Lipases Immobilization for Effective Synthesis of Biodiesel Starting from Coffee Waste Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Lipases and Immobilization Supports

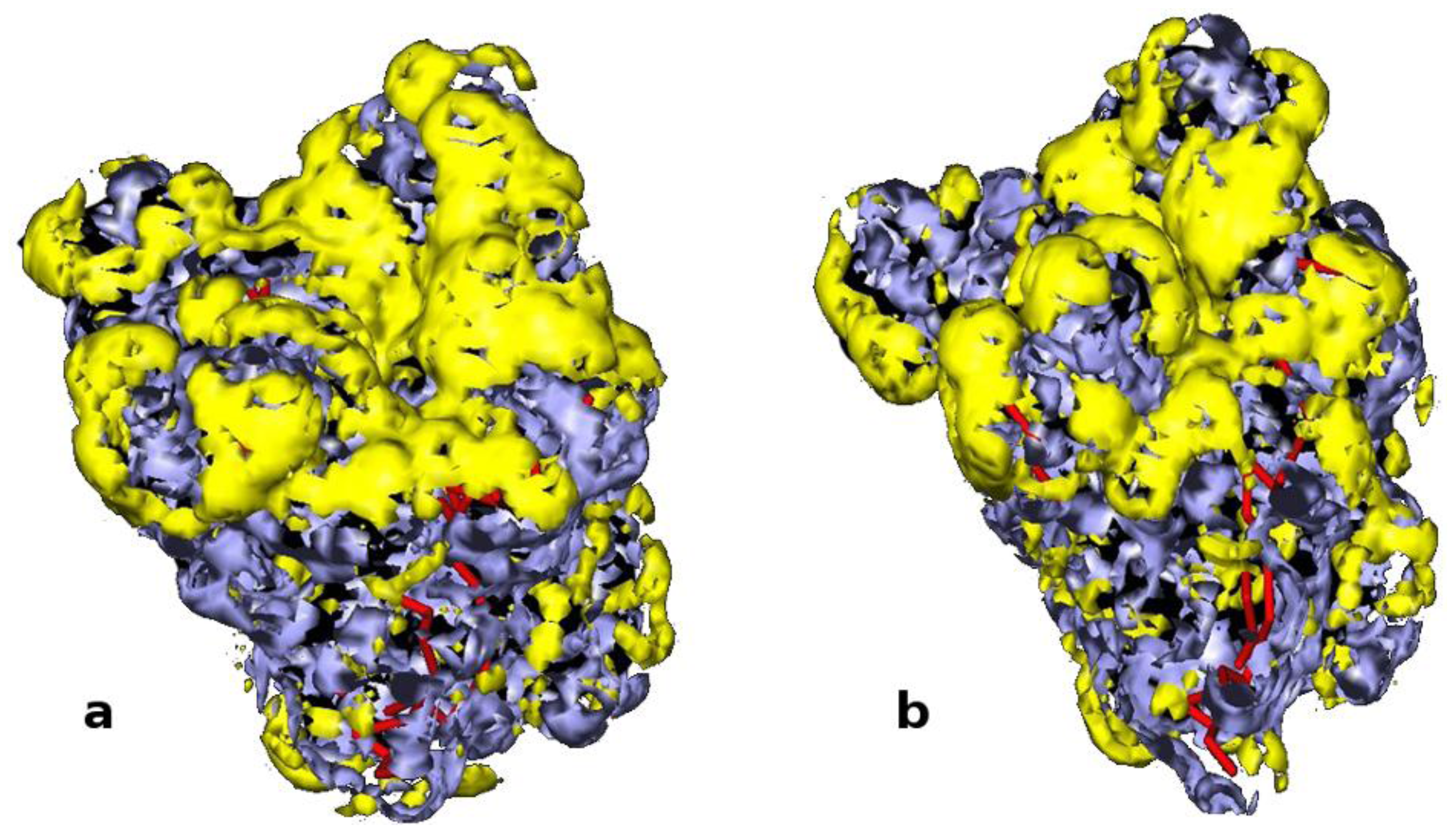

| Immobilized formulation | Carrier | Hydrophylicity | Particle size | Residualwater a | Synthetic activity | Hydrolitic activity d |
|---|---|---|---|---|---|---|
| (%) | (U/g dry) | (U/g dry) | ||||
| PcL-S | Styrenic Porous | --- | Beads, 300–500 μm | <5 | 159 b | 463 |
| PcL PS-IM | Siliceous Non porous | ++ | Powder <20 μm | <5 | 820 b | 15,400 |
| CaL-S | Styrenic Porous | -- | Beads, 300–500 μm | <5 | 3450 c | 978 |
| CaL-M | Methacrylic Porous | - | Beads, 300–500 μm | <5 | 3020 c | 490 |
| CaLB on Celite® R-640 | Siliceous Porous | +++ | Rods 5 × 3 mm | 15 | 2050 c | 274 |
2.2. Methanolysis of Triolein and Effect of Methanol Concentration
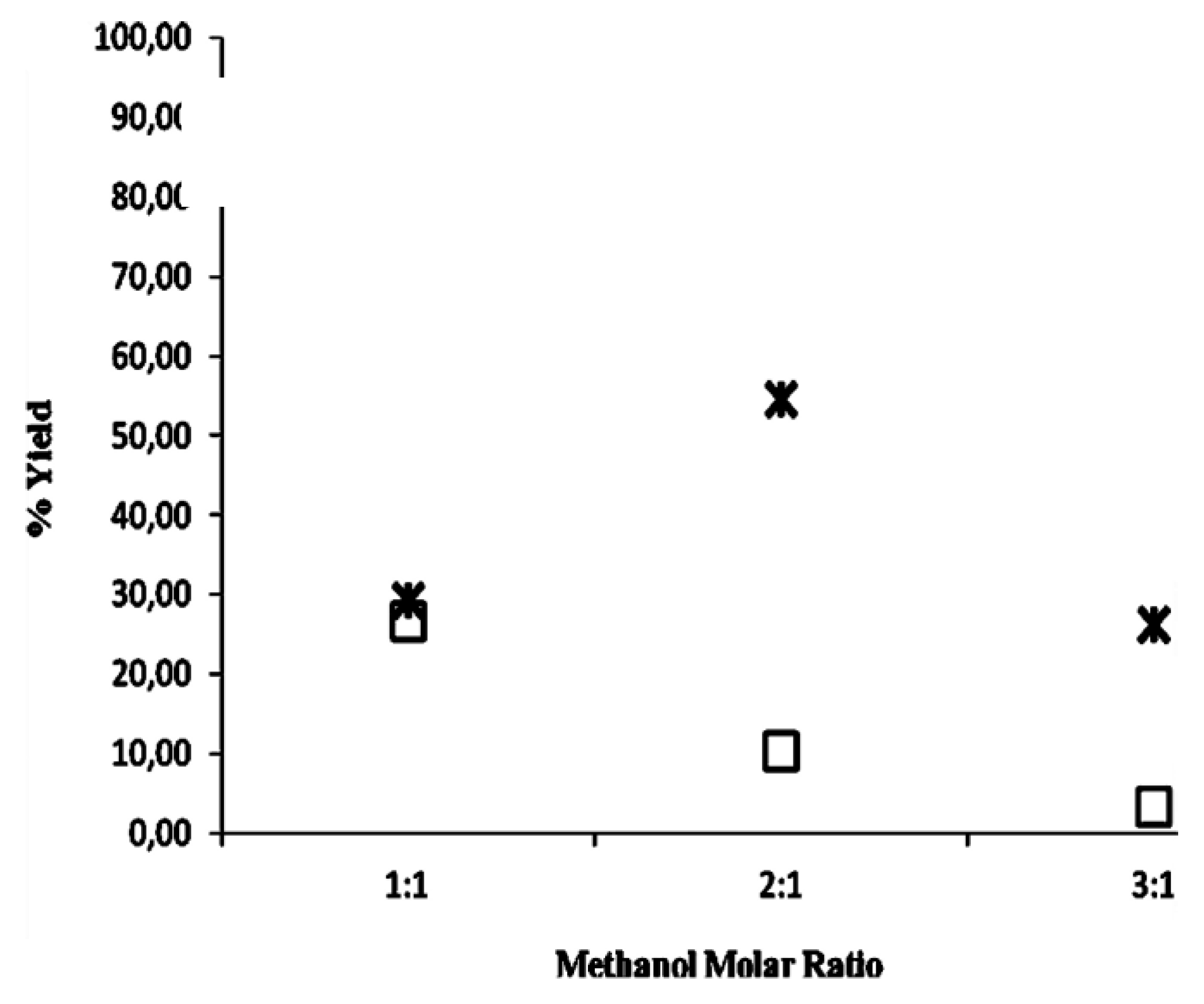

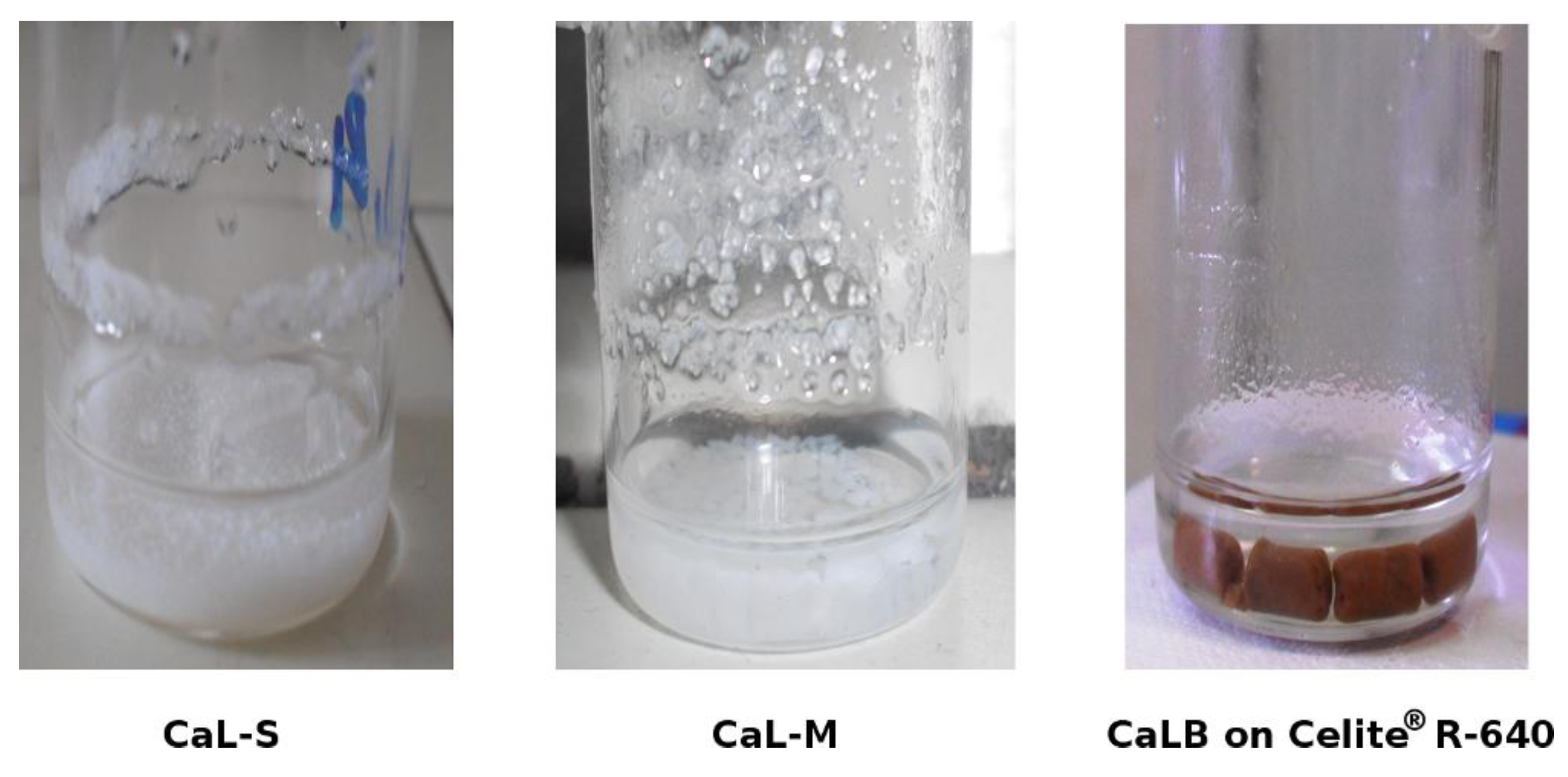
| Reaction System | Water activity (aw) |
|---|---|
| Reactants mixture | 0.64 |
| CaL-S | 0.48 |
| CaL-M | 0.27 |
| CaLB on Celite® R-640 | 0.27 |
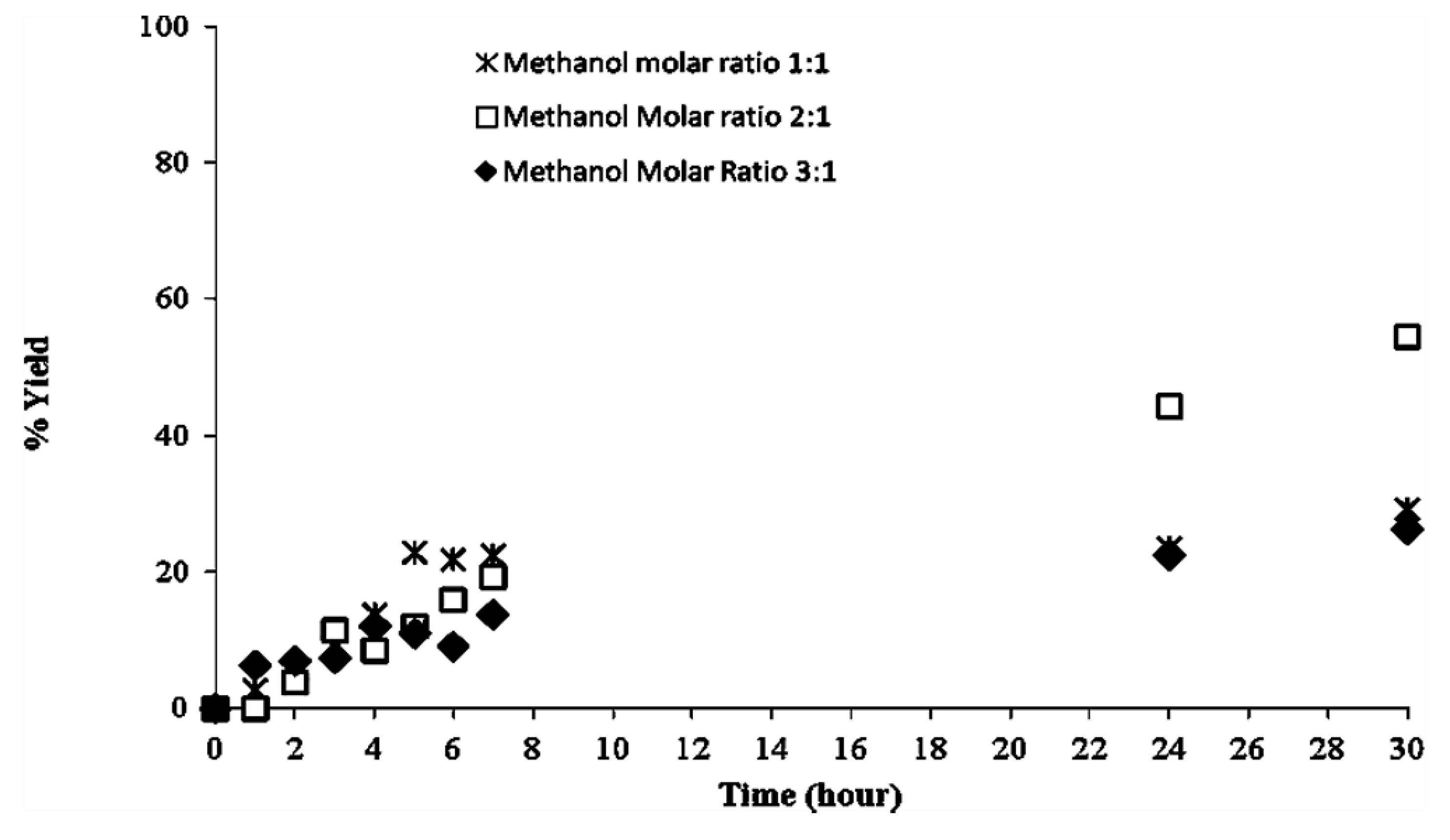
2.3. Recyclability of Cal-S

2.4. Enzymatic Methanolysis of Oil Extracted from Espresso Spent Coffee Ground
| Fatty acids | Oil from spent coffee ground as in [8] | Oil from espresso spent coffee ground |
|---|---|---|
| C14 | n.d. | n.d. |
| C16 | 51.4 | 58.29 |
| C18 | 8.3 | 8.79 |
| C18:1 | n.d. | 3.41 |
| C18:2 | 40.3 | 27.80 |
| C18:3 | n.d. | 0.09 |
