Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem
Abstract
1. Introduction
2. Materials and Methods
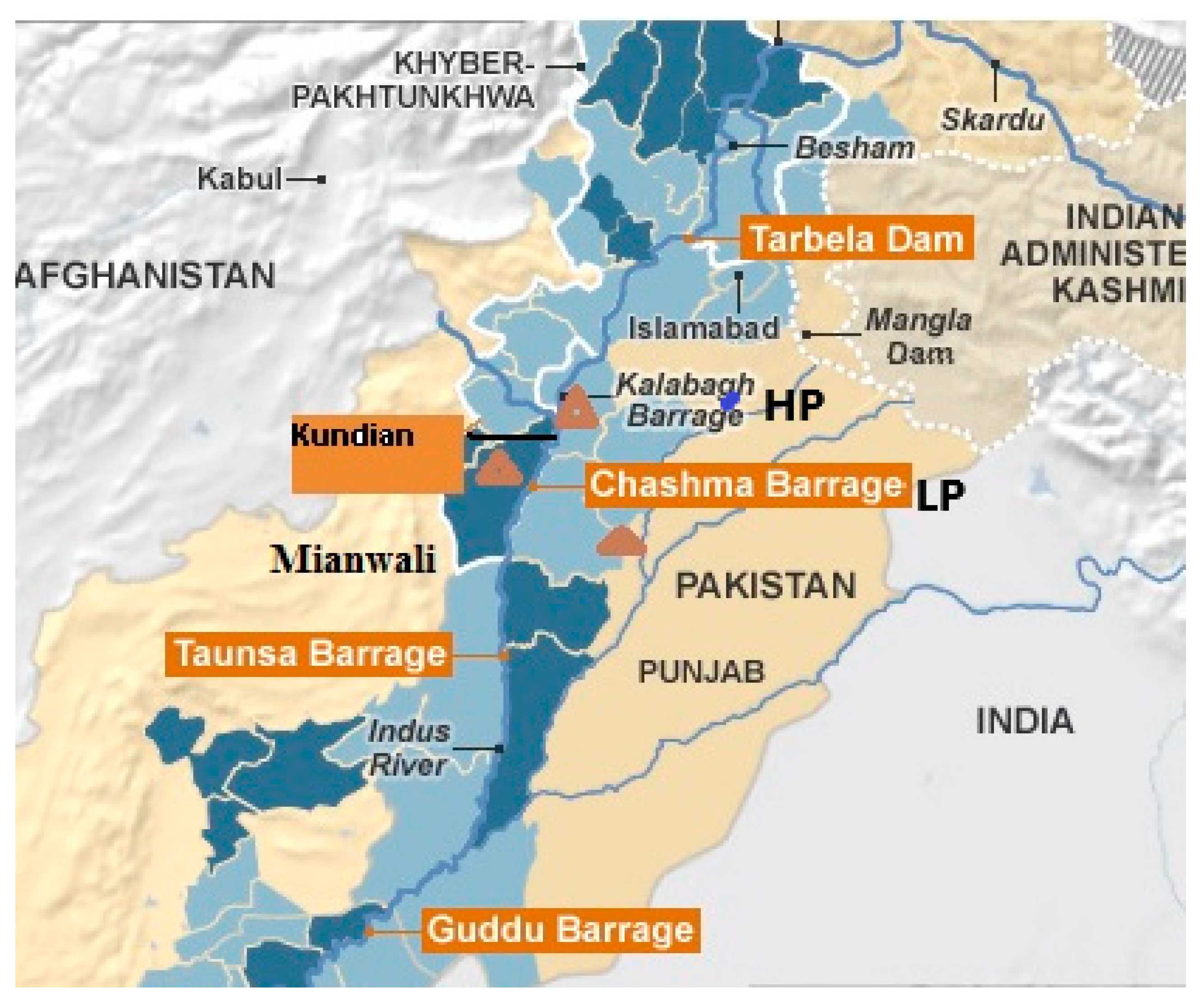
2.1. Study Area
2.2. Collection and Preparation of Fish Samples
2.3. Analysis of Water Samples
2.4. Periphyton Sampling
2.5. Zoobenthos Sampling
2.6. Fatty Acid Profiling
2.7. Statistical Analysis
3. Results
3.1. Physico-Chemical Factors and Heavy Metals
3.2. Fatty Acids Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beasley, G.; Kneale, P. Investigating the influence of heavy metals on macroinvertebrate assemblages using Partial Canonical Correspondence Analysis (pCCA). Hydrol. Earth Syst. Sci. 2003, 7, 221–233. [Google Scholar] [CrossRef][Green Version]
- Dahl, J.; Johnson, R.K.; Sandin, L. Detection of organic pollution of streams in southern Sweden using benthic macroinvertebrates. Hydrobiologia 2004, 516, 161–172. [Google Scholar] [CrossRef]
- Korkmaz, G.F.; Keser, R.; Akcay, N.; Dizman, S. Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere 2012, 87, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, Z.; Teodrorovic, V.; Dimitrijevic, M.; Borozan, S.; Beukovic, M.; Milicevic, D. Environmental Cd and Zn concentration in liver and kidney of European hare from different Serbian region: Age and tissue difference. Bull. Environ. Contamin. Toxicol. 2013, 90, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Riberio, O.C.A.; Vollaire, Y.; Sanchez-Chardi, A.; Roche, H. Bioaccumulation and the effects of organochlorine pesticides PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat. Toxicol. 2005, 74, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Nhiwatiwa, T.; Barson, M.; Harrison, A.P.; Utete, B.; Cooper, R.G. Metal concentrations in water, sediment and sharp tooth catfish Clarias gariepinus from three periurban rivers in the upper Manyame catchment, Zimbabwe. Afr. J. Aquat. Sci. 2011, 36, 243–252. [Google Scholar] [CrossRef]
- Annabi, A.; Said, K.; Messaoudi, I. Cadmium: Bioaccumulation, histopathology and detoxifying mechanisms in fish. Am. J. Res. Commun. 2013, 1, 60–79. [Google Scholar]
- Canli, M. Natural occurrence of metallothionein like proteins in the hepatopancreas of the Norway lobster, Nephros norvegicus and effects of Cd, Cu and Zn exposures on levels of the metal bound on metallothionein. Turk. J. Zool. 1995, 19, 313–321. [Google Scholar]
- Hussain, B.; Sultana, T.; Sultana, S.; Iqbal, Z.; Nadeem, S.; Mahboob, S. Habitat induced mutational effects and fatty acid profile changes in bottom dweller Cirrhinus mrigala inhabitant of river Chenab. Grasas y Aceites 2015, 66, e075. [Google Scholar] [CrossRef]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar]
- Kiron, V.; Fukuda, H.; Takeuchi, T.; Watanabe, T. Essential fatty acid nutrition and defense mechanisms in rainbow trout Oncorhynchus mykiss. Comp. Biochem. Physiol. A Physiol. 1995, 111, 361–367. [Google Scholar] [CrossRef]
- Bec, A.; Martin-Creuzburg, D.; von Elert, E. Trophic upgrading of autotrophic picoplankton by the heterotrophic nano-flagellate Paraphysomonas sp. Limnol. Oceanogr. 2006, 51, 1699–1707. [Google Scholar] [CrossRef]
- Klein Breteler, W.C.M.; Schogt, N.; Baas, M.; Schouten, S.; Kraay, G.W. Trophic upgrading of food quality by protozoans enhancing copepod growth: Role of essential lipids. Mar. Biol. 1999, 135, 191–198. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Borgå, K.; Fisk, A.T.; Hoekstra, P.F.; Muir, D.C.G. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in arctic marine food webs. Environ. Toxicol. Chem. 2004, 23, 367–2385. [Google Scholar] [CrossRef]
- Kainz, M.; Telmer, K.; Mazumder, A. Bioaccumulation patterns of methyl mercury and essential fatty acids in the planktonic food web and fish. Sci. Total Environ. 2006, 368, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.L.; Pan, Y. Benthic algal communities as biological indicators. In Algal Ecology: Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 705–739. [Google Scholar]
- Jabeen, F.; Chaudhry, A.S. Nutritional composition of seven fish species and the use of cluster analysis as a tool for their classification. J. Anim. Plant Sci. 2016, 26, 282–290. [Google Scholar]
