N-Terminal Pro-B-Type Natriuretic Peptide and Cardiac Troponin T in Stable Renal Transplant Recipients and All-Cause Mortality, Cardiovascular, and Renal Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Outcomes
2.2. Follow-Up
2.3. Statistical Analyses
2.3.1. Thin-Plate Spline Explanation
2.3.2. Winsorisation Explanation
- •
- Standardized point HR (population-standardized, point-specific): the hazard ratio predicted if everyone were set to a specific exposure value (e.g., quartile cut-point or model-derived cutoff), averaged over the cohort’s covariate distribution.
- •
- Subgroup-average HR (interval) (as-observed): the average of model-predicted hazard ratios among participants whose observed exposure falls within a given interval (e.g., below vs. above the cutoff; quartiles).
3. Results
3.1. Study Population Characteristics
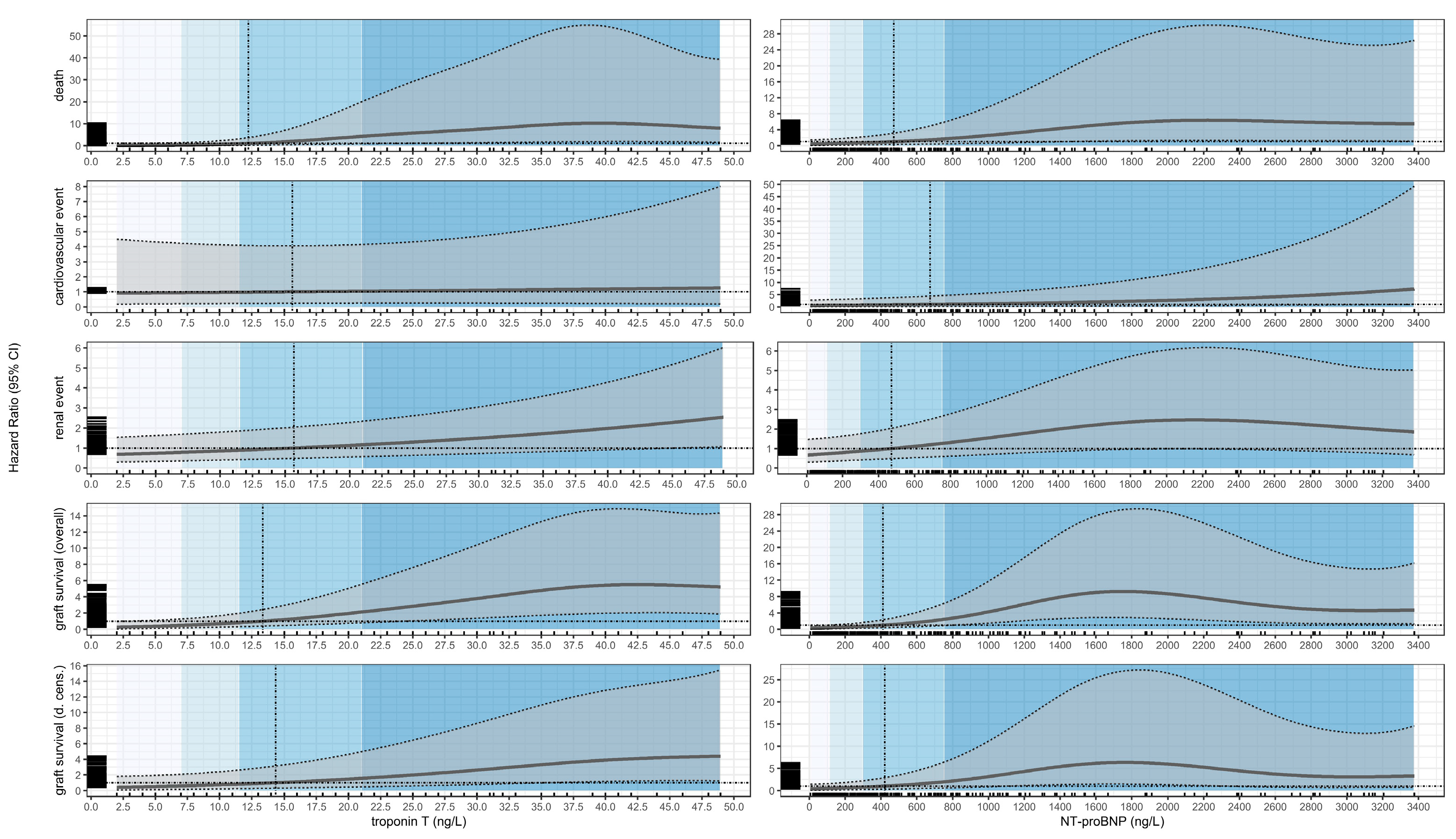
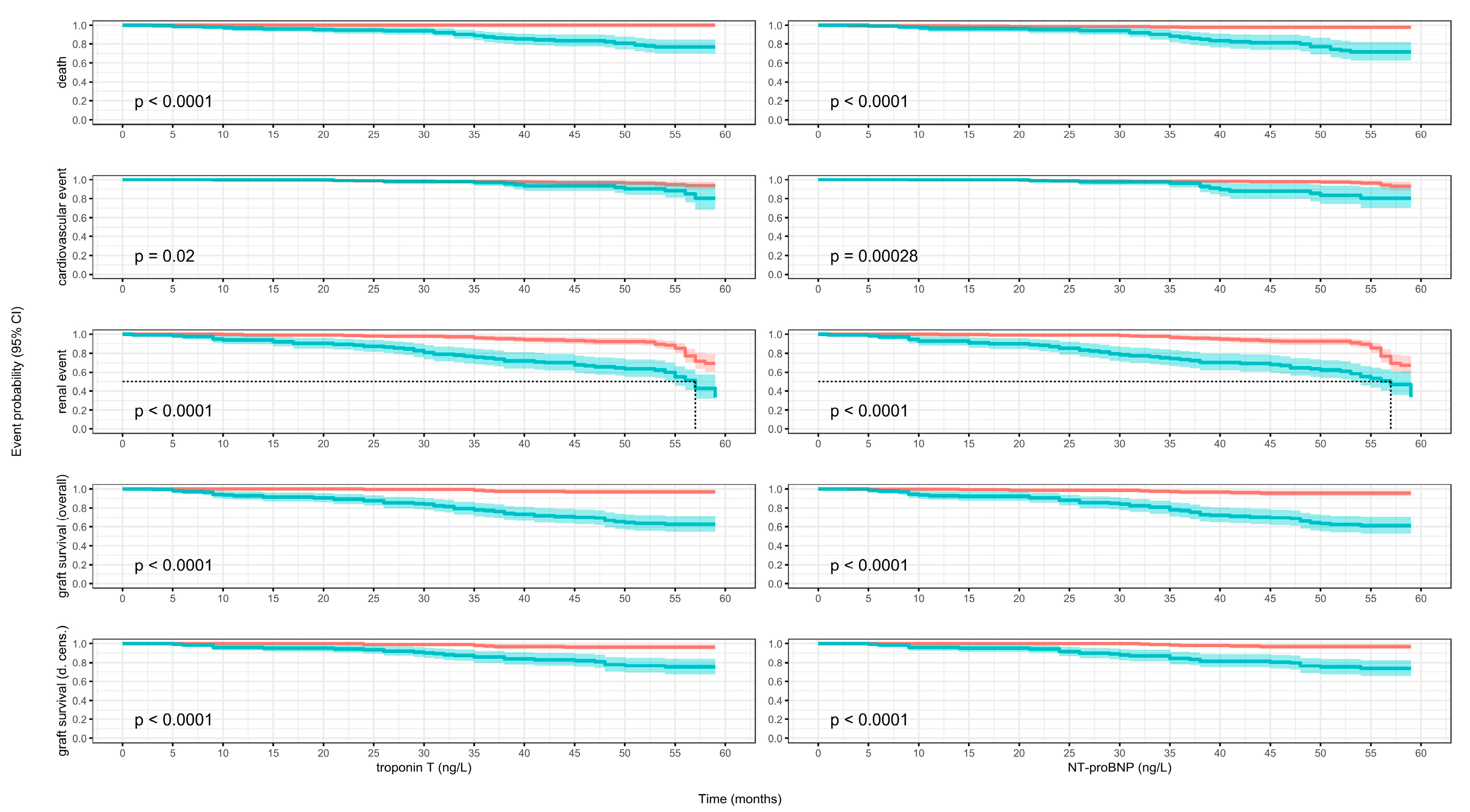
3.2. Death
3.3. Cardiovascular Events
3.4. Renal Events
3.5. Graft Loss (Overall Survival)
3.6. Graft Loss (Death-Censored)
3.7. Kaplan–Meier Models
3.8. Time-Dependent Discrimination
4. Discussion
4.1. Limitations
4.2. Pro
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RTRs | Renal transplant recipients |
| cTnT | Cardiac Troponin T |
| NTproBNP | N-terminal pro-B-type natriuretic peptide |
| cTnI | Cardiac Troponin I |
| CVD | Cardio-vascular disease |
| CKD | Chronic kidney disease |
| BNP | B-type natriuretic peptide |
| HR | Hazard ratio |
| eGFR | Estimated glomerular filtration rate |
| HF | Heart failure |
| NPs | Natriuretic peptides |
References
- Knoll, G. Trends in kidney transplantation over the past decade. Drugs 2008, 6 (Suppl. 1), 3–10. [Google Scholar] [CrossRef]
- Kabani, R.; Quinn, R.R.; Palmer, S.; Lewin, A.M.; Yilmaz, S.; Tibbles, L.A.; Lorenzetti, D.L.; Strippoli, G.F.; McLaughlin, K.; Ravani, P. Risk of death following kidney allograft failure: A systematic review and meta-analysis of cohort studies. Nephrol. Dial. Transplant. 2014, 29, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Mayrdorfer, M.; Liefeldt, L.; Wu, K.; Rudolph, B.; Zhang, Q.; Friedersdorff, F.; Lachmann, N.; Schmidt, D.; Osmanodja, B.; Naik, M.G.; et al. Exploring the Complexity of Death-Censored Kidney Allograft Failure. J. Am. Soc. Nephrol. 2021, 32, 1513–1526. [Google Scholar] [CrossRef]
- Mayrdorfer, M.; Liefeldt, L.; Osmanodja, B.; Naik, M.G.; Schmidt, D.; Duettmann, W.; Hammett, C.; Schrezenmeier, E.; Friedersdorff, F.; Wu, K.; et al. A single centre in-depth analysis of death with a functioning kidney graft and reasons for overall graft failure. Nephrol. Dial. Transplant. 2023, 38, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef]
- Stoumpos, S.; Jardine, A.G.; Mark, P.B. Cardiovascular morbidity and mortality after kidney transplantation. Transpl. Int. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Heleniak, Z.; Illersperger, S.; Brakemeier, S.; Dębska-Ślizień, A.; Budde, K.; Halleck, F. Obesity, Fat Tissue Parameters, and Arterial Stiffness in Renal Transplant Recipients. Transplant. Proc. 2020, 52, 2341–2346. [Google Scholar] [CrossRef]