| C20 | n.d. | 1.61 |
| C22 | n.d. | n.d. |

3. Experimental Sections
3.1. Enzymes
3.2. Immobilization of CalB on Celite®R-640
3.3. Chemicals
3.4. Monitoring the Formation of Fatty acid Methyl Ester (Biodiesel)
3.5. Synthetic Activity of PcL
3.6. Synthetic Activity of CaLB:
3.7. Hydrolytics Activity of Lipases
3.8. Water Activity
3.9. Monitoring the Methanolysis by HPLC
3.10. Lipase Catalyzed Methanolysis
3.11. Recycling of CaL-S
3.12. 1H-NMR
3.13. Extraction of Oil and GC-MS Analysis of Fatty Acids
3.14. Computational Study: Molecular Dynamic Simulations
3.15. Computational Study: GRID Mapping of Surface of Proteins
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References
- Medina, A.R.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef]
- Mittelbach, M.; Remschmidt, C. Biodiesel - The Comprehensive Handbook, 1st ed.; Börsedruk Ges. m.b.H: Vienna, 2004. [Google Scholar]
- Uma, B.H.; Kim, Y.S. Review: A chance for Korea to advance algal-biodiesel technology. J. Ind. Eng. Chem. 2009, 15, 1–7. [Google Scholar]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, S.; Gupta, M.N. Biodiesel preparation by lipase-catalyzed transesterification of jatropha oil. Energy Fuels 2004, 18, 154–159. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Camargos, R.R.S.; Ferraz, V.P. Coffee oil as a potential feedstock for biodiesel production. Bioresour. Technol. 2008, 99, 3244–3250. [Google Scholar] [CrossRef]
