Morin as a Preservative for Delaying Senescence of Banana
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials and Reagents
2.2. Fruit Treatment
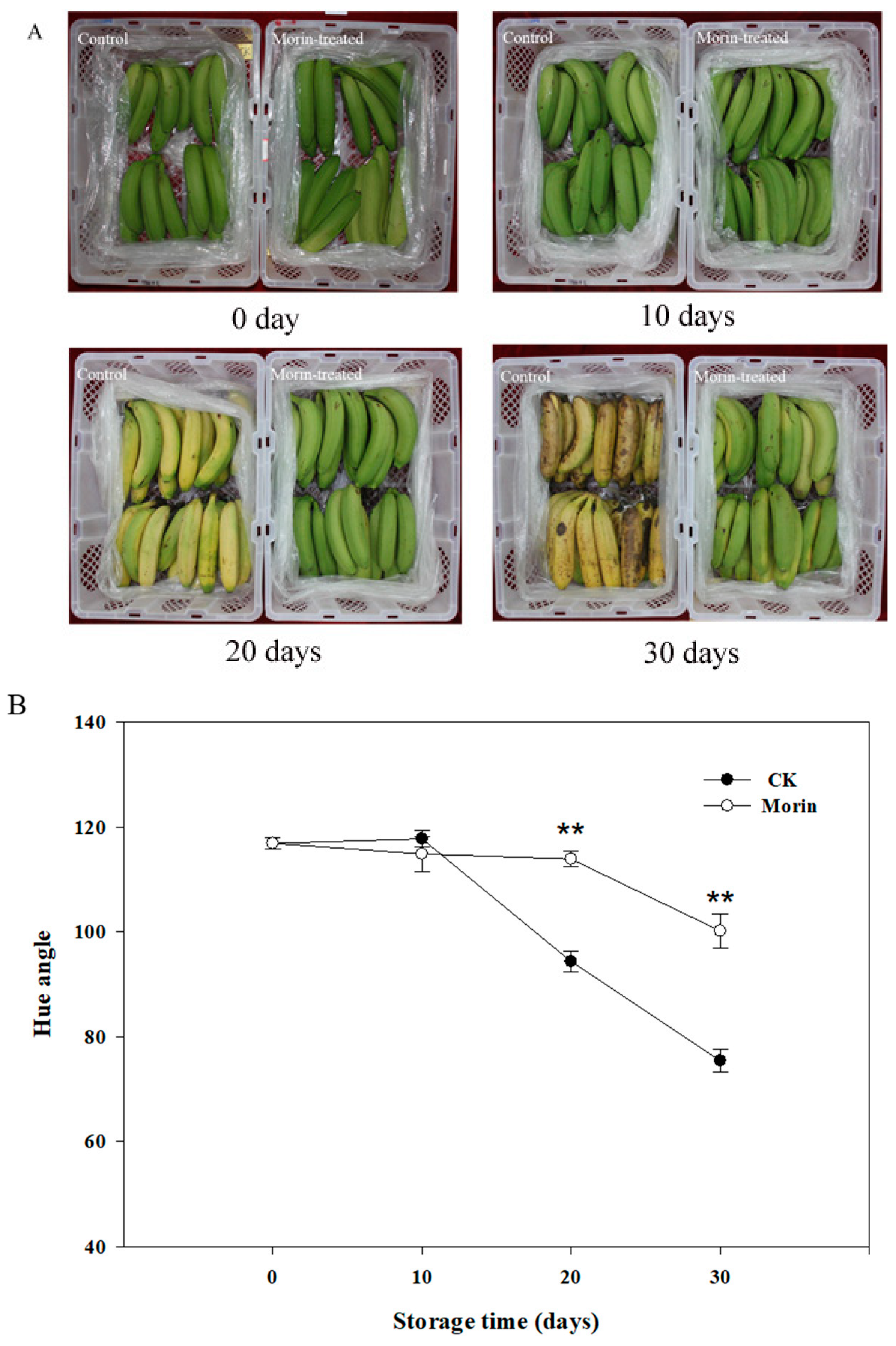
2.3. Measurement of Skin Color
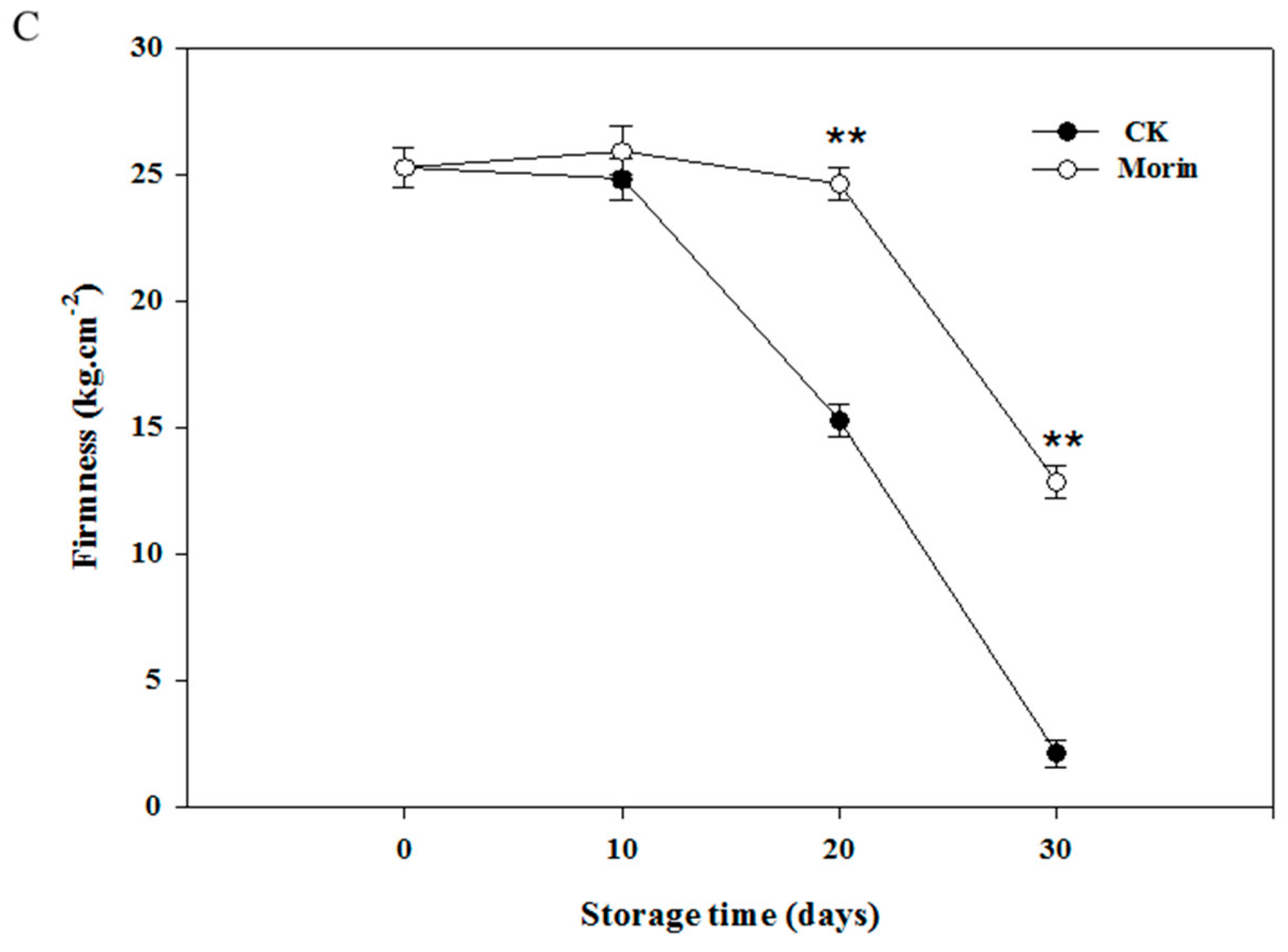
2.4. Determination of Fruit Firmness
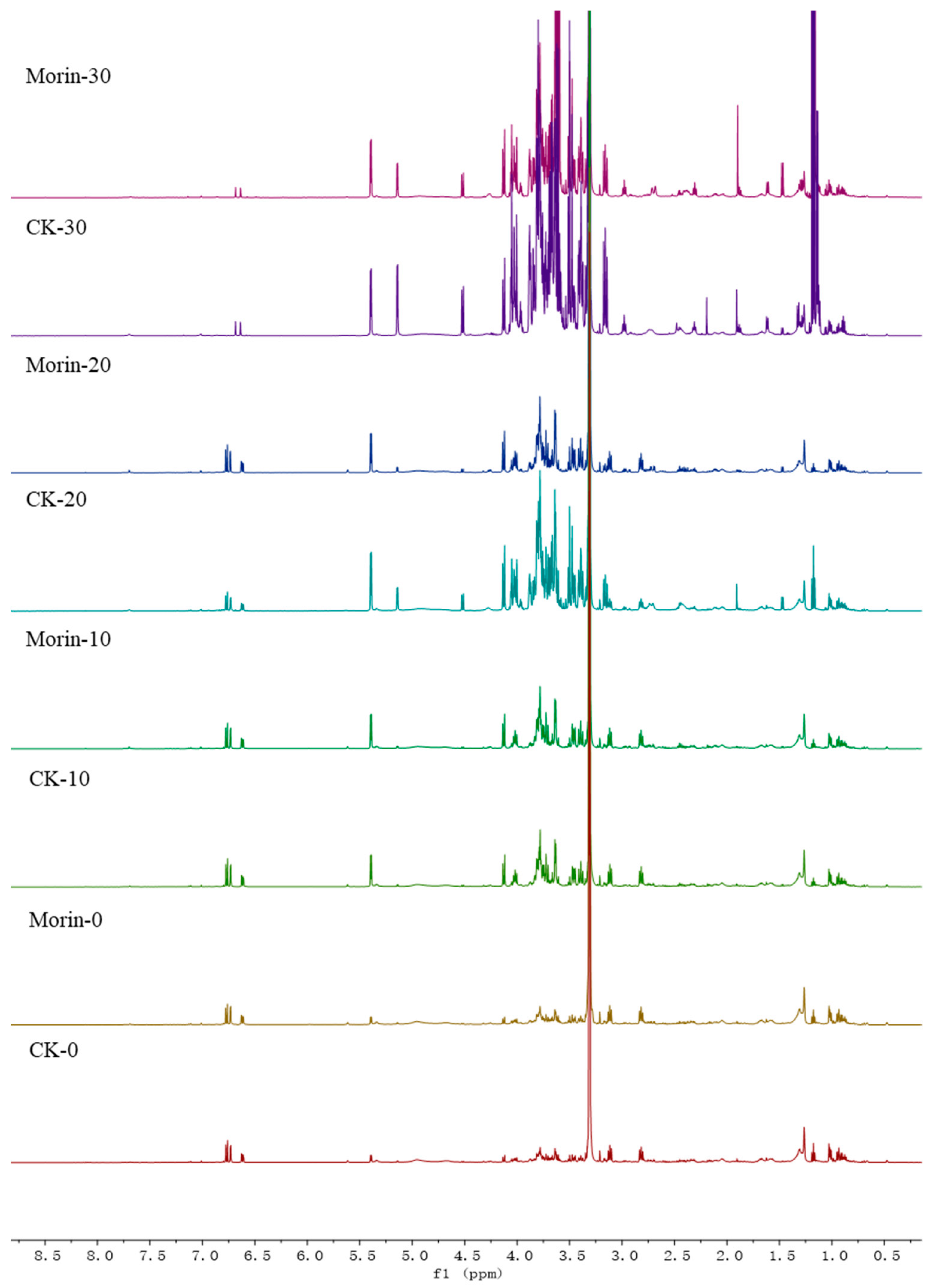
2.5. Analysis by NMR Spectroscopy
2.6. Processing and Analysis of NMR Data
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes of Hue Angle and Firmness
3.2. Identification of Metabolites in Banana Peel Extract by NMR
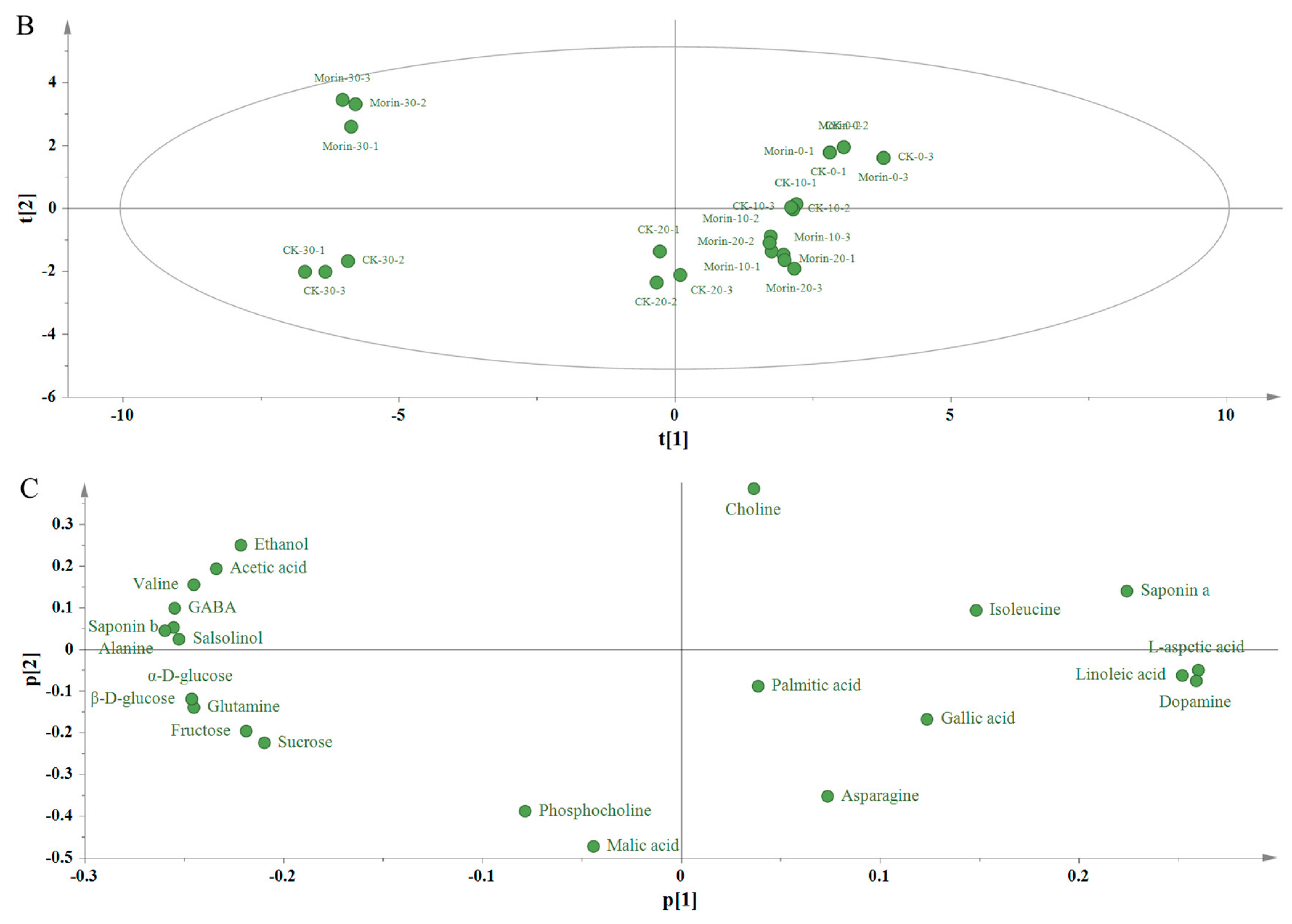
3.3. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.; Jing, G.X.; Wang, H.; Duan, X.W.; Qu, H.X.; Jiang, Y.M. The combined effects of phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Sci. Hortic. 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Best, R.; Lewis, D.A.; Nasser, N. The anti-ulcerogenic activity of the unripe plantain banana (musa species). Br. J. Pharmacol. 1984, 82, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Utrillacoello, R.G.; Agamaacevedo, E.; Osoriodíaz, P.; Tovar, J.; Bellopérez, L.A.; Tester, R. Composition and starch digestibility of whole grain bars containing maize or unripe banana flours. Starch-Starke 2011, 63, 416–423. