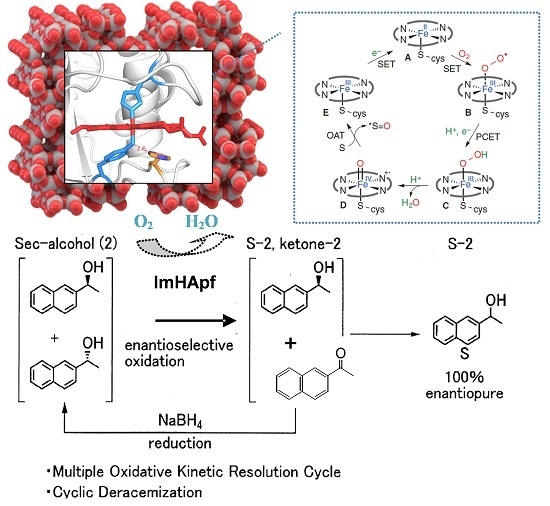
Continuous Reusability using Immobilized HasApf in Chemoenzymatic Deracemization: A New Heterogeneous Enzyme Catalysis
Abstract
:1. Introduction
2. Results
2.1. Application to the Immobilization of HasApf
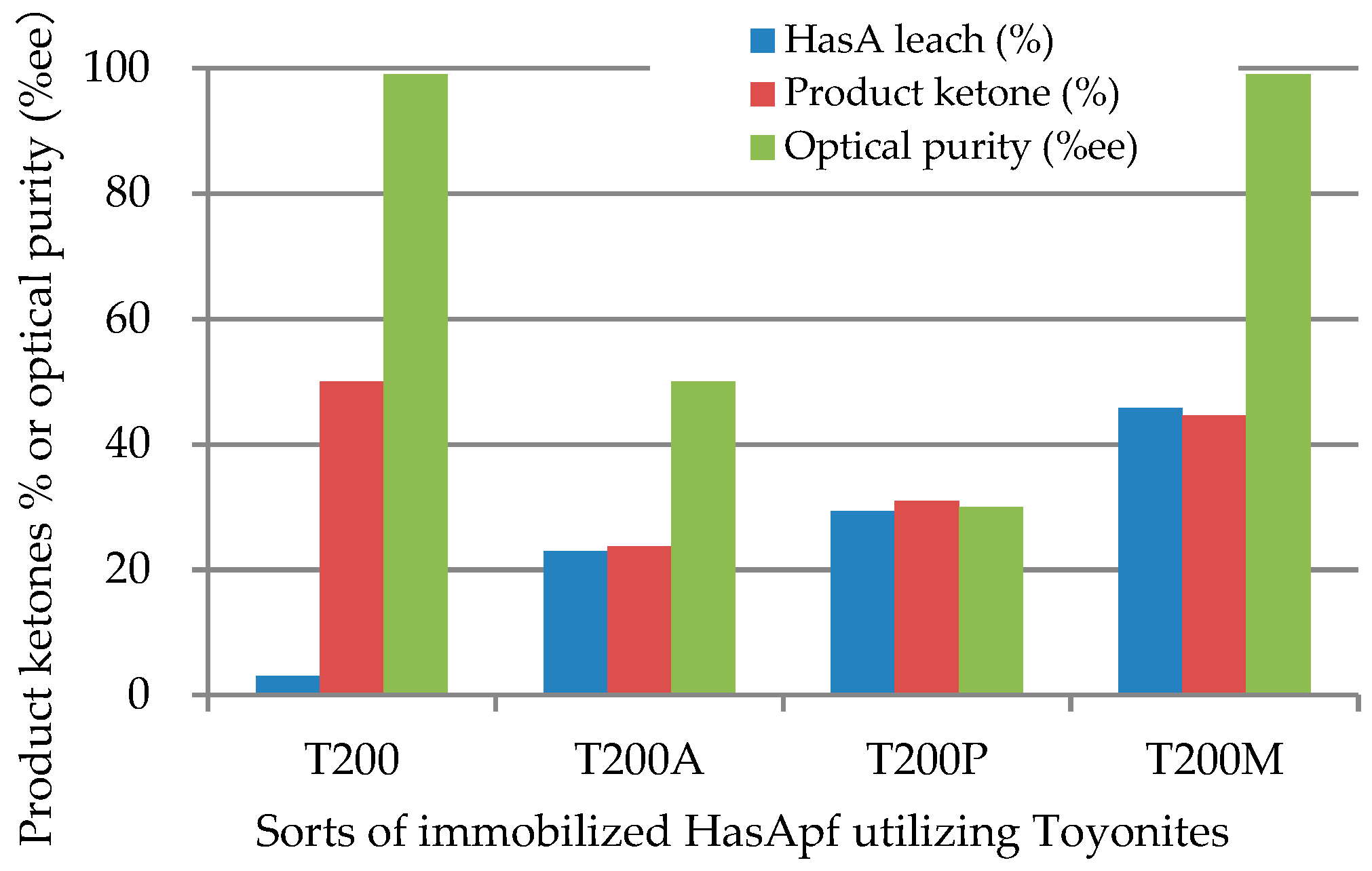
2.1.1. Immobilization of HasApf with a Toyonite
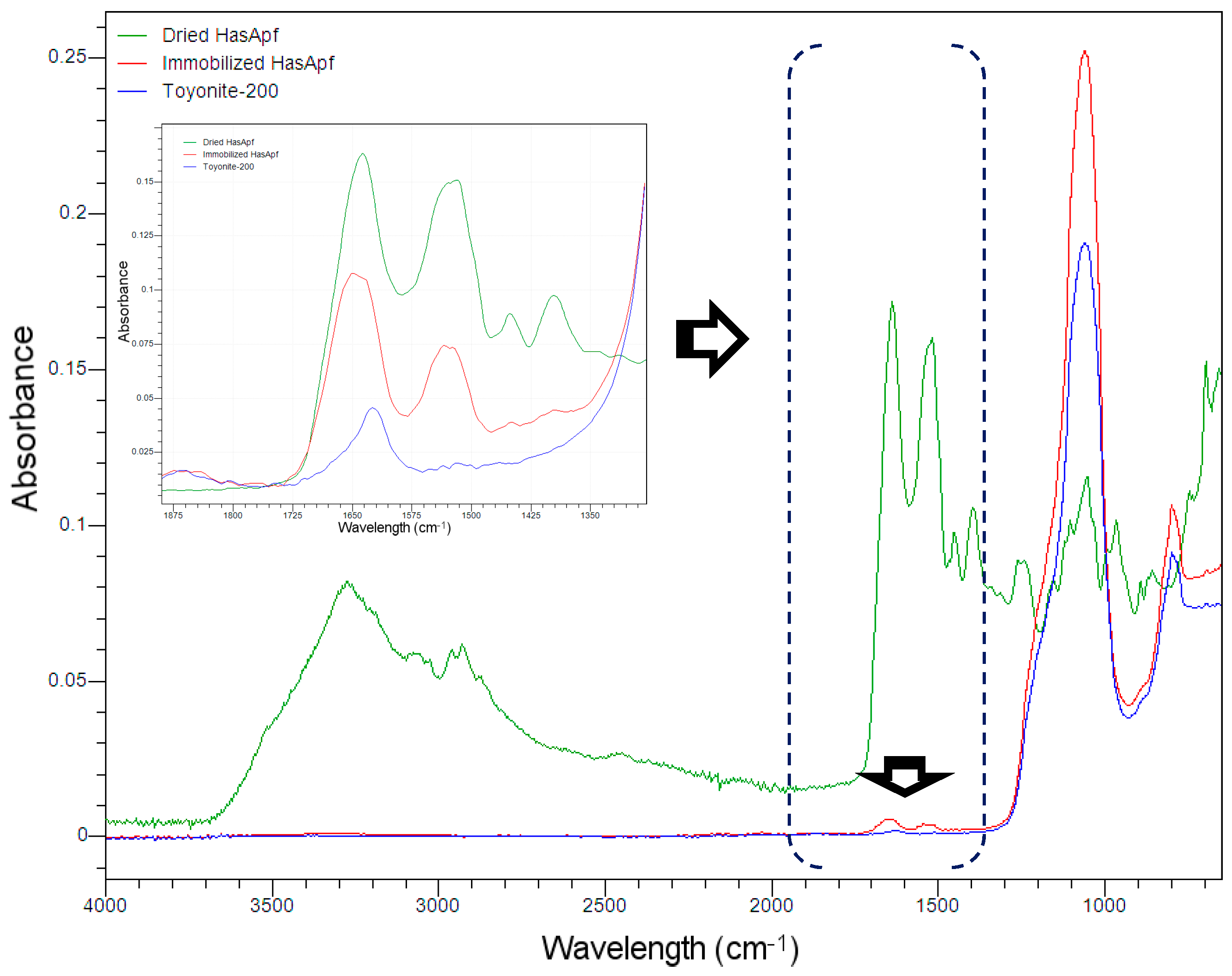
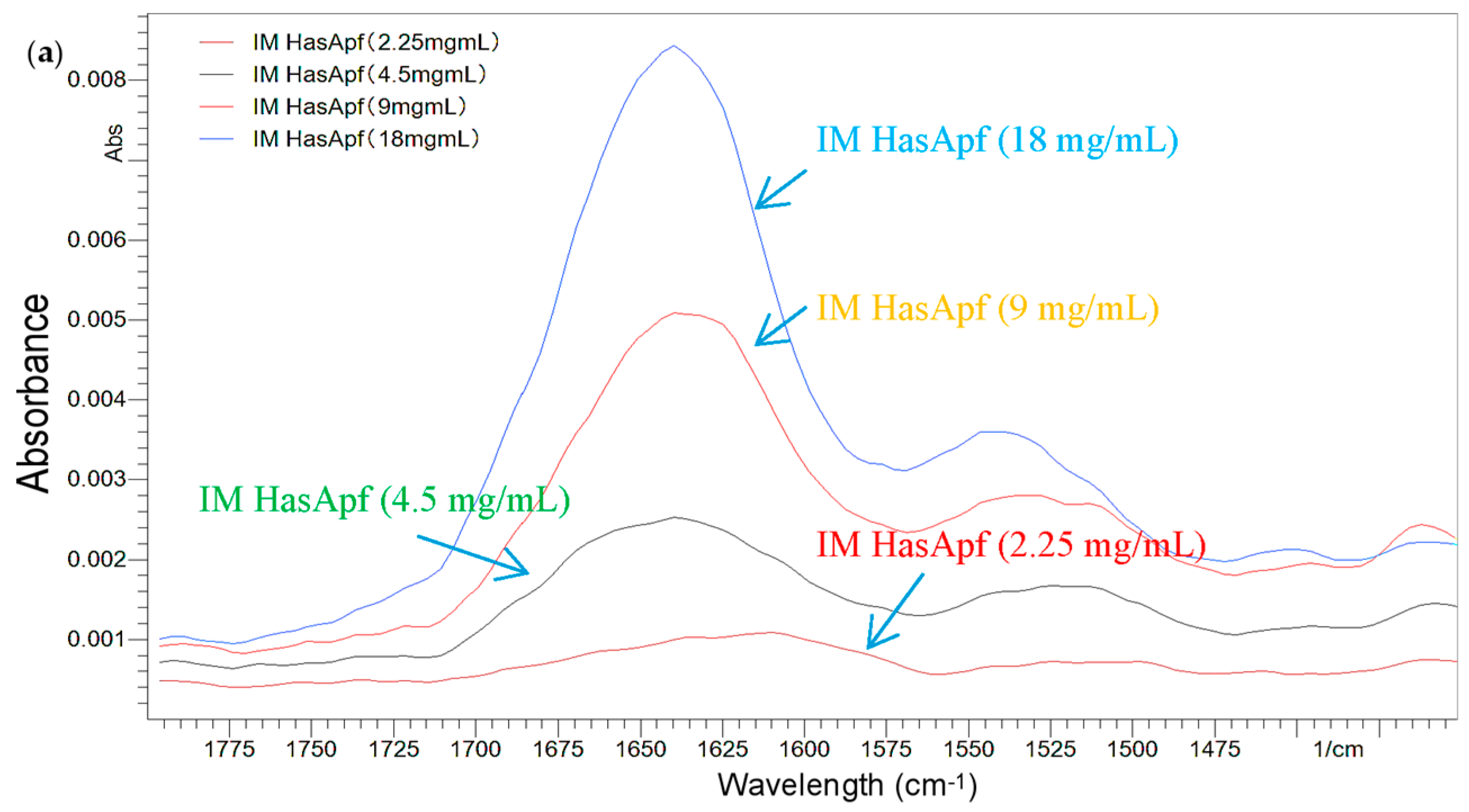
2.1.2. FTIR Analysis for the Detection of HasA Spectra in ImHApf
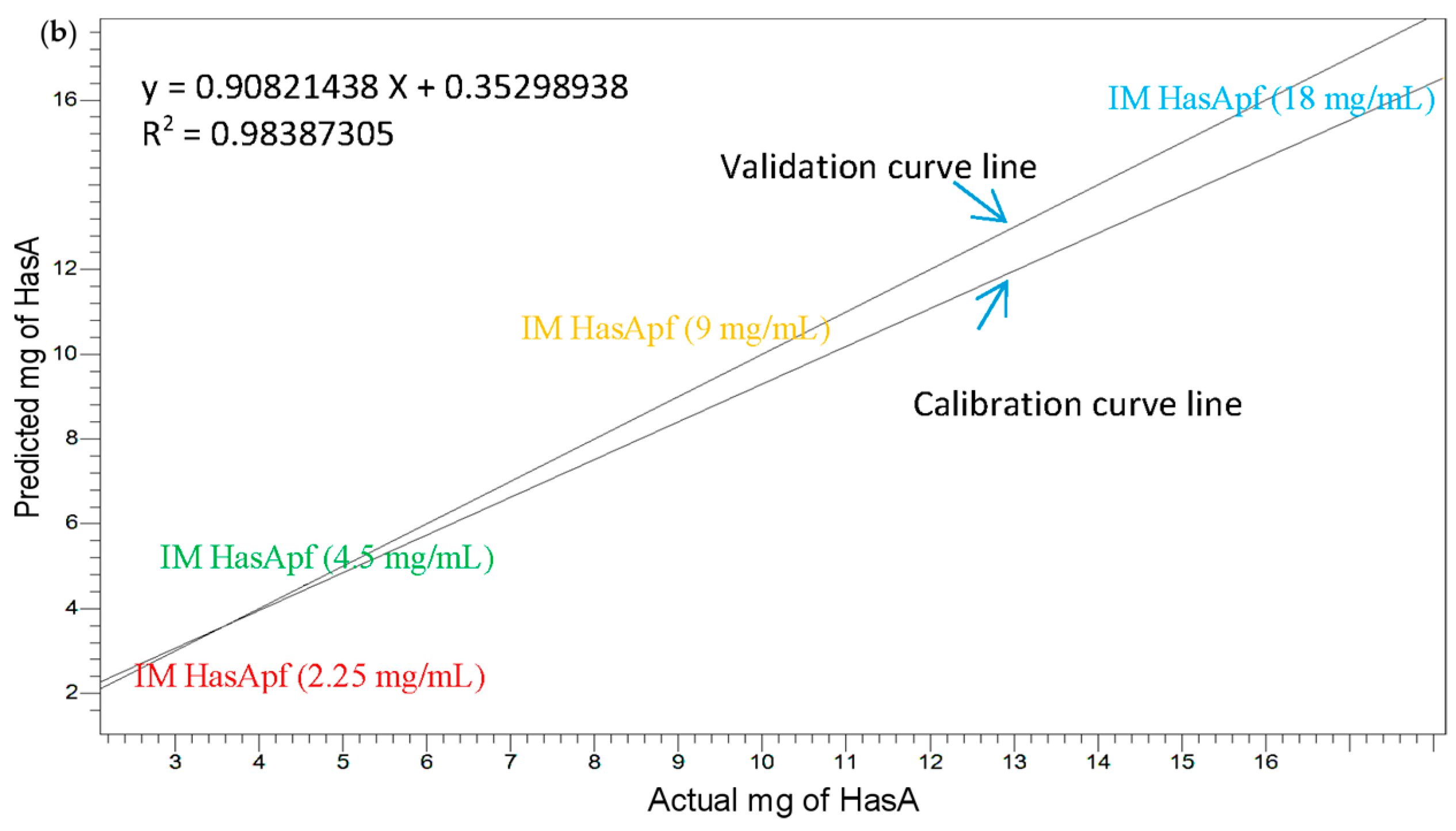
2.1.3. Construction of the HasA Calibration Curve in ImHApf
2.2. Catalytic Efficiency of ImHApf
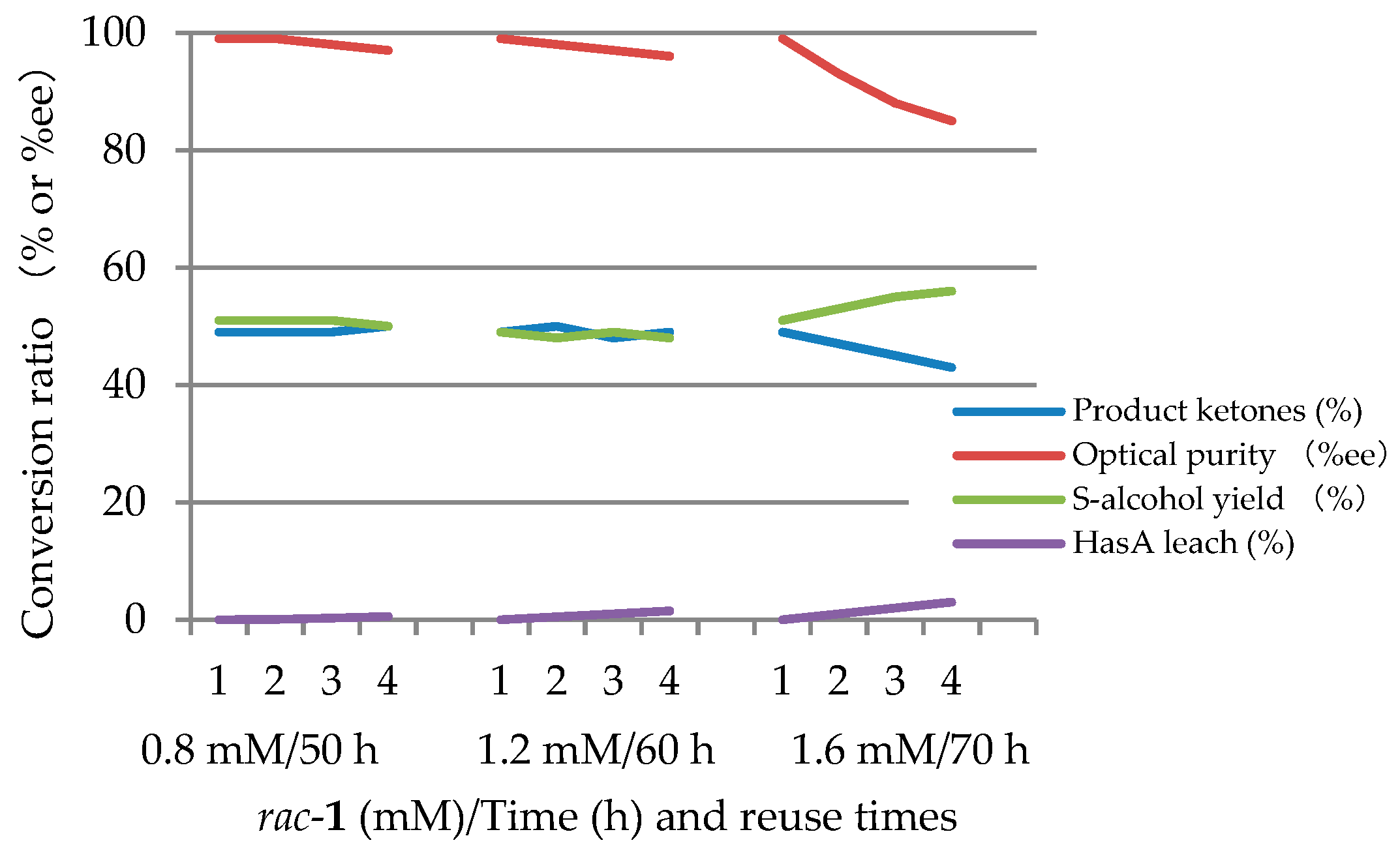
2.2.1. Continuous Reuse of ImHApf
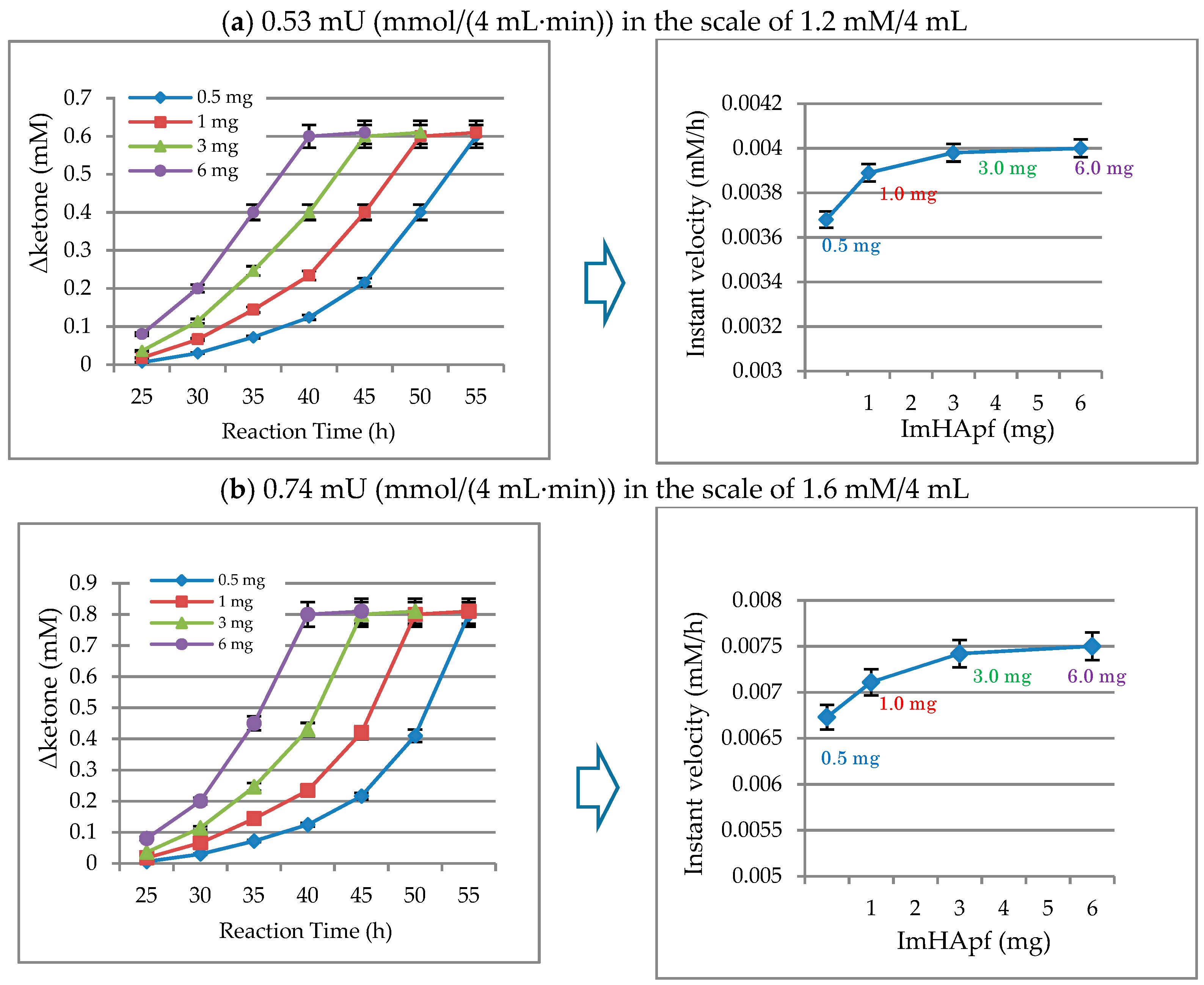
2.2.2. Unit of Activity Regarding ImHApf
2.3. Kinetic Resolution
2.3.1. Substrate-Specific Reactivities to the m- and p-Substituted Groups
2.3.2. ImHApf-Cyclic Deracemization Using NaBH4 (50 mg × 3)
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.1.1. ImHApf Preparation with HasApf Protein Expression/Purification
4.1.2. General Scale of the Reactions Using HasApf and ImHApf
4.1.3. Enantiomeric Excess of Isolated Sec-Alcohols