- Calabrò, V.; Ricca, E.; de Paola, M.G.; Curcio, S.; Iorio, G. Kinetics of enzymatic trans-esterification of glycerides for biodiesel production. Bioprocess Biosyst. Eng. 2010, 33, 701–710. [Google Scholar] [CrossRef]
- Kondamundi, N.; Mohapatra, S.K.; Misra, M. Spent coffee grounds as a versatile source of green energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.M.; Mata, T.M. Valorization of coffee grounds for biodiesel production. Chem. Eng. Trans. 2012, 26, 267–272. [Google Scholar]
- Khan, N.A.; Brown, J.B. The composition of coffee oil and its component fatty acids. J. Am. Oil Chem. Soc. 1953, 30, 606–609. [Google Scholar] [CrossRef]
- Nunes, A.A.; Franca, A.S.; Oliveira, L.S. Activated carbons from waste biomass: An alternative use for biodiesel production solid residues. Bioresour. Technol. 2009, 100, 1786–1792. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhata, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Takayuki, S. Antioxidative activities of fractions obtained from brewed coffee. J. Agricolture Food Chem. 2004, 52, 592–596. [Google Scholar] [CrossRef]
- Campo, P.; Zhao, Y.; Suidan, M.T.; Venosa, A.D.; Sorial, G.A. Biodegradation kitetics and toxicity of vegetable oils triacylglycelols under aerobic conditions. Chemosphere 2007, 68, 2054–2062. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzyme with attractive application. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Mittelbach, M. Lipase-catalyzed alcoholysis of sunflower oil. J. Am. Chem. Soc. 1990, 67, 168–170. [Google Scholar]
