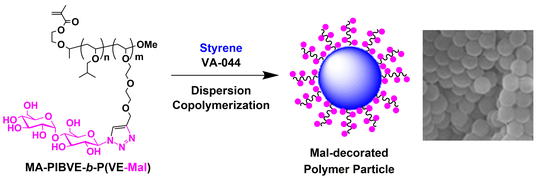
Protecting Group-Free Synthesis of Glycopolymer-Type Amphiphilic Macromonomers and Their Use for the Preparation of Carbohydrate-Decorated Polymer Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Methods
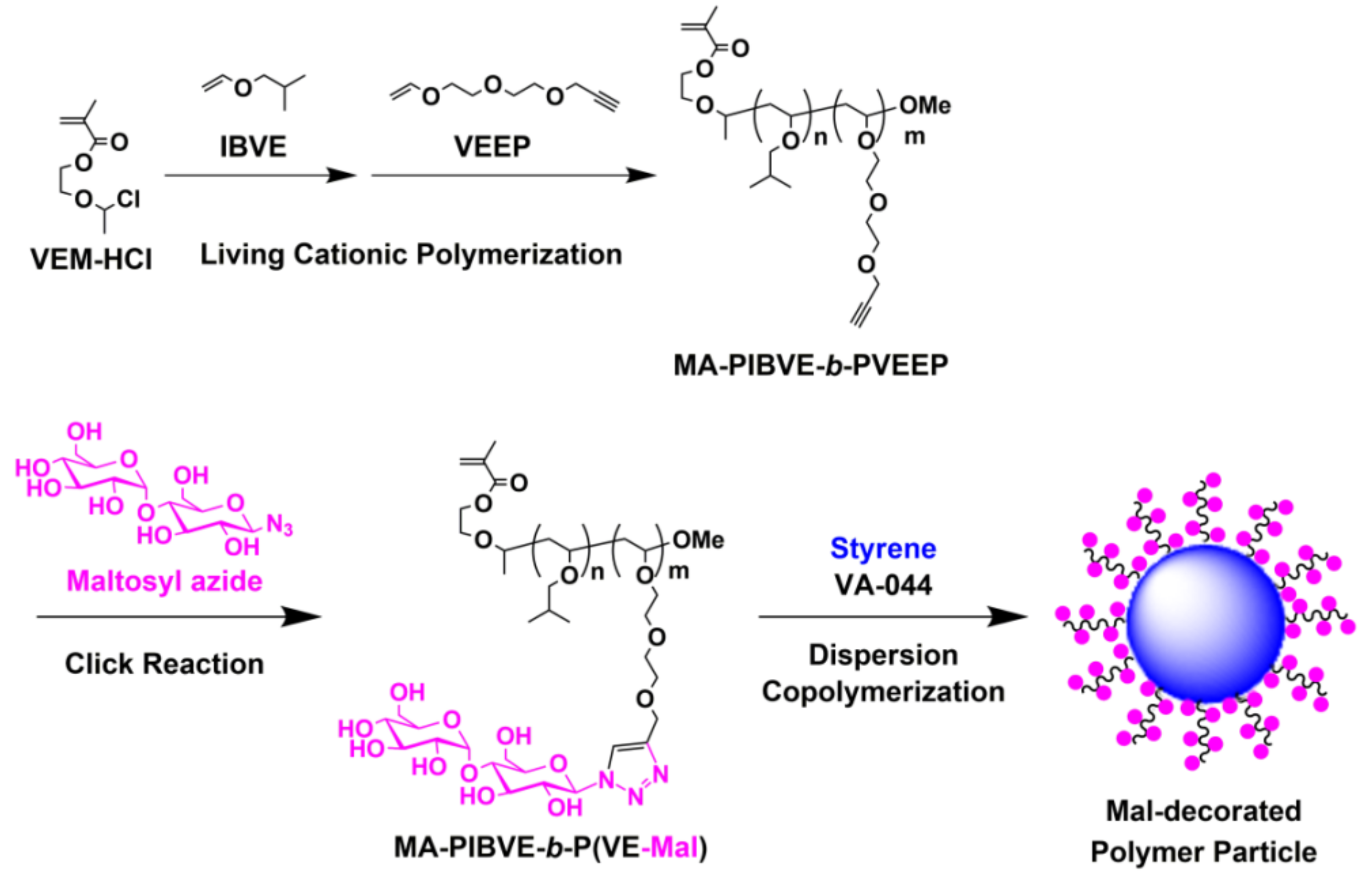
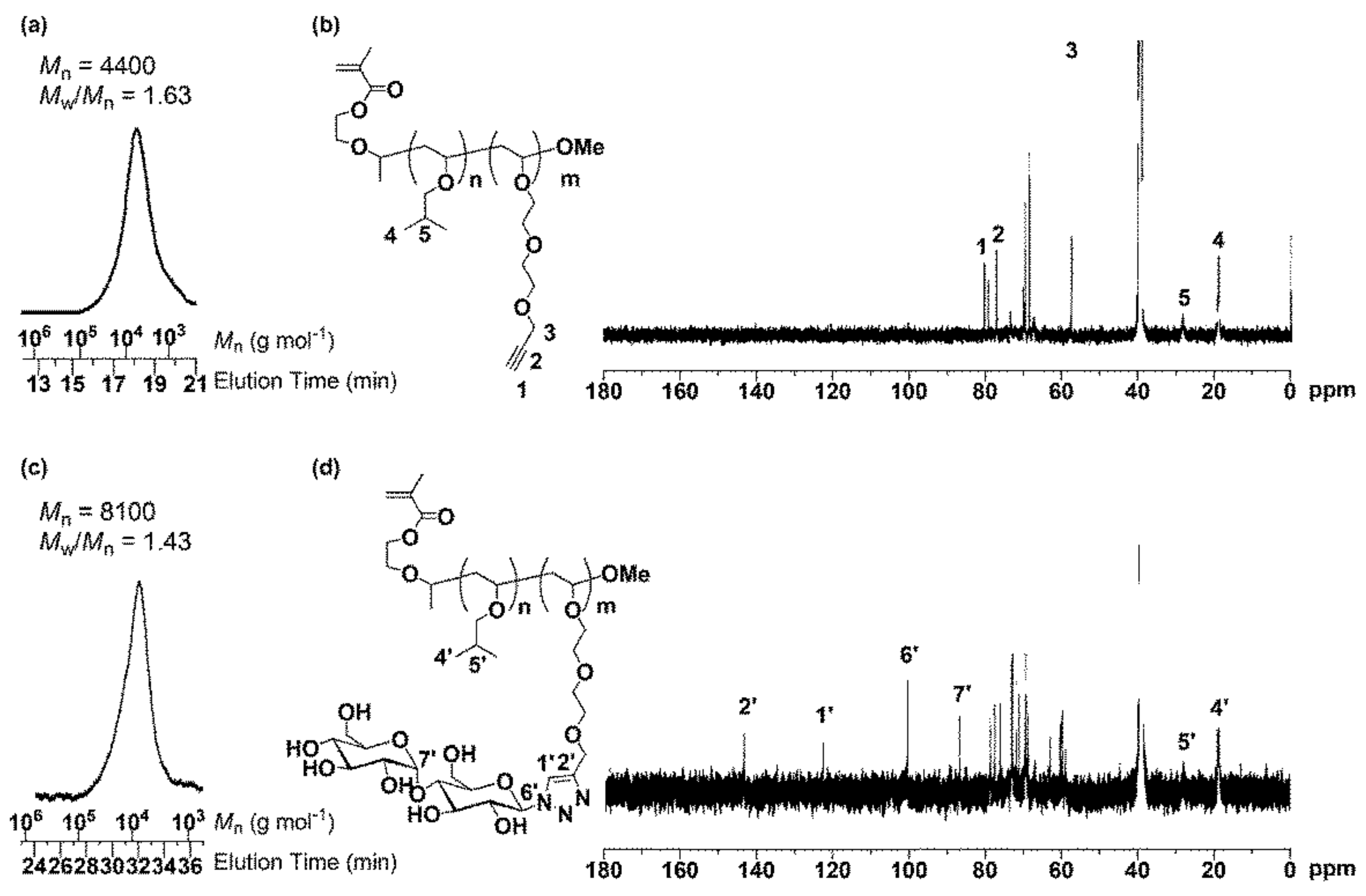
2.3. Synthesis of MA-PIBVE-b-PVEEP
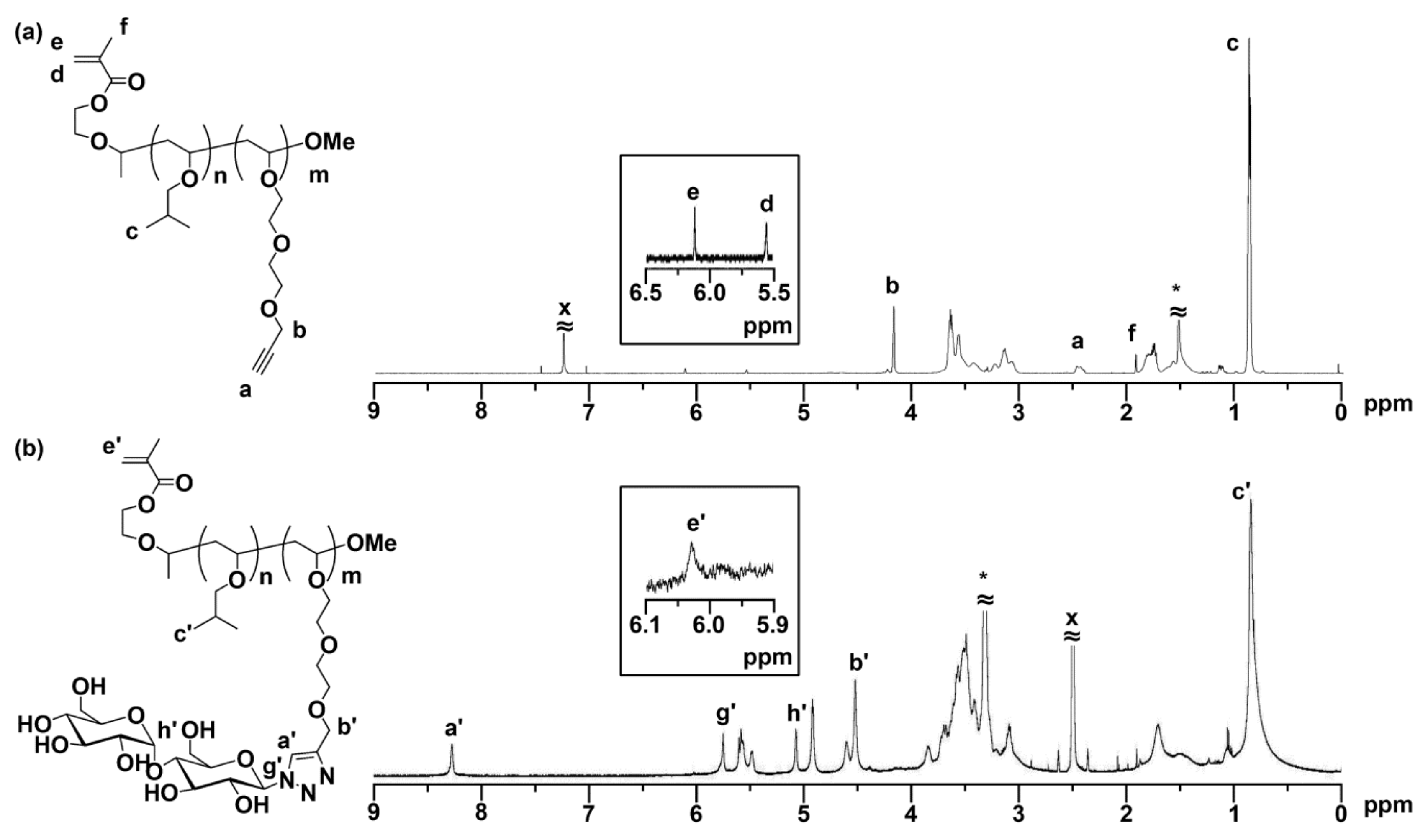
2.4. Synthesis of MA-PIBVE-b-P(VE-Mal)
2.5. Preparation of Mal-Decorated Polymer Particles
2.6. Lectin Binding Assay
2.7. Determination of Maltose Density on Polymer Particles
3. Results and Discussion
3.1. Synthesis of MA-PIBVE-b-PVEEP
3.2. Synthesis of MA-PIBVE-b-P(VE-Mal)
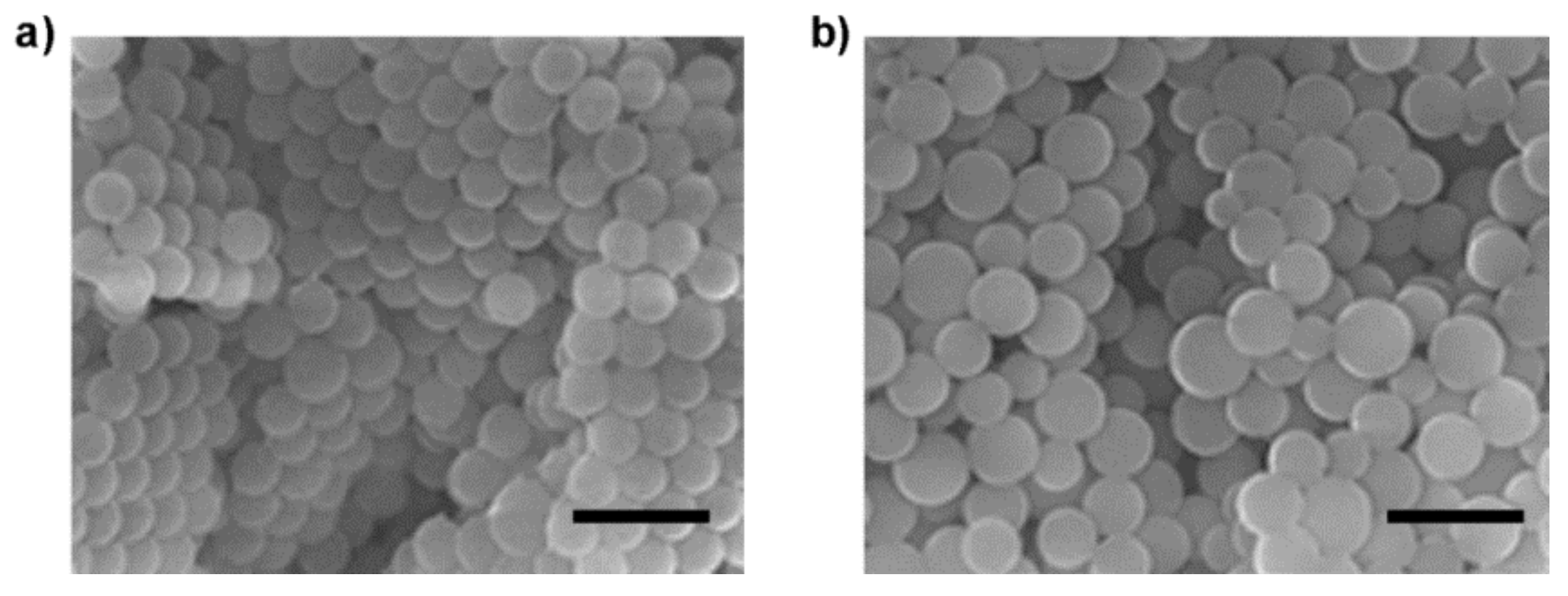
3.3. Preparation of Mal-Decorated Polymer Particles
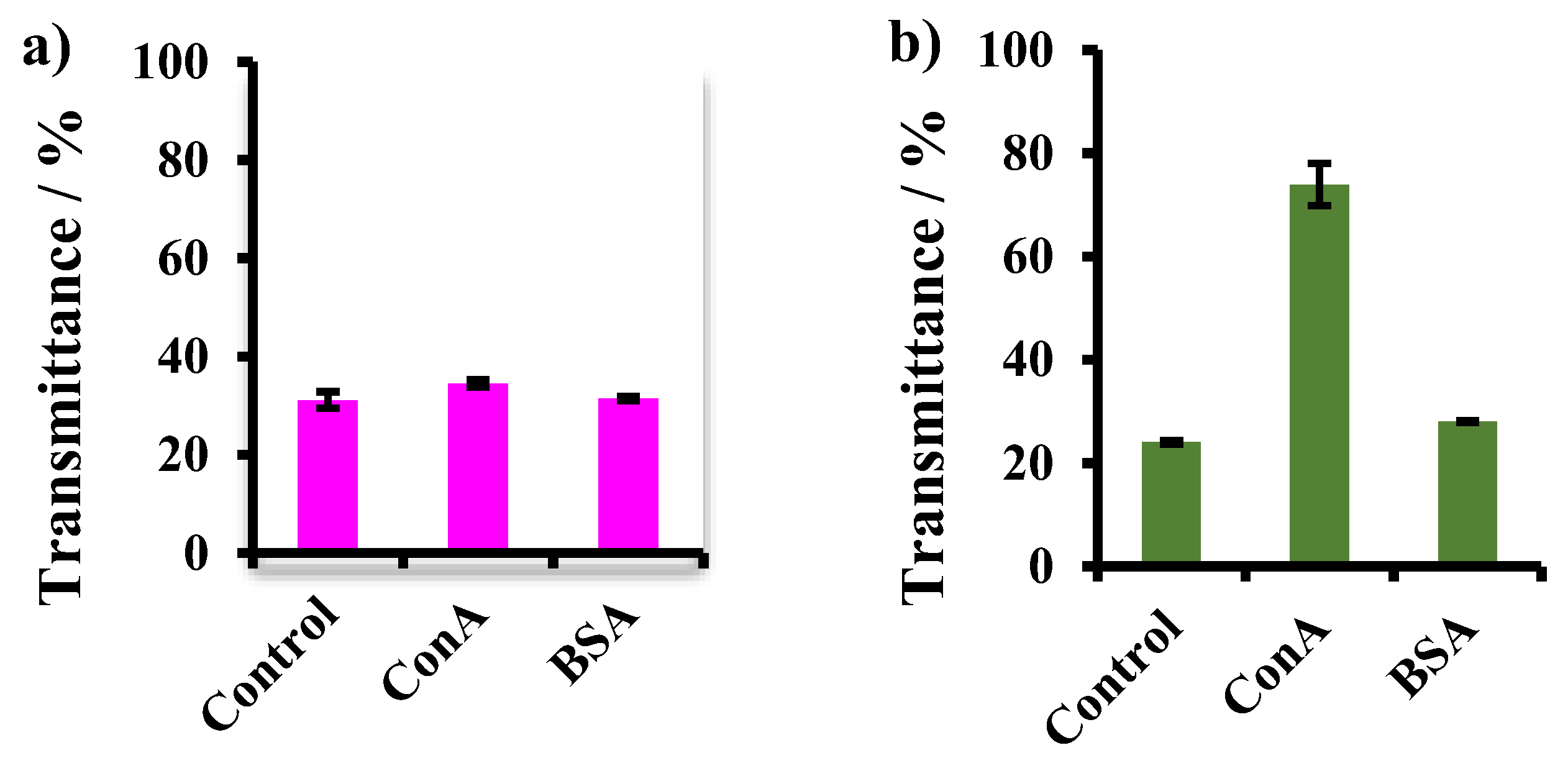
3.4. Lectin Binding Assay and Estimation of Maltose Density
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Itoh, T.; Kojima, K.; Shimomoto, H.; Ihara, E. Control of lengths and densities of surface-attached chains on polymer particles prepared by dispersion polymerization using macromonomer stabilizer. Polymer. 2018, 158, 158–165. [Google Scholar] [CrossRef]
- Kawaguchi, H. Functional polymer microspheres. Prog. Polym. Sci. 2000, 25, 1171–1210. [Google Scholar] [CrossRef]
- Barner, L. Synthesis of microspheres as versatile functional scaffolds for materials science applications. Adv. Mater. 2009, 21, 2547–2553. [Google Scholar] [CrossRef]