- Heleniak, Z.; Illersperger, S.; Małgorzewicz, S.; Dębska-Ślizień, A.; Budde, K.; Halleck, F. Arterial Stiffness as a Cardiovascular Risk Factor After Successful Kidney Transplantation in Diabetic and Nondiabetic Patients. Transplant. Proc. 2022, 54, 2205–2211. [Google Scholar] [CrossRef]
- Liefeldt, L.; Budde, K. Risk factors for cardiovascular disease in renal transplant recipients and strategies to minimize risk. Transpl. Int. 2010, 23, 1191–1204. [Google Scholar] [CrossRef]
- van der Linden, N.; Klinkenberg, L.J.; Bekers, O.; van Loon, L.J.; van Dieijen-Visser, M.P.; Zeegers, M.P.; Meex, S.J. Prognostic value of basal high-sensitive cardiac troponin levels on mortality in the general population: A meta-analysis. Medicine 2016, 95, e5703. [Google Scholar] [CrossRef] [PubMed]
- York, M.K.; Gupta, D.K.; Reynolds, C.F.; Farber-Eger, E.; Wells, Q.S.; Bachmann, K.N.; Xu, M.; Harrell, F.E.; Wang, T.J. Natriuretic Peptide Levels and Mortality in Patients with and Without Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 2079–2088. [Google Scholar] [CrossRef]
- Rehman, S.U.; Mueller, T.; Januzzi, J.L. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J. Am. Coll. Cardiol. 2008, 52, 1458–1465. [Google Scholar] [CrossRef]
- Jarolim, P. High sensitivity cardiac troponin assays in the clinical laboratories. Clin. Chem. Lab. Med. 2015, 53, 635–652. [Google Scholar] [CrossRef]
- Apple, F.S.; Murakami, M.M.; Pearce, L.A.; Herzog, C.A. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002, 106, 2941–2945. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Funk, G.-C.; Marti, G.; Leichtle, A.B.; Fiedler, G.M.; Schwarz, C.; Exadaktylos, A.K.; Lindner, G. Diagnostic performance of high-sensitive troponin T in patients with renal insufficiency. Am. J. Cardiol. 2013, 112, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.T.; Nambi, V.; de Lemos, J.A.; Chambless, L.E.; Virani, S.S.; Boerwinkle, E.; Hoogeveen, R.C.; Liu, X.; Astor, B.C.; Mosley, T.H.; et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011, 123, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Arolim, P.; Claggett, B.L.; Conrad, M.J.M.; Carpenter, M.A.; Ivanova, A.; Bostom, A.G.; Kusek, J.W.; Hunsicker, L.G.; Jacques, P.F.D.; Gravens-Mueller, L.; et al. B-Type Natriuretic Peptide and Cardiac Troponin I Are Associated With Adverse Outcomes in Stable Kidney Transplant Recipients. Transplantation 2017, 101, 182–190. [Google Scholar] [CrossRef]
- Keddis, M.T.; El-Zoghby, Z.M.; El Ters, M.; Rodrigo, E.; Pellikka, P.A.; Jaffe, A.S.; Cosio, F.G. Cardiac troponin T before and after kidney transplantation: Determinants and implications for posttransplant survival. Am. J. Transplant. 2013, 13, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Heleniak, Z.; Illersperger, S.; Brakemeier, S.; Bach, P.; Dębska-Ślizień, A.; Budde, K.; Halleck, F. The renin-angiotensin-aldosterone system blockade and arterial stiffness in renal transplant recipients—A cross-sectional prospective observational clinical study. Acta Biochim. Pol. 2020, 67, 613–622. [Google Scholar] [CrossRef]
- Schmidt, D.; Osmanodja, B.; Pfefferkorn, M.; Graf, V.; Raschke, D.; Duettmann, W.; Naik, M.G.; Gethmann, C.J.; Mayrdorfer, M.; Halleck, F.; et al. TBase—An Integrated Electronic Health Record and Research Database for Kidney Transplant Recipients. J. Vis. Exp. 2021, 13, 170. [Google Scholar]