- Dawood, M.A.O.; Koshio, S. Recent advances in the role of probiotics and prebiotics in carp aquaculture: A review. Aquaculture 2016, 454, 243–251. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Doan, H.V. Probiotic application for sustainable aquaculture. Rev. Aquac. 2018. [Google Scholar] [CrossRef]
- ElShehawy, S.M.; Gab-Alla, A.A.; Mutwally, H.M. Amino acids pattern and fatty acids composition of the most important fish species of Saudi Arabia. Int. J. Food Sci. Nutr. Eng. 2016, 6, 32–41. [Google Scholar]
- Razak, Z.K.A.; Basri, M.; Dzulkefly, K.; Razak, C.N.A.; Salleh, A.B. Extraction and characterization of fish oil from Monopterus albus. Malay J. Anal. Sci. 2001, 7, 217–220. [Google Scholar]
- Zhang, W.; Wang, W.X. Large-scale spatial and interspecies differences in trace elements and stable isotopes in marine wild fish from Chinese waters. J. Hazard. Mater. 2012, 215, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Pont, D.; Hugueny, B.; Beier, U.; Goffaux, D.; Melcher, A.; Noble, R.; Rogers, C.; Roset, N.; Schmutz, S. Assessing river biotic condition at a continental scale: A European approach using functional metrics and fish assemblages. J. Appl. Ecol. 2006, 43, 70–80. [Google Scholar] [CrossRef]
- Kainz, M.J.; Fisk, A.T. Integrating lipids and contaminants in aquatic ecology and ecotoxicology. In Lipids in Aquatic Ecosystems; Kainz, M., Brett, M., Arts, M., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kelly, E.N.; Schindler, D.W.; St. Louis, V.L.; Donald, D.B.; Vlaclicka, K.E. Forest fire increases mercury accumulation by fishes via food web restructuring and increased mercury inputs. Proc. Natl. Acad. Sci. 2006, 103, 19380–19385. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanim, K.A.; Mahboob, S.; Seema, S.; Sultana, S.; Sultana, T.; Al-Misned, F.; Ahmed, Z. Monitoring of trace metals in tissues of Wallago attu (lanchi) from the Indus River as an indicator of environmental pollution. Saudi J. Biol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Chaudhry, A.S. Monitoring trace metals in different tissues of Cyprinus carpio from the Indus River in Pakistan. Environ. Monit. Assess. 2010, 170, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S.; Al-Balwai, H.F.A.; Al-Misned, F.; Ahmad, Z. Investigation on the genotoxicity of mercuric chloride to freshwater Clarias gariepinus. Pak. Vet. J. 2014, 34, 100–103. [Google Scholar]
- Boyd, E.C. Water Quality in Warm Water Fishponds, 2nd ed.; Craftmaster Printers Inc.: Auburn, AL, USA, 1981; pp. 213–247. [Google Scholar]
- Gladyshev, M.I.; Anishchenko, O.V.; Sushchnik, N.N.; Kalacheva, G.S.; Gribovskaya, I.V.; Ageev, A.V. Influence of anthropogenic pollution on content of essential polyunsaturated fatty acids in links of food chain of river ecosystem. Contemp. Probl. Ecol. 2012, 5, 376–385. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanely, S.G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Bell, J.G.; McVicar, A.H.; Park, M.T.; Sargent, R.J. Effect of high dietary linoleic acid on fatty acid compositions of individual phospholipids from tissues of Atlantic salmon (Salmo salar): Association with a novel cardiac lesion. J. Nutr. 1991, 121, 1163–1172. [Google Scholar] [CrossRef]
- Kiessling, A.; Pickova, J.; Johansson, L.; Asgard, T.; Storebakken, T.; Kiessling, K.H. Changes in fatty acid composition in muscle and adipose tissue of farmed rainbow trout (Oncorhynchus mykiss) in relation to ration and age. Food Chem. 2001, 73, 271–284. [Google Scholar] [CrossRef]
- Hussain, B.; Sultana, T.; Sultana, S.; Al-Ghanim, K.A.; Mahboob, S. Effect of pollution on DNA damage and essential fatty acid profile in Cirrhinus mrigala from River Chenab. Chin. J. Oceanol. Limnol. 2016. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis Systems; S.A.S. Institute Inc.: Cary, NC, USA, 1995. [Google Scholar]
- United States Environmental Protection Agency. Drinking Water and Health. 2002. Available online: http://water.epa.gov/drink/index.cfm (accessed on 20 October 2013).
