MiR-92 Controls Synaptic Development Through Glial Vha55 Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Drosophila Strains and Genetics
2.2. Bioinformatics
2.3. Microarray
2.4. Immunohistochemistry and Quantification of NMJ Development
2.5. Image Analysis and Quantification
2.6. Electrophysiology
2.7. Dextran Fluorescence
2.8. Potassium Stimulation Assay
2.9. Transmission Electron Microscopy
2.10. Summary of Statistical Analysis
3. Results
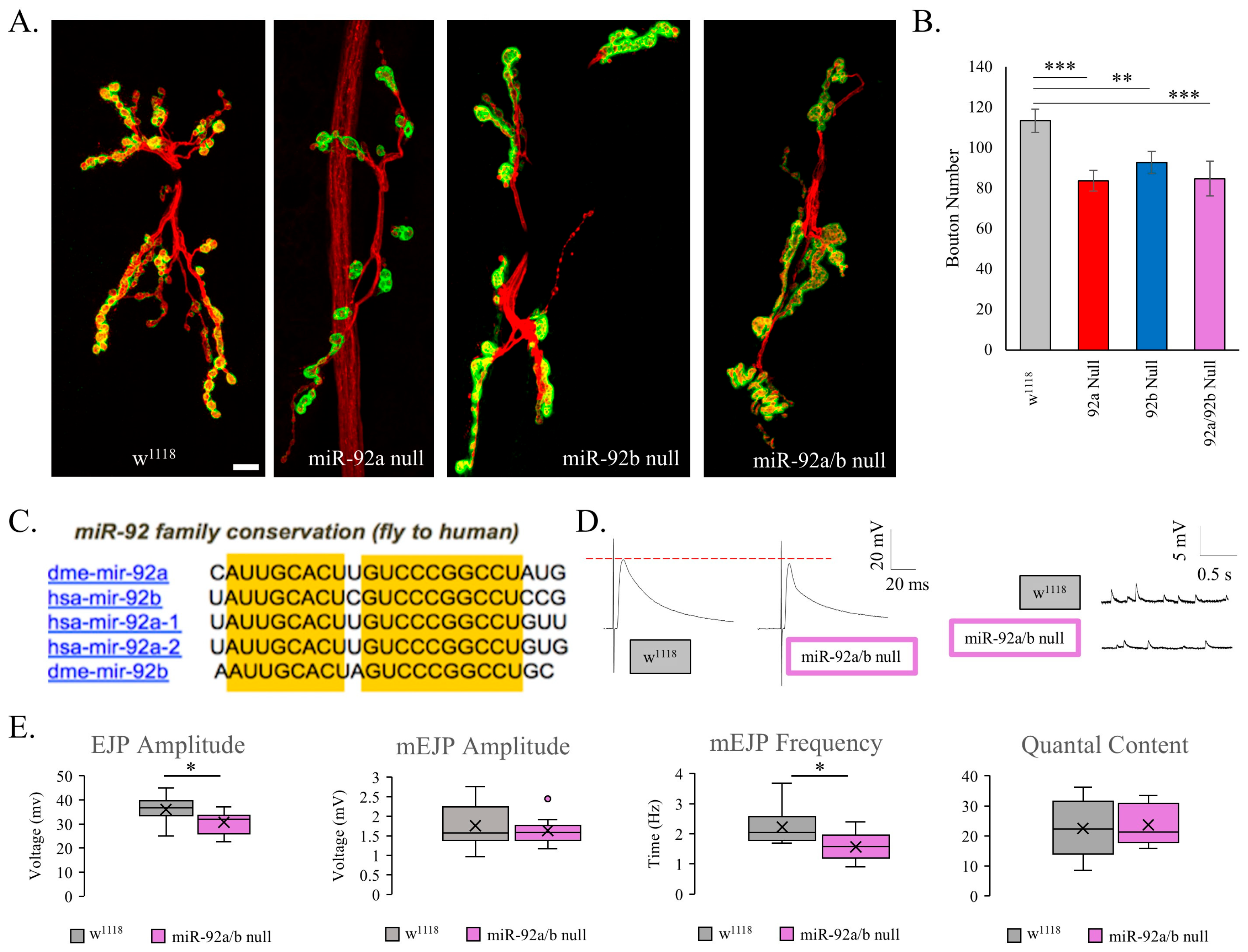
3.1. Elucidating the Role of miR-92 in Synaptogenesis
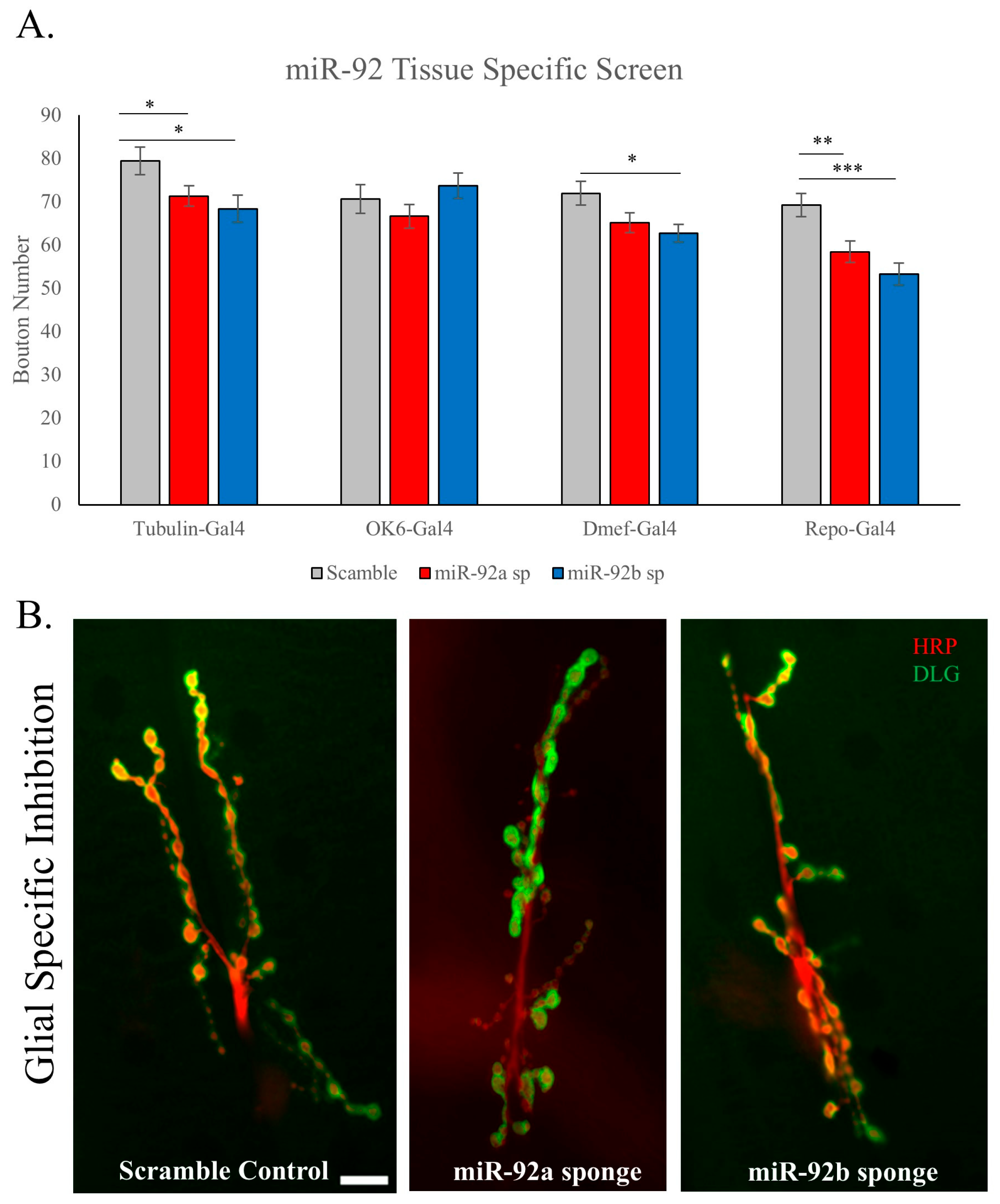
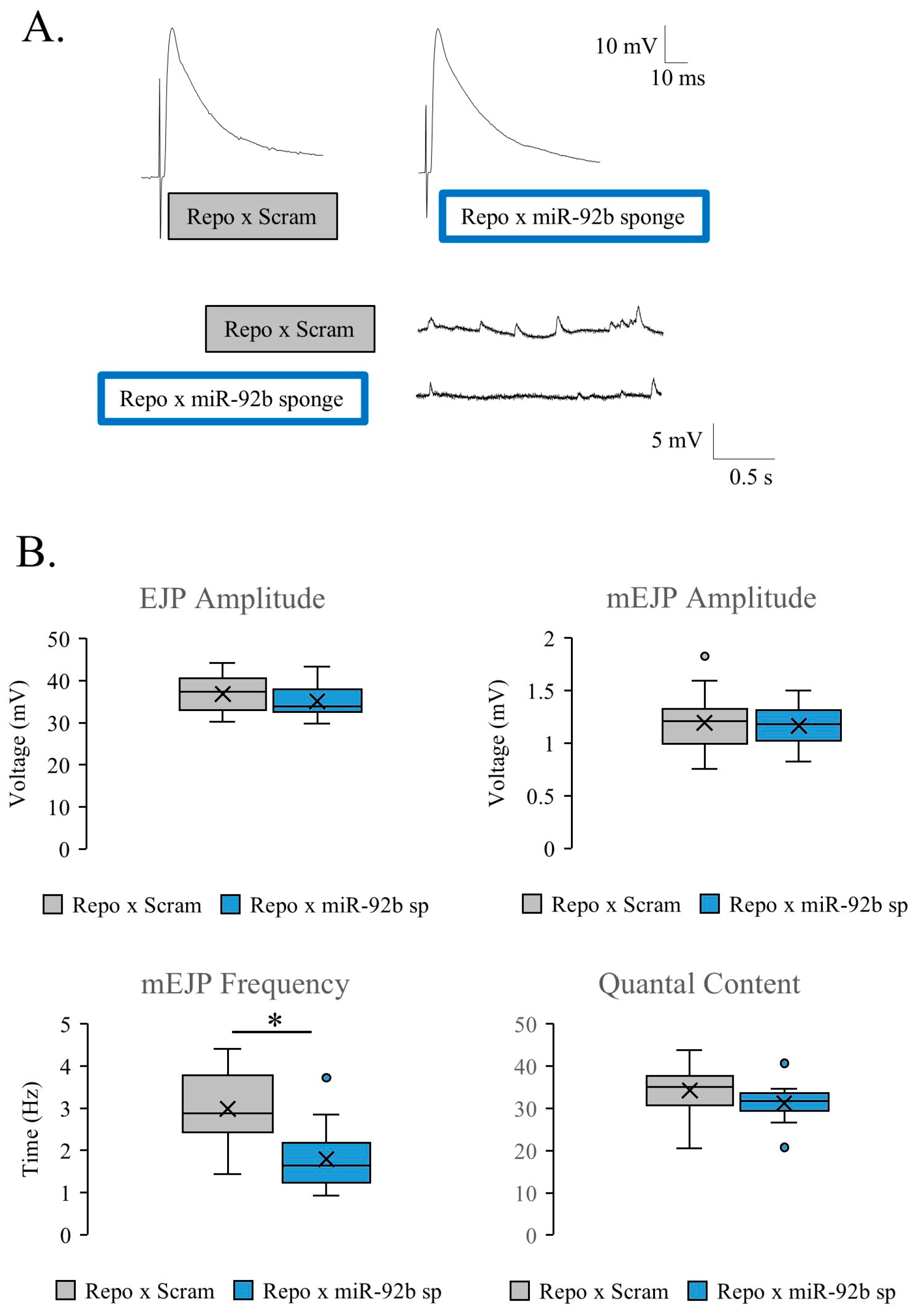
3.2. Glial-Specific Activity of miR-92
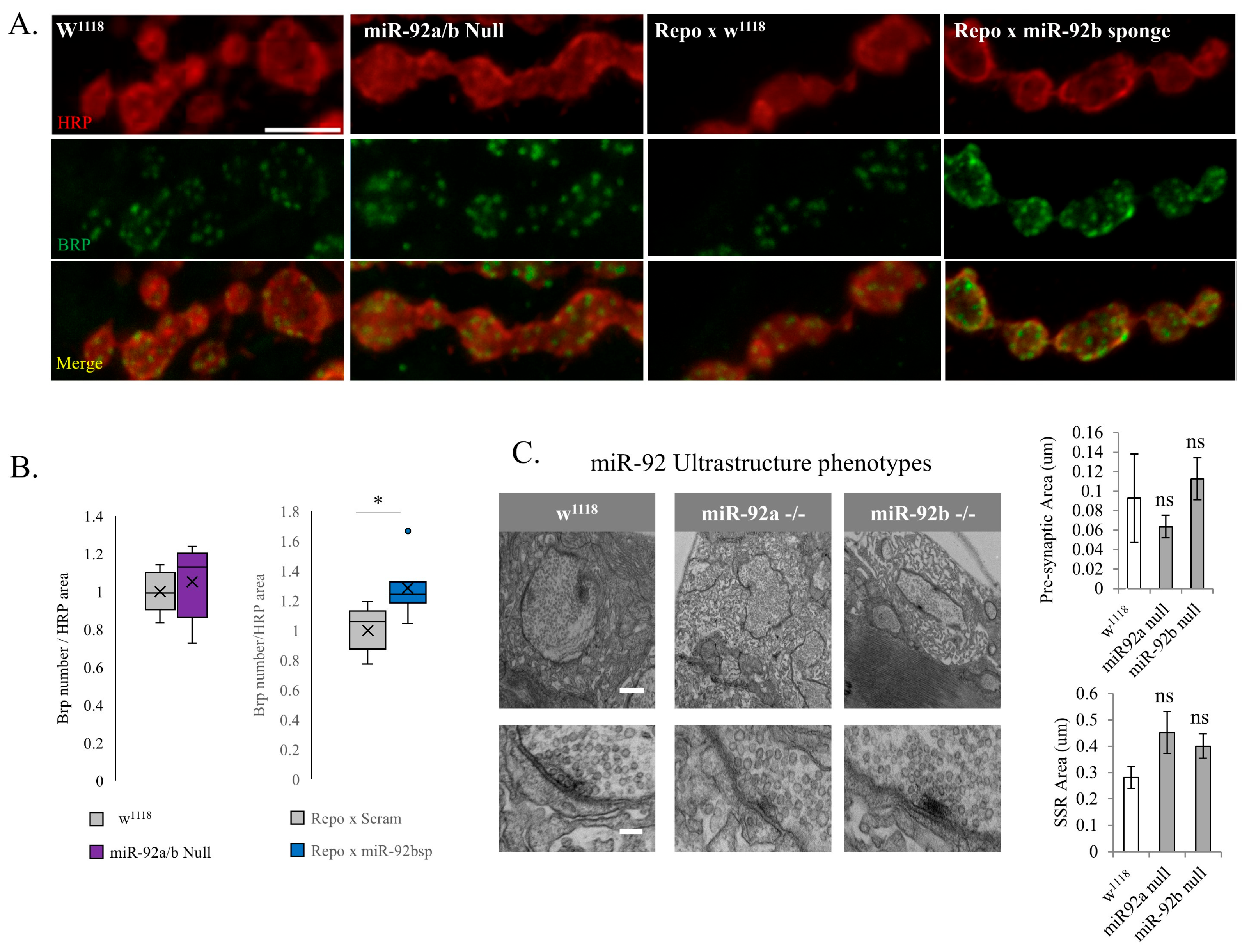
3.3. Synaptic Machinery and Ultrastructure in miR-92 Loss
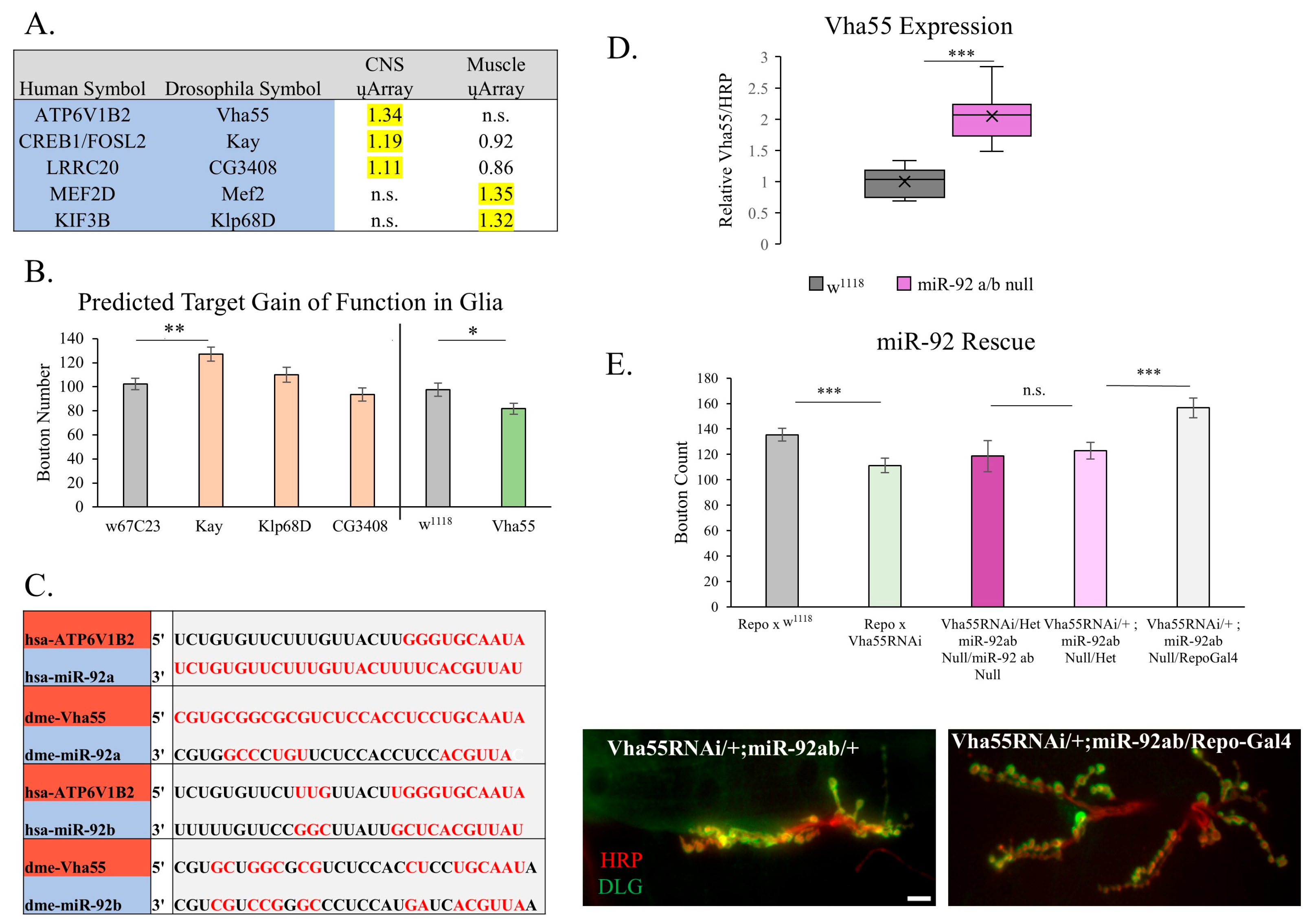
3.4. Targets of miR-92
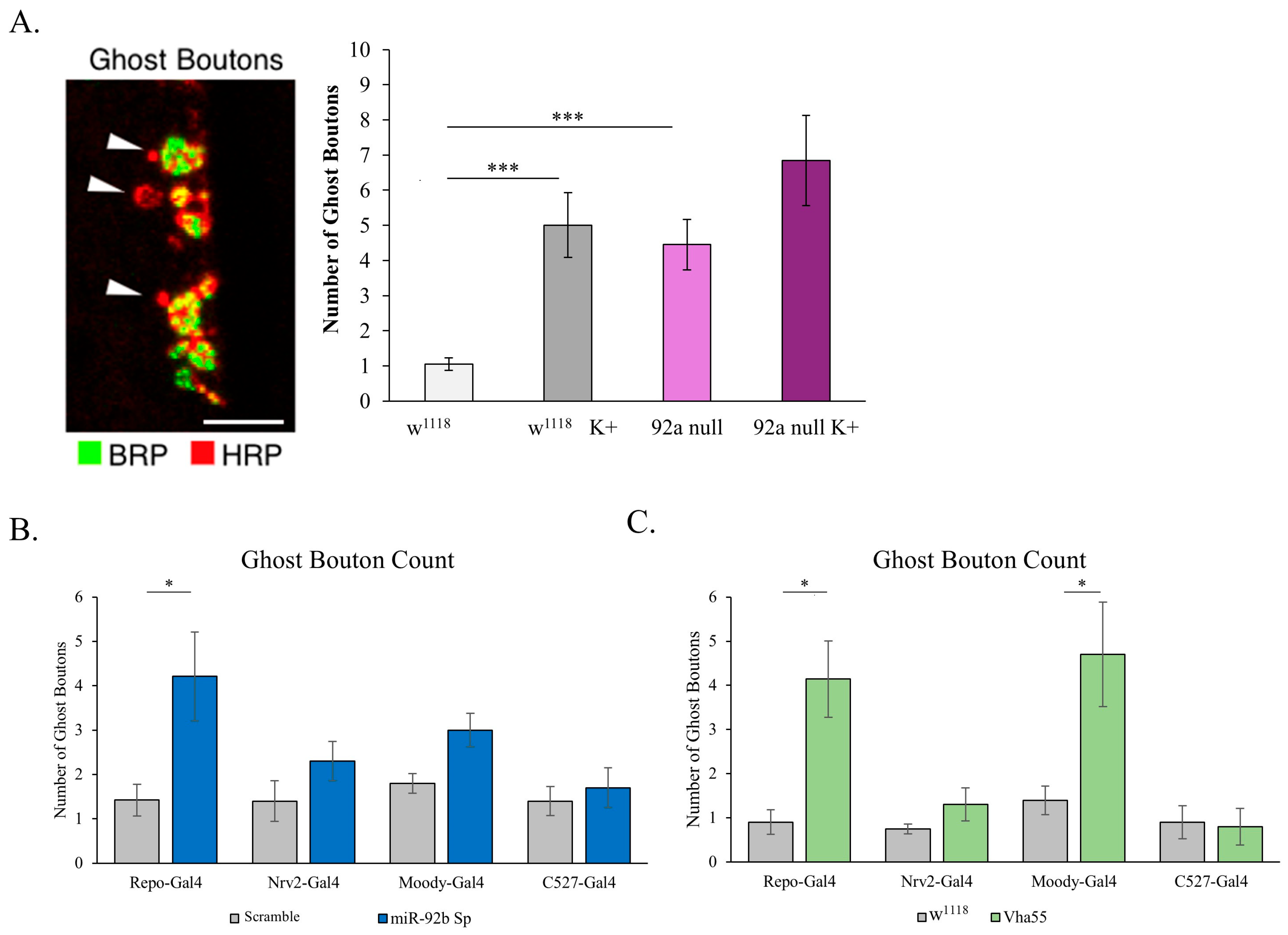
3.5. Disruption of Glial Barrier Capabilities
3.6. miR-92 Glial Subtype Specificity
3.7. Modifications in Bouton Addition
4. Discussion
4.1. Glial Involvement in Synaptic Development
4.2. miR-92 Regulation of NMJ Morphology and Function
4.3. Potential Targets of miR-92
4.4. Involvement of the Subperineurial Glia
4.5. Human Disease
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood brain barrier |
| NMJ | neuromuscular junction |
| BRP | bruchpilot |
| GPCR | G-protein coupled receptor |
| SPG | subperineurial glia |
| PG | perineurial glia |
| WG | wrapping glia |
| RRP | readily releasable pool |
| CNS | central nervous system |
| PNS | peripheral nervous system |
| HRP | horseradish peroxidase |
References
- Konopaske, G.T.; Lange, N.; Coyle, J.T.; Benes, F.M. Prefrontal Cortical Dendritic Spine Pathology in Schizophrenia and Bipolar Disorder. JAMA Psychiatry 2014, 71, 1323–1331. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. MicroRNA Functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Rodrigues, B.; Fernandes, J.; Santos, S.D.; Carreto, L.; Santos, M.A.S.; Pinheiro, P.; Carvalho, A.L. MicroRNA-186-5p Controls GluA2 Surface Expression and Synaptic Scaling in Hippocampal Neurons. Proc. Natl. Acad. Sci. USA 2019, 116, 5727–5736. [Google Scholar] [CrossRef] [PubMed]
