et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget 2017, 8, 47454–47473. [Google Scholar] [CrossRef]
- Cazet, A.; Groux-Degroote, S.; Teylaert, B.; Kwon, K.M.; Lehoux, S.; Slomianny, C.; Kim, C.H.; Le Bourhis, X.; Delannoy, P. GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol. Chem. 2009, 390, 601–609. [Google Scholar] [CrossRef]
- Cazet, A.; Lefebvre, J.; Adriaenssens, E.; Julien, S.; Bobowski, M.; Grigoriadis, A.; Tutt, A.; Tulasne, D.; Le Bourhis, X.; Delannoy, P. GD₃ synthase expression enhances proliferation and tumor growth of MDA-MB-231 breast cancer cells through c-Met activation. Mol. Cancer. Res. 2010, 8, 1526–1535. [Google Scholar] [CrossRef]
- Cazet, A.; Bobowski, M.; Rombouts, Y.; Lefebvre, J.; Steenackers, A.; Popa, I.; Guérardel, Y.; Le Bourhis, X.; Tulasne, D.; Delannoy, P. The ganglioside G(D2) induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the G(D3) synthase. Glycobiology 2012, 22, 806–816. [Google Scholar] [CrossRef]
- Furukawa, K.; Kambe, M.; Miyata, M.; Ohkawa, Y.; Tajima, O.; Furukawa, K. Ganglioside GD3 induces convergence and synergism of adhesion and hepatocyte growth factor/Met signals in melanomas. Cancer Sci. 2014, 105, 52–63. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Miyazaki, S.; Miyata, M.; Hamamura, K.; Furukawa, K.; Furukawa, K. Essential roles of integrin-mediated signaling for the enhancement of malignant properties of melanomas based on the expression of GD3. Biochem. Biophys. Res. Commun. 2008, 373, 14–19. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Miyazaki, S.; Hamamura, K.; Kambe, M.; Miyata, M.; Tajima, O.; Ohmi, Y.; Yamauchi, Y.; Furukawa, K.; Furukawa, K. Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J. Biol. Chem. 2010, 285, 27213–27223. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Tsuji, M.; Hotta, H.; Ohkawa, Y.; Takahashi, M.; Shibuya, H.; Nakashima, H.; Yamauchi, Y.; Hashimoto, N.; Hattori, H.; et al. Functional activation of Src family kinase Yes protein is essential for the enhanced malignant properties of human melanoma cells expressing ganglioside GD3. J. Biol. Chem. 2011, 286, 18526–18537. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Itoh, M.I.; Haraguchi, M.; Okajima, T.; Inoue, M.; Oishi, H.; Matsuda, Y.; Iwamoto, T.; Kawano, T.; Fukumoto, S.; et al. b-series Ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 2002, 277, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic acids in the brain: Gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Yuhsuke, O.; Orie, T.; Yuji, K.; Ji, S.; Noboru, H.; Furukawa, K. Gangliosides in Inflammation and Neurodegeneration. Prog. Mol. Biol. Transl. Sci. 2018, 156, 265–287. [Google Scholar] [CrossRef]
- Kasahara, K.; Watanabe, Y.; Yamamoto, T.; Sanai, Y. Association of Src family tyrosine kinase Lyn with ganglioside GD3 in rat brain. Possible regulation of Lyn by glycosphingolipid in caveolae-like domains. J. Biol. Chem. 1997, 272, 29947–29953. [Google Scholar] [CrossRef] [PubMed]
- Kristal, B.S.; Brown, A.M. Apoptogenic ganglioside GD3 Directly induces the mitochondrial permeability transition. J. Biol. Chem. 1999, 274, 23169–23175. [Google Scholar] [CrossRef] [PubMed]
- Sa, G.; Das, T.; Moon, C.; Hilston, C.M.; Rayman, P.A.; Rini, B.I.; Tannenbaum, C.S.; Finke, J.H. GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res. 2009, 69, 3095–3104. [Google Scholar] [CrossRef]
- Furukawa, K.; Aixinjueluo, W.; Kasama, T.; Ohkawa, Y.; Yoshihara, M.; Ohmi, Y.; Tajima, O.; Suzumura, A.; Kittaka, D.; Furukawa, K. Disruption of GM2/GD2 synthase gene resulted in overt expression of 9-O-acetyl GD3 irrespective of Tis21. J. Neurochem. 2008, 105, 1057–1066. [Google Scholar] [CrossRef]
- Malisan, F.; Franchi, L.; Tomassini, B.; Ventura, N.; Condò, I.; Rippo, M.R.; Rufini, A.; Liberati, L.; Nachtigall, C.; Kniep, B.; et al. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 2002, 196, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Kniep, B.; Kniep, E.; Ozkucur, N.; Barz, S.; Bachmann, M.; Malisan, F.; Testi, R.; Rieber, E.P. 9-O-acetyl GD3 protects tumor cells from apoptosis. Int. J. Cancer 2006, 119, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Arming, S.; Wipfler, D.; Mayr, J.; Merling, A.; Vilas, U.; Schauer, R.; Schwartz-Albiez, R.; Vlasak, R. The human Cas1 protein: A sialic acid-specific O-acetyltransferase? Glycobiology 2011, 21, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Baumann, A.M.; Bakkers, M.J.; Buettner, F.F.; Hartmann, M.; Grove, M.; Langereis, M.A.; de Groot, R.J.; Mühlenhoff, M. 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat. Commun. 2015, 6, 7673. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.W.; Choi, H.J.; Kwak, C.H.; Song, K.H.; Suh, S.J.; Kwon, K.M.; Chang, Y.C.; Park, Y.G.; Chang, H.W.; et al. Ganglioside GM3 participates in the TGF-β1-induced epithelial-mesenchymal transition of human lens epithelial cells. Biochem. J. 2013, 449, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Shi, Y.; Evans, K.W.; Wang, R.Y.; Spaeth, E.L.; Jacamo, R.O.; Guerra, R.; Sahin, A.A.; Marini, F.C.; Hortobagyi, G.; et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Invest. 2012, 122, 2066–2078. [Google Scholar] [CrossRef]