- Wilcox, R. Trimming and Winsorization. In Encyclopedia of Biostatistics; Armitage, P., Colton, T., Eds.; John Wiley and Sons: Chichester, UK, 2005; pp. 5531–5533. [Google Scholar]
- Firth, C.; Shamoun, F.; Cha, S.; Zhang, N.; Patel, S.; Wennberg, P.; Amer, H.; Wadei, H.; Heilman, R.; Keddis, M. Cardiac Troponin T Risk Stratification Model Predicts All-Cause Mortality Following Kidney Transplant. Am. J. Nephrol. 2018, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Cosio, F.G.; El-Zoghby, Z.M.; Gloor, J.M.; Kremers, W.K.; Stegall, M.D.; Griffin, M.D.; Jaffe, A.S. Survival of patients on the kidney transplant wait list: Relationship to cardiac troponin T. Am. J. Transplant. 2008, 8, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Connolly, G.M.; Cunningham, R.; McNamee, P.T.; Young, I.S.; Maxwell, A.P. Troponin T is an independent predictor of mortality in renal transplant recipients. Nephrol. Dial. Transplant. 2008, 23, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- A Abbas, N.; John, R.I.; Webb, M.C.; E Kempson, M.; Potter, A.N.; Price, C.P.; Vickery, S.; Lamb, E.J. Cardiac troponins and renal function in nondialysis patients with chronic kidney disease. Clin. Chem. 2005, 51, 2059–2066. [Google Scholar] [CrossRef]
- Hassan, H.C.; Howlin, K.; Jefferys, A.; Spicer, S.T.; Aravindan, A.N.; Suryanarayanan, G.; Hall, B.M.; Cleland, B.D.; Wong, J.K.; Suranyi, M.G.; et al. High-sensitivity troponin as a predictor of cardiac events and mortality in the stable dialysis population. Clin. Chem. 2014, 60, 389–398. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Baliga, R.; Kaplan, B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation 2003, 27, 1291–1295. [Google Scholar] [CrossRef]
- Cosio, F.G.; Hickson, L.J.; Griffin, M.D.; Stegall, M.D.; Kudva, Y. Patient survival and cardiovascular risk after kidney transplantation: The challenge of diabetes. Am. J. Transplant. 2008, 8, 593–599. [Google Scholar] [CrossRef]
- Adamo, M.; Gardner, R.S.; McDonagh, T.A.; Metra, M. The ‘Ten Commandments’ of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2022, 43, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef]
- Han, X.; Zhang, S.; Chen, Z.; Adhikari, B.K.; Zhang, Y.; Zhang, J.; Sun, J.; Wang, Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin. Chim. Acta 2020, 510, 298–310. [Google Scholar] [CrossRef]
- Wei, T.; Jin, L.; Lv, L.; Zhang, B.; Wang, L. Changesf in plasma B-type natriuretic peptide after allograft renal transplantation. Nephrology 2007, 12, 102–106. [Google Scholar] [CrossRef]
- Bodlaj, G.; Hubmann, R.; Saleh, K.; Biesenbach, G.; Pohanka, E.; Stojakovic, T.; Berg, J. Serum levels of N-terminal pro-B-type natriuretic peptide are associated with allograft function in recipients of renal transplants. Wien. Klin. Wochenschr. 2009, 121, 631–637. [Google Scholar] [CrossRef] [PubMed]
- E Emrich, I.; Scheuer, A.L.; Rogacev, K.S.; Mahfoud, F.; Wagenpfeil, S.; Fliser, D.; Schirmer, S.H.; Böhm, M.; Heine, G.H. Plasma biomarkers outperform echocardiographic measurements for cardiovascular risk prediction in kidney transplant recipients: Results of the HOME ALONE study. Clin. Kidney J. 2021, 15, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Moon, Y.; Kim, K.; Shin, W.; Huh, I.; Jun, I.; Song, J.; Hwang, G. Prognostic Value of B Type Natriuretic Peptide in Liver Transplant Patients: Implication in Posttransplant Mortality. Hepatology 2021, 74, 336–350. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall, N = 342 a | [0, 7], N = 108 a | [7, 11.5], N = 63 a | [11.5, 21], N = 86 a | [21, 48.9], N = 85 a | p-Value b |