4.1.4. FTIR Analyses Using ImHApf
4.1.5. Specific Activity and Turnover Efficiency on ImHApf
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nagaoka, H. Application of a heme-binding protein eluted from encapsulated biomaterials to the catalysis of enantioselective oxidation. ACS Catal. 2014, 4, 553–565. [Google Scholar] [CrossRef]
- Ozaki, S.; Nakahara, A.; Sato, T. Mechanism of heme uptake by heme acquisition system A. Chem. Lett. 2011, 40, 362–363. [Google Scholar] [CrossRef]
- Ozaki, S.; Sato, A.; Migita, T.C.; Uchida, T.; Ishimori, K. Spectroscopic studies on HasA from Yersinia pseudotuberculosis. J. Inorg. Biochem. 2014, 138, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.; Huché, F.; Diederichs, K.; Izadi-Pruneyre, N.; Lecroisey, A.; Wandersman, P.C. Heme uptake across the outer membrane as revealed by crystal structures of the receptor–hemophore complex. PNAS 2009, 106, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Kamori, M.; Hori, T.; Yamashita, Y.; Hirose, Y.; Naoshima, Y. Immobilization of lipase on a new inorganic ceramics support, toyonite, and the reactivity and enantioselectivity of the immobilized lipase. J. Mol. Catal. B Enzym. 2000, 9, 269–274. [Google Scholar] [CrossRef]
