The Emerging Role of Mitochondrial Dysfunction in Thyroid Cancer: Mediating Tumor Progression, Drug Resistance, and Reshaping of the Immune Microenvironment
Abstract
1. Introduction
2. Key Concepts and Review Methodology
2.1. Key Concepts
2.2. Review Methodology
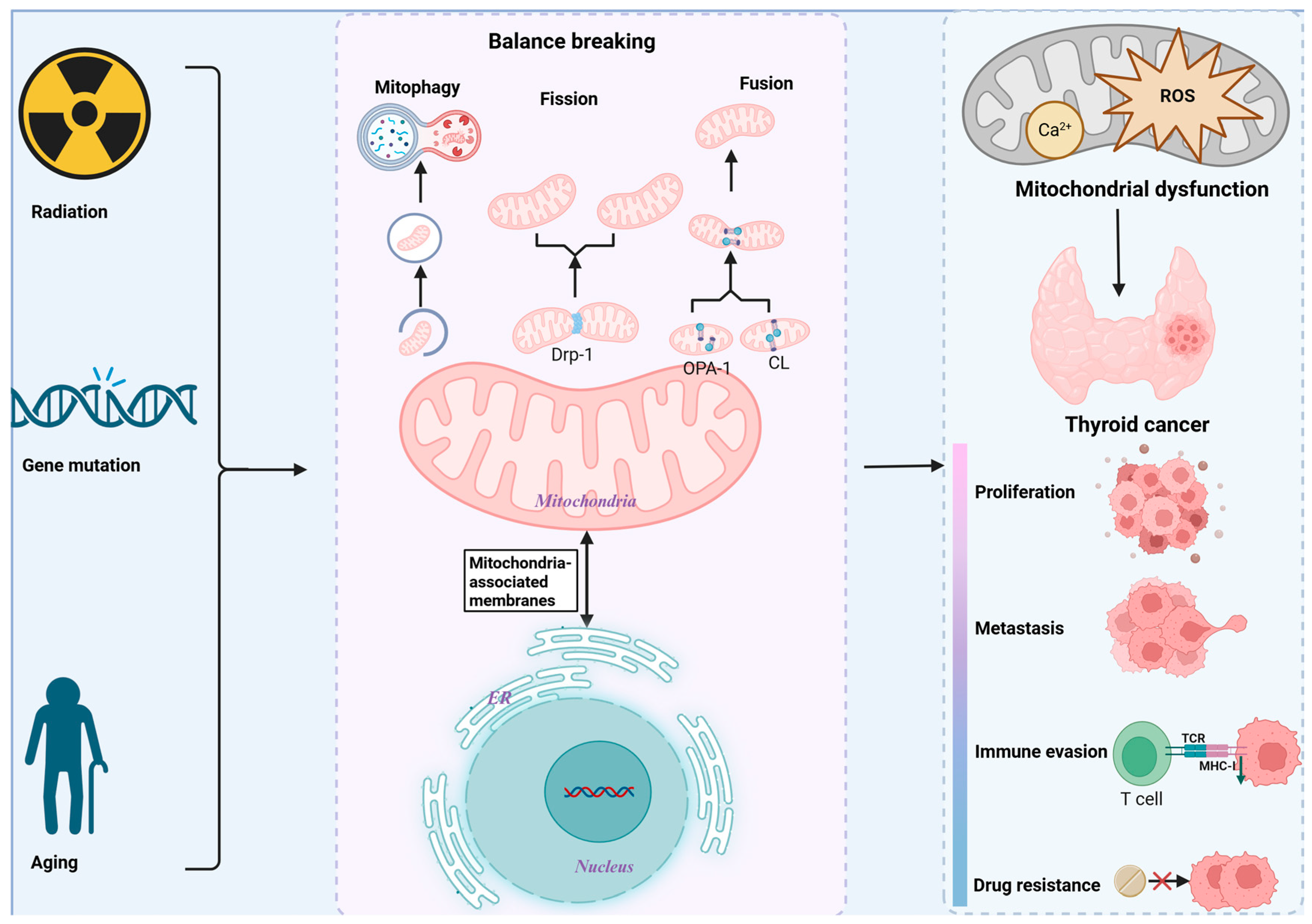
3. Processes and Mechanisms of Mitochondrial Homeostasis Regulation
4. Mitochondrial Dysfunction in TC
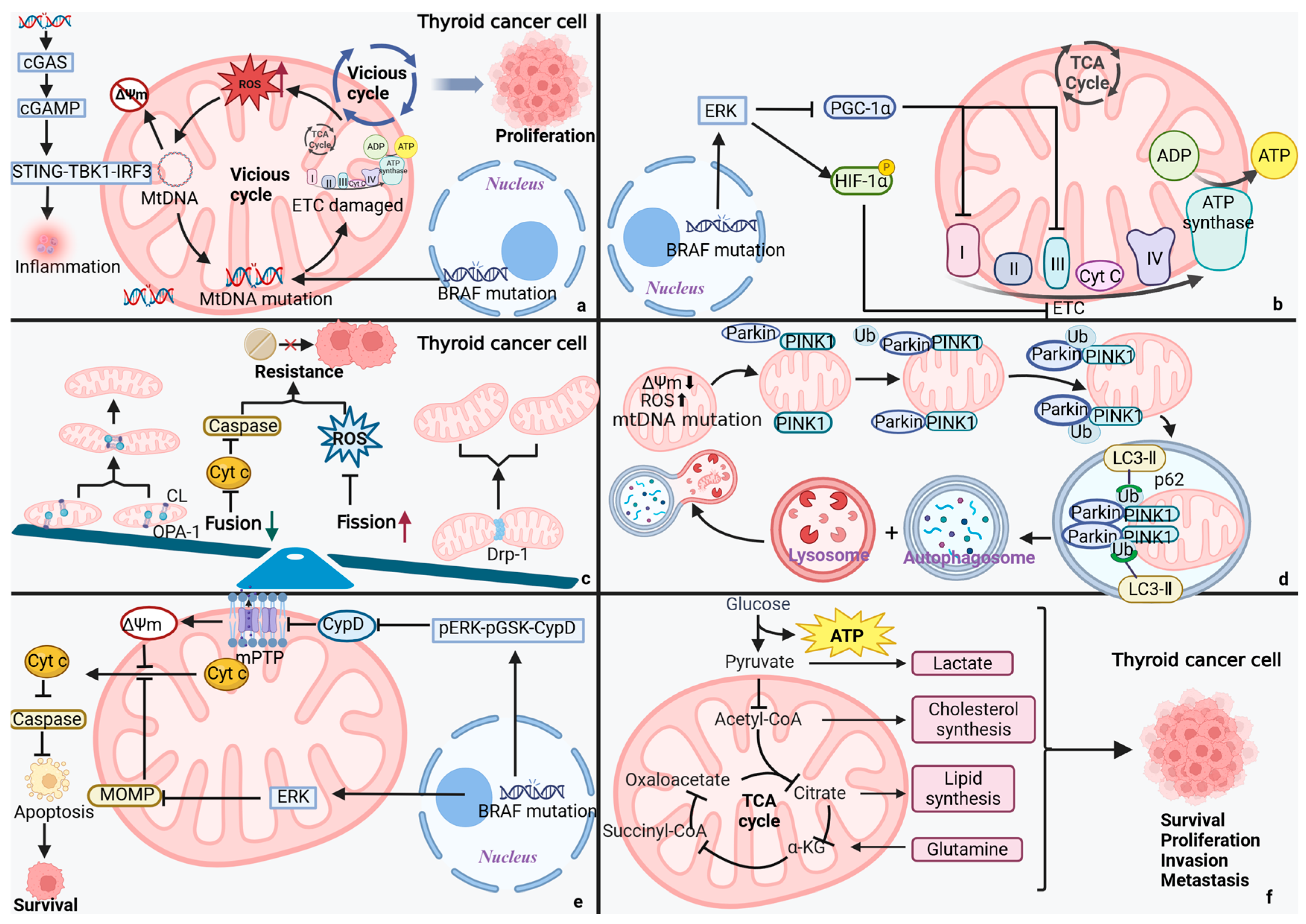
4.1. MtDNA Mutations: The Saboteur of TC
4.2. Mitochondrial Dynamics: A Lever for TC Cells’ Fate
4.3. Mitochondrial Autophagy: The Guardian of TC
4.4. Mitochondrial Permeability: A Switch for Programmed Cell Death in TC Cells
4.5. Disorders of Mitochondrial Metabolism
4.6. Mitochondrial Dysfunction and the TC Tumor Microenvironment (TME)
4.7. Mitochondrial Dysfunction and TC Stem Cells (TCSCs)
4.8. Mitochondrial Dysfunction and TC Treatment Resistance
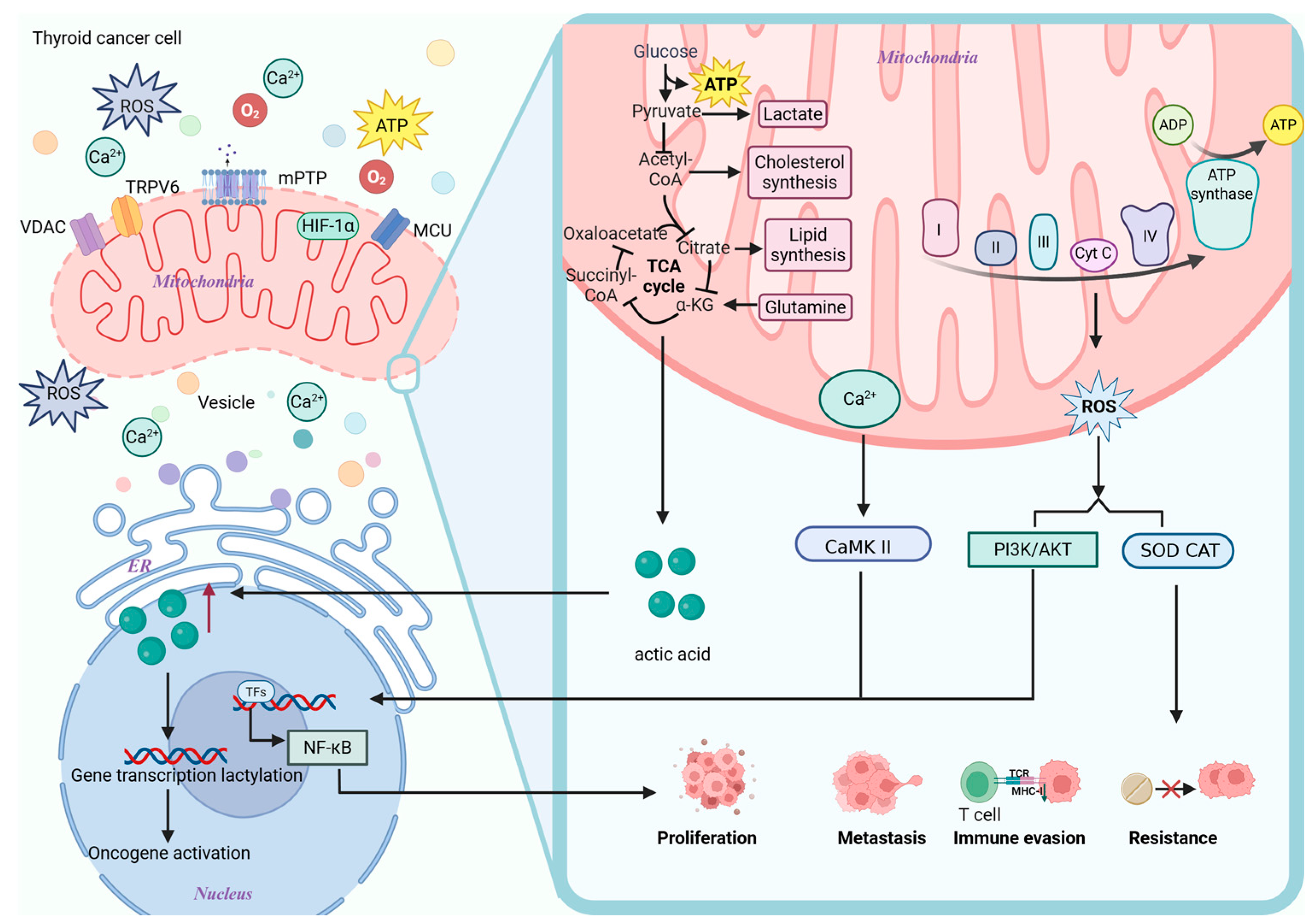
5. Mitochondrial Information Processing Networks: Bridging Mitochondrial Dysfunction and TC Progression
5.1. Calcium
5.2. ROS
5.3. Product of Metabolism
6. Key Regulatory Pathways of Abnormal Mitochondrial Functions in TC
6.1. AMPK-PGC-1α-NRF1
6.2. PI3K-AKT-mTOR
6.3. Wnt/β-Catenin
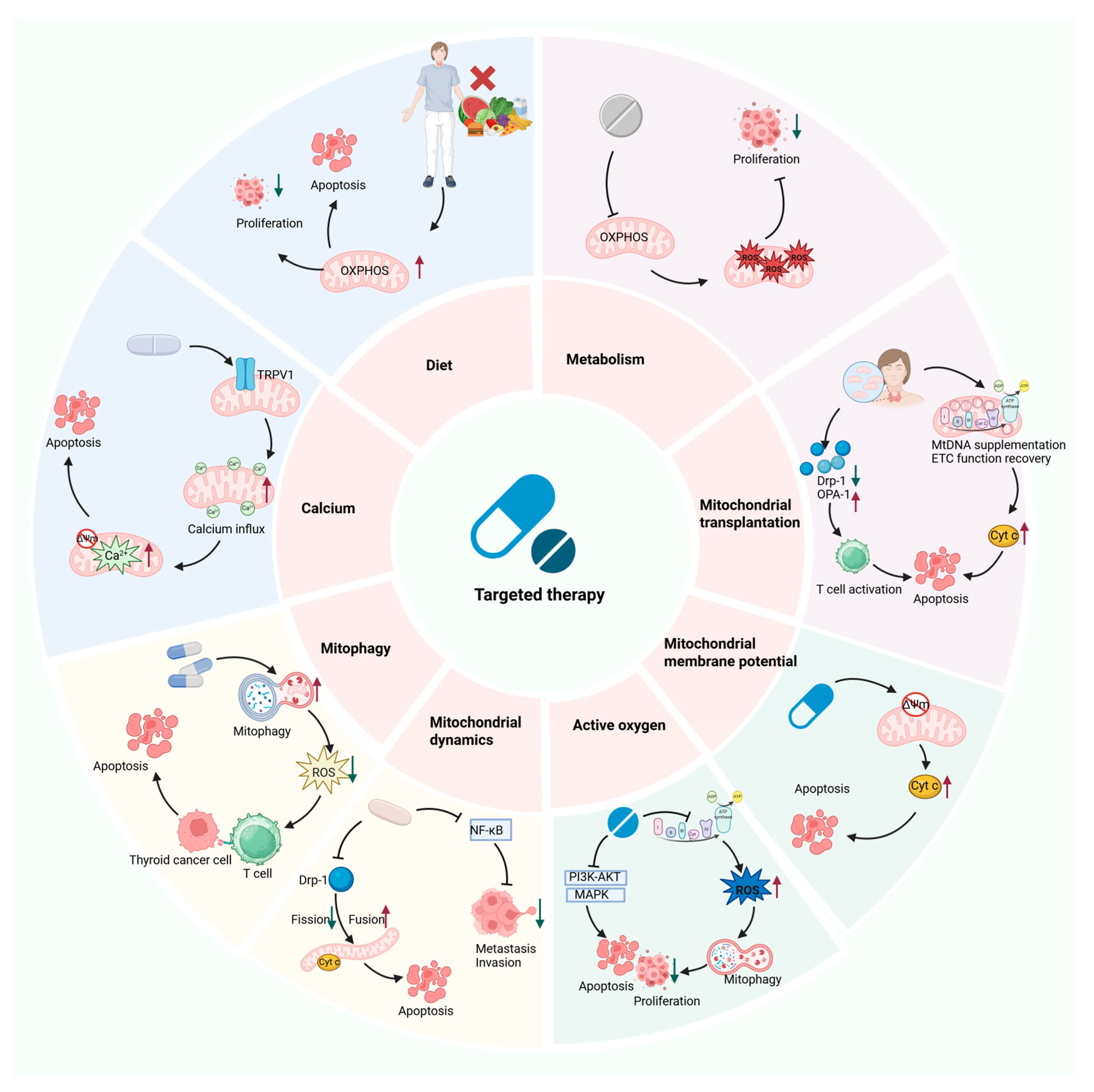
7. The Potential of Mitochondrial Dysfunction as a Therapeutic Target for the Treatment of TC
7.1. Targeting Mitochondrial Metabolism
7.2. Targeting Mitochondrial Dynamics
7.3. Targeting Mitochondrial Oxidative Stress
7.4. Targeting Mitochondrial Membrane Potential
7.5. Targeting Mitochondrial Autophagy
7.6. Targeting Mitochondrial Calcium Homeostasis
7.7. Mitochondrial Transplantation
7.8. Combination Therapy
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-DG | 2-Deoxy-D-glucose |
| 27-HC | 27 hydroxycholesterol |
| ACC | Acetyl-CoA carboxylase |
| AE | Adverse event |
| AML | Acute myeloid leukemia |
| ATC | Anaplastic thyroid cancer |
| CaMKII | Calcium/calmodulin-dependent kinase II |
| CAP | Capsaicin |
| CAT | Catalase |
| CL | Cardiolipin |
| CSCs | Cancer stem cells |
| CypD | Cyclophilin D |
| EMT | Epithelial–mesenchymal transition |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| FASN | Fatty acid synthase |
| FMD | Fasting mimicking diet |
| FTC | Follicular thyroid cancer |
| IGF-1 | Insulin-Like Growth Factor 1 |
| ILR | Interleukin receptor |
| LDLR | Low-density lipoprotein receptor |
| LRP | Low-density lipoprotein receptor-related protein |
| MAMs | Mitochondria-associated membranes |
| MHC-I | Major histocompatibility complex 1 |
| MIPS | Mitochondrial information processing system |
| mPTP | Mitochondrial permeability transition pore |
| MQC | Mitochondrial quality control |
| MTC | Medullary thyroid cancer |
| MtDNA | Mitochondrial DNA |
| MtDNA-CN | MtDNA copy number |