- McNeill, E.M.; Warinner, C.; Alkins, S.; Taylor, A.; Heggeness, H.; DeLuca, T.F.; Fulga, T.A.; Wall, D.P.; Griffith, L.C.; Van Vactor, D. The Conserved MicroRNA MiR-34 Regulates Synaptogenesis via Coordination of Distinct Mechanisms in Presynaptic and Postsynaptic Cells. Nat. Commun. 2020, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Loya, C.M.; Lu, C.S.; Van Vactor, D.; Fulga, T.A. Transgenic MicroRNA Inhibition with Spatiotemporal Specificity in Intact Organisms. Nat. Methods 2009, 6, 897–903. [Google Scholar] [CrossRef]
- Ma, J.; Shang, S.; Wang, J.; Zhang, T.; Nie, F.; Song, X.; Zhao, H.; Zhu, C.; Zhang, R.; Hao, D. Identification of MiR-22-3p, MiR-92a-3p, and MiR-137 in Peripheral Blood as Biomarker for Schizophrenia. Psychiatry Res. 2018, 265, 70–76. [Google Scholar] [CrossRef]
- Yuva-Aydemir, Y.; Xu, X.L.; Aydemir, O.; Gascon, E.; Sayin, S.; Zhou, W.; Hong, Y.; Gao, F.B. Downregulation of the Host Gene Jigr1 by MiR-92 Is Essential for Neuroblast Self-Renewal in Drosophila. PLoS Genet. 2015, 11, e1005264. [Google Scholar] [CrossRef]
- Guven-Ozkan, T.; Busto, G.U.; Jung, J.Y.; Drago, I.; Davis, R.L. Mir-92a Suppresses Mushroom Body-Dependent Memory Consolidation in Drosophila. eNeuro 2020, 7, ENEURO.0224–20.2020. [Google Scholar] [CrossRef]
- Bai, X.; Hua, S.; Zhang, J.; Xu, S. The MicroRNA Family Both in Normal Development and in Different Diseases: The MiR-17-92 Cluster. BioMed Res. Int. 2019, 2019, 9450240. [Google Scholar] [CrossRef]
- Donelson, N.C.; Dixit, R.; Pichardo-Casas, I.; Chiu, E.Y.; Ohman, R.T.; Slawson, J.B.; Klein, M.; Fulga, T.A.; Van Vactor, D.; Griffith, L.C. MicroRNAs Regulate Multiple Aspects of Locomotor Behavior in Drosophila. G3 Genes Genomes Genet. 2020, 10, 43–55. [Google Scholar] [CrossRef]
