The First Heterozygous TWNK Nonsense Mutation Associated with Progressive External Ophthalmoplegia: Evidence for a New Piece in the Puzzle of Mitochondrial Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals and Patient Consents
2.2. Morphological Analysis of Muscle Biopsies
2.3. DNA Analysis
2.4. Three-Dimensional (3D) Modeling
3. Results
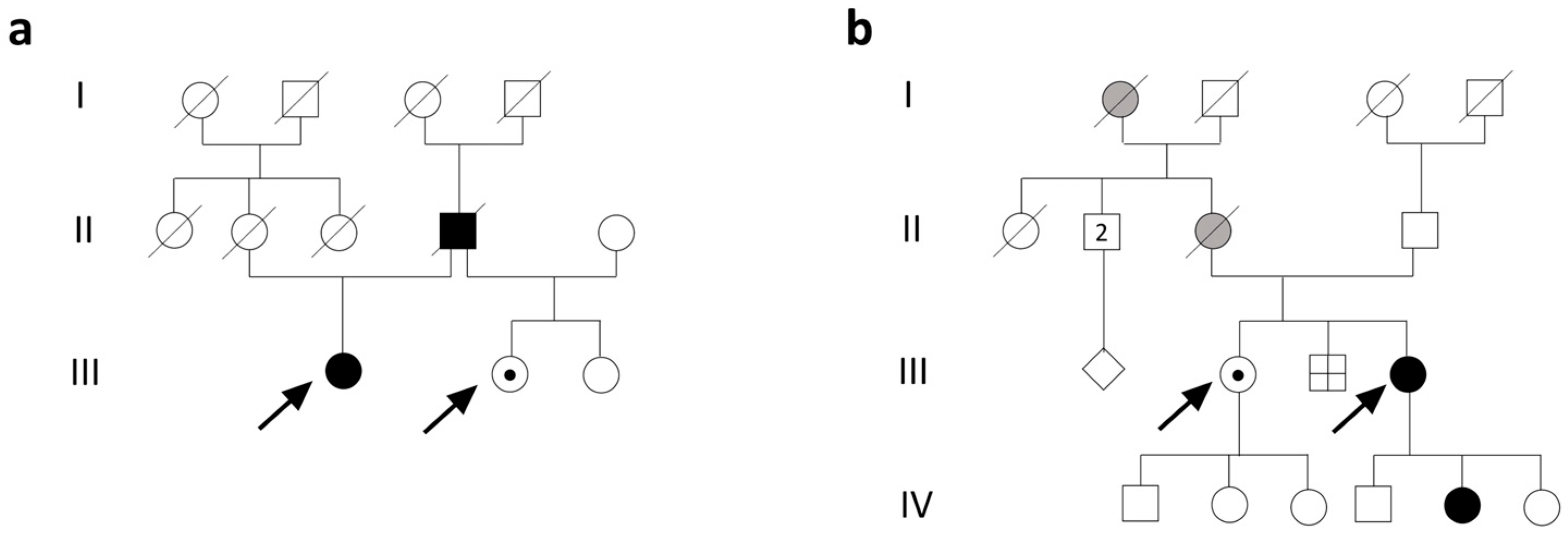
3.1. Clinical Description
3.1.1. Family 1
3.1.2. Family 2
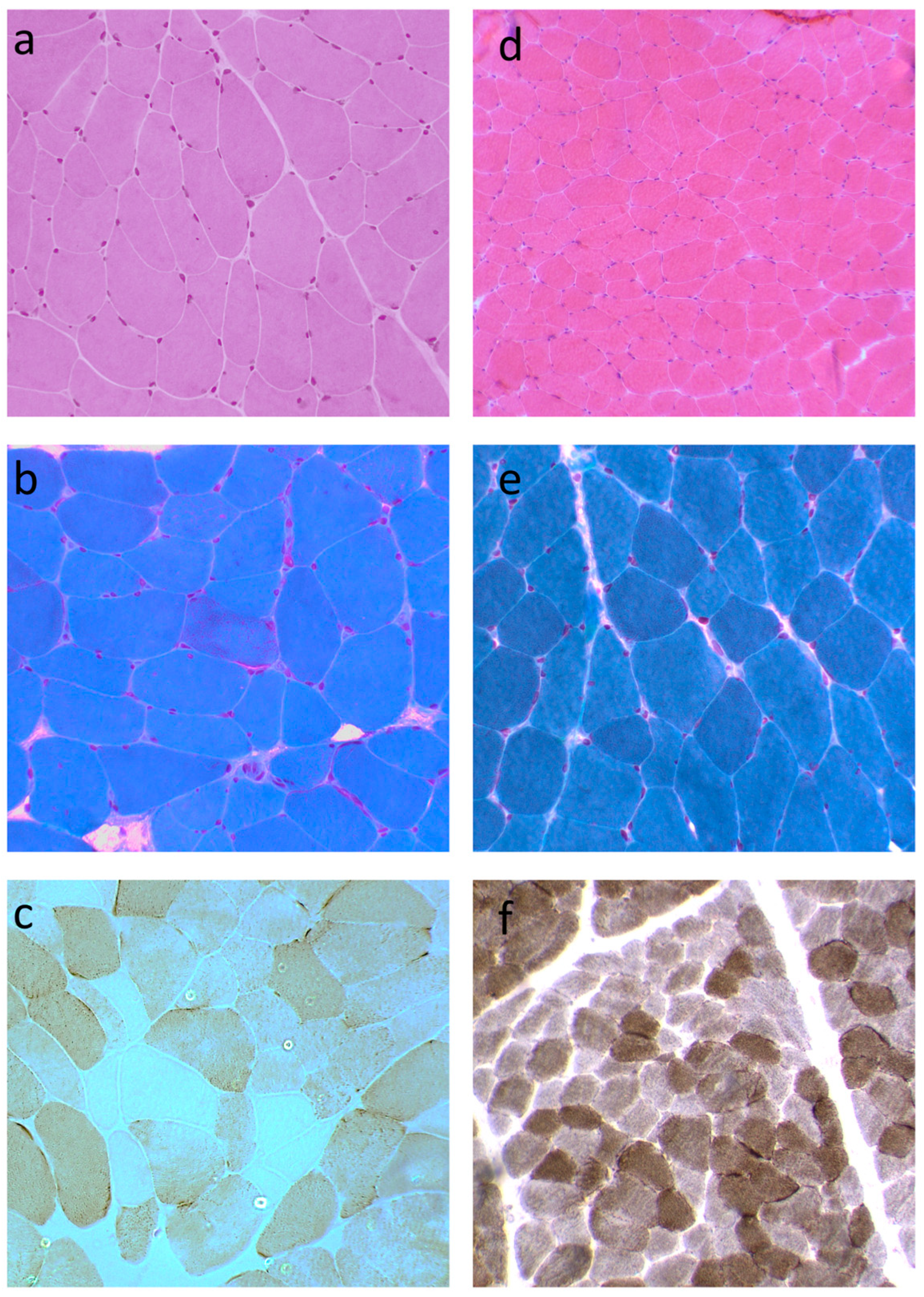
3.2. Histopathological Features
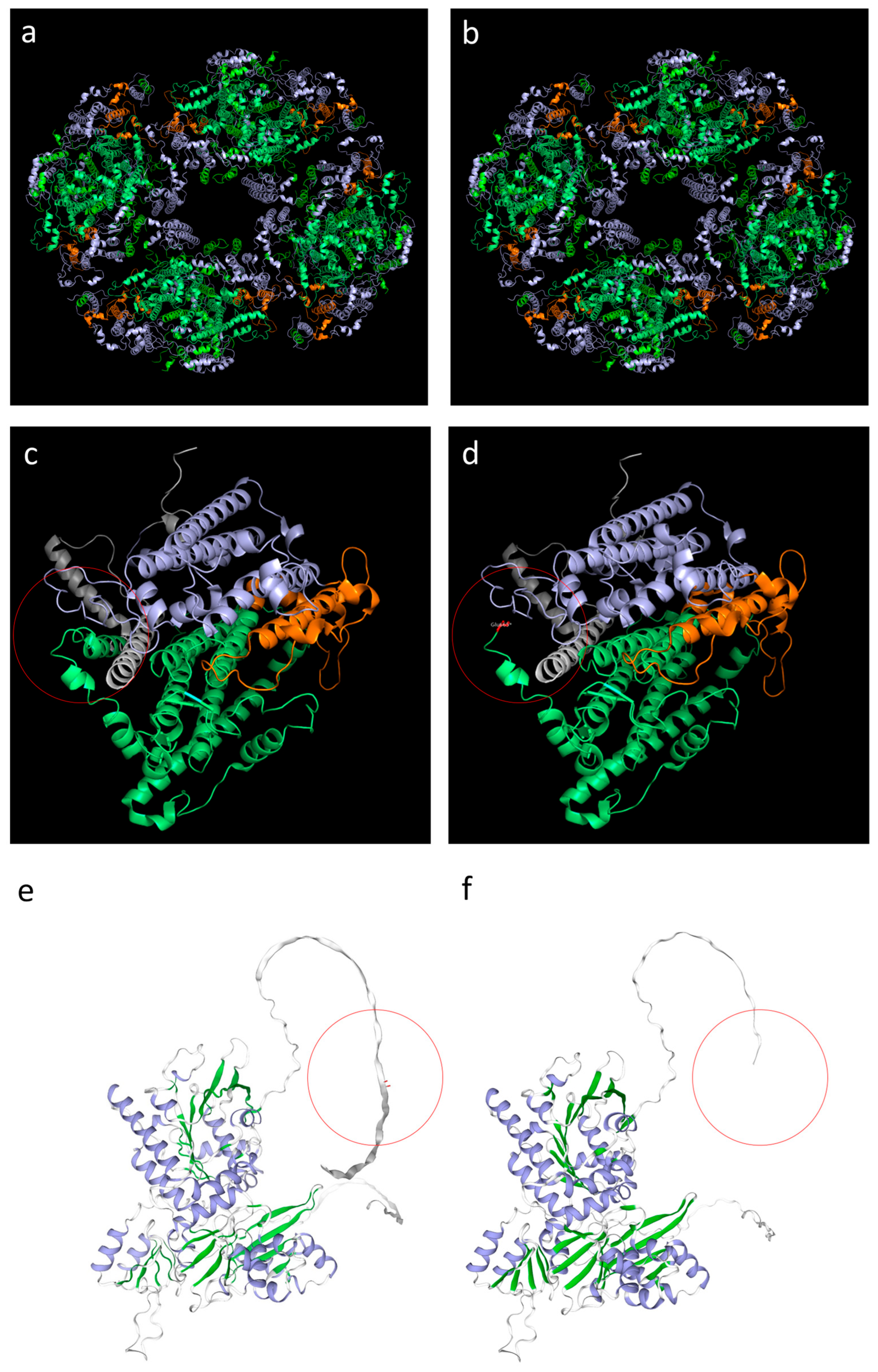
3.3. Genetic Analysis and 3D Structure Modeling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and Organization of the Human Mitochondrial Genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Fratter, C.; Gorman, G.S.; Stewart, J.D.; Buddles, M.; Smith, C.; Evans, J.; Seller, A.; Poulton, J.; Roberts, M.; Hanna, M.G.; et al. The Clinical, Histochemical, and Molecular Spectrum of PEO1 (Twinkle)-Linked AdPEO. Neurology 2010, 74, 1619–1626. [Google Scholar] [CrossRef]
- Spelbrink, J.N.; Li, F.Y.; Tiranti, V.; Nikali, K.; Yuan, Q.P.; Tariq, M.; Wanrooij, S.; Garrido, N.; Comi, G.; Morandi, L.; et al. Human Mitochondrial DNA Deletions Associated with Mutations in the Gene Encoding Twinkle, a Phage T7 Gene 4-like Protein Localized in Mitochondria. Nat. Genet. 2001, 28, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.A.; Gaspari, M.; Falkenberg, M. TWINKLE Has 5′ → 3′ DNA Helicase Activity and Is Specifically Stimulated by Mitochondrial Single-Stranded DNA-Binding Protein. J. Biol. Chem. 2003, 278, 48627–48632. [Google Scholar] [CrossRef]
- Falkenberg, M. Mitochondrial DNA Replication in Mammalian Cells: Overview of the Pathway. Essays Biochem. 2018, 62, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.G.; Gustafsson, C.M.; Falkenberg, M. Mechanisms and Regulation of Human Mitochondrial Transcription. Nat. Rev. Mol. Cell Biol. 2024, 25, 119–132. [Google Scholar] [CrossRef]