| NIS | Sodium iodide symporter |
| NRF1 | Nuclear respiratory factor 1 |
| ORR | Overall relief rate |
| OXPHOS | Oxidative phosphorylation |
| PDT | Photodynamic therapy |
| PFS | Progression-free survival |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1 α |
| PSA | Prostate-specific antigen |
| PTC | Papillary thyroid cancer |
| RAI | Radioactive iodine |
| RIRR | ROS-induced ROS release |
| ROS | Reactive oxygen species |
| SHMT1 | Serine hydroxymethyltransferase 1 |
| SOC | Standard of Care |
| SOD | Superoxide dismutase |
| SOR-C13 | Soricimed Biopharma Compound 13 |
| TAMs | Tumor-associated macrophages |
| TCR | T cell receptors |
| TC | Thyroid cancer |
| TME | Tumor microenvironment |
| TNFR | Tumor necrosis factor receptor |
| TRPV6 | Transient receptor potential vanilloid type 6 |
| UA | Urolithin A |
| VDAC | Voltage-dependent anion channels |
References
- Iovine, J.C.; Claypool, S.M.; Alder, N.N. Mitochondrial Compartmentalization: Emerging Themes in Structure and Function. Trends Biochem. Sci. 2021, 46, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in Health, Disease, and Aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.-X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Das, S.; Zea, M.P.; Russon, M.P.; Xing, Z.; Torregrosa-Allen, S.; Cervantes, H.E.; Harper, H.A.; Elzey, B.D.; Tran, E.J. Supinoxin Blocks Small Cell Lung Cancer Progression by Inhibiting Mitochondrial Respiration through DDX5Supinoxin. iScience 2025, 28, 112219. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Chen, T.-H.; Lin, S.-H.; Lee, M.-Y.; Wang, H.-C.; Tsai, K.-F.; Chou, C.-K. Mitochondrial Alterations and Signatures in Hepatocellular Carcinoma. Cancer Metastasis Rev. 2025, 44, 34. [Google Scholar] [CrossRef]
- Fu, Y.; Ricciardiello, F.; Yang, G.; Qiu, J.; Huang, H.; Xiao, J.; Cao, Z.; Zhao, F.; Liu, Y.; Luo, W.; et al. The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells. Cells 2021, 10, 497. [Google Scholar] [CrossRef]
- Huang, A.; Xue, H.; Xie, T.; Xiang, L.; Chen, Z.; Ma, A.; Yan, H.; Yuan, J. A Review of the Pathogenesis of Mitochondria in Breast Cancer and Progress of Targeting Mitochondria for Breast Cancer Treatment. J. Transl. Med. 2025, 23, 70. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.; Cheng, M.; Wang, S.; Ma, K. The Multifaceted Mechanisms of Ellagic Acid in the Treatment of Tumors: State-of-the-Art. Biomed. Pharmacother. 2023, 165, 115132. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Zhang, Y.; Sheng, C.; Huang, Y.; Zhang, Q.; Chen, K. Global Burden of Thyroid Cancer in 2022: Incidence and Mortality Estimates from GLOBOCAN. Chin. Med. J. 2024, 137, 2567–2576. [Google Scholar] [CrossRef]
- UNSD—Methodology. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 28 August 2025).