- Magnan, E.; Catarino, I.; Paolucci-Jeanjean, D.; Belleville, M.P. Immobilization of lipase on a ceramic membrane: Activity and stability. J. Membr. Sci. 2004, 241, 161–167. [Google Scholar] [CrossRef]
- Shoji, O.; Fujishiro, T.; Nakajima, H.; Kim, M.; Nagano, S.; Shiro, Y.; Watanabe, Y. Hydrogen peroxide dependent monooxygenations by tricking the substrate recognition of cytochrome P450BSβ. Angew. Chem. Int. Ed. 2007, 46, 3656–3659. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H. Chiral resolution function with immobilized food proteins. Biotechnol. Prog. 2003, 19, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H. Ability of different biomaterials to enantioselectively catalyze oxidation and reduction reactions. Biotechnol. Prog. 2004, 20, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Udagawa, K.; Kirimura, K. Cross-linked protein complex exhibiting asymmetric oxidation activities in the absence of added cofactor. Biotechnol. Prog. 2012, 28, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H. The application of a cytochrome P450 enzyme eluted from encapsulated for the catalysis of enantioselective oxidation. RSC Adv. 2014, 4, 16333–16344. [Google Scholar] [CrossRef]
- Thakur, V.V.; Sudalai, A. Enantioselective synthesis of (S)-α-arylpropionic acids via Pd-catalyzed kinetic resolution of benzylic alcohols. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 2005, 44B, 557–561. [Google Scholar] [CrossRef]
- Gillam, E.M.; Hayes, M.A. The evolution of cytochrome P450 enzymes as biocatalysts in drug discovery and development. Curr. Top. Med. Chem. 2013, 13, 2254–2280. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, I.T.; Press, C.M.; Ravel, J.; Kobayashi, D.Y.; Myers, G.S.; Mavrodi, D.V.; DeBoy, R.T.; Seshadri, R.; Ren, Q.; Madupu, R.; et al. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat. Biotechnol. 2005, 23, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H. An HASApf-Redoxin Complex causing asymmetric catalytic oxidation via the regenerative formation of a reactive oxygen species. Dalton Trans. 2015, 44, 13384–13393. [Google Scholar] [CrossRef] [PubMed]