- Mogilyansky, E.; Rigoutsos, I. The MiR-17/92 Cluster: A Comprehensive Update on Its Genomics, Genetics, Functions and Increasingly Important and Numerous Roles in Health and Disease. Cell Death Differ. 2013, 20, 1603–1614. [Google Scholar] [CrossRef]
- Frank, C.A.; James, T.D.; Müller, M. Homeostatic Control of Drosophila Neuromuscular Junction Function. Synapse 2020, 74, e22133. [Google Scholar] [CrossRef]
- Bykhovskaia, M.; Vasin, A. Electrophysiological Analysis of Synaptic Transmission in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e277. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.B. GAL4 System in Drosophila: A Fly Geneticist’s Swiss Army Knife. Genesis 2002, 34, 1–15. [Google Scholar] [CrossRef]
- Fulga, T.A.; McNeill, E.M.; Binari, R.; Yelick, J.; Blanche, A.; Booker, M.; Steinkraus, B.R.; Schnall-Levin, M.; Zhao, Y.; Deluca, T.; et al. A Transgenic Resource for Conditional Competitive Inhibition of Conserved Drosophila MicroRNAs. Nat. Commun. 2015, 6, 7279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Song, S.; Weng, R.; Verma, P.; Kugler, J.M.; Buescher, M.; Rouam, S.; Cohen, S.M. Systematic Study of Drosophila MicroRNA Functions Using a Collection of Targeted Knockout Mutations. Dev. Cell 2014, 31, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-MicroT Web Server v5.0: Service Integration into MiRNA Functional Analysis Workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef]
- Chatziioannou, A.; Moulos, P.; Kolisis, F.N. Gene ARMADA: An Integrated Multi-Analysis Platform for Microarray Data Implemented in MATLAB. BMC Bioinform. 2009, 10, 354. [Google Scholar] [CrossRef]
- Imlach, W.; McCabe, B.D. Electrophysiological Methods for Recording Synaptic Potentials from the NMJ of Drosophila Larvae. J. Vis. Exp. 2009, 6, e1109. [Google Scholar] [CrossRef]
- Hatan, M.; Shinder, V.; Israeli, D.; Schnorrer, F.; Volk, T. The Drosophila Blood Brain Barrier Is Maintained by GPCR-Dependent Dynamic Actin Structures. J. Cell Biol. 2011, 192, 307–319. [Google Scholar] [CrossRef]