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nadumane, V.K.; Timsina, B. Anti-cancer potential of banana flower extract: An in vitro study. Bangladesh J. Pharmacol. 2014, 9, 628–635. [Google Scholar] [CrossRef]
- Mohapatra, D.; Mishra, S.; Singh, C.B.; Jayas, D.S. Post-harvest processing of banana: Opportunities and challenges. Food Bioprocess Technol. 2011, 4, 327–339. [Google Scholar] [CrossRef]
- Ergun, M.; Sargent, S.A.; Fox, A.J.; Crane, J.H.; Huber, D.J. Ripening and quality responses of mamey sapote fruit to postharvest wax and 1-methylcyclopropene treatments. Postharvest Biol. Technol. 2005, 36, 127–134. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Joyce, D.C.; Macnish, A.J. Extension of the shelf life of banana fruit by 1-methylcyclopropene in combination with polyethylene bags. Postharvest Biol. Technol. 1999, 16, 187–193. [Google Scholar] [CrossRef]
- Boonyaritthongchai, P.; Kanlayanarat, S. Effect of 1-MCP treatment on the postharvest quality of banana fruit ‘kluai kai’. In Proceedings of the VI International Postharvest Symposium, Antalya, Turkey, 8–12 April 2009; Erkan, M., Aksoy, U., Eds.; 2010; Volume 877, pp. 359–364. [Google Scholar]
- Jung, H.J.; Kim, S.J.; Song, Y.S.; Park, E.H.; Lim, C.J. Evaluation of the antiangiogenic, anti-inflammatory, and antinociceptive activities of morin. Planta Med. 2010, 76, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Lee, H.S. Evaluation of antioxidant and antibacterial activities of morin isolated from mulberry fruits (Morus alba L.). J. Korean Soc. Appl. Biol. Chem. 2012, 55, 485–489. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Zhao, Y.P.; Yang, J.L.; Jiang, Y.M.; Lu, F.; Jia, Y.X.; Yang, B. Metabolomic analyses of banana during postharvest senescence by 1H-high resolution-NMR. Food Chem. 2017, 218, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, Y.F.; Wu, Q.X.; Zhao, Y.P.; Jiang, Y.M.; John, A.; Wen, L.R.; Li, T.T.; Jian, Q.J.; Yang, B. Analyses of quality and metabolites levels of okra during postharvest senescence by 1H-high resolution NMR. Postharvest Biol. Technol. 2017, 132, 171–178. [Google Scholar] [CrossRef]
