In Vitro Analysis of an Alkalihalobacillus clausii Spore-Based Probiotic Formulation Clarifies the Mechanisms Underlying Its Beneficial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Product, Strains, and Culture Conditions
2.2. Quantification of the Microorganisms Contained in the Probiotic Product
2.3. Viability of Spores and Growth Evaluation in Simulated Gastrointestinal Fluids
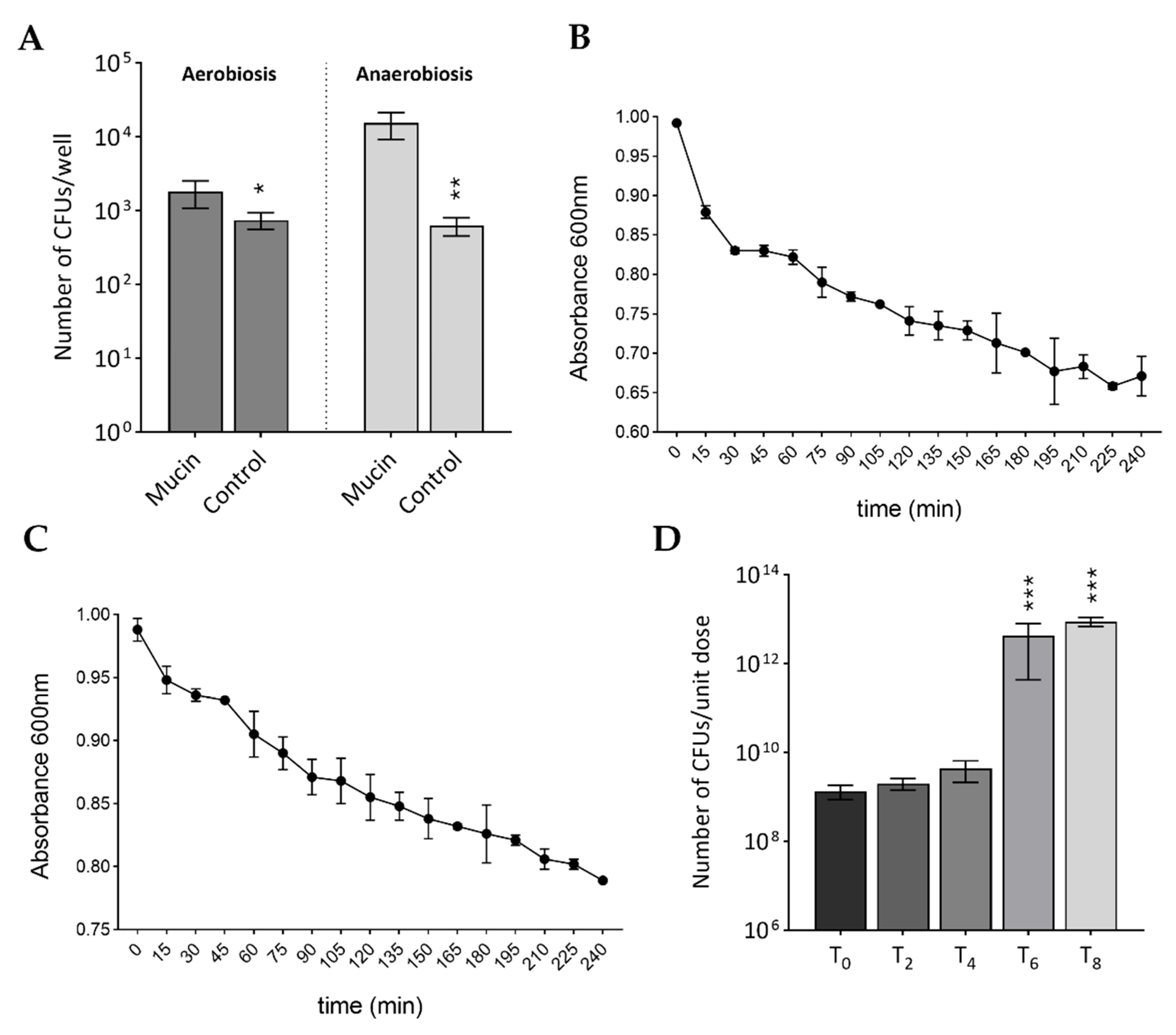
2.4. Mucin Adhesion Assay
2.5. Spore Germination in the Intestinal Fluid
2.6. Quantification of β-Galactosidase
2.7. Preparation of Cell Lysates and Culture Supernatants
2.8. Quantification of Catalase (CAT) and Superoxide Dismutase (SOD)
2.9. Quantification of Vitamin B2, B8, B9, and B12
2.10. Quantification of Short-Chain Fatty Acids
2.11. Evaluation of D-Lactic Acid Production
2.12. Statistical Analysis
3. Results and Discussion
3.1. Total Count
3.2. Behavior in Simulated Gastrointestinal Conditions: Survival, Adhesion, and Germination
3.3. Production of Beneficial Enzymes
3.4. Production of Vitamins and SCFAs
3.5. Production of D-Lactate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ròdenas, C.C.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera-deGuise, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and prebiotics. J. Clin. Gastroenterol. 2024, 58, 533–553. [Google Scholar] [CrossRef]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef]
- Zawistowska-Rojek, A.; Zaręba, T.; Tyski, S. Microbiological testing of probiotic preparations. Int. J. Environ. Res. Public Health 2022, 19, 5701. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food. Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Hong, H.A.; Duc, L.H.; Cutting, S.M. The use of bacterial spore formers as probiotics. FEMS. Microbiol. Rev. 2005, 29, 813–835. [Google Scholar] [CrossRef]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Gueye, S.A.; Lupetti, A.; Senesi, S. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 2015, 119, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Ramlucken, U.; Ramchuran, S.O.; Moonsamy, G.; Jansen van Rensburg, C.; Thantsha, M.S.; Lalloo, R. Production and stability of a multi-strain Bacillus based probiotic product for commercial use in poultry. Biotechnol. Rep. 2021, 29, e00575. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Tosca, M.A.; Milanese, M.; Caligo, G.; Ricca, V. Cytokines evaluation in nasal lavage of allergic children after Bacillus clausii administration: A pilot study. Pediatr. Allergy Immunol. 2004, 15, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Nista, E.C.; Candelli, M.; Cremonini, F.; Cazzato, I.A.; Zocco, M.A.; Franceschi, F.; Cammarota, G.; Gasbarrini, G.; Gasbarrini, A. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: Randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 2004, 20, 1181–1188. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Tosca, M.; Cirillo, I.; Licari, A.; Leone, M.; Marseglia, A.; Castellazzi, A.M.; Ciprandi, G. Efficacy of Bacillus clausii spores in the prevention of recurrent respiratory infections in children: A pilot study. Ther. Clin. Risk Manag. 2007, 3, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tewari, V.V.; Dubey, S.K.; Gupta, G. Bacillus clausii for prevention of late-onset sepsis in preterm infants: A randomized controlled trial. J. Trop. Pediatr. 2015, 61, 377–385. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Henning, A.L.; Bowman, E.M.; Gary, M.A.; Carbajal, K.M. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 117–126. [Google Scholar] [CrossRef]