- Ataman, B.; Ashley, J.; Gorczyca, M.; Ramachandran, P.; Fouquet, W.; Sigrist, S.J.; Budnik, V. Rapid Activity-Dependent Modifications in Synaptic Structure and Function Require Bidirectional Wnt Signaling. Neuron 2008, 57, 705–718. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved Stability of Drosophila Larval Neuromuscular Preparations in Haemolymph-like Physiological Solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef]
- Koenig, J.H.; Kosaka, T.; Lkeda, K. The Relationship Between the Number of Synaptic Vesicles and the Amount of Transmitter Released. J. Neurosci. 1989, 9, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Vicidomini, R.; Ramos, C.I.; Wang, Q.; Nguyen, P.; Jarnik, M.; Lee, C.H.; Stawarski, M.; Hernandez, R.X.; Macleod, G.T.; et al. Neto-α Controls Synapse Organization and Homeostasis at the Drosophila Neuromuscular Junction. Cell Rep. 2020, 32, 107886. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.A.; Fetter, R.D.; Noordermeer, J.N.; Goodman, C.S.; Diantonio, A. Genetic Analysis of Glutamate Receptors in Drosophila Reveals a Retrograde Signal Regulating Presynaptic Transmitter Release. Neuron 1997, 19, 1237–1248. [Google Scholar] [CrossRef]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klämbt, C. Organization and Function of the Blood-Brain Barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Littleton, J.T.; Broadie, K.; Bhat, M.A.; Harbecke, R.; Lengyel, J.A.; Chiquet-Ehrismann, R.; Prokop, A.; Bellen, H.J. A Drosophila Neurexin Is Required for Septate Junction and Blood-Nerve Barrier Formation and Function. Cell 1996, 87, 1059–1068. [Google Scholar] [CrossRef]
- Chou, V.T.; Johnson, S.A.; Van Vactor, D. Synapse Development and Maturation at the Drosophila Neuromuscular Junction. Neural Dev. 2020, 15, 11. [Google Scholar] [CrossRef]
- Chen, T.-H.; Chen, J.-A. Multifaceted Roles of MicroRNAs: From Motor Neuron Generation in Embryos to Degeneration in Spinal Muscular Atrophy. eLife 2019, 8, e50848. [Google Scholar] [CrossRef]