- Nielsen, P.M.; Brask, J.; Fjerbaek, L. Enzymatic biodiesel production: Technical and economical considerations. Eur. J. Lipid Sci. Technol. 2008, 110, 692–700. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.Y.; Liu, H.; Li, Z.B. Study on acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil for biodiesel production. J. Mol. Catal. B Enzym. 2005, 37, 68–71. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Christensen, M.W.; Andersen, L.; Husum, T.L.; Kirk, O. Industrial lipase immobilization. Eur. J. Lipid Sci. Technol. 2003, 105, 318–321. [Google Scholar] [CrossRef]
- Ferrario, V.; Ebert, C.; Knapic, L.; Fattor, D.; Basso, A.; Spizzo, P.; Gardossi, L. Conformational changes of lipases in aqueous media: A comparative computational study and experimental implications. Adv. Synth. Catal. 2011, 353, 2466–2480. [Google Scholar] [CrossRef]
- Skjot, M.; de Maria, L.; Chatterjee, R.; Svendsen, A.; Patkar, S.A.; Ostergraad, P.R.; Brask, J. Understanding the plasticity of the alpha/beta hydrolase fold: Lid swapping on the Candida antarctica lipase B results in chimeras with interesting biocatalytic properties. ChemBioChem 2009, 10, 520–527. [Google Scholar] [CrossRef]
- Goodford, P.J. A computational procedure for determining energetically favorable binding sites on biologically important macromolecules. J. Med. Chem. 1985, 28, 849–857. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Liu, D. Study on the kinetics of enzymatic interesterification of triglycerides for biodiesel production with methyl acetate as the acyl acceptor. J. Mol. Catal. B Enzym. 