- Viant, M.R.; Bearden, D.W.; Bundy, J.G.; Burton, I.W.; Collette, T.W.; Ekman, D.R.; Ezernieks, V.; Karakach, T.K.; Lin, C.Y.; Rochfort, S. International NMR-based environmental metabolomics intercomparison exercise. Environ. Sci. Technol. 2009, 43, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.M.; Klein, M.S.; Hochrein, J.; Oefner, P.J.; Spang, R.; Gronwald, W. State-of-the art data normalization methods improve NMR-based metabolomic analysis. Metabolomics 2012, 8, S146–S160. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Tapias, E.C.; Kim, H.K.; Lefeber, A.W.; Erkelens, C.; Verhoeven, J.T.; Brzin, J.; Zel, J.; Verpoorte, R. Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol. 2004, 135, 2398–2410. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Wu, D.; Jiang, Y.M.; Prasad, K.N.; Lin, S.; Jiang, G.X.; He, J.R.; Zhao, M.M.; Luo, W.; Yang, B. Identification of flavonoids in litchi (Litchi chinensis sonn.) leaf and evaluation of anticancer activities. J. Funct. Foods 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Riggin, R.M.; Mccarthy, M.J.; Kissinger, P.T. Identification of salsolinol as a major dopamine metabolite in the banana. J. Agric. Food Chem. 1976, 24, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochernical properties, and digestibility—A review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Lii, C.Y.; Chang, S.M.; Young, Y.L. Investigation of the physical and chemical properties of banana starches. J. Food Sci. 2010, 47, 1493–1497. [Google Scholar] [CrossRef]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar] [CrossRef] [PubMed]
- Leif, H. The glutamate–glutamine (GABA) cycle: Importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front. Endocrinol. 2013, 4, 308–315. [Google Scholar]
- Thompson, J.E.; Froese, C.D.; Madey, E.; Smith, M.D.; Hong, Y. Lipid metabolism during plant senescence. Prog. Lipid Res. 1998, 37, 119–141. [Google Scholar] [CrossRef]
- Herlt, A.J.; Mander, L.N.; Pongoh, E.; Rumampuk, R.J.; Tarigan, P. Two major saponins from seeds of Barringtonia asiatica: Putative antifeedants toward Epilachna sp. Larvae. J. Nat. Prod. 2002, 65, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Sakakibara, H. High content of dopamine, a strong antioxidant, in Cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Bressan, R.A.; Crippa, J.A. The role of dopamine in reward and pleasure behaviour—Review of data from preclinical research. Acta Psychiatr. Scand. 2005, 111, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zanassi, P.; Paolillo, M.; Montecucco, A.; Avvedimento, E.V.; Schinelli, S. Pharmacological and molecular evidence for dopamine D1 receptor expression by striatal astrocytes in culture. J. Neurosci. Res. 2015, 58, 544–552. [Google Scholar] [CrossRef]
- Juricic, M.A.; Berríos-Cárcamo, P.A.; Acevedo, M.L.; Israel, Y.; Almodóvar, I.; Cassels, B.K. Salsolinol and isosalsolinol: Condensation products of acetaldehyde and dopamine. Separation of their enantiomers in the presence of a large excess of dopamine. J. Pharm. Biomed. Anal. 2012, 63, 170–174. [Google Scholar] [CrossRef] [PubMed]
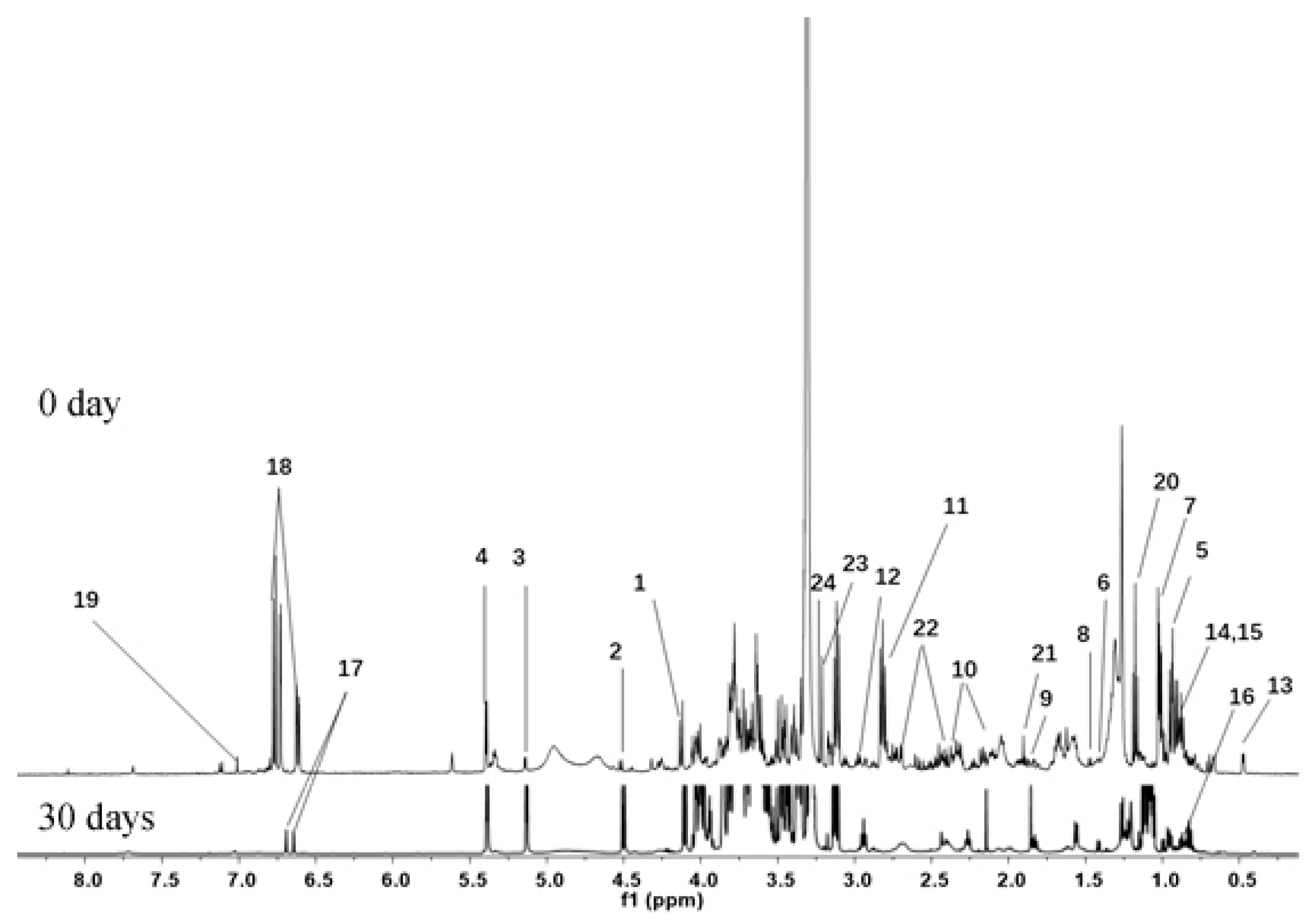
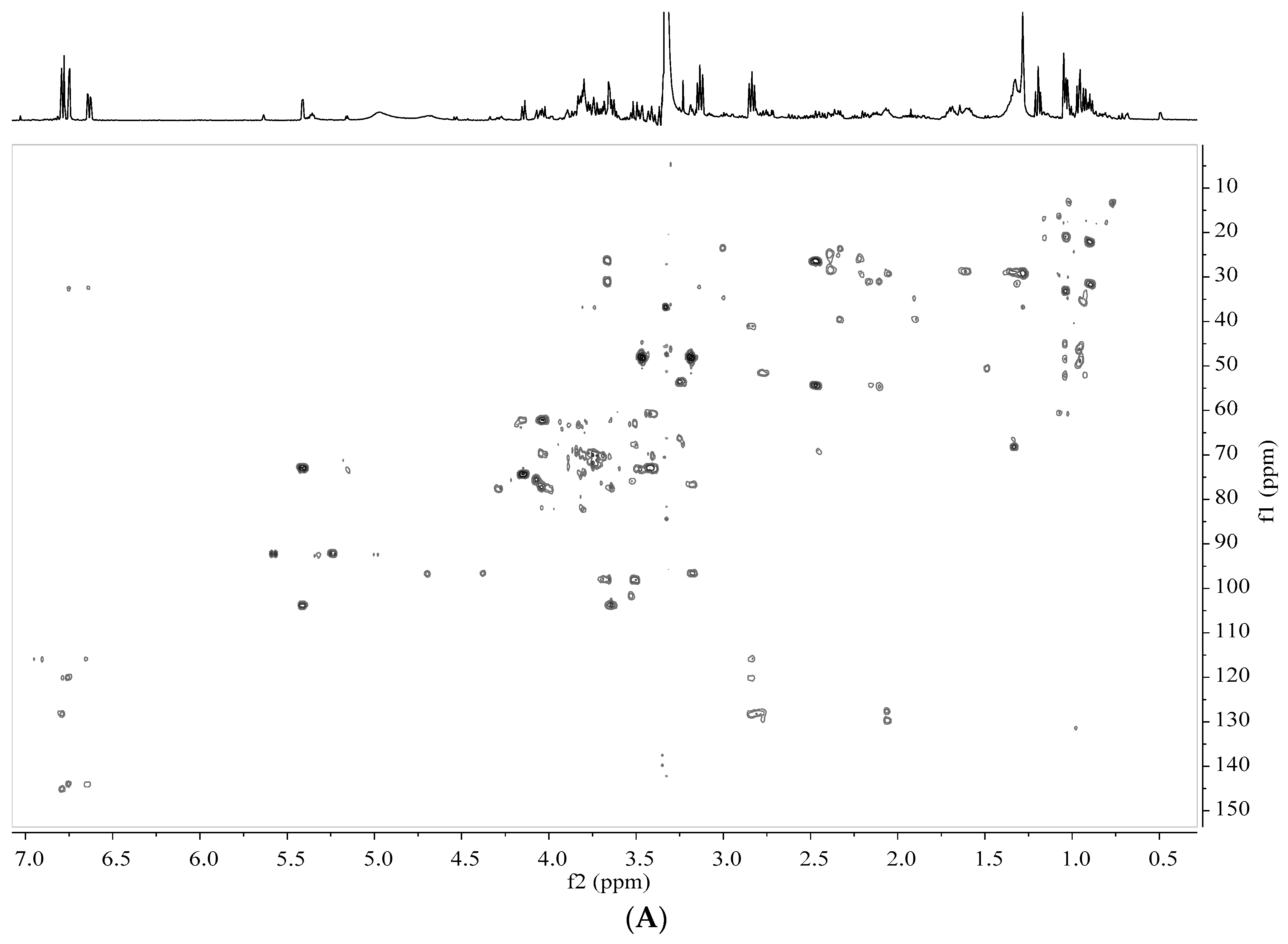
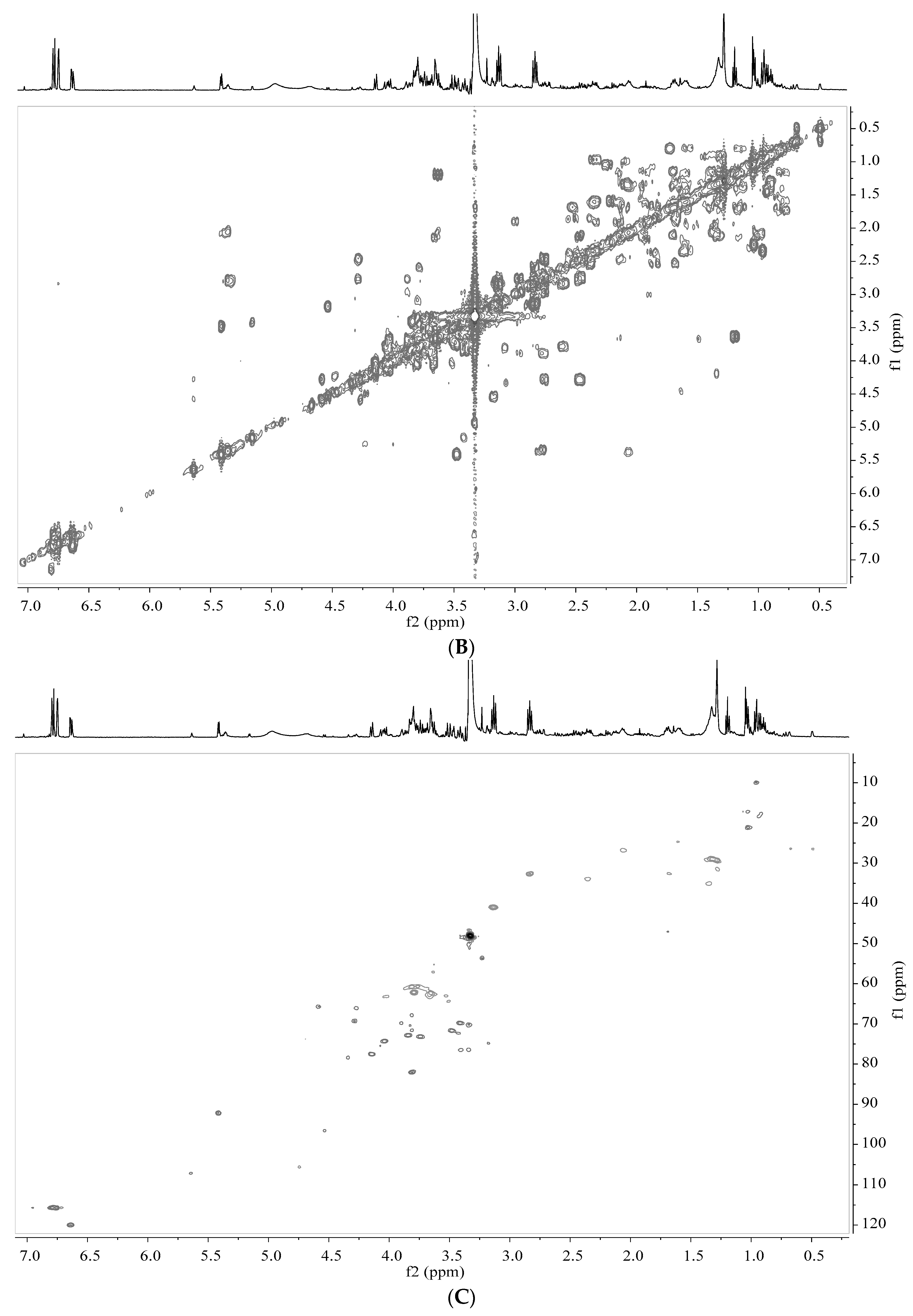
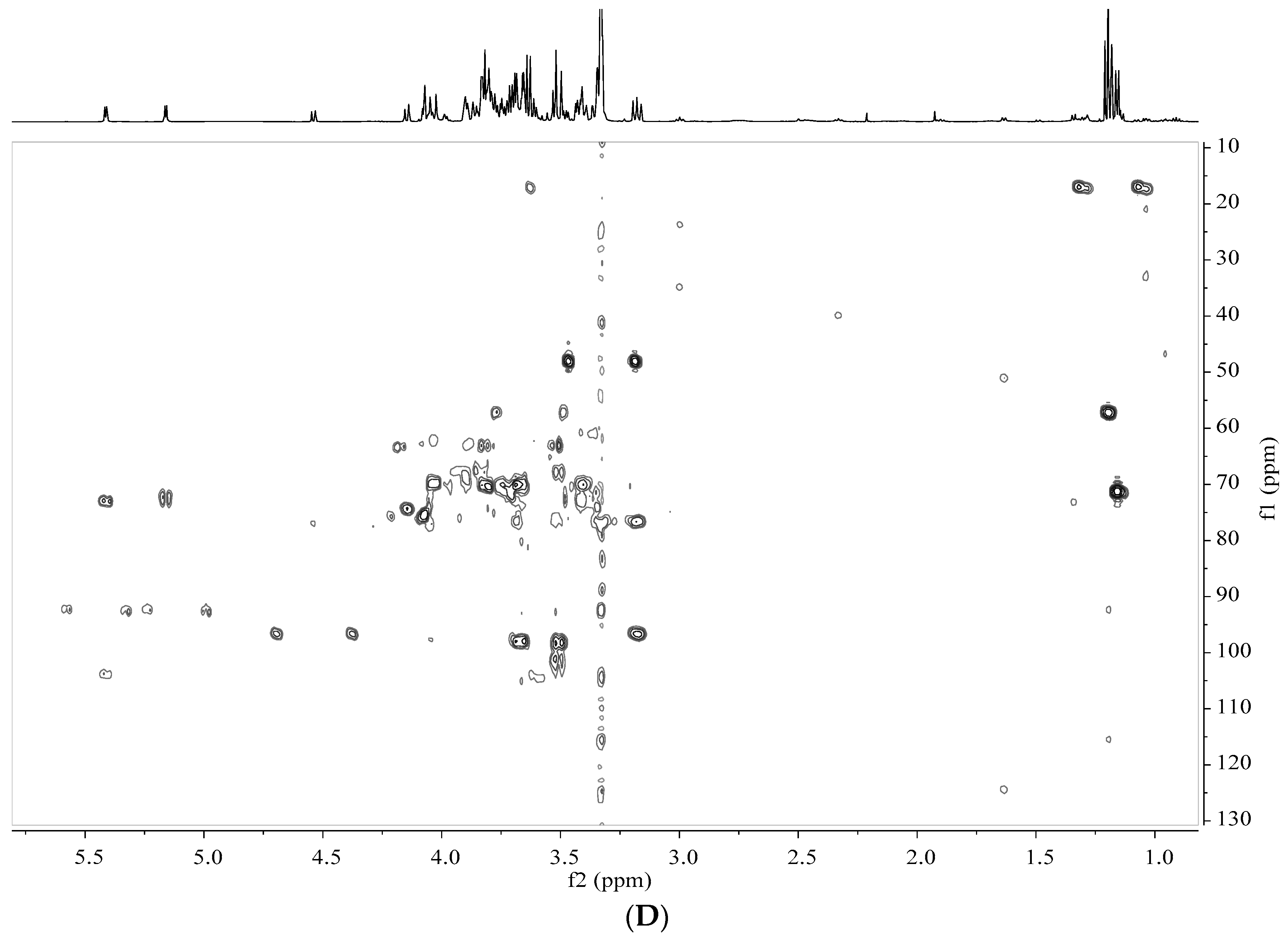
| No. | Metabolites | Assignment of Proton Signals |
|---|---|---|
| Carbohydrates | ||
| 1 | Fructose | 4.13 (d, J = 8.6 Hz) |