- Hussain, B.; Sultana, T.; Sultana, S.; Al-Ghanim, K.A.; Al-Misned, F.; Mahboob, S. Influence of habitat degradation on the fatty acid profiles of fish, microalgae, and zoobenthos in a river ecosystem. Proc. Saf. Environ. Prot. 2019, 122, 24–32. [Google Scholar] [CrossRef]
- Daly, H.V. General classification and key to the orders of aquatic and semi aquatic insects. In An Introduction to the Aquatic Insects of North America; Merrit, R.W., Cummins, K.W., Eds.; Kendall/Hunt: Dubuque, IA, USA, 1984; pp. 76–81. [Google Scholar]
- Carol, J.; Benejam, L.; Alcaraj, C.; Vila-Gispert, A.; Zamora, L.; Navarro, E.; Armengol, J.; Garcia-Berthou, E. The effects of limnological features on fish assemblages of 14 Spanish reservoirs. Ecol. Freshw. Fish. 2006, 15, 66–77. [Google Scholar] [CrossRef]
- Gundersen, P.; Olsvik, P.A.; Steinnes, E. Variations in heavy metal concentrations and speciation in two mining-polluted streams in central Norway. Environ. Toxicol. Chem. 2001, 20, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Heavy metals in commercial fish in New Jersey. Environ. Res. 2005, 99, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Staels, B.; Dallongeville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 19, 2088–2093. [Google Scholar] [CrossRef]
- Gervois, P.; Torra, I.P.; Furchart, J.C.; Staels, B. Regulation of lipid and lipoprotein metabolism by PPAR activators. Clin. Chem. Lab. Med. 2000, 38, 3–11. [Google Scholar] [CrossRef]
- Suga, T. Hepatocarcinogenesis by peroxisome proliferators. J. Toxicol. Sci. 2004, 29, 1e12. [Google Scholar] [CrossRef][Green Version]
- Olivares-Rubio, H.F.; Vega-Lopez, A. Fatty acid metabolism in fish species as a biomarker for environmental Monitoring: Review. Environ. Pollut. 2016, 218, 297e312. [Google Scholar] [CrossRef]
- Campbell, L.M.; Norstrom, R.J.; Hobson, K.A.; Muir, D.C.G.; Backus, S.; Fisk, A.T. Mercury and other trace elements in a pelagic Arctic marine food web (North water Polynya, Baffin Bay). Sci. Total Environ. 2005, 351, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.M.; Fisk, A.T.; Wang, X.; Köck, G.; Muir, D.C.G. Evidence of biomagnification of rubidium in aquatic and marine food webs. Can. J. Fish. Aquat. Sci. 2005, 62, 1161–1167. [Google Scholar] [CrossRef]
- Harwood, J.L. Membrane lipids in algae. In Lipids in Photosynthesis: Structure, Function and Genetics; Siegenthaler, P.-A., Murata, N., Eds.; Kluwer: Dordrecht, The Netherlands, 1998; pp. 53–64. [Google Scholar]
- Guckert, J.B.; Cooksey, K.E. Triglyceride accumulation and fatty acid profile changes in Chlorella (Chlorophyta) during high pH-induced cell cycle inhibition. J. Phycol. 1990, 26, 72–79. [Google Scholar] [CrossRef]
- Hartwich, M.; Straile, D.; Gaedke, U.; Wacker, A. Use of ciliate and phytoplankton taxonomic composition for the estimation of eicosapentaenoic acid concentration in lakes. Freshw. Biol. 2012, 57, 1385–1398. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N.; Dubovskaya, O.P.; Kravchuk, E.S.; Kalachova, G.S. Correlations between fatty acid composition of seston and zooplankton and effects of environmental parameters in a eutrophic Siberian reservoir. Limnologica 2010, 40, 343–357. [Google Scholar] [CrossRef]