|---|---|---|---|---|---|---|
| Age (years) | 53 (14) | 45 (12) | 51 (14) | 55 (13) | 62 (11) | <0.001 |
| Sex | - | - | - | - | - | <0.001 |
| Female | 129/342 (38%) | 59/108 (55%) | 22/63 (35%) | 28/86 (33%) | 20/85 (24%) | |
| Male | 213/342 (62%) | 49/108 (45%) | 41/63 (65%) | 58/86 (67%) | 65/85 (76%) | |
| BMI (kg/m2) | 25.6 (4.8) | 25.0 (4.7) | 25.9 (5.0) | 25.4 (4.6) | 26.4 (4.8) | 0.086 |
| Total weight (kg) | 76 (17) | 73 (17) | 77 (16) | 76 (17) | 81 (16) | 0.010 |
| DM type 2 (yes) | 45/342 (13%) | 7/108 (6.5%) | 10/63 (16%) | 10/86 (12%) | 18/85 (21%) | 0.023 |
| NODAT (yes) | 21/342 (6.1%) | 7/108 (6.5%) | 3/63 (4.8%) | 6/86 (7.0%) | 5/85 (5.9%) | >0.9 |
| CAD (yes) | 77/342 (23%) | 2/108 (1.9%) | 9/63 (14%) | 16/86 (19%) | 50/85 (59%) | <0.001 |
| Heart failure (yes) | 90/342 (26%) | 19/108 (18%) | 11/63 (17%) | 26/86 (30%) | 34/85 (40%) | 0.001 |
| Hypertension (yes) | 296/342 (87%) | 92/108 (85%) | 53/63 (84%) | 76/86 (88%) | 75/85 (88%) | 0.8 |
| Glomerulonephritis (yes) | 185/342 (54%) | 67/108 (62%) | 31/63 (49%) | 50/86 (58%) | 37/85 (44%) | 0.052 |
| Polycystic kidney disease (yes) | 55/342 (16%) | 12/108 (11%) | 11/63 (17%) | 14/86 (16%) | 18/85 (21%) | 0.3 |
| Tubulointerstitial nephritis (yes) | 69/342 (20%) | 27/108 (25%) | 12/63 (19%) | 12/86 (14%) | 18/85 (21%) | 0.3 |
| Hypertensive nephropathy (yes) | 18/342 (5.3%) | 1/108 (0.9%) | 2/63 (3.2%) | 6/86 (7.0%) | 9/85 (11%) | 0.013 |
| Unknown etiology (yes) | 10/342 (2.9%) | 1/108 (0.9%) | 7/63 (11%) | 2/86 (2.3%) | 0/85 (0%) | <0.001 |
| Time of RRT (months) | 59 (61) | 36 (53) | 67 (76) | 74 (60) | 68 (51) | <0.001 |
| Time post KTx (months) | 93 (80) | 100 (78) | 79 (83) | 89 (77) | 98 (81) | 0.13 |
| Preemptive KTX (yes) n (%) | 47/342 (14%) | 27/108 (25%) | 9/63 (14%) | 4/86 (4.7%) | 7/85 (8.2%) | <0.001 |
| Creatinine (mg/dL) | 1.67 (0.81) | 1.29 (0.37) | 1.51 (0.51) | 1.69 (0.76) | 2.27 (1.06) | <0.001 |
| eGFR CKDEPI (mL/min/1.73 m2) | 50 (20) | 62 (17) | 54 (18) | 48 (18) | 35 (17) | <0.001 |
| NT pro BNP (ng/L) | 684 (934) | 231 (357) | 411 (661) | 673 (816) | 1473 (1196) | <0.001 |
| Cyclosporine (yes) | 72/342 (21%) | 24/108 (22%) | 11/63 (17%) | 17/86 (20%) | 20/85 (24%) | 0.8 |
| Tacrolimus (yes) | 212/342 (62%) | 75/108 (69%) | 43/63 (68%) | 57/86 (66%) | 37/85 (44%) | <0.001 |
| Steroids (yes) | 176/342 (51%) | 38/108 (35%) | 37/63 (59%) | 45/86 (52%) | 56/85 (66%) | <0.001 |
| MMF (yes) | 150/342 (44%) | 45/108 (42%) | 23/63 (37%) | 44/86 (51%) | 38/85 (45%) | 0.3 |
| MPS (yes) | 170/342 (50%) | 59/108 (55%) | 35/63 (56%) | 40/86 (47%) | 36/85 (42%) | 0.3 |
| Betalacept (yes) | 41/342 (12%) | 8/108 (7.4%) | 6/63 (9.5%) | 9/86 (10%) | 18/85 (21%) | 0.023 |
| Calcium channel blocker (yes) | 157/342 (46%) | 37/108 (34%) | 29/63 (46%) | 48/86 (56%) | 43/85 (51%) | 0.018 |
| ACE inhibitor (yes) | 89/342 (26%) | 25/108 (23%) | 16/63 (25%) | 27/86 (31%) | 21/85 (25%) | 0.6 |