- García, I.; Gallo, J.; Marradi, M.; Penadés, S. Glyconanoparticles: New Nanoparticles for Biological Applications. In Engineered Carbohydrate-Based Materials for Biomedical Applications; Narain, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 213–259. ISBN 978-0-470-47235-4. [Google Scholar]
- De La Fuente, J.M.; Barrientos, A.G.; Rojas, T.C.; Rojo, J.; Cañada, J.; Fernández, A.; Penadés, S. Gold Glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew. Chem. Int. Ed. 2001, 40, 2258–2261. [Google Scholar] [CrossRef]
- Housni, A.; Cai, H.; Liu, S.; Pun, S.H.; Narain, R. Facile preparation of glyconanoparticles and their bioconjugation to streptavidin. Langmuir 2007, 23, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Sato, M.; Abo, F.; Inada, T.; Kasuya, M.; Taniguchi, T.; Nakahira, T. Preparation and lectin binding specificity of polystyrene particles grafted with glycopolymers bearing S-linked carbohydrates. Eur. Polym. J. 2011, 47, 2351–2360. [Google Scholar] [CrossRef]
- Amalvy, J.L.; Wanless, E.J.; Li, Y.; Michailidou, V.; Armes, S.P.; Duccini, Y. Synthesis and Characterization of Novel pH-responsive microgels based on tertiary amine methacrylates. Langmuir 2004, 20, 8992–8999. [Google Scholar] [CrossRef] [PubMed]