- Mayzaud, P.; Claustre, H.; Augier, P. Effect of variable nutrient supply on fatty acid composition of phytoplankton grown in an enclosed experimental ecosystem. Mar. Ecol. Prog. Ser. 1990, 60, 123–140. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; Wehr, J.D.; Perrone, A.A. Trophic relations in a stream foodweb: Importance of fatty acids for macroinvertebrate consumers. J. N. Am. Benthol. Soc. 2007, 26, 509–522. [Google Scholar] [CrossRef]
- Mathias, A.C.; Lombardi, A.T.; Grac, M.D.; Mela, G.; Parrish, C.C. Effects of cadmium and nitrogen on lipid composition of Chlorella vulgaris (Trebouxiophyceae, Chlorophyta). Eur. J. Phycol. 2013, 48, 1–11. [Google Scholar]
- Descroix, A.; Bec, A.; Bourdier, G.; Sargos, D.; Sauvanet, J.; Misson, B.; Desvilettes, C. Fatty acids as biomarkers to indicate main carbon sources of four major invertebrate families in a large River (the Allier, France). Fundam. Appl. Limnol. 2010, 177, 39–55. [Google Scholar] [CrossRef]
- Masclaux, H.; Bec, A.; Kainz, M.J.; Desvilettes, C.; Jouve, L.; Bourdier, G. Combined effects of food quality and temperature on somatic growth and reproduction of two freshwater cladocerans. Limnol. Oceanog. 2009, 54, 1323–1332. [Google Scholar] [CrossRef]
- Taipale, S.; Kankaala, P.; Hamalainen, H.; Jones, R.I. Seasonal shifts in the diet of lake zooplankton revealed by phospholipid fatty acid analysis. Freshw. Biol. 2009, 54, 90–104. [Google Scholar] [CrossRef]
- Kandemir, Ş.; Polat, N. Seasonal variation of total lipid and total fatty acid in muscle and liver of rainbow trout (Oncorhynchus mykiss) reared in Derbent Dam Lake. Turk. J. Fish. Aquat. Sci. 2007, 7, 27–31. [Google Scholar]
- Koca, S.; Koca, Y.B.; Yildiz, S.; Gürcü, B. Genotoxic and histopathological effects of water pollution on two fish species, Barbus capito pectoralis and Chondrostoma nasus in the Büyük Menderes River. Turk. Biol. Trace Elem. Res. 2008, 122, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Kwetegyeka, J.; Mpango, O.G.; Niesen, G. Variation in fatty acid composition in muscle and heart tissues among species and populations of tropical fish in Lakes Victoria and Kyoga. Lipids 2008, 43, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Masa, P.; Ogwok, J.H.; Muyonga, J.; Kwetegyeka, V.; Makokha, D. Fatty acid composition of muscle, liver and adipose tissue of freshwater fish from Lake Victoria, Uganda. J. Aquat. Food Prod. Technol. 2011, 20, 64–72. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, X. Fish and its multiple human health effects in times of threat to sustainability and affordability: Are there alternatives? APJCN 2009, 218, 553–563. [Google Scholar]
| Water Quality Characteristics | HP Site | LP Site | NP Site | Permissible Limits |
|---|---|---|---|---|
| pH | 12.1 ± 0.36 a | 8.5 ± 0.12 b | 8.1 ± 0.08 b | D: 6.5–8.5, P: ** |
| BOD (mg/L) | 81.2 ± 1.10 a | 48.8 ± 0.41 b | 36.7 ± 0.77 c | †D: 30 mg/L, P: ** |
| COD (mg/L) | 195.8 ± 1.16 a | 71.2 ± 0.90 b | 65.5 ± 0.58 c | †D: 250 mg/L, P: ** |
| TDS (mg/L) | 2444.5 ± 8.41 a | 1319.8 ±10.62 b | 340.3 ± 7.24 c | D: 500 mg/L, P: 2000 mg/L |
| TSS (mg/L) | 329.6 ± 6.41 a | 218.6 ± 5.15 b | 190.6 ± 4.24 c | D: 100mg/L, P: ** |
| Salinity (mg/L) | 1951.2 ±18.31 a | 458.5 ± 7.22 b | 242.3± 4.90 b | P: <100 mg/L |
| Conductivity µS/cm) | 4.1 ± 0.22 a | 1.55 ± 0.11 b | 0.42 ± 0.051 c | D:650 µS/cm, P: 1055 µS/cm |
| Phenols (mg/L) | 2.49 ± 0.18 a | 0.84 ± 0.04 b | 0.21 ± 0.01 c | D: 0.001 mg/L, P: 0.002 mg/L |