- Liu, S.; Yan, X.; Yang, Y.; Xia, Y.; Zhang, P. Knowledge Mapping of Anaplastic Thyroid Cancer Treatments: A Bibliometric Analysis (2000–2023). Front. Oncol. 2024, 14, 1330030. [Google Scholar] [CrossRef]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid Cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Boucai, L.; Zafereo, M.; Cabanillas, M.E. Thyroid Cancer: A Review. JAMA 2024, 331, 425. [Google Scholar] [CrossRef]
- Lin, L.; Almont, T.; Beaubrun, M.; Macni, J.; Pierre-Louis, A.; Zabulon, A.; Draganescu, C.; Lin, L.; Sabbah, N.; Drame, M.; et al. Overall Survival of Patients with Thyroid Cancer in Martinique (2008–2018). BMC Cancer 2023, 23, 739. [Google Scholar] [CrossRef] [PubMed]
- Raue, F.; Frank-Raue, K. Thyroid Cancer: Risk-Stratified Management and Individualized Therapy. Clin. Cancer Res. 2016, 22, 5012–5021. [Google Scholar] [CrossRef]
- Guarino, V.; Castellone, M.D.; Avilla, E.; Melillo, R.M. Thyroid Cancer and Inflammation. Mol. Cell Endocrinol. 2010, 321, 94–102. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Guo, S.; Qi, G.; Huang, J.; Gao, J.; Zhao, J.; Kang, L.; Li, Q. A Review of Advances in Mitochondrial Research in Cancer. Cancer Control 2024, 31, 10732748241299072. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.Y.; Choi, H.; Paeng, J.C.; Cheon, G.J.; Chung, J.-K.; Kang, K.W. Comprehensive Gene Expression Analysis for Exploring the Association between Glucose Metabolism and Differentiation of Thyroid Cancer. BMC Cancer 2019, 19, 1260. [Google Scholar] [CrossRef]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative Stress in Thyroid Carcinomas: Biological and Clinical Significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Nikiforov, N.G.; Zhuravlev, A.D.; Orekhov, N.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Altered Mitochondrial Metabolism in Thyroid Cancer Development and Mitochondria-Targeted Thyroid Cancer Treatment. Int. J. Mol. Sci. 2021, 23, 460. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.; Segaran, A.; Lord, S. Targeting OXPHOS and the Electron Transport Chain in Cancer; Molecular and Therapeutic Implications. Semin. Cancer Biol. 2022, 86, 851–859. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpoor, M.; Hedayati, M. Regulators of Glucose Uptake in Thyroid Cancer Cell Lines. Cell Commun. Signal. 2020, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of Reactive Oxygen Species in Inflammation and Cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef]
- Shan, G.; Wang, W.; Li, X.; Zhai, Z.; Liu, M.; Peng, R.; Sun, Q.; Zhang, H.; Guo, T.; He, X. Epigenetic-Targeted Biomimetic Nanomedicine Modulates Epithelial Mesenchymal Transition to Enhance Chemosensitivity in Heterogeneous Tumors. Biomaterials 2026, 324, 123529. [Google Scholar] [CrossRef]
- Taniguchi, N.; Ohkawa, Y.; Kuribara, T.; Abe, J.; Harada, Y.; Takahashi, M. Roles of Glyco-Redox in Epithelial Mesenchymal Transition and Mesenchymal Epithelial Transition, Cancer, and Various Diseases. Antioxid. Redox Signal. 2024, 41, 910–926. [Google Scholar] [CrossRef]
- Vasileiou, P.V.S.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.I.; Chronopoulos, E.; Passias, P.-G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial Homeostasis and Cellular Senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between Mitochondrial Biogenesis and Mitophagy to Maintain Mitochondrial Homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Jiang, D.; Hu, X.; Du, W.; Ji, L.; Yang, Y.; Li, X.; Sho, T.; Wang, X.; Li, Y.; et al. Mitocytosis, a Migrasome-Mediated Mitochondrial Quality-Control Process. Cell 2021, 184, 2896–2910.e13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The Role of Mitochondrial Quality Surveillance in Skin Aging: Focus on Mitochondrial Dynamics, Biogenesis and Mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Liu, B.-H.; Xu, C.-Z.; Liu, Y.; Lu, Z.-L.; Fu, T.-L.; Li, G.-R.; Deng, Y.; Luo, G.-Q.; Ding, S.; Li, N.; et al. Mitochondrial Quality Control in Human Health and Disease. Mil. Med. Res. 2024, 11, 32. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Tait, S.W.G. Mitochondrial Quality Control: From Molecule to Organelle. Cell Mol. Life Sci. 2021, 78, 3853–3866. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Tay, E.X.Y.; Ong, D.S.T.; Taneja, R. Mitochondrial Dysfunction at the Center of Cancer Therapy. Antioxid. Redox Signal. 2020, 32, 309–330. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the Tumor Microenvironment: Removing Obstruction to Anticancer Immune Responses and Immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; He, K.; Younes, A.I.; Barsoumian, H.B.; Chen, D.; Ozgen, T.; Mosaffa, S.; Patel, R.R.; Gu, M.; Novaes, J.; et al. Role of Mitochondria in Cancer Immune Evasion and Potential Therapeutic Approaches. Front. Immunol. 2020, 11, 573326. [Google Scholar] [CrossRef] [PubMed]
- Leuthner, T.C.; Meyer, J.N. Mitochondrial DNA Mutagenesis: Feature of and Biomarker for Environmental Exposures and Aging. Curr. Env. Health Rep. 2021, 8, 294–308. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Guo, Y.; Shi, X.; Chen, X.; Feng, W.; Wu, L.-L.; Zhang, J.; Yu, S.; Wang, Y.; et al. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int. J. Biol. Sci. 2023, 19, 897–915. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Perrone, M.; Genovese, I.; Pinton, P.; Giorgi, C. Cancer Metabolism and Mitochondria: Finding Novel Mechanisms to Fight Tumours. EBioMedicine 2020, 59, 102943. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Pedro, J.M.B.-S.; Kroemer, G.; Galluzzi, L. Mitochondrial Metabolism and Cancer. Cell Res. 2018, 28, 265–280. [Google Scholar] [CrossRef]
- Cai, X.; Liang, C.; Zhang, M.; Dong, Z.; Weng, Y.; Yu, W. Mitochondrial DNA Copy Number and Cancer Risks: A Comprehensive Mendelian Randomization Analysis. Int. J. Cancer 2024, 154, 1504–1513. [Google Scholar] [CrossRef]
- Tsybrovskyy, O.; De Luise, M.; de Biase, D.; Caporali, L.; Fiorini, C.; Gasparre, G.; Carelli, V.; Hackl, D.; Imamovic, L.; Haim, S.; et al. Papillary Thyroid Carcinoma Tall Cell Variant Shares Accumulation of Mitochondria, Mitochondrial DNA Mutations, and Loss of Oxidative Phosphorylation Complex I Integrity with Oncocytic Tumors. J. Pathol. Clin. Res. 2022, 8, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Máximo, V.; Lima, J.; Prazeres, H.; Soares, P.; Sobrinho-Simões, M. The Biology and the Genetics of Hurthle Cell Tumors of the Thyroid. Endocr. Relat. Cancer 2012, 19, R131–R147. [Google Scholar] [CrossRef] [PubMed]
- You, M.-H.; Jeon, M.J.; Kim, S.R.; Lee, W.K.; Cheng, S.-Y.; Jang, G.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Kim, W.G. Mitofusin-2 Modulates the Epithelial to Mesenchymal Transition in Thyroid Cancer Progression. Sci. Rep. 2021, 11, 2054. [Google Scholar] [CrossRef]
- Kashatus, J.A.; Nascimento, A.; Myers, L.J.; Sher, A.; Byrne, F.L.; Hoehn, K.L.; Counter, C.M.; Kashatus, D.F. Erk2 Phosphorylation of Drp1 Promotes Mitochondrial Fission and MAPK-Driven Tumor Growth. Mol. Cell 2015, 57, 537–551. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Wieder, S.Y.; Renault, T.T.; Elkholi, R.; Asciolla, J.J.; Yao, J.L.; Jabado, O.; Hoehn, K.; Kageyama, Y.; Sesaki, H.; et al. Mitochondrial Division Is Requisite to RAS-Induced Transformation and Targeted by Oncogenic MAPK Pathway Inhibitors. Mol. Cell 2015, 57, 521–536. [Google Scholar] [CrossRef]