- Elsesser, M.T.; Hollingsworth, A.D. Revisiting the synthesis of a well-known comb-graft copolymer stabilizer and its application to the dispersion polymerization of poly(methyl methacrylate) in organic media. Langmuir 2010, 26, 17989–17996. [Google Scholar] [CrossRef] [PubMed]
- McKee, J.R.; Ladmiral, V.; Niskanen, J.; Tenhu, H.; Armes, S.P. Synthesis of sterically-stabilized polystyrene latexes using well-defined thermoresponsive poly(N-isopropylacrylamide) macromonomers. Macromolecules 2011, 44, 7692–7703. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Nagura, K.; Sawada, K.; Miki, S.; Minoda, M. Synthesis of an amphiphilic macromonomer bearing a terminal fluorene moiety and its application to the preparation of fluorescent polymer particles. Chem. Lett. 2012, 41, 1487–1488. [Google Scholar] [CrossRef]
- Motoyanagi, J.; Tan, N.M.; Minoda, M. Synthesis of well-defined poly(vinyl ether)-based macromonomers having pendant glycerols via living cationic polymerization and their application to the preparation of core−shell polymer particles. Polym. Int. 2014, 63, 459–465. [Google Scholar] [CrossRef]
- Bonilla, A.M.; Heuts, J.P.; Garcia, M.F. Glycoparticles and bioactive films prepared by emulsion polymerization using a well-defined block glycopolymer stabilizer. Soft Matter 2011, 7, 2493–2499. [Google Scholar] [CrossRef]
- Tanaka, T.; Nguyen, M.T.; Minoda, M. Amphiphilic glycopolymer-type macromonomers for the preparation of carbohydrate-decorated polymer particles. Chem. Lett. 2018, 47, 1519–1521. [Google Scholar] [CrossRef]