| Statines (yes) | 142/342 (42%) | 34/108 (31%) | 22/63 (35%) | 44/86 (51%) | 42/85 (49%) | 0.011 |
| AT1-R antagonists (yes) | 111/342 (32%) | 28/108 (26%) | 17/63 (27%) | 31/86 (36%) | 35/85 (41%) | 0.093 |
| Characteristic | Overall, N = 342 a | [0, 113], N = 87 a | [113, 298], N = 84 a | [298, 754], N = 85 a | [754, 3376], N = 86 a | p-Value b |
|---|---|---|---|---|---|---|
| Age | 53 (14) | 46 (12) | 50 (13) | 56 (13) | 58 (14) | <0.001 |
| Sex | 0.008 | |||||
| Female | 129/342 (38%) | 20/87 (23%) | 33/84 (39%) | 40/85 (47%) | 36/86 (42%) | |
| Male | 213/342 (62%) | 67/87 (77%) | 51/84 (61%) | 45/85 (53%) | 50/86 (58%) | |
| BMI (kg/m2) | 25.6 (4.8) | 25.8 (4.2) | 25.9 (5.0) | 25.1 (4.7) | 25.6 (5.3) | 0.6 |
| Total weight (kg) | 76 (17) | 79 (16) | 77 (17) | 74 (16) | 75 (18) | 0.2 |
| DM type 2 (yes) | 45/342 (13%) | 11/87 (13%) | 9/84 (11%) | 9/85 (11%) | 16/86 (19%) | 0.4 |
| NODAT (yes) | 21/342 (6.1%) | 8/87 (9.2%) | 5/84 (6.0%) | 3/85 (3.5%) | 5/86 (5.8%) | 0.5 |
| CAD (yes) | 77/342 (23%) | 3/87 (3.4%) | 9/84 (11%) | 22/85 (26%) | 43/86 (50%) | <0.001 |
| Heart failure (yes) | 90/342 (26%) | 17/87 (20%) | 12/84 (14%) | 26/85 (31%) | 35/86 (41%) | <0.001 |
| Hypertension (yes) | 296/342 (87%) | 78/87 (90%) | 69/84 (82%) | 71/85 (84%) | 78/86 (91%) | 0.3 |
| Glomrulonephritis (yes) | 185/342 (54%) | 48/87 (55%) | 55/84 (65%) | 36/85 (42%) | 46/86 (53%) | 0.027 |
| Polycystic kidney disease (yes) | 55/342 (16%) | 11/87 (13%) | 14/84 (17%) | 20/85 (24%) | 10/86 (12%) | 0.14 |
| Tubulointerstitial nephritis (yes) | 69/342 (20%) | 23/87 (26%) | 10/84 (12%) | 17/85 (20%) | 19/86 (22%) | 0.12 |
| Hypertensive nephropathy (yes) | 18/342 (5.3%) | 2/87 (2.3%) | 2/84 (2.4%) | 7/85 (8.2%) | 7/86 (8.1%) | 0.12 |
| Unknown etiology (yes) | 10/342 (2.9%) | 1/87 (1.1%) | 3/84 (3.6%) | 6/85 (7.1%) | 0/86 (0%) | 0.020 |
| Time of RRT (months) | 59 (61) | 46 (69) | 59 (64) | 62 (54) | 71 (52) | <0.001 |
| Time post KTx (months) | 93 (80) | 97 (85) | 86 (77) | 84 (73) | 103 (82) | 0.4 |
| Preempitve KTX (yes) | 47/342 (14%) | 23/87 (26%) | 12/84 (14%) | 8/85 (9.4%) | 4/86 (4.7%) | <0.001 |
| Creatinine (mg/dL) | 1.67 (0.81) | 1.38 (0.38) | 1.40 (0.44) | 1.60 (0.63) | 2.31 (1.14) | <0.001 |
| eGFR CKDEPI (mL/min/1.73 m2) | 50 (20) | 61 (16) | 57 (17) | 48 (19) | 34 (16) | <0.001 |
| Troponin T (ng/L) | 16 (13) | 8 (5) | 12 (10) | 17 (12) | 27 (14) | <0.001 |
| Cyclosporine (yes) | 72/342 (21%) | 15/87 (17%) | 17/84 (20%) | 18/85 (21%) | 22/86 (26%) | 0.6 |
| Tacrolimus (yes) | 212/342 (62%) | 61/87 (70%) | 53/84 (63%) | 54/85 (64%) | 44/86 (51%) | 0.077 |
| Steroids (yes) | 176/342 (51%) | 31/87 (36%) | 40/84 (48%) | 49/85 (58%) | 56/86 (65%) | <0.001 |
| MMF (yes) | 150/342 (44%) | 44/87 (51%) | 33/84 (39%) | 34/85 (40%) | 39/86 (45%) | 0.4 |
| MPS (yes) | 170/342 (50%) | 40/87 (46%) | 45/84 (54%) | 47/85 (55%) | 38/86 (44%) | 0.4 |
| Betalacept (yes) | 41/342 (12%) | 8/87 (9.2%) | 10/84 (12%) | 9/85 (11%) | 14/86 (16%) | 0.5 |
| Calcium channel blocker (yes) | 157/342 (46%) | 38/87 (44%) | 38/84 (45%) | 33/85 (39%) | 48/86 (56%) | 0.2 |
| ACE inhibitor (yes) | 89/342 (26%) | 19/87 (22%) | 21/84 (25%) | 24/85 (28%) | 25/86 (29%) | 0.7 |