- Du, J.F.; Li, W.; Li, L.; Wen, G.B.; Lin, Y.W.; Tan, X. Regulation the coordination state of a heme protein by a designed distal hydrogen-bonding network. ChemistryOpen 2015, 4, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R. Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd ed.; Kluwer Academic/Plenum: New York, NY, USA, 2004. [Google Scholar]
- Machii, K.; Watanabe, Y. Acylperoxo-Iron (III) porphyrin complexes: A new entry of potent oxidants for the alkene epoxidation. J. Am. Chem. Soc. 1995, 117, 6691–6697. [Google Scholar] [CrossRef]
- Jepkorir, G.; Rodríguez, J.C.; Rui, H.; Im, W.; Lovell, S.; Battaile, K.P.; Alontaga, A.Y.; Yukl, E.T.; Moënne-Loccoz, P.; Rivera, M. Structural, NMR spectroscopic, and computational investigation of hemin loading in the hemophore HasAp from Pseudomonas aeruginosa. Am. Chem. Soc. 2010, 132, 9857–9872. [Google Scholar] [CrossRef] [PubMed]
- Giles, M.N.; Watts, B.A.; Giles, M.N.; Watts, B.A.; Giles, I.G.; Fry, H.F.; Littlechild, A.J.; Jacob, C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Nagaoka, H. Heterogeneous asymmetric oxidation catalysis using hemophore HasApf. Application in the chemoenzymatic deracemization of sec-alcohols with sodium borohydride. Catalysts 2016, 6, 38. [Google Scholar] [CrossRef]