- Brink, D.L.; Gilbert, M.; Xie, X.; Petley-Ragan, L.; Auld, V.J. Glial Processes at the Drosophila Larval Neuromuscular Junction Match Synaptic Growth. PLoS ONE 2012, 7, e37876. [Google Scholar] [CrossRef]
- Kerr, K.S.; Fuentes-Medel, Y.; Brewer, C.; Barria, R.; Ashley, J.; Abruzzi, K.C.; Sheehan, A.; Tasdemir-Yilmaz, O.E.; Freeman, M.R.; Budnik, V. Glial Wingless/Wnt Regulates Glutamate Receptor Clustering and Synaptic Physiology at the Drosophila Neuromuscular Junction. J. Neurosci. 2014, 34, 2910–2920. [Google Scholar] [CrossRef]
- Kremer, M.C.; Jung, C.; Batelli, S.; Rubin, G.M.; Gaul, U. The Glia of the Adult Drosophila Nervous System. Glia 2017, 65, 606–638. [Google Scholar] [CrossRef]
- Wang, T.; Morency, D.T.; Harris, N.; Davis, G.W. Epigenetic Signaling in Glia Controls Presynaptic Homeostatic Plasticity. Neuron 2020, 105, 491–505.e3. [Google Scholar] [CrossRef]
- Pogue, A.I.; Cui, J.G.; Li, Y.Y.; Zhao, Y.; Culicchia, F.; Lukiw, W.J. Micro RNA-125b (MiRNA-125b) Function in Astrogliosis and Glial Cell Proliferation. Neurosci. Lett. 2010, 476, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-Neuron Transfer of MiRNAs via Extracellular Vesicles: A New Mechanism Underlying Inflammation-Induced Synaptic Alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-W.; Sung, H.-H.; Li, J.-C.; Yeh, C.-Y.; Chen, P.-Y.; Cheng, Y.-J.; Chen, C.-H.; Tsai, Y.-C.; Chien, C.-T. Glia-Derived Exosomal MiR-274 Targets Sprouty in Trachea and Synaptic Boutons to Modulate Growth and Responses to Hypoxia. Proc. Natl. Acad. Sci. USA 2019, 116, 24651–24661. [Google Scholar] [CrossRef]
- Melom, J.E.; Akbergenova, Y.; Gavornik, J.P.; Littleton, J.T. Spontaneous and Evoked Release Are Independently Regulated at Individual Active Zones. J. Neurosci. 2013, 33, 17253–17263. [Google Scholar] [CrossRef]
- Koles, K.; Budnik, V. Wnt Signaling in Neuromuscular Junction Development. Perspect. Biol. 2012, 4, a008045. [Google Scholar] [CrossRef]
- Alfonso-Gonzalez, C.; Riesgo-Escovar, J.R. Fos Metamorphoses: Lessons from Mutants in Model Organisms. Mech. Dev. 2018, 154, 73–81. [Google Scholar] [CrossRef]