- de Castro, J.A.; Guno, M.J.V.; Perez, M.O. Bacillus clausii as adjunctive treatment for acute community-acquired diarrhea among Filipino children: A large-scale, multicenter, open-label study (CODDLE). Trop. Dis. Travel. Med. Vaccines 2019, 5, 14. [Google Scholar] [CrossRef]
- Hamid, F.; Quaium, S.M.M.A.; Rahman, A. Comparative study of Bacillus clausii and multistrain probiotics in the management of acute diarrhoea in children. Int. J. Res. Med. Sci. 2019, 7, 1156–1160. [Google Scholar] [CrossRef]
- Nirmala, M.; Smitha, S.G.; Kamath, G.J. A study to assess the efficacy of local application of oral probiotic in treating recurrent aphthous ulcer and oral candidiasis. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Smiyan, O.I.; Smiian-Horbunova, K.O.; Bynda, T.P.; Loboda, A.M.; Popov, S.V.; Vysotsky, I.Y.; Moshchych, O.P.; Vasylieva, O.G.; Manko, Y.A.; Ovsianko, O.L.; et al. Optimization of the treatment of rotavirus infection in children by using Bacillus clausii. Wiad. Lek. 2019, 72, 1320–1323. [Google Scholar] [CrossRef]
- Soman, R.J.; Swamy, M.V. A prospective, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of SNZ TriBac, a three-strain Bacillus probiotic blend for undiagnosed gastrointestinal discomfort. Int. J. Colorectal Dis. 2019, 34, 1971–1978. [Google Scholar] [CrossRef]
- Sudha, M.R.; Jayanthi, N.; Pandey, D.C.; Verma, A.K. Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: A double blind placebo controlled study. Benef. Microbes. 2019, 10, 149–154. [Google Scholar] [CrossRef]
- Plomer, M.; Perez, M., 3rd; Greifenberg, D.M. Effect of Bacillus clausii capsules in reducing adverse effects associated with Helicobacter pylori eradication therapy: A randomized, double-blind, controlled trial. Infect. Dis. Ther. 2020, 9, 867–878. [Google Scholar] [CrossRef]
- Maity, C.; Gupta, A.K. Therapeutic efficacy of probiotic Alkalihalobacillus clausii 088AE in antibiotic-associated diarrhea: A randomized controlled trial. Heliyon 2021, 7, e07993. [Google Scholar] [CrossRef]
- Acosta-Rodríguez-Bueno, C.P.; Abreu, Y.A.A.T.; Guarner, F.; Guno, M.J.V.; Pehlivanoğlu, E.; Perez, M., 3rd. Bacillus clausii for gastrointestinal disorders: A narrative literature review. Adv. Ther. 2022, 39, 4854–4874. [Google Scholar] [CrossRef]
- Ghelardi, E.; Abreu, Y.A.A.T.; Marzet, C.B.; Álvarez Calatayud, G.; Perez, M., 3rd; Moschione Castro, A.P. Current progress and future perspectives on the use of Bacillus clausii. Microorganisms 2022, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, K.R.; Singh, R.; Apte, M.; Patil, M.; Taksande, A.; Varona, R.; Chatterjee, G.; Verma, M.; Brette, S.; Perez, M.I. Efficacy and safety of Bacillus clausii (O/C, N/R, SIN, T) probiotic combined with oral rehydration therapy and zinc in acute diarrhea in children: A randomized, double-blind, placebo-controlled study in India. Trop. Dis. Travel. Med. Vaccines 2022, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Frias, R.; Consuelo-Sánchez, A.; Acosta-Rodríguez-Bueno, C.P.; Blanco-Montero, A.; Robles, D.C.; Cohen, V.; Márquez, D.; Perez, M., 3rd. Efficacy and safety of the adjuvant use of probiotic Bacillus clausii strains in pediatric irritable bowel syndrome: A randomized, double-blind, placebo-controlled study. Pediatr. Drugs 2023, 25, 115–126. [Google Scholar] [CrossRef]