2005, 32, 241–245. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Conversion of degummed soybean oil to biodiesel fuel with immobilized Candida antartica lipase. J. Mol. Catal. B Enzym. 2002, 17, 151–155. [Google Scholar] [CrossRef]
- Anderson, E.; Larsson, K.; Kirk, O. One biocatalyst—many applications: The use of Candida antarctica. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Salis, A.; Pinna, M.; Monduzzi, M.; Solinas, V. Biodiesel production from triolein and short chain alcohols through biocatalysis. J. Biotechnol. 2005, 119, 291–299. [Google Scholar] [CrossRef]
- Marrink, S.J.; Tieleman, D.P. Perspective on the Martini model. Chem. Soc. Rev. 2013. [Google Scholar] [CrossRef]
- Friedrich, T.; Stuermer, R. Production of immobilized lipase from Pseudomonas and application for enantioselective reactions. U.S. Patent 6 596 520, 2003. [Google Scholar]
- Gardossi, L. Immobilization of Enzymes and Control of Water Activity in Low-Water Media: Properties and Applications of Celite R-640 (Celite Rods). In Methods in Biotechnology: Enzyme in Non-Aqueous Solvents: Methods and Protocols; Vulfson, E.N., Halling, P.J., Holland, H., Eds.; Humana Press, Inc.: Totowa, NJ, USA, 2001; pp. 151–172. [Google Scholar]
- Basso, A.; de Martin, L.; Ebert, C.; Gardossi, L.; Linda, P. High isolated yields in thermodynamically controlled peptide synthesis in toluene catalysed by thermolysin adsorbed on Celite R-640. Chem. Commun. 2000, 6, 467–468. [Google Scholar]