- Kim, S.M.; Kumar, V.; Lin, Y.Q.; Karunanithi, S.; Ramaswami, M. Fos and Jun Potentiate Individual Release Sites and Mobilize the Reserve Synaptic-Vesicle Pool at the Drosophila Larval Motor Synapse. Proc Natl Acad. Sci. USA 2009, 106, 4000–4005. [Google Scholar] [CrossRef]
- Hartwig, C.L.; Worrell, J.; Levine, R.B.; Ramaswami, M.; Sanyal, S. Normal Dendrite Growth in Drosophila Motor Neurons Requires the AP-1 Transcription Factor. Dev. Neurobiol. 2008, 68, 1225–1242. [Google Scholar] [CrossRef]
- Marshansky, V.; Futai, M. The V-Type H+-ATPase in Vesicular Trafficking: Targeting, Regulation and Function. Curr Opin Cell Biol. 2008, 20, 415–426. [Google Scholar] [CrossRef]
- Davies, S.A.; Goodwin, S.F.; Kelly, D.C.; Wang, Z.; Ali Sözen, M.; Kaiser, K.; Dow, J.A.T. Analysis and Inactivation of Vha55, the Gene Encoding the Vacuolar ATPase B-Subunit in Drosophila Melanogaster Reveals a Larval Lethal Phenotype. J. Biol. Chem. 1996, 271, 30677–30684. [Google Scholar] [CrossRef] [PubMed]
- Gonda, X.; Eszlari, N.; Anderson, I.M.; Deakin, J.F.W.; Bagdy, G.; Juhasz, G. Association of ATP6V1B2 Rs1106634 with Lifetime Risk of Depression and Hippocampal Neurocognitive Deficits: Possible Novel Mechanisms in the Etiopathology of Depression. Transl. Psychiatry 2016, 6, e945. [Google Scholar] [CrossRef]
- Du, J.; Kean, L.; Allan, A.K.; Southall, T.D.; Davies, S.A.; McInerny, C.J.; Dow, J.A.T. The SzA Mutations of the B Subunit of the Drosophila Vacuolar H+ ATPase Identify Conserved Residues Essential for Function in Fly and Yeast. J. Cell Sci. 2006, 119, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Mauvezin, C.; Nagy, P.; Juhász, G.; Neufeld, T.P. Autophagosome-Lysosome Fusion Is Independent of V-ATPase-Mediated Acidification. Nat. Commun. 2015, 6, 7007. [Google Scholar] [CrossRef]