| Statines (yes) | 142/342 (42%) | 35/87 (40%) | 35/84 (42%) | 29/85 (34%) | 43/86 (50%) | 0.2 |
| AT1-R antagonists (yes) | 111/342 (32%) | 23/87 (26%) | 24/84 (29%) | 32/85 (38%) | 32/86 (37%) | 0.3 |
| Substance | Event | Quartile | Estimate1 | p-Value1 | Cut-Off | Estimate2 | p-Value2 |
|---|---|---|---|---|---|---|---|
| Overall HR | |||||||
| troponin T (ng/L) | death | 7 | 0.30 (0.07; 1.27) | 0.10 | 12.25 | 0.98 (0.27; 3.50) | >0.9 |
| 11.5 | 0.83 (0.24; 2.94) | 0.8 | |||||
| 21 | 4.11 (0.87; 19.38) | 0.074 | |||||
| 48.95 | 7.74 (1.56; 38.41) | 0.012 | |||||
| Conditional HR | |||||||
| troponin T (ng/L) | death | [2.00, 7.00) | 0.07 (0.01; 0.39) | 0.003 | [2.00, 12.25) | 0.19 (0.05; 0.74) | 0.017 |
| [7.00, 11.50) | 0.36 (0.10; 1.35) | 0.13 | |||||
| [11.50, 21.00) | 2.05 (0.54; 7.79) | 0.3 | [12.25, 48.95] | 6.98 (1.70; 28.72) | 0.007 | ||
| [21.00, 48.95] | 15.98 (3.45; 74.04) | <0.001 | |||||
| Overall HR | |||||||
| NT-proBNP (ng/L) | death | 113 | 0.67 (0.21; 2.15) | 0.5 | 471.53 | 1.36 (0.44; 4.19) | 0.6 |
| 298.5 | 0.97 (0.32; 2.91) | >0.9 | |||||
| 753.5 | 2.31 (0.67; 7.95) | 0.2 | |||||
| 3376.05 | 7.43 (1.54; 35.83) | 0.013 | |||||
| Conditional HR | |||||||
| NT-proBNP (ng/L) | death | [6.00, 113.00) | 0.34 (0.09; 1.31) | 0.12 | [6.00, 471.53) | 0.56 (0.17; 1.79) | 0.3 |
| [113.00, 298.50) | 0.58 (0.19; 1.77) | 0.3 | |||||
| [298.50, 753.50) | 1.56 (0.54; 4.56) | 0.4 | [471.53, 3376.05] | 8.26 (2.51; 27.22) | <0.001 | ||
| [753.50, 3376.05] | 11.45 (3.24; 40.48) | <0.001 | |||||
| Overall HR | |||||||
| troponin T (ng/L) | cardiovascular event | 7 | 1.33 (0.30; 5.96) | 0.7 | 15.63 | 1.41 (0.35; 5.72) | 0.6 |
| 11.5 | 1.37 (0.33; 5.77) | 0.7 | |||||
| 21 | 1.47 (0.37; 5.84) | 0.6 | |||||
| 48.95 | 1.78 (0.28; 11.31) | 0.5 | |||||
| Conditional HR | |||||||
| troponin T (ng/L) | cardiovascular event | [2.00, 7.00) | 0.62 (0.16; 2.45) | 0.5 | [2.00, 15.63) | 0.91 (0.23; 3.62) | 0.9 |
| [7.00, 11.50) | 1.08 (0.25; 4.65) | >0.9 | |||||
| [11.50, 21.00) | 1.62 (0.39; 6.67) | 0.5 | [15.63, 48.95] | 3.30 (0.69; 15.69) | 0.13 | ||
| [21.00, 48.95] | 3.59 (0.73; 17.63) | 0.11 | |||||
| Overall HR | |||||||
| NT-proBNP (ng/L) | cardiovascular event | 113 | 1.06 (0.24; 4.63) | >0.9 | 673.94 | 1.61 (0.37; 6.95) | 0.5 |
| 298.5 | 1.22 (0.28; 5.28) | 0.8 | |||||
| 753.5 | 1.70 (0.39; 7.38) | 0.5 | |||||
| 3376.05 | 11.68 (1.73; 78.93) | 0.012 | |||||
| Conditional HR | |||||||
| NT-proBNP (ng/L) | cardiovascular event | [6.00, 113.00) | 0.80 (0.17; 3.71) | 0.8 | [6.00, 673.94) | 1.03 (0.24; 4.35) | >0.9 |
| [113.00, 298.50) | 1.03 (0.26; 4.17) | >0.9 | |||||
| [298.50, 753.50) | 1.48 (0.35; 6.31) | 0.6 | [673.94, 3376.05] | 5.24 (1.03; 26.77) | 0.046 | ||
| [753.50, 3376.05] | 5.57 (1.07; 29.01) | 0.041 | |||||
| Overall HR | |||||||
| troponin T (ng/L) | renal event | 7 | 3.15 (1.47; 6.76) | 0.003 | 15.68 | 4.02 (1.98; 8.18) | <0.001 |
| 11.5 | 3.58 (1.72; 7.43) | <0.001 | |||||
| 21 | 4.67 (2.32; 9.37) | <0.001 | |||||
| 48.95 | 10.21 (4.31; 24.17) | <0.001 | |||||
| Conditional HR | |||||||
| troponin T (ng/L) | renal event | [2.00, 7.00) | 1.81 (0.90; 3.62) | 0.094 | [2.00, 15.68) | 2.49 (1.23; 5.02) | 0.011 |
| [7.00, 11.50) | 2.80 (1.34; 5.85) | 0.006 | |||||
| [11.50, 21.00) | 3.93 (1.91; 8.10) | <0.001 | [15.68, 48.95] | 10.13 (4.66; 22.04) | <0.001 | ||