| Sulfates (mg/L) | 452.3 ± 7.62 a | 341.21± 0.08 b | 97.4 ± 3.90 c | D: 0.001 mg/L, P: 0.002 mg/L |
| Heavy Metal Contamination | ||||
| Sn (mg/L) | 0.54 ± 0.02 a | 0.03 ± 0.0 b | 0.01 ± 0.0 b | D: 0.01 mg/L, P: ** |
| Cr (mg/L) | 0.72 ± 0.03 a | 0.36 ± 0.02 b | 0.05 ± 0.00 c | D: 0.05 mg/L, P: ** |
| Pb (mg/L) | 3.02 ± 0.07 a | 0.21 ± 0.02 b | 0.14 ± 0.01 c | D: 0.05 mg/L, P: ** |
| Zn (mg/L) | 0.56 ± 0.02 a | 0.251 ± 0.03 b | 0.05 ±0.00 a | D: 5 mg/L, P: 15 mg/L |
| Mn (mg/L) | 2.81 ± 0.12 a | 2.05 ± 0.06 a | 0.41 ± 0.01 c | D: 0.1 mg/L, P: 0.3 mg/L |
| Cu (mg/L) | 2.05 ± 0.05 a | 0.99 ± 0.11 b | 0.08 ± 0.00 c | D: 0.05 mg/L, P: 1.5 mg/L |
| Cd (mg/L) | 0.29 ± 0.02 a | 0.03 ± 0.00 b | 0.00 ± 0.00 b | D: 0.01 mg/L, P: ** |
| Hg (mg/L0 | 1.51 ± 0.04 b | 0.05 ± 0.01 c | < 0.001 | D: 0.001 mg/L, P: ** |
| Parameter | HP Site | LP Site | NP Site |
|---|---|---|---|
| Periphyton | |||
| Sn | 17.61 ± 0.90 a | 11.34 ± 0.77 b | 4.88 ± 0.61 c |
| Cr | 6.10 ± 0.70 a | 1.98 ± 0.55 b | 0.15 ± 0.23 c |
| P b | 1.68 ± 0.67 a | 0.41 ± 0.10 b | 0.15 ± 0.03 c |
| Zn | 14.46 ± 1.35 b | 23.11 ± 1.66 a | 8.19 ± 0.1.05 c |
| Mn | 20.66 ± 1.41 a | 11.44 ± 1.33 a | 5.90 ± 0.88 c |
| Cu | 18.43 ± 1.44 a | 6.97 ± 0.92 b | 4.02 ± 0.55 c |
| Cd | 2.41 ± 0.31 a | 0.62 ± 0.12 b | 0.16 ± 0.02 c |
| Hg | 3.78 ± 0.40 b | 1.70 ± 0.18 c | 0.17 ± 0.00 c |
| Zoobenthos | |||
| Sn | 3.43 ± 0.31 a | 2.90 ± 0.70 b | 1.11 ± 0.40 c |
| Cr | 2.80 ± 0.42 a | 1.22 ± 0.18 b | 0.26 ± 0.03 c |
| P b | 1.21 ± 0.05 a | 0.38 ± 0.01 b | 0.15 ± 0.02 c |
| Zn | 6.94 ± 0.77 b | 8.06 ± 1.0 a | 3.80 ±0.22 c |
| Mn | 3.91 ± 0.48 a | 1.30 ± 0.26 b | 0.92 ± 0.01 c |
| Cu | 6.02 ± 0.72 a | 1.40 ± 0.21 b | 0.33 ± 0.03 c |
| Cd | 1.99 ± 0.18 a | 0.89 ± 0.02 b | 0.36 ± 0.07 c |
| Hg | 1.92 ± 0.31 b | 0.86 ± 0.18 c | 0.09 ± 0.00 c |
| Hypophthalmichthys molitrix | |||
| Sn | 1.95 ± 0.41 a | 1.32 ± 0.36 b | 0.67± 0.16 c |
| Cr | 3.01 ± 0.60 a | 1.71 ± 0.22 b | 0.82 ± 0.15 c |
| P b | 0.81 ± 0.16 a | 0.49 ± 0.08 b | 0.21 ± 0.01 c |
| Zn | 5.67 ± 0.67 a | 3.62 ± 0.41 b | 1.62 ± 0.33 c |
| Mn | 2.70 ± 0.41 a | 1.92 ± 0.38 b | 0.79 ± 0.08 c |
| Cu | 4.93 ± 0.62 a | 1.08 ± 0.15 b | 0.49 ± 0.05 c |
| Cd | 1.41 ± 0.28 a | 0.76 ± 0.12 b | 0.35 ± 0.05 c |
| Hg | 1.37 ± 0.22 a | 0.87 ± 0.09 b | 0.06 ± 0.00 c |
| Ctenopharyngodon idella | |||
| Sn | 1.81 ± 0.3 a | 1.12 ± 0.18 b | 0.61 ± 0.06 c |
| Cr | 2.28 ± 0.27 a | 1.57 ± 0.20 b | 0.71 ± 0.15 c |
| P b | 0.74 ± 0.10 a | 0.49 ± 0.06 b | 0.23 ± 0.01 c |
| Zn | 5.62 ± 0.72 a | 3.01 ± 0.47 b | 1.52 ± 0.31 c |
| Mn | 2.50 ± 0.38 a | 1.62 ± 0.21 b | 0.69 ± 0.17 c |
| Cu | 4.70 ± 0.52 a | 1.02 ± 0.20 b | 0.42 ± 0.05 c |
| Cd | 1.34 ± 0.31 a | 0.70 ± 0.21 b | 0.31 ± 0.06 c |
| Hg | 1.41 ± 0.22 a | 0.87 ± 0.16 b | 0.09 ± 0.00 c |
| Cyprinus carpio | |||
| Sn | 2.41 ± 0.4 a | 1.61 ± 0.17 b | 0.72 ± 0.06 c |
| Cr | 3.44 ± 0.40 a | 1.90 ± 0.23 b | 0.95 ± 0.16 c |
| P b | 0.98 ± 0.12 a | 0.69± 0.07 b | 0.40 ± 0.03 c |
| Zn | 6.75 ± 0.88 a | 3.96 ± 0.60 b | 1.88 ± 0.44 c |
| Mn | 2.90 ± 0.40 a | 1.80 ± 0.24 b | 0.77 ± 0.18 c |
| Cu | 5.48 ± 0.80 a | 1.57 ± 0.28 b | 0.58 ± 0.02 c |
| Cd | 1.62 ± 0.40 a | 0.88 ± 0.16 b | 0.45 ± 0.05 c |
| Hg | 1.69 ± 0.21 a | 0.92 ± 0.18 b | 0.22 ± 0.01 c |
| Pytoperiphyton | |||
|---|---|---|---|