- Steinreiber, J.; Faber, K.; Griengl, H. De-racemization of enantiomers versus de-epimerization of diastereomers--classification of dynamic kinetic asymmetric transformations (DYKAT). Chem. Eur. J. 2008, 14, 8060–8072. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J. Deracemisation methods. Curr. Opin. Chem. Biol. 2010, 14, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bowler, B.E.; Lynch, K.; Eaton, S.S.; Eaton, G.R. Interspin distances in spin-labeled metmyoglobin variants determine by saturation recovery EPR. Biophys. J. 2000, 79, 1039–1052. [Google Scholar] [CrossRef]
- Gruber, C.C.; Lavandera, I.; Faber, K.; Kroutil, W. From a Racemate to a Single Enantiomer: Deracemization by Stereoinversion. Adv. Synth. Catal. 2006, 348, 1789–1805. [Google Scholar] [CrossRef]
- Auld, D.S.; Bergman, T. The role of zinc for alcohol dehydrogenase structure and function. Cell Mol. Life Sci. 2008, 65, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Liu, J.; Hahn, M.; Qiao, S.; Middelberg, A.P.J.; He, L. Encapsulation of lipase in mesoporous silica yolk–shell spheres with enhanced enzyme stability. RSC Adv. 2013, 3, 22008–22013. [Google Scholar] [CrossRef]