- Basso, A.; Braiuca, P.; Cantone, S.; Ebert, C.; Linda, P.; Spizzo, P.; Caimi, P.; Hanefeld, U.; Degrassi, G.; Gardossi, L. In silico analysis of enzyme surface and glycosylation effect as a tool for efficient covalent immobilization of CalB and PGA on Sepabeads®. Adv. Synth. Catal. 2007, 349, 877–886. [Google Scholar] [CrossRef]
- Tanaka, A.; Sugimoto, H.; Muta, Y.; Mizuno, T.; Senoo, K.; Obata, H.; Inouye, K. Differential scanning calorimetry of the effects of Ca2+ on the thermal unfolding of Pseudomonas cepacia lipase. Biosci. Biotechnol. Biochem. 2003, 67, 207–210. [Google Scholar] [CrossRef]
- Pirozzi, D. Improvement of lipase stability in the presence of commercial triglycerides. Eur. J. Lipid Sci. Technol. 2003, 105, 608–613. [Google Scholar] [CrossRef]
- Ulijn, R.V.; de Martin, L.; Halling, P.J.; Janssen, A.E.M.; Gardossi, L.; Moore, B.D. Solvent selection for solid-to-solid synthesis. Biotechnol. Bioeng. 2002, 80, 509–515. [Google Scholar] [CrossRef]
- Kaeida, M.; Samukawa, T.; Kondo, A.; Fukuda, H. Effect of methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J. Biosci. Bioeng. 2001, 91, 12–15. [Google Scholar]
- Ferrari, M.; Ravera, F.; De Angelis, E.; Suggi Liverani, F.; Navarini, L. Interfacial properties of coffee oils. Colloids Surf. A Physicochem. Eng. Aspects 2010, 365, 79–82. [Google Scholar]
- Holcapek, M.; Jandera, P.; Fischer, J.; Prokes, B. Analytical monitoring of the production of biodiesel by high-performance liquid chromatography with various detection methods. J. Chromatogr. A 1999, 858, 13–31. [Google Scholar] [CrossRef]