| Fatty Acids | HP Site | LP Site | NP Site |
| SFAs | |||
| C8:0 | ---------- | ---------- | ---------- |
| C10:0 | 3.33 ± 0.11 c | 6.78 ± 0.88 b | 8.12 ± 0.98 a |
| C12:0 | ---------- | --------- | --------- |
| C14:0 | 1.67 ± 0.08 c | 4.41 ± 0.16 b | 5.69 ± 0.77 a |
| C16:0 | 16.69 ± 2.77 c | 19.63 ± 2.55 b | 21.89 ± 2.44 a |
| C18:0 | 12.47 ± 1.63 c | 13.12 ± 1.20 b | 15.76 ± 2.89 a |
| C20:0 | ---------- | --------- | 0.67 ± 0.11 |
| MUFAs | |||
| C16:1(n-7) | --------- | --------- | --------- |
| C16:1(n-9) | --------- | --------- | --------- |
| C18:1(n-7) | --------- | ---------- | 1.99± 0.16 a |
| C18:1(n-9) | --------- | --------- | 0.97 ± 0.11 |
| C20:1(n-9) | --------- | --------- | --------- |
| C22:1(n-9) | 3.11± 0.13 c | 5.12 ± 0.34 b | 7.89 ± 0.25 a |
| PUFAs | |||
| C18:2(n-6) | 0.43 ± 0.04 c | 0.66 ± 0.19 b | 1.15 ± 0.22 a |
| C18:3(n-3) | 8.31 ± 0.70 b | 9.11 ± 0.90 a | 9.44 ± 0.88 a |
| C18:4(n-3) | --------- | --------- | --------- |
| C20:2(n-6) | --------- | ----------- | 2.24 ± 0.05 a |
| C20:4(n-6) | 6.11 ± 0.55 c | 8.02 ± 0.71 b | 9.98 ± 0.41 a |
| C20:5(n-6) | --------- | 4.11 ± 0.22 b | 5.23 ± 0.28 a |
| C20:5(n-3) | 6.78 ± 0.90 a | 5.97 ± 0.60 a | 4.66 ± 0.70 c |
| C22:4(n-6) | --------- | --------- | --------- |
| C22:5(n-6) | ----------- | ---------- | 2.79 ± 0.24 a |
| C22:5(n-3) | 6.57 ± 0.41 c | 7.33 ± 0.70 b | 8.95± 0.66 a |
| C22:6(n-3) | 5.44 ± 0.25 a | 4.76 ± 0.41 a | 3.77 ± 0.33 b |
| Zoobenthos | |||
| Fatty acids | Highly polluted water | Less polluted water | Non-polluted site |
| SFAs | |||
| C8:0 | ---------- | ---------- | ---------- |
| C10:0 | 1.44 ± 0.23 c | 4.11 ± 0.71 b | 6.32 ± 0.71 a |
| C12:0 | ---------- | --------- | --------- |
| C14:0 | 0.99 ± 0.08 c | 2.98 ± 0.27 b | 4.22 ± 0.55 a |
| C16:0 | 13.22 ± 1.66 c | 15.38 ± 2.80 b | 18.45 ± 2.88 a |
| C18:0 | 7.23 ± 1.66 b | 10.45 ± 1.76 b | 14.77 ± 2.18 a |
| C20:0 | ---------- | --------- | 0.1 ± 0.11 |
| MUFAs | |||
| C16:1(n-7) | --------- | --------- | --------- |
| C16:1(n-9) | --------- | --------- | --------- |
| C18:1(n-7) | --------- | --------- | 1.66 ± 0.07 a |
| C18:1(n-9) | --------- | --------- | 0.77 ± 0.08 |
| C20:1(n-9) | --------- | --------- | --------- |
| C22:1(n-9) | 1.72 ± 0.14 c | 3.77 ± 0.53 b | 4.99 ± 0.66 a |
| PUFAs | |||
| C18:2(n-6) | 0.20 ± 0.02 c | 0.41 ± 0.28 b | 0.81 ± 0.30 a |
| C18:3(n-3) | 4.10 ± 0.7 c | 6.22 ± 0.41 b | 7.69 ± 0.44 a |
| C18:4(n-3) | --------- | --------- | --------- |
| C20:2(n-6) | --------- | ----------- | 2.89 ± 0.14 |
| C20:4(n-6) | 3.00 ± 0.49 c | 4.77 ± 0.79 b | 6.67 ± 0.51 a |
| C20:5(n-6) | --------- | 1.90 ± 0.27 b | 3.69 ± 0.47 a |
| C20:5(n-3) | 4.89 ± 0.71 a | 3.81 ± 0.20 a | 2.44 ± 0.22 b |
| C22:4(n-6) | --------- | --------- | --------- |
| C22:5(n-6) | 0.60 ± 0.09 b | 1.00 ± 0.11 b | 1.89 ± 0.47 a |
| C22:5(n-3) | 4.12 ± 0.70 c | 6.22 ± 0.60 b | 7.52 ± 0.70 a |
| C22:6(n-3) | 4.78 ± 0.31 a | 3.83 ± 0.54 a | 2.77 ± 0.40 c |
| Less Polluted Site (LP) | |||
|---|---|---|---|
| Fatty Acids | H. molitrix | C. idella | C. carpio |
| SFAs | |||
| C8:0 | -------- | ---------- | ------------- |
| C10:0 | 1.02 ± 0.07 a | 0.41 ± 0.01a b | 1.44 ± 0.03 a |
| C12:0 | 0.01 ± 0.00 a b | 0.01± 0.03 a | 0.74 ± 0.06 a |
| C14:0 | 1.54 ± 0.04 a | 1.37 ± 0.03 a | 0.53 ± 0.02 b |
| C16:0 | 41.22 ± 2.70 a | 37.66 ± 2.61 a | 43.98 ± 2.14 a b |