- Geng, J.; Mantovani, G.; Tao, L.; Nicolas, J.; Chen, G.; Wallis, R.; Mitchell, D.A.; Johnson, B.R.G.; Evans, S.D.; Haddleton, D.M. Site-directed conjugation of “clicked” glycopolymers to form glycoprotein mimics: Binding to mammalian lectin and induction of immunological function. J. Am. Chem. Soc. 2007, 129, 15156–15163. [Google Scholar] [CrossRef] [PubMed]
- Iha, R.K.; Wooley, K.L.; Nyström, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of orthogonal “click” chemistries in the synthesis of functional soft materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.R.S.; Chen, G.; Stenzel, M.H. Synthesis of glycopolymers and their multivalent recognitions with lectins. Polym. Chem. 2010, 1, 1392–1412. [Google Scholar] [CrossRef]
- Ouchi, M.; Terashima, T.; Sawamoto, M. Transition metal-catalyzed living radical polymerization: Toward Perfection in catalysis and precision polymer synthesis. Chem. Rev. 2009, 109, 4963–5050. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Davis, T.P.; Ladmiral, V.; Liu, J.; Perrier, S.B. Bioapplications of RAFT polymerization. Chem. Rev. 2009, 109, 5402–5436. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- Yamada, K.; Minoda, M.; Miyamoto, T. Controlled synthesis of glycopolymers with pendant d-glucosamine residues by living cationic polymerization. J. Polym. Sci. Part A: Polym. Chem. 1997, 35, 751–757. [Google Scholar] [CrossRef]