- Dang, H.T.; Tran, D.M.; Phung, T.T.B.; Bui, A.T.P.; Vu, Y.H.; Luong, M.T.; Nguyen, H.M.; Trinh, H.T.; Nguyen, T.T.; Nguyen, A.H.; et al. Promising clinical and immunological efficacy of Bacillus clausii spore probiotics for supportive treatment of persistent diarrhea in children. Sci. Rep. 2024, 14, 6422. [Google Scholar] [CrossRef] [PubMed]
- Sadrimovahed, M.; Ulusoy, B.H. Bacillus clausii: A review into story of its probiotic success and potential food applications. Fermentation 2024, 10, 522. [Google Scholar] [CrossRef]
- Ripert, G.; Racedo, S.M.; Elie, A.M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.S.; França, W.W.M.; de Arújo, H.D.A.; Ximenes, E.; de Souza, V.M.; Albuquerque, M.; Aires, A.L.; Costa, V.M.A. In vitro and in vivo evaluation of Bacillus clausii against Schistosoma mansoni. Acta. Trop. 2022, 235, 106669. [Google Scholar] [CrossRef]
- Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Lupetti, A.; Ghelardi, E. In vitro assessment of probiotic attributes for strains contained in commercial formulations. Sci. Rep. 2022, 12, 21640. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, E.; Colom, J.; Simon, A.; Mazhar, S.; García-Lainez, G.; Llopis, S.; Gonzalez, N.; Enrique-López, M.; Álvarez, B.; Martorell, P.; et al. Immunomodulatory and antioxidant properties of a novel potential probiotic Bacillus clausii CSI08. Microorganisms 2023, 11, 240. [Google Scholar] [CrossRef]
- Saroj, D.B.; Ahire, J.J.; Shukla, R. Genetic and phenotypic assessments for the safety of probiotic Bacillus clausii 088AE. 3 Biotech. 2023, 13, 238. [Google Scholar] [CrossRef]
- Urdaci, M.C.; Bressollier, P.; Pinchuk, I. Bacillus clausii probiotic strains: Antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004, 38, S86–S90. [Google Scholar] [CrossRef]
- Bouhss, A.; Al-Dabbagh, B.; Vincent, M.; Odaert, B.; Aumont-Nicaise, M.; Bressolier, P.; Desmadril, M.; Mengin-Lecreulx, D.; Urdaci, M.C.; Gallay, J. Specific interactions of clausin, a new lantibiotic, with lipid precursors of the bacterial cell wall. Biophys. J. 2009, 97, 1390–1397. [Google Scholar] [CrossRef]
- Villéger, R.; Saad, N.; Grenier, K.; Falourd, X.; Foucat, L.; Urdaci, M.C.; Bressollier, P.; Ouk, T.S. Characterization of lipoteichoic acid structures from three probiotic Bacillus strains: Involvement of D-alanine in their biological activity. Antonie Van Leeuwenhoek 2014, 106, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Tokatlı, M.; Gülgör, G.; Bağder Elmacı, S.; Arslankoz İşleyen, N.; Özçelik, F. In vitro properties of potential probiotic indigenous lactic acid bacteria originating from traditional pickles. Biomed. Res. Int. 2015, 2015, 315819. [Google Scholar] [CrossRef]
- Bédard, J.; Lefebvre, G.M. L-alanine and inosine enhancement of glucose triggering in Bacillus megaterium spores. Can. J. Microbiol. 1989, 35, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Senesi, S.; Cercignani, G.; Freer, G.; Batoni, G.; Barnini, S.; Ota, F. Structural and stereospecific requirements for the nucleoside-triggered germination of Bacillus cereus spores. J. Gen. Microbiol. 1991, 137, 399–404. [Google Scholar] [CrossRef][Green Version]