- D’Amelio, N.; de Angelis, E.; Navarini, L.; Schievano, E.; Mammi, S. Green coffee oil analysis by high-resolution nuclear magnetic resonance spectroscopy. Talanta 2013, 110, 118–127. [Google Scholar] [CrossRef]
- Available online: http://md.chem.rug.nl/cgmartini/index.php/home (accessed on 12/05/2010).
- Wolfgang, K.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferrario, V.; Veny, H.; De Angelis, E.; Navarini, L.; Ebert, C.; Gardossi, L. Lipases Immobilization for Effective Synthesis of Biodiesel Starting from Coffee Waste Oils. Biomolecules 2013, 3, 514-534. https://doi.org/10.3390/biom3030514
Ferrario V, Veny H, De Angelis E, Navarini L, Ebert C, Gardossi L. Lipases Immobilization for Effective Synthesis of Biodiesel Starting from Coffee Waste Oils. Biomolecules. 2013; 3(3):514-534. https://doi.org/10.3390/biom3030514
Chicago/Turabian StyleFerrario, Valerio, Harumi Veny, Elisabetta De Angelis, Luciano Navarini, Cynthia Ebert, and Lucia Gardossi. 2013. "Lipases Immobilization for Effective Synthesis of Biodiesel Starting from Coffee Waste Oils" Biomolecules 3, no. 3: 514-534. https://doi.org/10.3390/biom3030514
APA StyleFerrario, V., Veny, H., De Angelis, E., Navarini, L., Ebert, C., & Gardossi, L. (2013). Lipases Immobilization for Effective Synthesis of Biodiesel Starting from Coffee Waste Oils. Biomolecules, 3(3), 514-534. https://doi.org/10.3390/biom3030514