- Kiura, K.; Watarai, S.; Ueoka, H.; Tabata, M.; Gemba, K.; Aoe, K.; Yamane, H.; Yasuda, T.; Harada, M. An alteration of ganglioside composition in cisplatin-resistant lung cancer cell line. Anticancer Res. 1998, 18, 2957–2960. [Google Scholar]
- Noguchi, M.; Kabayama, K.; Uemura, S.; Kang, B.W.; Saito, M.; Igarashi, Y.; Inokuchi, J. Endogenously produced ganglioside GM3 endows etoposide and doxorubicin resistance by up-regulating Bcl-2 expression in 3LL Lewis lung carcinoma cells. Glycobiology 2006, 16, 641–650. [Google Scholar] [CrossRef]
- Luen, S.J.; Savas, P.; Fox, S.B.; Salgado, R.; Loi, S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 2017, 49, 141–155. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Kudo, D.; Rayman, P.; Horton, C.; Cathcart, M.K.; Bukowski, R.M.; Thornton, M.; Tannenbaum, C.; Finke, J.H. Gangliosides expressed by the renal cell carcinoma cell line SK-RC-45 are involved in tumor-induced apoptosis of T cells. Cancer Res. 2003, 63, 1676–1683. [Google Scholar] [PubMed]
- Shenoy, G.N.; Loyall, J.; Berenson, C.S.; Kelleher, R.J., Jr.; Iyer, V.; Balu-Iyer, S.V.; Odunsi, K.; Bankert, R.B. Sialic Acid-Dependent Inhibition of T Cells by Exosomal Ganglioside GD3 in Ovarian Tumor Microenvironments. J. Immunol. 2018, 201, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.J.; Li, X.; Giuntoli, R.L., 2nd; Lopez, P.H.; Heuser, C.; Schnaar, R.L.; Tsuji, M.; Kurts, C.; Oelke, M.; Schneck, J.P. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 2012, 72, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Valle-Argos, B.; Gómez-Nicola, D.; Nieto-Sampedro, M. Glioma growth inhibition by neurostatin and O-But GD1b. Neuro Oncol. 2010, 12, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, J.; Wang, S.; Unger, J.M.; Arias-Fuenzalida, J.; Shi, Y.; Li, J.; Gao, Y.; Shi, W.; Wang, X.; et al. The Effects of Ganglioside-Monosialic Acid in Taxane-induced Peripheral Neurotoxicity in Patients with Breast Cancer: A Randomized Trial. J. Natl. Cancer Inst. 2019. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.N.; Mintzer, D.; Cordon-Cardo, C.; Welt, S.; Fliegel, B.; Vadhan, S.; Carswell, E.; Melamed, M.R.; Oettgen, H.F.; Old, L.J. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: A phase I trial in patients with malignant melanoma. Proc. Natl. Acad. Sci. USA 1985, 82, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Dinutuximab: First Global Approval. Drugs 2015, 75, 923–927. [Google Scholar] [CrossRef]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef]
- Durbas, M.; Horwacik, I.; Boratyn, E.; Kamycka, E.; Rokita, H. GD2 ganglioside specific antibody treatment downregulates PI3K/Akt/mTOR signaling network in human neuroblastoma cell lines. Int. J. Oncol. 2015, 47, 1143–1159. [Google Scholar] [CrossRef]
- Doronin, I.I.; Vishnyakova, P.A.; Kholodenko, I.V.; Ponomarev, E.D.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014, 14, 295. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, Y.; Zhou, Y.; Peng, D. Endothelin A receptor antagonism enhances inhibitory effects of anti-ganglioside GD2 monoclonal antibody on invasiveness and viability of human osteosarcoma cells. PLoS ONE 2014, 9, e93576. [Google Scholar] [CrossRef] [PubMed]
- Horwacik, I.; Durbas, M.; Boratyn, E.; Węgrzyn, P.; Rokita, H. Targeting GD2 ganglioside and aurora A kinase as a dual strategy leading to cell death in cultures of human neuroblastoma cells. Cancer Lett. 2013, 341, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Jpn. J. Cancer Res. 2002, 93, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Mao, X.; Wang, W.; Chen, Y.; Li, D.; Li, H.; Dou, P. Anti-ganglioside GD2 monoclonal antibody synergizes with cisplatin to induce endoplasmic reticulum-associated apoptosis in osteosarcoma cells. Pharmazie 2018, 73, 80–86. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. https://doi.org/10.3390/biom9080311
Cavdarli S, Groux-Degroote S, Delannoy P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules. 2019; 9(8):311. https://doi.org/10.3390/biom9080311
Chicago/Turabian StyleCavdarli, Sumeyye, Sophie Groux-Degroote, and Philippe Delannoy. 2019. "Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors" Biomolecules 9, no. 8: 311. https://doi.org/10.3390/biom9080311
APA StyleCavdarli, S., Groux-Degroote, S., & Delannoy, P. (2019). Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules, 9(8), 311. https://doi.org/10.3390/biom9080311