- Senesi, S.; Freer, G.; Batoni, G.; Barnini, S.; Capaccioli, A.; Cercignani, G. Role of spore coats in the germinative response of Bacillus cereus to adenosine and its analogues. Can. J. Microbiol. 1992, 38, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mazzantini, D.; Fonnesu, R.; Celandroni, F.; Calvigioni, M.; Vecchione, A.; Mrusek, D.; Bange, G.; Ghelardi, E. GTP-dependent FlhF homodimer supports secretion of a hemolysin in Bacillus cereus. Front. Microbiol. 2020, 11, 879. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory: New York, NY, USA, 1972. [Google Scholar]
- Calvigioni, M.; Bertolini, A.; Codini, S.; Mazzantini, D.; Panattoni, A.; Massimino, M.; Celandroni, F.; Zucchi, R.; Saba, A.; Ghelardi, E. HPLC-MS-MS quantification of short-chain fatty acids actively secreted by probiotic strains. Front. Microbiol. 2023, 14, 1124144. [Google Scholar] [CrossRef]
- Mayeur, C.; Gratadoux, J.J.; Bridonneau, C.; Chegdani, F.; Larroque, B.; Kapel, N.; Corcos, O.; Thomas, M.; Joly, F. Faecal D/L lactate ratio is a metabolic signature of microbiota imbalance in patients with short bowel syndrome. PLoS ONE 2013, 8, e54335. [Google Scholar] [CrossRef]
- Soares, M.B.; Almada, C.N.; Pereira, E.P.R.; Ferreira, B.M.; Balthazar, C.F.; Khorshidian, N.; Rocha, R.S.; Xavier-Santos, D.; Cruz, A.G.; Ranadheera, C.S.; et al. Spore-forming probiotic bacteria: Characteristics, health benefits, and technological aspects for their applications in foods and beverages. Trends Food Sci. Technol. 2023, 138, 453–469. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Svensson, B. Dietary nutrients, proteomes, and adhesion of probiotic lactobacilli to mucin and host epithelial cells. Microorganisms 2018, 6, 90. [Google Scholar] [CrossRef]
- Ringot-Destrez, B.; D’Alessandro, Z.; Lacroix, J.-M.; Mercier-Bonin, M.; Léonard, R.; Robbe-Masselot, C. A sensitive and rapid method to determine the adhesion capacity of probiotics and pathogenic microorganisms to human gastrointestinal mucins. Microorganisms 2018, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Saggese, A.; Giglio, R.; D’Anzi, N.; Baccigalupi, L.; Ricca, E. Comparative genomics and physiological characterization of two aerobic spore formers isolated from human ileal samples. Int. J. Mol. Sci. 2022, 23, 14946. [Google Scholar] [CrossRef]
- Cenci, G.; Trotta, F.; Caldini, G. Tolerance to challenges miming gastrointestinal transit by spores and vegetative cells of Bacillus clausii. J. Appl. Microbiol. 2006, 101, 1208–1215. [Google Scholar] [CrossRef]
- Vecchione, A.; Celandroni, F.; Mazzantini, D.; Senesi, S.; Lupetti, A.; Ghelardi, E. Compositional quality and potential gastrointestinal behavior of probiotic products commercialized in Italy. Front. Med. 2018, 5, 59. [Google Scholar] [CrossRef]
- Ahire, J.J.; Kashikar, M.S.; Madempudi, R.S. Survival and germination of Bacillus clausii UBBC07 spores in in vitro human gastrointestinal tract simulation model and evaluation of clausin production. Front. Microbiol. 2020, 11, 1010. [Google Scholar] [CrossRef]
- Ghelardi, E.; Mazzantini, D.; Celandroni, F.; Calvigioni, M.; Panattoni, A.; Lupetti, A.; Bois De Fer, B.; Perez, M., 3rd. Analysis of the microbial content of probiotic products commercialized worldwide and survivability in conditions mimicking the human gut environment. Front. Microbiol. 2023, 14, 1127321. [Google Scholar] [CrossRef]