- Yamada, K.; Minoda, M.; Miyamoto, T. Controlled synthesis of amphiphilic block copolymers with pendant N-acetyl-d-glucosamine residues by living cationic polymerization and their interaction with WGA lectin. Macromolecules 1999, 32, 3553–3558. [Google Scholar] [CrossRef]
- Yamada, K.; Minoda, M.; Fukuda, T.; Miyamoto, T. Amphiphilic block and statistical copolymers with pendant glucose residues: Controlled synthesis by living cationic polymerization and the effect of copolymer architecture on their properties. J. Polym. Sci. Part A: Polym. Chem. 2001, 39, 459–467. [Google Scholar] [CrossRef]
- Dai, X.H.; Wang, Z.M.; Huang, Y.F.; Pan, J.M.; Yan, Y.S.; Liu, D.M.; Sun, L. Biomimetic star-shaped poly(ε-caprolactone)-b-glycopolymer block copolymers with porphyrin-core for targeted photodynamic therapy. RSC Adv. 2014, 4, 42486–42493. [Google Scholar] [CrossRef]
- Yamada, K.; Miyazaki, M.; Ohno, K.; Fukuda, T.; Minoda, M. Atom transfer radical polymerization of poly(vinyl ether) macromonomers. Macromolecules 1999, 32, 290–293. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S. One-step conversion of unprotected sugars to β-glycosyl azides using 2-chloroimidazolinium salt in aqueous solution. Chem. Commun. 2009, 3378–3379. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishitani, H.; Miura, Y.; Oishi, K.; Takahashi, T.; Suzuki, T.; Shoda, S.; Kimura, Y. Protecting-group-free synthesis of glycopolymers bearing sialyloligosaccharide and their high binding with the influenza virus. ACS Macro Lett. 2014, 3, 1074–1078. [Google Scholar] [CrossRef]
- Tan, N.M.; Mori, R.; Tanaka, T.; Motoyanagi, J.; Minoda, M. Living cationic polymerization of a vinyl ether with an unprotected pendant alkynyl group and their use for the protecting group-free synthesis of macromonomer-type glycopolymers via CuAAC with maltosyl azides. J. Polym. Sci. A Polym. Chem. 2019, accepted. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate–protein interactions: Basis of glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
| Entry | Precursor Macromonomer | CuAAC Click Reaction Product | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DPn of PIBVE 1 | DPn of PVEEP 1 | Yield (%) 4 | Degree of Substitution (%) 1 | |||||||
| 1 | P1 | 4400 | 1.63 | 25 | 10 | P3 | 8100 | 1.43 | 20 | Quant. |
| 2 | P2 | 7800 | 1.60 | 25 | 30 | P4 | 18,800 | 2.13 | 15 | Quant. |
| Particle | Yield (%) 1 | Dn (nm) 2 | PDI 2 |
|---|---|---|---|
| P3-Particle | 55 | 460 ± 80 | 1.19 |
| P4-Particle | 55 | 550 ± 110 | 1.24 |
| Particle | Absorbance at 630 nm 1 | Concentration of Free Glucose (μg/mL) | Amount of Free Glucose (mg/8.1 mg) | Amount of Maltose (mg/8.1 mg) | Dn (nm) 2 | Surface Area of the Polymer Particle (cm2) × 10−10 | Surface Density of Maltose Moieties on the Particles (µg/cm2) |