- Lee, J.; Ham, S.; Lee, M.H.; Kim, S.J.; Park, J.H.; Lee, S.E.; Chang, J.Y.; Joung, K.H.; Kim, T.Y.; Kim, J.M.; et al. Dysregulation of Parkin-Mediated Mitophagy in Thyroid Hürthle Cell Tumors. Carcinogenesis 2015, 36, 1407–1418. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, D.; Wang, F.; Zhang, D.; Li, P.; Wang, K. BRAF V600E Protect from Cell Death via Inhibition of the Mitochondrial Permeability Transition in Papillary and Anaplastic Thyroid Cancers. J. Cell Mol. Med. 2022, 26, 4048–4060. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.; Yi, S.; Choi, N.R.; Lim, M.A.; Chang, J.W.; Won, H.-R.; Kim, J.R.; Ko, H.M.; Chung, E.-J.; et al. Unraveling the Role of the Mitochondrial One-Carbon Pathway in Undifferentiated Thyroid Cancer by Multi-Omics Analyses. Nat. Commun. 2024, 15, 1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, X.; Pan, Y.; Liu, Y.; Zhang, Y. Pyruvate Carboxylase Promotes Thyroid Cancer Aggressiveness through Fatty Acid Synthesis. BMC Cancer 2021, 21, 722. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, M.; Zwara, A.; Kowalski, J.; Mika, A.; Hellmann, A. The Role of Lipid Metabolism Disorders in the Development of Thyroid Cancer. Int. J. Mol. Sci. 2024, 25, 7129. [Google Scholar] [CrossRef]
- Wan, Y.; Li, G.; Cui, G.; Duan, S.; Chang, S. Reprogramming of Thyroid Cancer Metabolism: From Mechanism to Therapeutic Strategy. Mol. Cancer 2025, 24, 74. [Google Scholar] [CrossRef]
- Gammage, P.A.; Frezza, C. Mitochondrial DNA: The Overlooked Oncogenome? BMC Biol. 2019, 17, 53. [Google Scholar] [CrossRef]
- Kim, M.; Mahmood, M.; Reznik, E.; Gammage, P.A. Mitochondrial DNA Is a Major Source of Driver Mutations in Cancer. Trends Cancer 2022, 8, 1046–1059. [Google Scholar] [CrossRef]
- Smith, A.L.M.; Whitehall, J.C.; Greaves, L.C. Mitochondrial DNA Mutations in Ageing and Cancer. Mol. Oncol. 2022, 16, 3276–3294. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.R.; Foster, C.; Rigoutsos, I. Roles of Mitochondrial Genetics in Cancer Metastasis. Trends Cancer 2022, 8, 1002–1018. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and Cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Stewart, J.B.; Alaei-Mahabadi, B.; Sabarinathan, R.; Samuelsson, T.; Gorodkin, J.; Gustafsson, C.M.; Larsson, E. Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers. PLoS Genet. 2015, 11, e1005333. [Google Scholar] [CrossRef]
- Hertweck, K.L.; Dasgupta, S. The Landscape of mtDNA Modifications in Cancer: A Tale of Two Cities. Front. Oncol. 2017, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Alzahrani, A.S.; Zou, M.; Shi, Y. High Frequency of Somatic Mitochondrial DNA Mutations in Human Thyroid Carcinomas and Complex I Respiratory Defect in Thyroid Cancer Cell Lines. Oncogene 2005, 24, 1455–1460. [Google Scholar] [CrossRef]
- Chen, S.; Bao, X.; Chen, H.; Jia, M.; Li, W.; Zhang, L.; Fan, R.; Fang, H.; Jin, L. Thyroid Cancer-Associated Mitochondrial DNA Mutation G3842A Promotes Tumorigenicity via ROS-Mediated ERK1/2 Activation. Oxid. Med. Cell Longev. 2022, 2022, 9982449. [Google Scholar] [CrossRef]
- Su, X.; Wang, W.; Ruan, G.; Liang, M.; Zheng, J.; Chen, Y.; Wu, H.; Fahey, T.J.; Guan, M.; Teng, L. A Comprehensive Characterization of Mitochondrial Genome in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2016, 17, 1594. [Google Scholar] [CrossRef] [PubMed]
- Alwehaidah, M.S.; Al-Awadhi, R.; Roomy, M.A.; Baqer, T.A. Mitochondrial DNA Copy Number and Risk of Papillary Thyroid Carcinoma. BMC Endocr. Disord. 2024, 24, 138. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Chan, D.C. Mitochondrial Dynamics and Its Involvement in Disease. Annu. Rev. Pathol. 2020, 15, 235–259. [Google Scholar] [CrossRef]
- He, J.; Liu, K.; Fu, C. Recent Insights into the Control of Mitochondrial Fission. Biochem. Soc. Trans. 2024, 52, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.R.; Jongens, T.A.; Holzbaur, E.L.F. Mitochondrial Dynamics: Shaping and Remodeling an Organelle Network. Curr. Opin. Cell Biol. 2021, 68, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Ferraz, L.S. Therapeutic Potential of Targeting Mitochondrial Dynamics in Cancer. Biochem. Pharmacol. 2020, 182, 114282. [Google Scholar] [CrossRef]
- Ferreira-da-Silva, A.; Valacca, C.; Rios, E.; Pópulo, H.; Soares, P.; Sobrinho-Simões, M.; Scorrano, L.; Máximo, V.; Campello, S. Mitochondrial Dynamics Protein Drp1 Is Overexpressed in Oncocytic Thyroid Tumors and Regulates Cancer Cell Migration. PLoS ONE 2015, 10, e0122308. [Google Scholar] [CrossRef]
- Choe, S.C.; Hamacher-Brady, A.; Brady, N.R. Autophagy Capacity and Sub-Mitochondrial Heterogeneity Shape Bnip3-Induced Mitophagy Regulation of Apoptosis. Cell Commun. Signal. 2015, 13, 37. [Google Scholar] [CrossRef]
- Schofield, J.H.; Schafer, Z.T. Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid. Redox Signal. 2021, 34, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liao, X.; Wu, H.; Li, Y.; Zhu, Y.; Chen, Q. Mitophagy and Its Contribution to Metabolic and Aging-Associated Disorders. Antioxid. Redox Signal. 2020, 32, 906–927. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.-W.; Zhao, G. The Mitophagy Pathway and Its Implications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wang, Y.; Li, B.; Shen, K.; Li, Q.; Ni, Y.; Huang, L. Mitophagy in Carcinogenesis, Drug Resistance and Anticancer Therapeutics. Cancer Cell Int. 2021, 21, 350. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Xie, X.; Yang, B.; Wang, X.; Zhang, J.; Qiao, T.; Guan, J.; Qiu, Y.; Huang, Y.-X.; et al. PINK1-Mediated Mitophagy Promotes Oxidative Phosphorylation and Redox Homeostasis to Induce Drug-Tolerant Persister Cancer Cells. Cancer Res. 2023, 83, 398–413. [Google Scholar] [CrossRef]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The Emerging, Multifaceted Role of Mitophagy in Cancer and Cancer Therapeutics. Semin. Cancer Biol. 2020, 66, 45–58. [Google Scholar] [CrossRef]
- Hamada, K.; Kurashige, T.; Shimamura, M.; Arakawa, H.; Nakamura, Y.; Nagayama, Y. MIEAP and ATG5 Are Tumor Suppressors in a Mouse Model of BRAFV600E-Positive Thyroid Cancer. Front. Endocrinol. 2022, 13, 932754. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, L.; Xu, S.; Cheng, X.; Wu, J.; Wang, Y.; Gao, W.; Bao, J.; Yu, H. Curcumin Induces Mitophagy by Promoting Mitochondrial Succinate Dehydrogenase Activity and Sensitizes Human Papillary Thyroid Carcinoma BCPAP Cells to Radioiodine Treatment. Toxicol. Vitr. 2023, 93, 105669. [Google Scholar] [CrossRef]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular Mechanisms and Consequences of Mitochondrial Permeability Transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of Cyclophilin D Reveals a Critical Role for Mitochondrial Permeability Transition in Cell Death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-Dependent Mitochondrial Permeability Transition Regulates Some Necrotic but Not Apoptotic Cell Death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.Q.; Molkentin, J.D. Physiological and Pathological Roles of the Mitochondrial Permeability Transition Pore in the Heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Newman, L.E.; Shadel, G.S. Mitochondrial DNA Release in Innate Immune Signaling. Annu. Rev. Biochem. 2023, 92, 299–332. [Google Scholar] [CrossRef]
- Jiang, M.; Qi, L.; Li, L.; Li, Y. The Caspase-3/GSDME Signal Pathway as a Switch between Apoptosis and Pyroptosis in Cancer. Cell Death Discov. 2020, 6, 112. [Google Scholar] [CrossRef]
- Riley, J.S.; Quarato, G.; Cloix, C.; Lopez, J.; O’Prey, J.; Pearson, M.; Chapman, J.; Sesaki, H.; Carlin, L.M.; Passos, J.F.; et al. Mitochondrial Inner Membrane Permeabilisation Enables mtDNA Release during Apoptosis. EMBO J. 2018, 37, e99238. [Google Scholar] [CrossRef] [PubMed]
- Vringer, E.; Tait, S.W.G. Mitochondria and Cell Death-Associated Inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef]