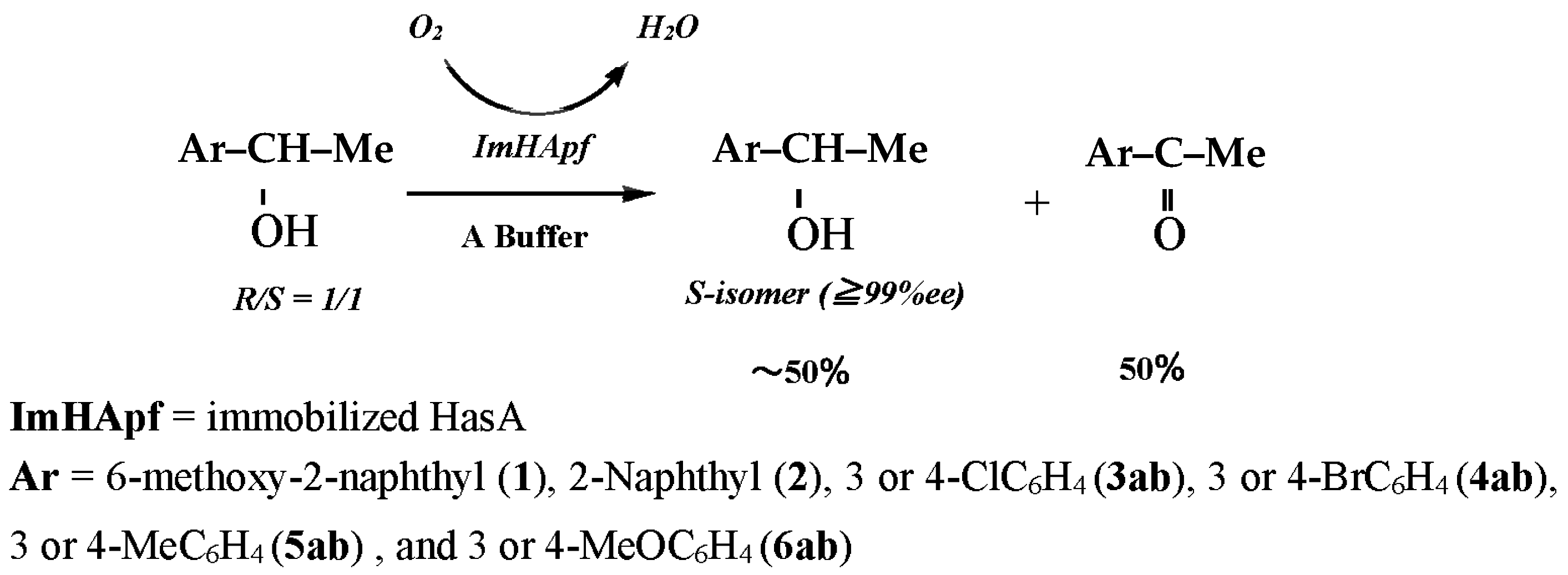
| Substrate | Reaction Time (h) | Origin | Product | ||||
|---|---|---|---|---|---|---|---|
| Racemic-ArCH(OH)R | Ar | R | Compound | OP (% ee) a | CY (%) b | ||
| 1 | 6-MeO-2-Np | Me | 50 | ImHApf c | (S)-1 | >99 | 50 |
| 2 | 2-Np | Me | 55 | ImHApf | (S)-2 | >99 | 50 |
| 3a | 3-Cl-Ar | Me | 50 | ImHApf | (S)-3a | >99 | 50 |
| 4a | 3-Br-Ar | Me | 55 | ImHApf | (S)-4a | >99 | 49 |
| 5a | 3-Me-Ar | Me | 60 | ImHApf | (S)-5a | >99 | 50 |
| 6a | 3-MeO-Ar | Me | 50 | ImHApf | (S)-6a | >99 | 50 |
| 3b | 4-Cl-Ar | Me | 50 | ImHApf | (S)-3b | >99 | 50 |
| 4b | 4-Br-Ar | Me | 55 | ImHApf | (S)-4b | >99 | 50 |
| 5b | 4-Me-Ar | Me | 60 | ImHApf | (S)-5b | >99 | 49 |
| 6b | 4-MeO-Ar | Me | 50 | ImHApf | (S)-6b | >99 | 50 |
| Deracemization: ImHApf (100 mg)/rac-1 (100 mg)/50 mM glycine–NaOH Buffer (300 mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| NaBH4 | Time (h) | % Ketone | % Alcohol a | % R-isomer | % S-isomer | Compound | % ee | |
| 1st | 0 | 60 | 50 | 50 | 1.6 | 98.4 | (S)-1 b | 96.8 |
| 1.2 | 98.8 | (S)-2 c | 97.6 | |||||