- Chelliah, R.; Kim, N.H.; Rubab, M.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Hirad, A.H.; Oh, D.H. Robust and safe: Unveiling Bacillus clausii OHRC1’s potential as a versatile probiotic for enhanced food quality and safety. LWT 2024, 203, 116291. [Google Scholar] [CrossRef]
- Khatri, I.; Sharma, G.; Subramanian, S. Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina, and insights into its probiotic properties. BMC Microbiol. 2019, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kataoka, F.; Nagaoka, S.; Kawai, Y.; Kitazawa, H.; Itoh, H.; Kimura, K.; Taketomo, N.; Yamazaki, Y.; Tateno, Y.; et al. β-Galactosidase, phospho-β-galactosidase and phospho-β-glucosidase activities in lactobacilli strains isolated from human faeces. Lett. Appl. Microbiol. 2007, 45, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-I.; Kim, M.S.; Park, D.G.; Han, B.K.; Kim, Y.J. Effects of probiotics administration on lactose intolerance in adulthood: A meta-analysis. J. Dairy Sci. 2023, 106, 4489–4501. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Fu, A.; Gong, L.; Li, W.; Li, Y. Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl. Microbiol. Biotechnol. 2017, 101, 3015–3026. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yen, C.L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef]
- Persichetti, E.; De Michele, A.; Codini, M.; Traina, G. Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 2014, 30, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; de Moreno de LeBlanc, A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. Biomed. Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef] [PubMed]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of vitamin B2 by microorganisms: An overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B vitamins: Functions and uses in medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, M.; Cronan, J.E. Pimelic acid, the first precursor of the Bacillus subtilis biotin synthesis pathway, exists as the free acid and is assembled by fatty acid synthesis. Mol. Microbiol. 2017, 104, 595–607. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate role of gut microbiota in vitamin B nutrition and its influences on human health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Du, G.; Fang, H.; Li, R.; Zhang, D. Advances and prospects in microbial production of biotin. Microb. Cell Fact. 2024, 23, 135. [Google Scholar] [CrossRef]
- Bretzel, W.; Schurter, W.; Ludwig, B.; Kupfer, E.; Doswald, S.; Pfister, M.; van Loon, A.P.G.M. Commercial riboflavin production by recombinant Bacillus subtilis: Down-stream processing and comparison of the composition of riboflavin produced by fermentation or chemical synthesis. J. Ind. Microbiol. Biotech. 1999, 22, 19–26. [Google Scholar] [CrossRef]
- Salvetti, S.; Celandroni, F.; Ghelardi, E.; Baggiani, A.; Senesi, S. Rapid determination of vitamin B2 secretion by bacteria growing on solid media. J. Appl. Microbiol. 2003, 95, 1255–1260. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Qi, X.; Gao, H.; Wang, M.; Guan, H.; Yu, B. Metabolic engineering of Bacillus subtilis for riboflavin production: A review. Microorganisms 2023, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Halczuk, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T.; Karmańska, A.; Cieślak, M. Vitamin B12-Multifaceted in vivo functions and in vitro applications. Nutrients 2023, 15, 2734. [Google Scholar] [CrossRef]