- Nikali, K.; Suomalainen, A.; Saharinen, J.; Kuokkanen, M.; Spelbrink, J.N.; Lönnqvist, T.; Peltonen, L. Infantile Onset Spinocerebellar Ataxia Is Caused by Recessive Mutations in Mitochondrial Proteins Twinkle and Twinky. Hum. Mol. Genet. 2005, 14, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, G.; Dermaut, B.; Löfgren, A.; Martin, J.J.; Van Broeckhoven, C. Mutation of POLG Is Associated with Progressive External Ophthalmoplegia Characterized by MtDNA Deletions. Nat. Genet. 2001, 28, 211–212. [Google Scholar] [CrossRef]
- Wanrooij, S.; Goffart, S.; Pohjoismäki, J.L.O.; Yasukawa, T.; Spelbrink, J.N. Expression of Catalytic Mutants of the MtDNA Helicase Twinkle and Polymerase POLG Causes Distinct Replication Stalling Phenotypes. Nucleic Acids Res. 2007, 35, 3238–3251. [Google Scholar] [CrossRef]
- Goh, V.; Helbling, D.; Biank, V.; Jarzembowski, J.; Dimmock, D. Next-Generation Sequencing Facilitates the Diagnosis in a Child with Twinkle Mutations Causing Cholestatic Liver Failure. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 291–294. [Google Scholar] [CrossRef]
- Domínguez-Ruiz, M.; García-Martínez, A.; Corral-Juan, M.; Pérez-Álvarez, Á.I.; Plasencia, A.M.; Villamar, M.; Moreno-Pelayo, M.A.; Matilla-Dueñas, A.; Menéndez-González, M.; Del Castillo, I. Perrault Syndrome with Neurological Features in a Compound Heterozygote for Two TWNK Mutations: Overlap of TWNK-Related Recessive Disorders. J. Transl. Med. 2019, 17, 290. [Google Scholar] [CrossRef]
- Hu, X.; Li, N.; Xu, Y.; Li, G.; Yu, T.; Yao, R.E.; Fu, L.; Wang, J.; Yin, L.; Yin, Y.; et al. Proband-Only Medical Exome Sequencing as a Cost-Effective First-Tier Genetic Diagnostic Test for Patients without Prior Molecular Tests and Clinical Diagnosis in a Developing Country: The China Experience. Genet. Med. 2018, 20, 1045–1053. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Tomelleri, G.; Palmucci, L.; Tonin, P.; Mongini, T.; Marini, M.; L’Erario, R.; Rizzuto, N.; Vattemi, G. SERCA1 and Calsequestrin Storage Myopathy: A New Surplus Protein Myopathy. Brain 2006, 129, 2085–2092. [Google Scholar] [CrossRef]