| 2nd | 50 mg | 80 | 32 | 68 | 1.1 | 98.9 | (S)-1 | 97.8 |
| 0.9 | 99.1 | (S)-2 | 98.2 | |||||
| 3rd | 50 mg | 90 | 17 | 83 | 0.7 | 99.3 | (S)-1 | 98.6 |
| 0.5 | 99.5 | (S)-2 | 99.0 | |||||
| 4th | 50 mg | 100 | 4 | 96 | 0.4 | 99.6 | (S)-1 | 99.2 |
| 0.2 | 99.8 | (S)-2 | 99.6 | |||||
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaoka, H. Continuous Reusability using Immobilized HasApf in Chemoenzymatic Deracemization: A New Heterogeneous Enzyme Catalysis. Biomolecules 2016, 6, 41. https://doi.org/10.3390/biom6040041
Nagaoka H. Continuous Reusability using Immobilized HasApf in Chemoenzymatic Deracemization: A New Heterogeneous Enzyme Catalysis. Biomolecules. 2016; 6(4):41. https://doi.org/10.3390/biom6040041
Chicago/Turabian StyleNagaoka, Hiroyuki. 2016. "Continuous Reusability using Immobilized HasApf in Chemoenzymatic Deracemization: A New Heterogeneous Enzyme Catalysis" Biomolecules 6, no. 4: 41. https://doi.org/10.3390/biom6040041
APA StyleNagaoka, H. (2016). Continuous Reusability using Immobilized HasApf in Chemoenzymatic Deracemization: A New Heterogeneous Enzyme Catalysis. Biomolecules, 6(4), 41. https://doi.org/10.3390/biom6040041