| [21.00, 48.95] | 12.49 (5.65; 27.60) | <0.001 | |||||
| Overall HR | |||||||
| NT-proBNP (ng/L) | renal event | 113 | 3.19 (1.52; 6.71) | 0.002 | 471.53 | 4.35 (2.14; 8.84) | <0.001 |
| 298.5 | 3.75 (1.85; 7.63) | <0.001 | |||||
| 753.5 | 5.49 (2.61; 11.54) | <0.001 | |||||
| 3376.05 | 8.07 (2.98; 21.83) | <0.001 | |||||
| Conditional HR | |||||||
| NT-proBNP (ng/L) | renal event | [6.00, 113.00) | 1.99 (0.96; 4.13) | 0.065 | [6.00, 471.53) | 2.62 (1.31; 5.25) | 0.007 |
| [113.00, 298.50) | 2.59 (1.31; 5.11) | 0.006 | |||||
| [298.50, 753.50) | 4.57 (2.26; 9.26) | <0.001 | [471.53, 3376.05] | 12.01 (5.54; 26.03) | <0.001 | ||
| [753.50, 3376.05] | 15.12 (6.72; 34.01) | <0.001 | |||||
| Overall HR | |||||||
| troponin T (ng/L) | graft survival (overall) | 7 | 0.64 (0.24; 1.67) | 0.4 | 13.37 | 1.32 (0.55; 3.21) | 0.5 |
| 11.5 | 1.07 (0.45; 2.58) | 0.9 | |||||
| 21 | 2.81 (1.08; 7.31) | 0.034 | |||||
| 48.95 | 6.92 (2.52; 19.00) | <0.001 | |||||
| Conditional HR | |||||||
| troponin T (ng/L) | graft survival (overall) | [2.00, 7.00) | 0.24 (0.09; 0.65) | 0.005 | [2.00, 13.37) | 0.47 (0.19; 1.14) | 0.10 |
| [7.00, 11.50) | 0.66 (0.27; 1.62) | 0.4 | |||||
| [11.50, 21.00) | 1.60 (0.65; 3.94) | 0.3 | [13.37, 48.95] | 5.85 (2.35; 14.56) | <0.001 | ||
| [21.00, 48.95] | 11.12 (4.25; 29.09) | <0.001 | |||||
| Overall HR | |||||||
| NT-proBNP (ng/L) | graft survival (overall) | 113 | 0.78 (0.32; 1.88) | 0.6 | 410.81 | 1.91 (0.84; 4.37) | 0.12 |
| 298.5 | 1.38 (0.62; 3.08) | 0.4 | |||||
| 753.5 | 4.66 (1.80; 12.10) | 0.002 | |||||
| 3376.05 | 8.98 (2.61; 30.92) | <0.001 | |||||
| Conditional HR | |||||||
| NT-proBNP (ng/L) | graft survival (overall) | [6.00, 113.00) | 0.45 (0.17; 1.18) | 0.10 | [6.00, 410.81) | 0.66 (0.29; 1.55) | 0.3 |
| [113.00, 298.50) | 0.71 (0.32; 1.59) | 0.4 | |||||
| [298.50, 753.50) | 2.21 (0.98; 4.98) | 0.055 | [410.81, 3376.05] | 11.18 (4.54; 27.51) | <0.001 | ||
| [753.50, 3376.05] | 19.61 (7.36; 52.25) | <0.001 | |||||
| Overall HR | |||||||
| troponin T (ng/L) | graft survival (d. cens.) | 7 | 1.27 (0.36; 4.41) | 0.7 | 14.36 | 2.14 (0.69; 6.70) | 0.2 |
| 11.5 | 1.75 (0.55; 5.57) | 0.3 | |||||
| 21 | 3.34 (1.05; 10.62) | 0.041 | |||||
| 48.95 | 9.46 (2.70; 33.13) | <0.001 | |||||
| Conditional HR | |||||||
| troponin T (ng/L) | graft survival (d. cens.) | [2.00, 7.00) | 0.54 (0.16; 1.85) | 0.3 | [2.00, 14.36) | 0.96 (0.31; 3.02) | >0.9 |
| [7.00, 11.50) | 1.22 (0.37; 4.00) | 0.7 | |||||
| [11.50, 21.00) | 2.08 (0.65; 6.64) | 0.2 | [14.36, 48.95] | 7.64 (2.36; 24.72) | <0.001 | ||
| [21.00, 48.95] | 13.87 (4.13; 46.55) | <0.001 | |||||
| Overall HR | |||||||
| NT-proBNP (ng/L) | graft survival (d. cens.) | 113 | 1.56 (0.50; 4.83) | 0.4 | 420.93 | 3.12 (1.09; 8.93) | 0.034 |
| 298.5 | 2.39 (0.85; 6.73) | 0.10 | |||||
| 753.5 | 6.15 (1.89; 19.99) | 0.003 | |||||
| 3376.05 | 10.25 (2.31; 45.51) | 0.002 | |||||
| Conditional HR | |||||||
| NT-proBNP (ng/L) | graft survival (d. cens.) | [6.00, 113.00) | 0.80 (0.24; 2.65) | 0.7 | [6.00, 420.93) | 1.17 (0.40; 3.43) | 0.8 |
| [113.00, 298.50) | 1.20 (0.43; 3.35) | 0.7 | |||||
| [298.50, 753.50) | 3.58 (1.26; 10.19) | 0.017 | [420.93, 3376.05] | 16.94 (5.35; 53.68) | <0.001 | ||