|---|---|---|---|---|---|---|---|
| P3-Particle | 0.328 | 23.8 | 0.298 | 0.595 | 480 | 18.0 | 1.90 |
| P4-Particle | 0.419 | 35.6 | 0.447 | 0.894 | 550 | 24.1 | 3.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motoyanagi, J.; Nguyen, M.T.; Tanaka, T.; Minoda, M. Protecting Group-Free Synthesis of Glycopolymer-Type Amphiphilic Macromonomers and Their Use for the Preparation of Carbohydrate-Decorated Polymer Particles. Biomolecules 2019, 9, 72. https://doi.org/10.3390/biom9020072
Motoyanagi J, Nguyen MT, Tanaka T, Minoda M. Protecting Group-Free Synthesis of Glycopolymer-Type Amphiphilic Macromonomers and Their Use for the Preparation of Carbohydrate-Decorated Polymer Particles. Biomolecules. 2019; 9(2):72. https://doi.org/10.3390/biom9020072
Chicago/Turabian StyleMotoyanagi, Jin, Minh Tan Nguyen, Tomonari Tanaka, and Masahiko Minoda. 2019. "Protecting Group-Free Synthesis of Glycopolymer-Type Amphiphilic Macromonomers and Their Use for the Preparation of Carbohydrate-Decorated Polymer Particles" Biomolecules 9, no. 2: 72. https://doi.org/10.3390/biom9020072
APA StyleMotoyanagi, J., Nguyen, M. T., Tanaka, T., & Minoda, M. (2019). Protecting Group-Free Synthesis of Glycopolymer-Type Amphiphilic Macromonomers and Their Use for the Preparation of Carbohydrate-Decorated Polymer Particles. Biomolecules, 9(2), 72. https://doi.org/10.3390/biom9020072