- Formichi, P.; Cardone, N.; Taglia, I.; Cardaioli, E.; Salvatore, S.; Gerfo, A.L.; Simoncini, C.; Montano, V.; Siciliano, G.; Mancuso, M.; et al. Fibroblast Growth Factor 21 and Grow Differentiation Factor 15 Are Sensitive Biomarkers of Mitochondrial Diseases Due to Mitochondrial Transfer-RNA Mutations and Mitochondrial DNA Deletions. Neurol. Sci. 2020, 41, 3653–3662. [Google Scholar] [CrossRef]
- Cardaioli, E.; Da Pozzo, P.; Malfatti, E.; Gallus, G.N.; Rubegni, A.; Malandrini, A.; Gaudiano, C.; Guidi, L.; Serni, G.; Berti, G.; et al. Chronic Progressive External Ophthalmoplegia: A New Heteroplasmic TRNALeu(CUN) Mutation of Mitochondrial DNA. J. Neurol. Sci. 2008, 272, 106–109. [Google Scholar] [CrossRef]
- Cardaioli, E.; Mignarri, A.; Cantisani, T.A.; Malandrini, A.; Nesti, C.; Rubegni, A.; Funel, N.; Federico, A.; Santorelli, F.M.; Dotti, M.T. Myoclonus Epilepsy, Retinitis Pigmentosa, Leukoencephalopathy and Cerebral Calcifications Associated with a Novel m.5513G>A Mutation in the MT-TW Gene. Biochem. Biophys. Res. Commun. 2018, 500, 158–162. [Google Scholar] [CrossRef]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and Revision of the Cambridge Reference Sequence for Human Mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Farge, G.; Mehmedovic, M.; Baclayon, M.; vandenWildenberg, S.M.J.L.; Roos, W.H.; Gustafsson, C.M.; Wuite, G.J.L.; Falkenberg, M. In Vitro-Reconstituted Nucleoids Can Block Mitochondrial DNA Replication and Transcription. Cell Rep. 2014, 8, 66–74. [Google Scholar] [CrossRef]
- Bermejo-Guerrero, L.; de Fuenmayor-Fernández de la Hoz, C.P.; Serrano-Lorenzo, P.; Blázquez-Encinar, A.; Gutiérrez-Gutiérrez, G.; Martínez-Vicente, L.; Galán-Dávila, L.; García-García, J.; Arenas, J.; Muelas, N.; et al. Clinical, Histological, and Genetic Features of 25 Patients with Autosomal Dominant Progressive External Ophthalmoplegia (Ad-PEO)/PEO-plus Due to TWNK Mutations. J. Clin. Med. 2022, 11, 22. [Google Scholar] [CrossRef]