| C18:0 | 0.47 ± 0.05 b | 24.66 ± 3.40 a b | 29.51 ± 2.67 b |
| C20:0 | ----------- | ------------ | 1.45 ± 0.03 |
| MUFAs | |||
| C16:1(n-7) | 0.44 ± 0.04 a | ---------- | ---------- |
| C16:1(n-9) | 0.71 ± 0.10 a b | 0.79 ± 0.02 b | 0.29 ± 0.01 a b |
| C18:1(n-7) | 0.14 ± 0.08 b | 0.08 ± 0.01a b | 0.40 ± 0.04 b |
| C18:1(n-9) | 3.95 ± 0.33 b | 4.79 ± 0.04 a | 0.11 ± 0.02 a b |
| C20:1(n-9) | 0.77 ± 0.03 | ---------- | ----------- |
| C22:1(n-9) | 0.90 ± 0.06 b | 0.41 ± 0.03 a | 0.32 ± 0.04 a b |
| PUFAs | |||
| C18:2(n-6) | 0.70 ± 0.06 a b | 1.29 ± 0.3 a | 0.41 ± 0.07 a b |
| C18:3(n-3) | 12.66 ± 0.79 a | 3.82 ± 0.04 a | 0.003 ± 0.00 a b |
| C18:4(n-3) | 3.24 ± 0.19 a | 0.44 ± 0.05 a | 2.60 ± 0.02 a b |
| C20:2(n-6) | ----------- | --------------- | --------------- |
| C20:4(n-6) | 0.87 ± 0.09 a | 0.39 ± 0.02 a | 0.82 ± 0.01 a |
| C20:5(n-6) | 12.06 ± 0.54 a | 8.02 ± 0.24 a | 9.16 ± 0.42 a |
| C20:5(n-3) | 2.42± 0.30 b | 0.37 ± 0.18 c | 3.61 ± 0.22 a |
| C22:4(n-6) | --------- | ----------- | ---------- |
| C22:5(n-6) | 16.77 ± 0.66 a | 4.70 ± 0.12 b | 5.44 ± 0.22 b |
| C22:5(n-3) | 1.17± 0.10 b | 0.02 ± 0.00 c | 1.88 ± 0.06 b |
| C22:6(n-3) | 4.02 ± 0.33 a b | 2.94 ± 0.22 a | 3.71 ± 0.14 a b |
| Highly polluted site (HP) | |||
| Fatty acids | H. moiltrix | C. idella | C. carpio |
| SFAs | |||
| C8:0 | ---------- | ---------- | ---------- |
| C10:0 | 0.18 ± 0.03 c | 1.97 ± 0.11 b | 6.93 ± 0.77 a |
| C12:0 | ---------- | --------- | --------- |
| C14:0 | 0.44 ± 0.05 c | 1.83 ± 0.21 b | 2.65 ± 0.22 a |
| C16:0 | 34.25 ± 4.66 c | 44.25 ±5.26 b | 50.12 ± 4.77 a |
| C18:0 | 27.18 ± 3.16 b | 35.69 ± 3.75 a | 12.24 ± 1.77 c |
| C20:0 | ---------- | 0.25 ± 0.01 | --------- |
| MUFAs | |||
| C16:1(n-7) | --------- | --------- | --------- |
| C16:1(n-9) | --------- | --------- | --------- |
| C18:1(n-7) | 5.22 ± 0.52 | --------- | --------- |
| C18:1(n-9) | 0.62 ± 0.06 a | --------- | 0.49 ± 0.01 a |
| C20:1(n-9) | --------- | --------- | --------- |
| C22:1(n-9) | 0.32 ± 0.02 c | 5.61 ± 0.17 a | 4.11 ± 0.22 b |
| PUFAs | |||
| C18:2(n-6) | --------- | --------- | --------- |
| C18:3(n-3) | 16.55 ± 0.54 a | 3.66 ± 0.32 b | 0.24 ± 0.01 c |
| C18:4(n-3) | --------- | --------- | --------- |
| C20:2(n-6) | --------- | 0.87 ± 0.11 a | 0.59 ± 0.02 b |
| C20:4(n-6) | 4.72 ± 0.33 b | 7.42 ± 0.22 a | 3.13 ± 0.07 c |
| C20:5(n-6) | --------- | --------- | 0.92 ± 0.11 |
| C20:5(n-3) | 0.68± 0.07 | --------- | --------- |
| C22:4(n-6) | --------- | --------- | --------- |
| C22:5(n-6) | 0.52 ± 0.11 b | 0.17 ± 0.01 c | 0.88 ± 0.11 a |
| C22:5(n-3) | 6.12 ± 0.44 a | 4.12 ± 0.22 b | --------- |
| C22:6(n-3) | 3.62 ± 0.11 a | 0.21 ± 0.01 c | 1.76 ± 0.22 b |
| Non-polluted site (NP) | |||
| Fatty acids | H. moiltrix | C. idella | C. carpio |
| SFAs | |||
| C8:0 | 0.80 ± 0.05 a | 0.01 ± 0.01 a | ---------- |
| C10:0 | 0.31 ± 0.00 b | 3.15 ± 0.03 a | 3.67 ± 0.08 a |
| C12:0 | 1.21± 0.15 c | 3.10 ± 0.40 a | 2.16 ± 0.05 b |
| C14:0 | 0.45 ± 0.01 c | 4.68 ± 0.55 b | 7.79± 0.44 a |
| C16:0 | 17.10 ± 0.61 a | 14.78 ± 1.12 b | 12.42 ± 0.55 b |
| C18:0 | 6.01 ± 0.41 a | 3.80 ± 0.25 c | 8.90 ± 0.75 a |
| C20:0 | 1.42 ± 0.17 a | 0.32 ± 0.01 b | 0.38 ± 0.01 b |
| MUFAs | |||
| C16:1(n-7) | -------- | -------- | 0.72 ± 0.04 |
| C16:1(n-9) | 4.75 ± 0.16 a | 2.41 ± 0.04 b | 2.77 ± 0.05 b |