| 2 | β-d-glucose | 4.52 (d, J = 8.0 Hz), 3.16 (m) |
| 3 | α-d-glucose | 5.14 (d, J = 3.72 Hz) |
| 4 | Sucrose | 5.40 (d, J = 3.8 Hz), 3.84 (m), 3.74 (m), 3.47 (m), 3.41 (m) |
| Amino acids | ||
| 5 | Leucine | 0.92 (d, J = 7.0 Hz), 0.94 (d, J = 7.0 Hz) |
| 6 | Valine | 1.00 (d, J = 7.0 Hz), 1.05 (d, J = 7.0 Hz) |
| 7 | Isoleucine | 1.02 (d, J = 7.0 Hz), 0.95 (t, J = 7.5 Hz) |
| 8 | Alanine | 1.47 (d, J = 7.2 Hz), 3.64 (m) |
| 9 | γ-Amino-butyrate (GABA) | 1.89 (m), 2.31 (t, J = 7.5 Hz), 2.98 (t, J = 7.5 Hz) |
| 10 | Glutamine | 2.12 (m), 2.47 (m), 3.66 (m) |
| 11 | l-aspartic acid | 2.57 (m), 2.80 (m), 3.78 (m) |
| 12 | Asparagine | 2.73 (m), 2.93 (m), 3.87 (m) |
| Lipids | ||
| 13 | Saponin a | 0.48 (d, J = 3.9 Hz), 0.66 (d, J = 4.0 Hz) |
| 14 | Palmitic acid | 0.89 (t, J = 7.3 Hz), 1.20–1.28 (m), 1.54–1.58 (m), 2.24 (t, J = 7.5 Hz) |
| 15 | Linoleic acid | 0.89 (t, J = 7.3 Hz), 1.30–1.33 (m), 1.58–1.64 (m), 2.38 (t, J = 7.5 Hz) |
| 16 | Saponin b | 0.89 (s), 1.30–1.33 (m), 1.62 (d, J = 6.7 Hz) |
| Phenolics | ||
| 17 | Salsolinol | 6.68 (s), 6.64 (s) |
| 18 | Dopamine | 6.78 (d, J = 8.0 Hz), 6.73 (d, J = 2.3 Hz), 6.62 (d, J = 8.0, 2.3 Hz) |