| [753.50, 3376.05] | 28.52 (8.21; 99.04) | <0.001 | |||||
| Substance | Event | Month | AUC | 95% CI |
|---|---|---|---|---|
| troponin T (ng/L) | death | 12.00 | 77.96 | 75.28; 80.65 |
| 24.00 | 78.57 | 75.8; 81.34 | ||
| 36.00 | 80.04 | 77.18; 82.89 | ||
| 48.00 | 81.76 | 78.81; 84.72 | ||
| NT-proBNP (ng/L) | 12.00 | 74.26 | 56.61; 91.91 | |
| 24.00 | 65.96 | 48.95; 82.96 | ||
| 36.00 | 73.96 | 63.14; 84.77 | ||
| 48.00 | 77.46 | 68.76; 86.15 | ||
| troponin T (ng/L) | cardiovascular event | 12.00 | 33.74 | 31.2; 36.29 |
| 24.00 | 46.52 | 25.08; 67.97 | ||
| 36.00 | 57.10 | 38.45; 75.74 | ||
| 48.00 | 60.52 | 46.64; 74.4 | ||
| NT-proBNP (ng/L) | 12.00 | 37.12 | 34.74; 39.49 | |
| 24.00 | 50.15 | 28.73; 71.58 | ||
| 36.00 | 60.86 | 42.25; 79.47 | ||
| 48.00 | 72.86 | 59.54; 86.17 | ||
| troponin T (ng/L) | renal event | 12.00 | 77.66 | 65.85; 89.48 |
| 24.00 | 75.23 | 65.26; 85.2 | ||
| 36.00 | 74.14 | 66.48; 81.8 | ||
| 48.00 | 72.17 | 65.29; 79.05 | ||
| NT-proBNP (ng/L) | 12.00 | 84.41 | 81.89; 86.94 | |
| 24.00 | 79.08 | 70.57; 87.6 | ||
| 36.00 | 76.47 | 69.12; 83.83 | ||
| 48.00 | 73.12 | 66.25; 79.98 | ||
| troponin T (ng/L) | graft survival (overall) | 12.00 | 80.34 | 77.69; 82.98 |
| 24.00 | 80.94 | 78.22; 83.66 | ||
| 36.00 | 77.43 | 71.68; 83.18 | ||
| 48.00 | 77.48 | 71.83; 83.13 | ||
| NT-proBNP (ng/L) | 12.00 | 77.02 | 67.36; 86.67 | |
| 24.00 | 72.39 | 61.9; 82.89 | ||
| 36.00 | 74.77 | 67.63; 81.91 | ||
| 48.00 | 76.17 | 69.87; 82.47 | ||
| troponin T (ng/L) | graft survival (d. cens.) | 12.00 | 81.55 | 78.94; 84.17 |
| 24.00 | 82.08 | 79.4; 84.77 | ||
| 36.00 | 73.58 | 64.59; 82.58 | ||
| 48.00 | 73.06 | 64.67; 81.46 | ||
| NT-proBNP (ng/L) | 12.00 | 82.32 | 79.73; 84.91 | |
| 24.00 | 82.74 | 80.07; 85.4 | ||
| 36.00 | 76.85 | 68.69; 85.01 | ||
| 48.00 | 75.50 | 67.46; 83.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heleniak, Z.; Naik, M.G.; Eleftheriadis, G.; Madej, T.; Halleck, F.; Dębska-Ślizień, A.; Budde, K. N-Terminal Pro-B-Type Natriuretic Peptide and Cardiac Troponin T in Stable Renal Transplant Recipients and All-Cause Mortality, Cardiovascular, and Renal Events. Biomolecules 2025, 15, 1298. https://doi.org/10.3390/biom15091298
Heleniak Z, Naik MG, Eleftheriadis G, Madej T, Halleck F, Dębska-Ślizień A, Budde K. N-Terminal Pro-B-Type Natriuretic Peptide and Cardiac Troponin T in Stable Renal Transplant Recipients and All-Cause Mortality, Cardiovascular, and Renal Events. Biomolecules. 2025; 15(9):1298. https://doi.org/10.3390/biom15091298
Chicago/Turabian StyleHeleniak, Zbigniew, Marcel G. Naik, Georgios Eleftheriadis, Tomasz Madej, Fabian Halleck, Alicja Dębska-Ślizień, and Klemens Budde. 2025. "N-Terminal Pro-B-Type Natriuretic Peptide and Cardiac Troponin T in Stable Renal Transplant Recipients and All-Cause Mortality, Cardiovascular, and Renal Events" Biomolecules 15, no. 9: 1298. https://doi.org/10.3390/biom15091298
APA StyleHeleniak, Z., Naik, M. G., Eleftheriadis, G., Madej, T., Halleck, F., Dębska-Ślizień, A., & Budde, K. (2025). N-Terminal Pro-B-Type Natriuretic Peptide and Cardiac Troponin T in Stable Renal Transplant Recipients and All-Cause Mortality, Cardiovascular, and Renal Events. Biomolecules, 15(9), 1298. https://doi.org/10.3390/biom15091298