- Kapse, N.G.; Engineer, A.S.; Gowdaman, V.; Wagh, S. Genome profiling for health promoting and disease preventing traits unraveled probiotic potential of Bacillus clausii B106. Microbiol. Biotechnol. Lett. 2018, 46, 334–345. [Google Scholar] [CrossRef]
- D’Aimmo, M.R.; Satti, M.; Scarafile, D.; Modesto, M.; Pascarelli, S.; Biagini, S.A.; Luiselli, D.; Mattarelli, P.; Andlid, T. Folate-producing bifidobacteria: Metabolism, genetics, and relevance. Microbiome Res. Rep. 2024, 3, 11. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; Stapleton, D.I.; Butt, H.L.; De Meirleir, K.L. Increased D-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
- Rao, S.S.C.; Rehman, A.; Yu, S.; Andino, N.M. Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clin. Transl. Gastroenterol. 2018, 9, 162. [Google Scholar] [CrossRef]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.; Hörmannsperger, G.; Huys, G.; Levy, D.D.; Lutgendorff, F.; Mack, D.; et al. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Kowlgi, N.G.; Chhabra, L. D-lactic acidosis: An underrecognized complication of short bowel syndrome. Gastroenterol. Res. Pract. 2015, 2015, 476215. [Google Scholar] [CrossRef]
| Production of Group B Vitamins (ng/mL) | Mean ± SD | |
| Vitamin B2 | Supernatant | 22.86 ± 1.61 |
| Vitamin B8 | Cell lysate | 5.89 ± 0.03 |
| Supernatant | 3.93 ± 0.01 | |
| Vitamin B9 | Cell lysate | 3.11 ± 0.32 |
| Supernatant | 2.01 ± 0.12 | |
| Vitamin B12 | Cell lysate | 1.48 ± 0.15 |
| Supernatant | 1.17 ± 0.64 | |
| Production of enzymes with antioxidant activity (ng/mL) | ||
| Superoxide dismutase | Cell lysate | 1000.82 ± 102.15 |
| Supernatant | 661.95 ± 171.47 | |
| Catalase | Cell lysate | 0.02 ± 0.00 |
| Supernatant | 0.18 ± 0.08 | |
| Production of β-galactosidase(Miller units) | ||
| β-galactosidase | 61.76 ± 13.49 | |
| Secretion of short-chain fatty acids (ng/mL) | ||
| Acetic acid | Supernatant | 586.67 ± 39.37 |
| Propionic acid | Supernatant | 0.79 ± 0.14 |
| Butyric acid | Supernatant | 2.85 ± 0.46 |
| Secretion of D-lactic acid | ||
| D-lactate | Supernatant | Not produced |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzantini, D.; Calvigioni, M.; Celandroni, F.; Saba, A.; Ghelardi, E. In Vitro Analysis of an Alkalihalobacillus clausii Spore-Based Probiotic Formulation Clarifies the Mechanisms Underlying Its Beneficial Properties. Biomolecules 2025, 15, 1294. https://doi.org/10.3390/biom15091294
Mazzantini D, Calvigioni M, Celandroni F, Saba A, Ghelardi E. In Vitro Analysis of an Alkalihalobacillus clausii Spore-Based Probiotic Formulation Clarifies the Mechanisms Underlying Its Beneficial Properties. Biomolecules. 2025; 15(9):1294. https://doi.org/10.3390/biom15091294
Chicago/Turabian StyleMazzantini, Diletta, Marco Calvigioni, Francesco Celandroni, Alessandro Saba, and Emilia Ghelardi. 2025. "In Vitro Analysis of an Alkalihalobacillus clausii Spore-Based Probiotic Formulation Clarifies the Mechanisms Underlying Its Beneficial Properties" Biomolecules 15, no. 9: 1294. https://doi.org/10.3390/biom15091294
APA StyleMazzantini, D., Calvigioni, M., Celandroni, F., Saba, A., & Ghelardi, E. (2025). In Vitro Analysis of an Alkalihalobacillus clausii Spore-Based Probiotic Formulation Clarifies the Mechanisms Underlying Its Beneficial Properties. Biomolecules, 15(9), 1294. https://doi.org/10.3390/biom15091294