| 19 | Gallic acid | 7.01 (s) |
| Other metabolites | ||
| 20 | Ethanol | 1.18 (t, J = 7.5 Hz), 3.62 (m) |
| 21 | Acetic acid | 1.91 (s) |
| 22 | Malic acid | 2.45 (m), 2.75 (m), 4.27 (m) |
| 23 | Choline | 3.21 (s) |
| 24 | Phosphocholine | 3.23 (s) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Yang, J.; Jiang, Y.; Zeng, J.; Zhou, X.; Hua, Y.; Yang, B. Morin as a Preservative for Delaying Senescence of Banana. Biomolecules 2018, 8, 52. https://doi.org/10.3390/biom8030052
Zhu H, Yang J, Jiang Y, Zeng J, Zhou X, Hua Y, Yang B. Morin as a Preservative for Delaying Senescence of Banana. Biomolecules. 2018; 8(3):52. https://doi.org/10.3390/biom8030052
Chicago/Turabian StyleZhu, Hong, Jiali Yang, Yueming Jiang, Jun Zeng, Xuesong Zhou, Yanglin Hua, and Bao Yang. 2018. "Morin as a Preservative for Delaying Senescence of Banana" Biomolecules 8, no. 3: 52. https://doi.org/10.3390/biom8030052
APA StyleZhu, H., Yang, J., Jiang, Y., Zeng, J., Zhou, X., Hua, Y., & Yang, B. (2018). Morin as a Preservative for Delaying Senescence of Banana. Biomolecules, 8(3), 52. https://doi.org/10.3390/biom8030052