- Virgilio, R.; Ronchi, D.; Hadjigeorgiou, G.M.; Bordoni, A.; Saladino, F.; Moggio, M.; Adobbati, L.; Kafetsouli, D.; Tsironi, E.; Previtali, S.; et al. Novel Twinkle (PEO1) Gene Mutations in Mendelian Progressive External Ophthalmoplegia. J. Neurol. 2008, 255, 1384–1391. [Google Scholar] [CrossRef]
- Fekete, B.; Pentelényi, K.; Rudas, G.; Gál, A.; Grosz, Z.; Illés, A.; Idris, J.; Csukly, G.; Domonkos, A.; Molnar, M.J. Broadening the Phenotype of the TWNK Gene Associated Perrault Syndrome. BMC Med. Genet. 2019, 20, 198. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; He, X.; Pu, S.; Yang, C.; Wu, Q.; Zhou, Z.; Cen, X.; Zhao, H. Mitochondrial Protein Dysfunction in Pathogenesis of Neurological Diseases. Front. Mol. Neurosci. 2022, 15, 974480. [Google Scholar] [CrossRef]
- Wen, H.; Deng, H.; Li, B.; Chen, J.; Zhu, J.; Zhang, X.; Yoshida, S.; Zhou, Y. Mitochondrial Diseases: From Molecular Mechanisms to Therapeutic Advances. Signal Transduct. Target. Ther. 2025, 10, 9. [Google Scholar] [CrossRef]
- Deschauer, M.; Kiefer, R.; Blakely, E.L.; He, L.; Zierz, S.; Turnbull, D.M.; Taylor, R.W. A Novel Twinkle Gene Mutation in Autosomal Dominant Progressive External Ophthalmoplegia. Neuromuscul. Disord. 2003, 13, 568–572. [Google Scholar] [CrossRef]
- Van Goethem, G.; Martin, J.J.; Dermaut, B.; Löfgren, A.; Wibail, A.; Ververken, D.; Tack, P.; Dehaene, I.; Van Zandijcke, M.; Moonen, M.; et al. Recessive POLG Mutations Presenting with Sensory and Ataxic Neuropathy in Compound Heterozygote Patients with Progressive External Ophthalmoplegia. Neuromuscul. Disord. 2003, 13, 133–142. [Google Scholar] [CrossRef]
- McFarland, R.; Taylor, R.W.; Turnbull, D.M. The Neurology of Mitochondrial DNA Disease. Lancet Neurol. 2002, 1, 343–351. [Google Scholar] [CrossRef]