- Vaccari, T.; Duchi, S.; Cortese, K.; Tacchetti, C.; Bilder, D. The Vacuolar ATPase Is Required for Physiological as Well as Pathological Activation of the Notch Receptor. Development 2010, 137, 1825–1832. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Selma-Soriano, E.; Ortega, A.; Cortes, R.; Redon, J. Small Rab Gtpases in Intracellular Vesicle Trafficking: The Case of Rab3a/Raphillin-3a Complex in the Kidney. Int. J. Mol. Sci. 2021, 22, 7679. [Google Scholar] [CrossRef]
- Yildirim, K.; Petri, J.; Kottmeier, R.; Klämbt, C. Drosophila Glia: Few Cell Types and Many Conserved Functions. Glia 2018, 67, 5–26. [Google Scholar] [CrossRef]
- Freeman, M.R. Drosophila Central Nervous System Glia. Cold Spring Harb. Perspect. Biol. 2015, 7, a020552. [Google Scholar] [CrossRef]
- Schmidt, I.; Thomas, S.; Kain, P.; Risse, B.; Naffin, E.; Klämbt, C. Kinesin Heavy Chain Function in Drosophila Glial Cells Controls Neuronal Activity. J. Neurosci. 2012, 32, 7466–7476. [Google Scholar] [CrossRef]
- Tiklová, K.; Senti, K.A.; Wang, S.; GräCurrency Signslund, A.; Samakovlis, C. Epithelial Septate Junction Assembly Relies on Melanotransferrin Iron Binding and Endocytosis in Drosophila. Nat. Cell Biol. 2010, 12, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Babatz, F.; Naffin, E.; Klämbt, C. The Drosophila Blood-Brain Barrier Adapts to Cell Growth by Unfolding of Pre-Existing Septate Junctions. Dev. Cell 2018, 47, 697–710.e3. [Google Scholar] [CrossRef] [PubMed]
- Böhme, M.A.; McCarthy, A.W.; Blaum, N.; Berezeckaja, M.; Ponimaskine, K.; Schwefel, D.; Walter, A.M. Glial Synaptobrevin Mediates Peripheral Nerve Insulation, Neural Metabolic Supply, and Is Required for Motor Function. Glia 2021, 69, 1897–1915. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.R.; Mori, M.; Kauwe, G.; Farnsworth, J.; Ulian-Benitez, S.; Maksoud, E.; Shore, J.; Haghighi, A.P. Delta/Notch signaling in glia maintains motor nerve barrier function and synaptic transmission by controlling matrix metalloproteinase expression. Proc. Natl. Acad. Sci. USA 2022, 119, e2110097119. [Google Scholar] [CrossRef]
- Futai, M.; Sun-Wada, G.H.; Wada, Y.; Matsumoto, N.; Nakanishi-Matsui, M. Vacuolar-Type ATPase: A Proton Pump to Lysosomal Trafficking. Proc. Jpn. Acad. Ser. B Phys Biol Sci. 2019, 95, 261–277. [Google Scholar] [CrossRef]