- Bikas, A.; Jensen, K.; Patel, A.; Costello, J.; Kaltsas, G.; Hoperia, V.; Wartofsky, L.; Burman, K.; Vasko, V. Mitotane Induces Mitochondrial Membrane Depolarization and Apoptosis in Thyroid Cancer Cells. Int. J. Oncol. 2019, 55, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Diebold, L.P.; Kong, H.; Schieber, M.; Huang, H.; Hensley, C.T.; Mehta, M.M.; Wang, T.; Santos, J.H.; Woychik, R.; et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol. Cell 2016, 61, 199–209. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Hsu, Y.-C.; Lee, J.-J.; Chen, M.-J.; Lin, C.-H.; Huang, S.-Y.; Cheng, S.-P. Targeting the Pentose Phosphate Pathway Increases Reactive Oxygen Species and Induces Apoptosis in Thyroid Cancer Cells. Mol. Cell Endocrinol. 2020, 499, 110595. [Google Scholar] [CrossRef] [PubMed]
- Revilla, G.; Pons, M.d.P.; Baila-Rueda, L.; García-León, A.; Santos, D.; Cenarro, A.; Magalhaes, M.; Blanco, R.M.; Moral, A.; Ignacio Pérez, J.; et al. Cholesterol and 27-Hydroxycholesterol Promote Thyroid Carcinoma Aggressiveness. Sci. Rep. 2019, 9, 10260. [Google Scholar] [CrossRef]
- Sun, W.Y.; Kim, H.M.; Jung, W.-H.; Koo, J.S. Expression of Serine/Glycine Metabolism-Related Proteins Is Different According to the Thyroid Cancer Subtype. J. Transl. Med. 2016, 14, 168. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor Microenvironment as a Therapeutic Target in Cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Ikeda, H.; Kawase, K.; Nishi, T.; Watanabe, T.; Takenaga, K.; Inozume, T.; Ishino, T.; Aki, S.; Lin, J.; Kawashima, S.; et al. Immune Evasion through Mitochondrial Transfer in the Tumour Microenvironment. Nature 2025, 638, 225–236. [Google Scholar] [CrossRef]
- Guido, C.; Whitaker-Menezes, D.; Lin, Z.; Pestell, R.G.; Howell, A.; Zimmers, T.A.; Casimiro, M.C.; Aquila, S.; Ando’, S.; Martinez-Outschoorn, U.E.; et al. Mitochondrial Fission Induces Glycolytic Reprogramming in Cancer-Associated Myofibroblasts, Driving Stromal Lactate Production, and Early Tumor Growth. Oncotarget 2012, 3, 798–810. [Google Scholar] [CrossRef]
- Behera, B.P.; Mishra, S.R.; Patra, S.; Mahapatra, K.K.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Klionsky, D.J.; Bhutia, S.K. Molecular Regulation of Mitophagy Signaling in Tumor Microenvironment and Its Targeting for Cancer Therapy. Cytokine Growth Factor Rev. 2025, in press. [CrossRef]
- Xia, J.; Jin, J.; Dai, S.; Fan, H.; Chen, K.; Li, J.; Luo, F.; Peng, X. Mitophagy: A Key Regulator of Radiotherapy Resistance in the Tumor Immune Microenvironment. Mol. Asp. Med. 2025, 105, 101385. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liang, H.; Wang, X.; Cao, Z.; Ma, Y.; Liu, X. Alterations in Mitochondrial Function and Energy Metabolism-Related Properties in Thyroid Cancer Stem Cells. Acta Biochim. Pol. 2021, 69, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Yamamoto, K.; Kurashige, T.; Nagayama, Y. Intracellular Redox Status Controls Spherogenicity, an in Vitro Cancer Stem Cell Marker, in Thyroid Cancer Cell Lines. Exp. Cell Res. 2018, 370, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Praharaj, P.P.; Panigrahi, D.P.; Bhol, C.S.; Patra, S.; Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Singh, A.; Patil, S.; Bhutia, S.K. Mitochondrial Rewiring through Mitophagy and Mitochondrial Biogenesis in Cancer Stem Cells: A Potential Target for Anti-CSC Cancer Therapy. Cancer Lett. 2021, 498, 217–228. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Chen, J.-J.; Sun, Y.-B.; Chen, T.-Q.; Wang, J.; Yu, S.-C. Mitochondria in Cancer Stem Cells: Achilles Heel or Hard Armor. Trends Cell Biol. 2023, 33, 708–727. [Google Scholar] [CrossRef]
- Guha, M.; Srinivasan, S.; Ruthel, G.; Kashina, A.K.; Carstens, R.P.; Mendoza, A.; Khanna, C.; Van Winkle, T.; Avadhani, N.G. Mitochondrial Retrograde Signaling Induces Epithelial-Mesenchymal Transition and Generates Breast Cancer Stem Cells. Oncogene 2014, 33, 5238–5250. [Google Scholar] [CrossRef]
- Aldea, M.; Andre, F.; Marabelle, A.; Dogan, S.; Barlesi, F.; Soria, J.-C. Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov. 2021, 11, 874–899. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Z.; Liu, T.; Tang, M.; Mi, L.; Zhu, J.; Wu, W.; Wei, T. Targeted Therapy and Drug Resistance in Thyroid Cancer. Eur. J. Med. Chem. 2022, 238, 114500. [Google Scholar] [CrossRef]
- Shen, H.; Zhu, R.; Liu, Y.; Hong, Y.; Ge, J.; Xuan, J.; Niu, W.; Yu, X.; Qin, J.-J.; Li, Q. Radioiodine-Refractory Differentiated Thyroid Cancer: Molecular Mechanisms and Therapeutic Strategies for Radioiodine Resistance. Drug Resist. Updates 2024, 72, 101013. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K. Drug Resistance Mechanisms in Cancers: Execution of pro-Survival Strategies. J. Biomed. Res. 2024, 38, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Dhanyamraju, P.K.; Schell, T.D.; Amin, S.; Robertson, G.P. Drug-Tolerant Persister Cells in Cancer Therapy Resistance. Cancer Res. 2022, 82, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Sandulache, V.C.; Skinner, H.D.; Wang, Y.; Chen, Y.; Dodge, C.T.; Ow, T.J.; Bankson, J.A.; Myers, J.N.; Lai, S.Y. Glycolytic Inhibition Alters Anaplastic Thyroid Carcinoma Tumor Metabolism and Improves Response to Conventional Chemotherapy and Radiation. Mol. Cancer Ther. 2012, 11, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Wei, Y.-H.; Shieh, D.-B.; Lin, L.-L.; Cheng, S.-P.; Wang, P.-W.; Chuang, J.-H. 2-Deoxy-d-Glucose Can Complement Doxorubicin and Sorafenib to Suppress the Growth of Papillary Thyroid Carcinoma Cells. PLoS ONE 2015, 10, e0130959. [Google Scholar] [CrossRef]
- Valvo, V.; Iesato, A.; Kavanagh, T.R.; Priolo, C.; Zsengeller, Z.; Pontecorvi, A.; Stillman, I.E.; Burke, S.D.; Liu, X.; Nucera, C. Fine-Tuning Lipid Metabolism by Targeting Mitochondria-Associated Acetyl-CoA-Carboxylase 2 in BRAFV600E Papillary Thyroid Carcinoma. Thyroid 2021, 31, 1335–1358. [Google Scholar] [CrossRef]
- Chen, X.; Glytsou, C.; Zhou, H.; Narang, S.; Reyna, D.E.; Lopez, A.; Sakellaropoulos, T.; Gong, Y.; Kloetgen, A.; Yap, Y.S.; et al. Targeting Mitochondrial Structure Sensitizes Acute Myeloid Leukemia to Venetoclax Treatment. Cancer Discov. 2019, 9, 890–909. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W.; Chen, Z.; Li, X.; Wang, M. Novel Tumor Therapy Strategies Targeting Endoplasmic Reticulum-Mitochondria Signal Pathways. Ageing Res. Rev. 2023, 88, 101951. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S. Mitochondrial Signal Transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Zhang, R.; Lyu, H.; Xiao, S.; Guo, D.; Ali, D.W.; Michalak, M.; Zhou, C.; Chen, X.-Z.; et al. The TRPV6 Calcium Channel and Its Relationship with Cancer. Biology 2024, 13, 168. [Google Scholar] [CrossRef]
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8323. [Google Scholar] [CrossRef]
- Yang, X.; Zhuang, J.; Song, W.; Shen, W.; Wu, W.; Shen, H.; Han, S. Mitochondria-associated Endoplasmic Reticulum Membrane: Overview and Inextricable Link with Cancer. J. Cell Mol. Med. 2023, 27, 906–919. [Google Scholar] [CrossRef]
- Stewart, J.M. TRPV6 as A Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef]
- Russo, E.; Salzano, M.; De Falco, V.; Mian, C.; Barollo, S.; Secondo, A.; Bifulco, M.; Vitale, M. Calcium/Calmodulin-Dependent Protein Kinase II and Its Endogenous Inhibitor α in Medullary Thyroid Cancer. Clin. Cancer Res. 2014, 20, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Sée, V.; Rajala, N.K.M.; Spiller, D.G.; White, M.R.H. Calcium-Dependent Regulation of the Cell Cycle via a Novel MAPK–NF-κB Pathway in Swiss 3T3 Cells. J. Cell Biol. 2004, 166, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Hirte, H.; Welch, S.; Ilenchuk, T.T.; Lutes, T.; Rice, C.; Fields, N.; Nemet, A.; Dugourd, D.; Piha-Paul, S.; et al. First-in-Human Phase I Study of SOR-C13, a TRPV6 Calcium Channel Inhibitor, in Patients with Advanced Solid Tumors. Invest. New Drugs 2017, 35, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, M.R.; Salzano, M.; Monaco, S.; Sapio, M.R.; Illario, M.; De Falco, V.; Santoro, M.; Campiglia, P.; Pastore, L.; Fenzi, G.; et al. The Ca2+-Calmodulin-Dependent Kinase II Is Activated in Papillary Thyroid Carcinoma (PTC) and Mediates Cell Proliferation Stimulated by RET/PTC. Endocr. Relat. Cancer 2010, 17, 113–123. [Google Scholar] [CrossRef]