- Korhonen, J.A.; Pham, X.H.; Pellegrini, M.; Falkenberg, M. Reconstitution of a Minimal MtDNA Replisome in Vitro. EMBO J. 2004, 23, 2423–2429. [Google Scholar] [CrossRef]
- Riccio, A.A.; Bouvette, J.; Perera, L.; Longley, M.J.; Krahn, J.M.; Williams, J.G.; Dutcher, R.; Borgnia, M.J.; Copeland, W.C. Structural Insight and Characterization of Human Twinkle Helicase in Mitochondrial Disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2207459119. [Google Scholar] [CrossRef]
- Fernández-Millán, P.; Lázaro, M.; Canslz-Arda, Ş.; Gerhold, J.M.; Rajala, N.; Schmitz, C.A.; Silva-Espiña, C.; Gil, D.; Bernadó, P.; Valle, M.; et al. The Hexameric Structure of the Human Mitochondrial Replicative Helicase Twinkle. Nucleic Acids Res. 2015, 43, 4284–4295. [Google Scholar] [CrossRef]
- Milenkovic, D.; Matic, S.; Kühl, I.; Ruzzenente, B.; Freyer, C.; Jemt, E.; Park, C.B.; Falkenberg, M.; Larsson, N.G. Twinkle Is an Essential Mitochondrial Helicase Required for Synthesis of Nascent D-Loop Strands and Complete MtDNA Replication. Hum. Mol. Genet. 2013, 22, 1983–1993. [Google Scholar] [CrossRef]
- Brown, T.A.; Cecconi, C.; Tkachuk, A.N.; Bustamante, C.; Clayton, D.A. Replication of Mitochondrial DNA Occurs by Strand Displacement with Alternative Light-Strand Origins, Not via a Strand-Coupled Mechanism. Genes. Dev. 2005, 19, 2466–2476. [Google Scholar] [CrossRef]
- Morino, H.; Pierce, S.B.; Matsuda, Y.; Walsh, T.; Ohsawa, R.; Newby, M.; Hiraki-Kamon, K.; Kuramochi, M.; Lee, M.K.; Klevit, R.E.; et al. Mutations in Twinkle Primase-Helicase Cause Perrault Syndrome with Neurologic Features. Neurology 2014, 83, 2054–2061. [Google Scholar] [CrossRef]
- Sen, D.; Patel, G.; Patel, S.S. Homologous DNA Strand Exchange Activity of the Human Mitochondrial DNA Helicase TWINKLE. Nucleic Acids Res. 2016, 44, 4200–4210. [Google Scholar] [CrossRef]