- Lautemann, J.; Bohrmann, J. Relating Proton Pumps with Gap Junctions: Colocalization of Ductin, the Channel-Forming Subunit c of V-ATPase, with Subunit a and with Innexins 2 and 3 during Drosophila Oogenesis. BMC Dev. Biol. 2016, 16, 24. [Google Scholar] [CrossRef]
- Coyle, J.T.; Brad Ruzicka, W.; Balu, D.T. Fifty Years of Research on Schizophrenia: The Ascendance of the Glutamatergic Synapse. Am. J. Psychiatry 2020, 177, 1119–1128. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Blood-Brain Barrier Associated Tight Junction Disruption Is a Hallmark Feature of Major Psychiatric Disorders. Transl. Psychiatry 2020, 10, 373. [Google Scholar] [CrossRef]
- Nakamura-Palacios, E.M.; Lopes, I.B.C.; Souza, R.A.; Klauss, J.; Batista, E.K.; Conti, C.L.; Moscon, J.A.; de Souza, R.S.M. Ventral Medial Prefrontal Cortex (VmPFC) as a Target of the Dorsolateral Prefrontal Modulation by Transcranial Direct Current Stimulation (TDCS) in Drug Addiction. J. Neural Transm. 2016, 123, 1179–1194. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moe, S.M.; Taylor, A.; Robertson, A.P.; Van Vactor, D.; McNeill, E.M. MiR-92 Controls Synaptic Development Through Glial Vha55 Regulation. Biomolecules 2025, 15, 1330. https://doi.org/10.3390/biom15091330
Moe SM, Taylor A, Robertson AP, Van Vactor D, McNeill EM. MiR-92 Controls Synaptic Development Through Glial Vha55 Regulation. Biomolecules. 2025; 15(9):1330. https://doi.org/10.3390/biom15091330
Chicago/Turabian StyleMoe, Simon M., Alicia Taylor, Alan P. Robertson, David Van Vactor, and Elizabeth M. McNeill. 2025. "MiR-92 Controls Synaptic Development Through Glial Vha55 Regulation" Biomolecules 15, no. 9: 1330. https://doi.org/10.3390/biom15091330
APA StyleMoe, S. M., Taylor, A., Robertson, A. P., Van Vactor, D., & McNeill, E. M. (2025). MiR-92 Controls Synaptic Development Through Glial Vha55 Regulation. Biomolecules, 15(9), 1330. https://doi.org/10.3390/biom15091330