- Wang, Y.; Su, X.; Wang, Q.; Zhang, L.; Yu, Y.; Zhao, Y.; Liu, Z. Bisphenol A Exposure Enhances Proliferation and Tumorigenesis of Papillary Thyroid Carcinoma through ROS Generation and Activation of NOX4 Signaling Pathways. Ecotoxicol. Environ. Saf. 2025, 292, 117946. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.-Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R.; Yang, D.-H.; Chen, Z.-S. Modulating ROS to Overcome Multidrug Resistance in Cancer. Drug Resist. Updat. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel Action of Paclitaxel against Cancer Cells: Bystander Effect Mediated by Reactive Oxygen Species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Q.; Hu, Y.; He, B.; Cao, T.; Tang, Y.; Zhou, X.; Lan, X.; Liu, S. quan Advances in the Interaction of Glycolytic Reprogramming with Lactylation. Biomed. Pharmacother. 2024, 177, 116982. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ying, T.; Yuan, J.; Wang, Y.; Su, X.; Chen, S.; Zhao, Y.; Zhao, Y.; Sheng, J.; Teng, L.; et al. BRAFV600E Restructures Cellular Lactylation to Promote Anaplastic Thyroid Cancer Proliferation. Endocr. Relat. Cancer 2023, 30, e220344. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-Activated/SNF1 Protein Kinases: Conserved Guardians of Cellular Energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Bergeron, R.; Ren, J.M.; Cadman, K.S.; Moore, I.K.; Perret, P.; Pypaert, M.; Young, L.H.; Semenkovich, C.F.; Shulman, G.I. Chronic Activation of AMP Kinase Results in NRF-1 Activation and Mitochondrial Biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1340–E1346. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of Mitochondrial Biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.M.; de Carvalho, D.P. Perspectives of the AMP-Activated Kinase (AMPK) Signalling Pathway in Thyroid Cancer. Biosci. Rep. 2014, 34, e00105. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/mTOR Signaling Pathway for Targeted Therapeutic Treatment in Human Cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Petrulea, M.S.; Plantinga, T.S.; Smit, J.W.; Georgescu, C.E.; Netea-Maier, R.T. PI3K/Akt/mTOR: A Promising Therapeutic Target for Non-Medullary Thyroid Carcinoma. Cancer Treat. Rev. 2015, 41, 707–713. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Zuo, K.; Li, D.; Ye, M.; Ding, L.; Cai, H.; Fu, D.; Fan, Y.; Lv, Z. Upregulated miR-155 in Papillary Thyroid Carcinoma Promotes Tumor Growth by Targeting APC and Activating Wnt/β-Catenin Signaling. J. Clin. Endocrinol. Metab. 2013, 98, E1305–E1313. [Google Scholar] [CrossRef]
- Wang, S.; Lloyd, R.V.; Hutzler, M.J.; Safran, M.S.; Patwardhan, N.A.; Khan, A. The Role of Cell Cycle Regulatory Protein, Cyclin D1, in the Progression of Thyroid Cancer. Mod. Pathol. 2000, 13, 882–887. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Y.; Gao, C.; Yuan, Q.; Lu, X. SEMA3C Promotes Thyroid Cancer via the Wnt/β-Catenin Pathway. Exp. Cell Res. 2025, 444, 114378. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Wang, L.; Wang, J.; Wang, D.; Jiang, J.; Zhang, J.; Zhou, Q. Mitochondrial Division Inhibitor (Mdivi-1) Inhibits Proliferation and Epithelial-Mesenchymal Transition via the NF-κB Pathway in Thyroid Cancer Cells. Toxicol. Vitr. 2023, 88, 105552. [Google Scholar] [CrossRef]
- Ha, T.K.; Jung, I.; Kim, M.E.; Bae, S.K.; Lee, J.S. Anti-Cancer Activity of Myricetin against Human Papillary Thyroid Cancer Cells Involves Mitochondrial Dysfunction-Mediated Apoptosis. Biomed. Pharmacother. 2017, 91, 378–384. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, Q.; Cai, Y.; Liu, Y.; Ma, W.; Li, Q.; Song, F.; Guo, Y.; Zhu, L.; Ge, J.; et al. Alantolactone Induces Concurrent Apoptosis and GSDME-Dependent Pyroptosis of Anaplastic Thyroid Cancer through ROS Mitochondria-Dependent Caspase Pathway. Phytomedicine 2023, 108, 154528. [Google Scholar] [CrossRef]
- Yang, Q.; Ji, M.; Guan, H.; Shi, B.; Hou, P. Shikonin Inhibits Thyroid Cancer Cell Growth and Invasiveness through Targeting Major Signaling Pathways. J. Clin. Endocrinol. Metab. 2013, 98, E1909–E1917. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.A.; Schiller, G.J.; Kambhampati, S.; Tallman, M.S.; Douer, D.; Minden, M.D.; Yee, K.W.; Gupta, V.; Brandwein, J.; Jitkova, Y.; et al. A Phase 1 Study of Intravenous Infusions of Tigecycline in Patients with Acute Myeloid Leukemia. Cancer Med. 2016, 5, 3031–3040. [Google Scholar] [CrossRef] [PubMed]
- Dao, K.-H.T.; Gotlib, J.; Deininger, M.M.N.; Oh, S.T.; Cortes, J.E.; Collins, R.H.; Winton, E.F.; Parker, D.R.; Lee, H.; Reister, A.; et al. Efficacy of Ruxolitinib in Patients with Chronic Neutrophilic Leukemia and Atypical Chronic Myeloid Leukemia. J. Clin. Oncol. 2020, 38, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.M.; Robinson-Papp, J.; Van, J.; Stoker, M.; Jacobs, H.; Snijder, R.J.; Schregardus, D.S.; Long, S.K.; Lambourg, B.; Katz, N. Capsaicin 8% Patch in Painful Diabetic Peripheral Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Study. J. Pain. 2017, 18, 42–53. [Google Scholar] [CrossRef]
- Wang, F.; He, M.-M.; Xiao, J.; Zhang, Y.-Q.; Yuan, X.-L.; Fang, W.-J.; Zhang, Y.; Wang, W.; Hu, X.-H.; Ma, Z.-G.; et al. A Randomized, Open-Label, Multicenter, Phase 3 Study of High-Dose Vitamin C Plus FOLFOX ± Bevacizumab versus FOLFOX ± Bevacizumab in Unresectable Untreated Metastatic Colorectal Cancer (VITALITY Study). Clin. Cancer Res. 2022, 28, 4232–4239. [Google Scholar] [CrossRef]
- Parikh, M.; Liu, C.; Wu, C.-Y.; Evans, C.P.; Dall’Era, M.; Robles, D.; Lara, P.N.; Agarwal, N.; Gao, A.C.; Pan, C.-X. Phase Ib Trial of Reformulated Niclosamide with Abiraterone/Prednisone in Men with Castration-Resistant Prostate Cancer. Sci. Rep. 2021, 11, 6377. [Google Scholar] [CrossRef]
- Nencioni, A.; Caffa, I.; Cortellino, S.; Longo, V.D. Fasting and Cancer: Molecular Mechanisms and Clinical Application. Nat. Rev. Cancer 2018, 18, 707–719. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Liu, L.; Jia, C.; Cai, H.; Yang, J.; Wu, B.; Lv, Z. Fasting Regulates Mitochondrial Function through lncRNA PRKCQ-AS1-Mediated IGF2BPs in Papillary Thyroid Carcinoma. Cell Death Dis. 2023, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.T.H.B.M.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting Mimicking Diet as an Adjunct to Neoadjuvant Chemotherapy for Breast Cancer in the Multicentre Randomized Phase 2 DIRECT Trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef]
- Dong, Z.; Abbas, M.N.; Kausar, S.; Yang, J.; Li, L.; Tan, L.; Cui, H. Biological Functions and Molecular Mechanisms of Antibiotic Tigecycline in the Treatment of Cancers. Int. J. Mol. Sci. 2019, 20, 3577. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, G.; Chwa, J.; Oh, M.E.; Abeywardana, T.; Yang, Y.; Wang, Q.A.; Jiang, L. Mitochondrial Division Inhibitor (Mdivi-1) Decreases Oxidative Metabolism in Cancer. Br. J. Cancer 2020, 122, 1288–1297. [Google Scholar] [CrossRef]