| C18:1(n-7) | 4.81 ± 0.45 a | 3.60 ± 0.02 b | 1.81 ± 0.11 b |
| C18:1(n-9) | 13.12 ± 0.82 a | 12.87 ± 0.66 a | 10.94 ± 0.42 a |
| C20:1(n-9) | 1.44 ± 0.07 b | 4.14 ± 0.51 a | 3.88 ± 0.22 a |
| C22:1(n-9) | 0.01 ± 0.00 c | 0.61 ± 0.01 a | 0.70 ± 0.02 a |
| PUFAs | |||
| C18:2(n-6) | 4.07 ± 0.06 a | 3.42 ± 0.11 a | 0.01 ± 0.00 b |
| C18:3(n-3) | 3.44 ± 0.23 b | 3.22 ± 0.22 b | 5.94 ± 0.41 a |
| C18:4(n-3) | 2.41 ± 0.11 a | 2.05 ± 0.07 a | 0.71 ± 0.02 b |
| C20:2(n-6) | 0.91 ± 0.02 a | 0.88 ± 0.01 a | 0.57 ± 0.02 b |
| C20:4(n-6) | 14.26 ± 0.87 a | 12.14 ± 0.28 b | 9.87 ± 0.71 c |
| C20:5(n-6) | 6.44 ± 0.28 a | 4.12 ± 0.20 b | 0.21 ± 0.01 c |
| C20:5(n-3) | 0.40 ± 0.01 c | 5.06 ± 0.31 b | 8.23 ± 0.60 a |
| C22:4(n-6) | 1.21 ± 0.02 a | 0.60 ± 0.00 b | 0.50 ± 0.00 b |
| C22:5(n-6) | 3.44 ± 0.32 b | 4.02 ± 0.09 b | 6.68 ± 0.25 a |
| C22:5(n-3) | 5.05 ± 0.33 a | 3.60 ± 0.22 b | 2.39 ± 0.04 c |
| Metals | HP-PP | LP-PP | NP-PP | HP-ZB | LP-ZB | NP-ZB | HP-HM | LP-HM | NP-HM | HP-GC | LP-GC | NP-GC | HP-CP | LP-CP | NP-CP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sn | −0.24 * | −0.13 | 0.07 | −0.37 * | −0.28 * | 0.05 | −0.46 ** | −0.24 * | 0.06 | −0.30 * | −0.31 * | 0.08 | −0.60 ** | −0.27 * | 0.05 |
| Cr | −0.56 ** | −0.33 * | 0.10 | −0.58 ** | −0.33 * | 0.02 | −0.33 * | −0.24 * | 0.04 | −0.58 ** | −0.31 * | −0.07 | −0.54 ** | −0.28 * | −0.11 |
| P b | −0.37 * | −0.26 * | −0.06 | −0.58 ** | −0.28 * | −0.05 | −0.44 ** | −0.25 * | 0.03 | −0.40 ** | −0.22 * | 0.01 | −0.560 ** | −0.27 * | −0.01 |
| Zn | −0.51 ** | −0.23 * | 0.03 | −0.53 ** | −0.25 * | −0.07 | −0.34 * | −0.23 * | 0.01 | −0.28 * | −0.27 * | −0.03 | −0.57 ** | −0.24 * | 0.09 |
| Mn | −0.60 ** | −0.28 * | 0.02 | 0.56 ** | −0.27 * | −0.09 | −0.48 ** | −0.24 * | −0.02 | −0.64 ** | −0.26 * | 0.04 | −0.57 ** | 0.28 * | −0.05 |
| Cu | −0.60 ** | −0.27 * | 0.05 | −0.57 ** | −0.44 ** | −0.08 | −0.53 ** | −0.25 * | 0.03 | −0.37 * | −0.28 * | −0.06 | −0.57 ** | −0.29 * | 0.04 |
| Cd | −0.27 * | −0.24 * | 0.10 | −0.33 * | −0.25 * | −0.07 | −0.41 * | −0.27 * | −0.12 | −0.42 ** | −0.28 * | 0.02 | −0.51 ** | −0.25 * | −0.05 |
| Hg | −0.34 * | −0.22 * | 0.01 | −0.27 * | 0.12 | 0.01 | −0.28 * | −0.22 * | 0.004 | −0.35 * | −0.27 * | −0.01 | −0.48 ** | −0.26 * | 0.003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Shahid, T.; Sultana, S.; Sultan, T.; Hussain, B.; Ahmed, Z. Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem. Biomolecules 2019, 9, 231. https://doi.org/10.3390/biom9060231
Mahboob S, Al-Ghanim KA, Al-Misned F, Shahid T, Sultana S, Sultan T, Hussain B, Ahmed Z. Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem. Biomolecules. 2019; 9(6):231. https://doi.org/10.3390/biom9060231
Chicago/Turabian StyleMahboob, Shahid, Khalid Abdullah Al-Ghanim, Fahad Al-Misned, Tehniat Shahid, Salma Sultana, Tayyaba Sultan, Bilal Hussain, and Zubair Ahmed. 2019. "Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem" Biomolecules 9, no. 6: 231. https://doi.org/10.3390/biom9060231
APA StyleMahboob, S., Al-Ghanim, K. A., Al-Misned, F., Shahid, T., Sultana, S., Sultan, T., Hussain, B., & Ahmed, Z. (2019). Impact of Water Pollution on Trophic Transfer of Fatty Acids in Fish, Microalgae, and Zoobenthos in the Food Web of a Freshwater Ecosystem. Biomolecules, 9(6), 231. https://doi.org/10.3390/biom9060231