- Li, Z.; Kaur, P.; Lo, C.Y.; Chopra, N.; Smith, J.; Wang, H.; Gao, Y. Structural and Dynamic Basis of DNA Capture and Translocation by Mitochondrial Twinkle Helicase. Nucleic Acids Res. 2022, 50, 11965–11978. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein Degradation and Protection against Misfolded or Damaged Proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Ziebarth, T.D.; Farr, C.L.; Kaguni, L.S. Modular Architecture of the Hexameric Human Mitochondrial DNA Helicase. J. Mol. Biol. 2007, 367, 1382–1391. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopergolo, D.; Berti, G.; Gallus, G.N.; Bianchi, S.; Santorelli, F.M.; Malandrini, A.; De Stefano, N. The First Heterozygous TWNK Nonsense Mutation Associated with Progressive External Ophthalmoplegia: Evidence for a New Piece in the Puzzle of Mitochondrial Diseases. Biomolecules 2025, 15, 1337. https://doi.org/10.3390/biom15091337
Lopergolo D, Berti G, Gallus GN, Bianchi S, Santorelli FM, Malandrini A, De Stefano N. The First Heterozygous TWNK Nonsense Mutation Associated with Progressive External Ophthalmoplegia: Evidence for a New Piece in the Puzzle of Mitochondrial Diseases. Biomolecules. 2025; 15(9):1337. https://doi.org/10.3390/biom15091337
Chicago/Turabian StyleLopergolo, Diego, Gianna Berti, Gian Nicola Gallus, Silvia Bianchi, Filippo Maria Santorelli, Alessandro Malandrini, and Nicola De Stefano. 2025. "The First Heterozygous TWNK Nonsense Mutation Associated with Progressive External Ophthalmoplegia: Evidence for a New Piece in the Puzzle of Mitochondrial Diseases" Biomolecules 15, no. 9: 1337. https://doi.org/10.3390/biom15091337
APA StyleLopergolo, D., Berti, G., Gallus, G. N., Bianchi, S., Santorelli, F. M., Malandrini, A., & De Stefano, N. (2025). The First Heterozygous TWNK Nonsense Mutation Associated with Progressive External Ophthalmoplegia: Evidence for a New Piece in the Puzzle of Mitochondrial Diseases. Biomolecules, 15(9), 1337. https://doi.org/10.3390/biom15091337