- Bordt, E.A.; Clerc, P.; Roelofs, B.A.; Saladino, A.J.; Tretter, L.; Adam-Vizi, V.; Cherok, E.; Khalil, A.; Yadava, N.; Ge, S.X.; et al. The Putative Drp1 Inhibitor Mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor That Modulates Reactive Oxygen Species. Dev. Cell 2017, 40, 583–594.e6. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.; Song, J.; Chen, C. Ruxolitinib Enhances Gastric Cancer to Chemotherapy by Suppressing JAK/STAT3 and Inducing Mitochondrial Dysfunction and Oxidative Stress. Immunopharmacol. Immunotoxicol. 2025, 47, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-W.; Zhu, L.; Duan, Y.-T.; Hu, Y.-Q.; Li, L.-B.; Fan, W.-J.; Song, F.-H.; Cai, Y.-F.; Liu, Y.-Y.; Zheng, G.-W.; et al. Ruxolitinib Induces Apoptosis and Pyroptosis of Anaplastic Thyroid Cancer via the Transcriptional Inhibition of DRP1-Mediated Mitochondrial Fission. Cell Death Dis. 2024, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Zhelev, Z.; Getsov, P.; Nikolova, B.; Aoki, I.; Higashi, T.; Bakalova, R. Vitamin K: Redox-Modulation, Prevention of Mitochondrial Dysfunction and Anticancer Effect. Redox Biol. 2018, 16, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Böttger, F.; Vallés-Martí, A.; Cahn, L.; Jimenez, C.R. High-Dose Intravenous Vitamin C, a Promising Multi-Targeting Agent in the Treatment of Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 343. [Google Scholar] [CrossRef]
- Su, X.; Shen, Z.; Yang, Q.; Sui, F.; Pu, J.; Ma, J.; Ma, S.; Yao, D.; Ji, M.; Hou, P. Vitamin C Kills Thyroid Cancer Cells through ROS-Dependent Inhibition of MAPK/ERK and PI3K/AKT Pathways via Distinct Mechanisms. Theranostics 2019, 9, 4461–4473. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, K.; Peng, B.; Xu, Z.; Peng, J.; Li, J.; Chen, X.; Zeng, S.; Hu, K.; Yan, Y. Alantolactone: A Natural Plant Extract as a Potential Therapeutic Agent for Cancer. Front. Pharmacol. 2021, 12, 781033. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.W.; Seok, K.H.; Hwang, C.W.; Ahn, J.-C.; Jin, J.-O.; Kang, H.W. Hypericin-Assisted Photodynamic Therapy against Anaplastic Thyroid Cancer. Photodiagn. Photodyn. Ther. 2018, 24, 15–21. [Google Scholar] [CrossRef]
- Kim, E.J.; Mangold, A.R.; DeSimone, J.A.; Wong, H.K.; Seminario-Vidal, L.; Guitart, J.; Appel, J.; Geskin, L.; Lain, E.; Korman, N.J.; et al. Efficacy and Safety of Topical Hypericin Photodynamic Therapy for Early-Stage Cutaneous T-Cell Lymphoma (Mycosis fungoides): The FLASH Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2022, 158, 1031–1039. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-Tumor Effects and Associated Molecular Mechanisms of Myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Riedmeier, M.; Antonini, S.R.R.; Brandalise, S.; Costa, T.E.J.B.; Daiggi, C.M.; de Figueiredo, B.C.; de Krijger, R.R.; De Sá Rodrigues, K.E.; Deal, C.; Del Rivero, J.; et al. International Consensus on Mitotane Treatment in Pediatric Patients with Adrenal Cortical Tumors: Indications, Therapy, and Management of Adverse Effects. Eur. J. Endocrinol. 2024, 190, G15–G24. [Google Scholar] [CrossRef]
- Yu, K.; Wang, T.; Li, Y.; Wang, C.; Wang, X.; Zhang, M.; Xie, Y.; Li, S.; An, Z.; Ye, T. Niclosamide Induces Apoptosis through Mitochondrial Intrinsic Pathway and Inhibits Migration and Invasion in Human Thyroid Cancer in Vitro. Biomed. Pharmacother. 2017, 92, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, B.; Wu, Q.; Wang, G. Combination of Niclosamide and Current Therapies to Overcome Resistance for Cancer: New Frontiers for an Old Drug. Biomed. Pharmacother. 2022, 155, 113789. [Google Scholar] [CrossRef]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II Trial to Investigate the Safety and Efficacy of Orally Applied Niclosamide in Patients with Metachronous or Sychronous Metastases of a Colorectal Cancer Progressing after Therapy: The NIKOLO Trial. BMC Cancer 2018, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Totiger, T.M.; Srinivasan, S.; Jala, V.R.; Lamichhane, P.; Dosch, A.R.; Gaidarski, A.A.; Joshi, C.; Rangappa, S.; Castellanos, J.; Vemula, P.K.; et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 301–311. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, Y.; Zhou, B.; Qian, F.; Liu, D.; Ye, D.; Zhou, X.; Fang, L. Urolithin A Inhibits Breast Cancer Progression via Activating TFEB-Mediated Mitophagy in Tumor Macrophages. J. Adv. Res. 2025, 69, 125–138. [Google Scholar] [CrossRef]
- Khan, S.; Karmokar, A.; Howells, L.; Britton, R.G.; Parrott, E.; Palacios-Gallego, R.; Tufarelli, C.; Cai, H.; Higgins, J.; Sylvius, N.; et al. An Old Spice with New Tricks: Curcumin Targets Adenoma and Colorectal Cancer Stem-like Cells Associated with Poor Survival Outcomes. Cancer Lett. 2025, 629, 217885. [Google Scholar] [CrossRef]
- Chinreddy, S.R.; Mashozhera, N.T.; Rashrash, B.; Flores-Iga, G.; Nimmakayala, P.; Hankins, G.R.; Harris, R.T.; Reddy, U.K. Unraveling TRPV1’s Role in Cancer: Expression, Modulation, and Therapeutic Opportunities with Capsaicin. Molecules 2024, 29, 4729. [Google Scholar] [CrossRef]
- Xu, S.; Cheng, X.; Wu, L.; Zheng, J.; Wang, X.; Wu, J.; Yu, H.; Bao, J.; Zhang, L. Capsaicin Induces Mitochondrial Dysfunction and Apoptosis in Anaplastic Thyroid Carcinoma Cells via TRPV1-Mediated Mitochondrial Calcium Overload. Cell. Signal. 2020, 75, 109733. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Q.; Xiang, L.; Chen, Y. Mitochondrial Transplantation: A Promising Therapy for Mitochondrial Disorders. Int. J. Pharm. 2024, 658, 124194. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Amador-Martinez, I.; Maycotte, P. Mitochondrial Transplantation Strategies in Multifaceted Induction of Cancer Cell Death. Life Sci. 2023, 332, 122098. [Google Scholar] [CrossRef]
- Chang, J.-C.; Chang, H.-S.; Wu, Y.-C.; Cheng, W.-L.; Lin, T.-T.; Chang, H.-J.; Kuo, S.-J.; Chen, S.-T.; Liu, C.-S. Mitochondrial Transplantation Regulates Antitumour Activity, Chemoresistance and Mitochondrial Dynamics in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 30. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Z.; Yu, Z.; Hou, Y.; Keerthiga, R.; Fu, A. Mitochondrial Transplantation Therapy Inhibits the Proliferation of Malignant Hepatocellular Carcinoma and Its Mechanism. Mitochondrion 2022, 65, 11–22. [Google Scholar] [CrossRef]
- Celik, A.; Orfany, A.; Dearling, J.; del Nido, P.J.; McCully, J.D.; Bakar-Ates, F. Mitochondrial Transplantation: Effects on Chemotherapy in Prostate and Ovarian Cancer Cells in Vitro and in Vivo. Biomed. Pharmacother. 2023, 161, 114524. [Google Scholar] [CrossRef] [PubMed]
- Ali Pour, P.; Kenney, M.C.; Kheradvar, A. Bioenergetics Consequences of Mitochondrial Transplantation in Cardiomyocytes. J. Am. Heart Assoc. 2020, 9, e014501. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Jiang, P.; Wang, Z.; Kong, W.; Feng, L. Mitochondrial Transplantation: A Novel Therapeutic Approach for Treating Diseases. MedComm 2025, 6, e70253. [Google Scholar] [CrossRef]
- Ramirez-Barbieri, G.; Moskowitzova, K.; Shin, B.; Blitzer, D.; Orfany, A.; Guariento, A.; Iken, K.; Friehs, I.; Zurakowski, D.; del Nido, P.J.; et al. Alloreactivity and Allorecognition of Syngeneic and Allogeneic Mitochondria. Mitochondrion 2019, 46, 103–115. [Google Scholar] [CrossRef]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What Are the Indications for Post-Surgical Radioiodine Therapy in Differentiated Thyroid Cancer? Eur. Thyroid. J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Moshtaghie, A.A.; Daneshpour, M.; Pishdad, R.; Farahani, A.; Hedayati, M. The Toxicological Role of Myricetin in the Progression of Human Anaplastic Thyroid Cancer SW1736 Cell Line. Food Chem. Toxicol. 2025, 195, 115137. [Google Scholar] [CrossRef]
- Montero-Conde, C.; Ruiz-Llorente, S.; Dominguez, J.M.; Knauf, J.A.; Viale, A.; Sherman, E.J.; Ryder, M.; Ghossein, R.A.; Rosen, N.; Fagin, J.A. Relief of Feedback Inhibition of HER3 Transcription by RAF and MEK Inhibitors Attenuates Their Antitumor Effects in BRAF-Mutant Thyroid Carcinomas. Cancer Discov. 2013, 3, 520–533. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Su, X.; Li, P.; Han, B.; Jia, H.; Liang, Q.; Wang, H.; Gu, M.; Cai, J.; Li, S.; Zhou, Y.; et al. Vitamin C Sensitizes BRAFV600E Thyroid Cancer to PLX4032 via Inhibiting the Feedback Activation of MAPK/ERK Signal by PLX4032. J. Exp. Clin. Cancer Res. 2021, 40, 34. [Google Scholar] [CrossRef]
- Gu, L.; Yi, Z.; Zhang, Y.; Ma, Z.; Zhu, Y.; Gao, J. Low Dose of 2-Deoxy-D-Glucose Kills Acute Lymphoblastic Leukemia Cells and Reverses Glucocorticoid Resistance via N-Linked Glycosylation Inhibition under Normoxia. Oncotarget 2017, 8, 30978–30991. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Sun, Z.; Li, Y.; Gu, X.; Xu, H. Research Progress in Strategies to Improve the Efficacy and Safety of Doxorubicin for Cancer Chemotherapy. Expert. Rev. Anticancer. Ther. 2021, 21, 1385–1398. [Google Scholar] [CrossRef]
- Ito, Y.; Onoda, N.; Ito, K.-I.; Sugitani, I.; Takahashi, S.; Yamaguchi, I.; Kabu, K.; Tsukada, K. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 1142–1148. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, F.; Chen, D.; Wang, L. Inhibition of Mitochondrial Respiration by Tigecycline Selectively Targets Thyroid Carcinoma and Increases Chemosensitivity. Clin. Exp. Pharmacol. Physiol. 2019, 46, 890–897. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Feng, W.; Du, S.; Ge, R.; Li, J.; Liu, Y.; Sun, H.; Zhang, D.; Zhang, H.; et al. Targeting Mitochondria with Au-Ag@Polydopamine Nanoparticles for Papillary Thyroid Cancer Therapy. Biomater. Sci. 2019, 7, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Kong, C.; Jiang, C.; Hou, R.; Zhao, X.; Li, J.; Wang, Y.; Gao, Y.; Zhang, H.; Yang, B.; et al. Polydopamine-Coated Au-Ag Nanoparticle-Guided Photothermal Colorectal Cancer Therapy through Multiple Cell Death Pathways. Acta Biomater. 2019, 83, 414–424. [Google Scholar] [CrossRef] [PubMed]

| Type of Dysfunction | Specificities | Impact on TC | References |
|---|---|---|---|
| MtDNA mutation | Increased mtDNA mutations and copy number | 1. Inhibits OXPHOS; increases ROS; induces metabolic reprogramming. 2. Promotes TC occurrence, proliferation, invasion, and metastasis. | [43,44,45] |
| Mitochondrial dynamics | Increased mitochondrial fission; Decreased mitochondrial fusion | 1. Inhibits OXPHOS; promotes EMT. 2. Promotes TC proliferation, invasion, and metastasis; poor prognosis. | [46,47,48] |
| Mitochondrial autophagy | Downregulation of Parkin | 1. Increases ROS; activates NF-κB. 2. Promotes metastasis, invasion, and drug resistance in thyroid Hürthle cell tumors. | [49] |
| Mitochondrial permeability | Decreased mitochondrial permeability | Inhibits apoptosis and pyroptosis in TC. | [50] |
| Disorders of mitochondrial metabolism | Promotes aerobic glycolysis and inhibits OXPHOS; Enhances glutamate, serine, and glycine metabolism; Increases fatty acid and cholesterol synthesis and uptake | Promotes TC survival, proliferation, invasion, and metastasis; poor prognosis. | [19,23,51,52,53,54] |
| Mitochondrial Dysfunction | Medicine | TC Subtype Tested | Studied Model | Primary Outcomes | Secondary Outcomes | Evidence Level | References |
|---|---|---|---|---|---|---|---|
| Mitochondrial autophagy | Curcumin | PTC, FTC and ATC | In vitro cell model (Nthy-ori-3.1, BCPAP, FTC133, and 8505C cells) | Decreased proliferation, and induced apoptosis in PTC, FTC, and ATC cells | Synergizes with RAI to kill TC cells | V | [125] |
| Mitochondrial fission | Mdivi-1 | PTC and ATC | In vitro cell model (K1 cells and 8505C cells) | Decreased proliferation, invasion, and induced apoptosis in PTC and ATC cells | Reversed EMT by inhibiting the NF-κB pathway | V | [146] |
| Mitochondrial membrane potential | Myricetin | PTC | In vitro cell model (SNU-790 cells) | Decreased proliferation and induced apoptosis in PTC cells | Induces a decrease in mitochondrial membrane potential | V | [147] |
| Oxidative stress | Alantolactone | ATC | In vitro cell model (KHM-5M, KMH-2, C643CAL-62, and 8505C cells) and xenograft mouse model | Decreased proliferation and induced apoptosis in ATC cells | - | V | [148] |
| Shikonin | PTC, FTC, and ATC | In vitro cell model (C643, 8305c, K1, BCPAP, TPC-1, IHH4, FTC133, HTori-3, and primaryTC cells) and xenograft mouse model | Decreased growth, migration, invasion, and induced apoptosis in TC cells | Reversed EMT by inhibiting multiple targets (e.g., Mdm2, Slug, MMPs) | V | [149] |
| Mitochondrial Dysfunction | Medicine | Test Stage | Studied Model/Population | Experimental Group Sample Size | Control Group Sample Size | Outcomes | Adverse Reaction | NCT Identifier | Evidence Level | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Mitochondrial metabolism | Tigecycline | Phase I trial (Single-Arm Trial) | Relapsed and refractory acute myeloid leukemia patients | 27 | - | Primary outcomes: maximal tolerated dose was 300 mg/day; Secondary outcomes: the t1/2 becomes shorter | The 300 mg/day dose was well-tolerated | NCT 01332786 * | IV | [150] |
| Mitochondrial fission | Ruxolitinib | Phase II trial (Single-Arm Trial) | Chronic neutrophilic leukemia and atypical chronic myeloid leukemia patients | 44 | - | Primary outcomes: ORR 32%; Secondary outcomes: CNL ORR 58%, aCML ORR 8% | Anemia (34%),Thrombocytopenia (14%) | NCT 02092324 * | II | [151] |
| Calcium regulation | Capsaicin | Phase III trial (Randomized Controlled Trial) | Patients with painful diabetic peripheral neuropathy | 186 | 183 | Primary outcomes: significant reduction in average daily pain; Secondary outcomes: proportion of responders ≥30% and ≥50% pain reduction | Application site pain (33.9%) | NCT 01533428 * | I | [152] |
| Oxidative stress | Vitamin C | Phase III trial (Randomized Controlled Trial) | Metastatic colorectal cancer patients | 221 | 221 | Primary outcomes: experimental group PFS 8.6 months, control group 8.3 months, HR 0.86; Secondary outcomes: experimental group and control group (ORR, 44.3% vs. 42.1%; OS, 20.7 vs. 19.7 months) | Neutropenia (14.9% vs. 15.4%), anemia (5.0% vs. 2.3%) | NCT 03146962 * | I | [153] |
| Mitochondrial membrane potential | Niclosamide | Phase Ib trial (Single-Arm Trial) | Castration-resistant prostate cancer patients | 9 | - | Primary outcomes: the maximal tolerated dose was 1200 mg, given three times a day; Secondary outcomes: 5 patients had ≥50% PSA response to treatment, and 2 of these patients had complete PSA response | The 1200 mg/day dose was well-tolerated | NCT 02807805 * | IV | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Han, H.; Zhang, T.; Zhang, T.; Ma, L.; Yang, Z.; Zhao, Y. The Emerging Role of Mitochondrial Dysfunction in Thyroid Cancer: Mediating Tumor Progression, Drug Resistance, and Reshaping of the Immune Microenvironment. Biomolecules 2025, 15, 1292. https://doi.org/10.3390/biom15091292
Zhang Y, Han H, Zhang T, Zhang T, Ma L, Yang Z, Zhao Y. The Emerging Role of Mitochondrial Dysfunction in Thyroid Cancer: Mediating Tumor Progression, Drug Resistance, and Reshaping of the Immune Microenvironment. Biomolecules. 2025; 15(9):1292. https://doi.org/10.3390/biom15091292
Chicago/Turabian StyleZhang, Yating, Hengtong Han, Tingting Zhang, Tianying Zhang, Libin Ma, Ze Yang, and Yongxun Zhao. 2025. "The Emerging Role of Mitochondrial Dysfunction in Thyroid Cancer: Mediating Tumor Progression, Drug Resistance, and Reshaping of the Immune Microenvironment" Biomolecules 15, no. 9: 1292. https://doi.org/10.3390/biom15091292
APA StyleZhang, Y., Han, H., Zhang, T., Zhang, T., Ma, L., Yang, Z., & Zhao, Y. (2025). The Emerging Role of Mitochondrial Dysfunction in Thyroid Cancer: Mediating Tumor Progression, Drug Resistance, and Reshaping of the Immune Microenvironment. Biomolecules, 15(9), 1292. https://doi.org/10.3390/biom15091292






