2. Methods
2.1. Study Design and Oversight
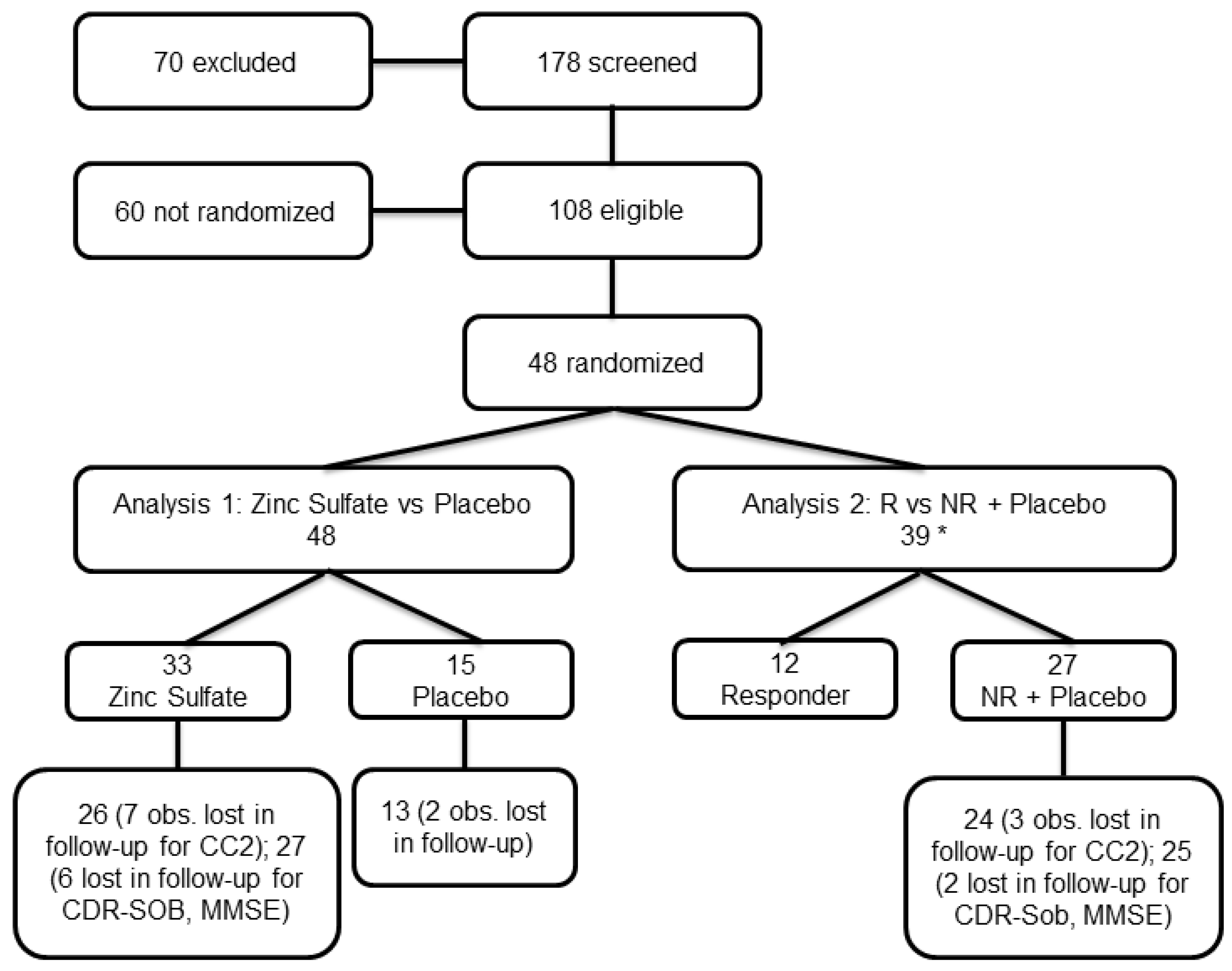
ZINCAiD was a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II trial aimed at evaluating the efficacy and safety of zinc therapy in MCI due to AD. Patients with MCI due to AD were recruited between June 2020 and May 2023 at the University Hospital of Brescia (Italy) and the Memory Clinic of the IRCCS Centro San Giovanni di Dio Fatebenefratelli in Brescia (Italy). Eligible participants were randomly assigned to one of the trial’s arms, consisting of a treatment group receiving zinc therapy and a placebo group taking identical placebo tablets in a 2:1 ratio.
2.2. Ethics Approval
The study protocol (PTC-19-602325; EudraCT No.: 2019-000604-15; registered on 26 March 2020) was approved by the local Regional Ethics Committees (approval codes: 25/2020, CE150186; 95/2020; and 02/2021), in accordance with Italian regulatory requirements, Agenzia Italiana del Farmaco (AIFA) guidelines, and the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants or their legal representatives. The trial was an investigator-initiated and academically sponsored study, supported by the Alzheimer’s Association (PTC-19-602325).
2.3. Participants and Selection Criteria
Eligible participants were aged 60 years or older, met internationally accepted criteria for MCI due to AD [
10,
11], and had a Clinical Dementia Rating (CDR) [
12] global score of 0.5. Additional inclusion criteria included the following: if women, to be in menopause since at least 2 years; stable presence of an informant family member; capacity of full compliance with the protocol requirements (i.e., assumption of the medicine per os, etc.); brain MRI performed within 12 months preceding or at the screening visit; evidence of cerebral amyloidosis (β-amyloid positivity) by scanning with Florbetapir (18F)-PET or positive for cerebrospinal fluid biomarkers of AD; nonceruloplasmin copper serum concentration > 1.6 μmol/L [
8,
13].
Exclusion criteria included diagnosis of concomitant severe or unstable diseases and disabilities that may interfere with copper metabolism, and with primary and secondary outcome evaluation, or may bias the assessment of the clinical or mental status of the subject or put the subject at special risk; concomitant severe or unstable cardiovascular diseases; concomitant primary neurodegenerative disorder besides AD, or neurological or psychiatric disorders of any etiology; clinically significant anemia; known hypersensitivity to zinc sulphate tablets including any of the components of the formulation.
2.4. Randomization and Masking
Participants were randomly assigned in a 2:1 ratio to receive zinc or placebo using a computer-generated permuted block randomization sequence, stratified by site. The random allocation sequence was prepared by an independent statistician who was not involved in participant enrolment or outcome assessment. Allocation was concealed using centralized communication with site pharmacies, and all investigators, participants, and outcome assessors were blinded to treatment assignment throughout the study.
Unlike prior open-label studies in copper-related disorders, where dosing was titrated to achieve pharmacodynamic targets [
7,
8], ZINCAiD employed a fixed-dose, double-blind design. Ceruloplasmin (Cp) was measured at prespecified time points solely for safety monitoring. Post hoc, after unblinding, participants with a ≥20% reduction in serum Cp at week 12 were classified as Zinc Responders to explore biological engagement with therapy.
2.5. Procedures
Participants assigned to the zinc treatment arm received oral elemental zinc in tablet form (200 mg zinc sulfate tablets). During the first week, participants received 67.5 mg/day of elemental zinc (administered as one and a half 200 mg zinc sulfate tablets daily). From week 2 to week 12, the dose was increased to 135 mg/day (three 200 mg tablets daily, divided into three doses). Beginning at week 13 and continuing through week 24, the dose was reduced to 67.5 mg/day (one and a half 200 mg tablets daily), administered in two doses (45 mg in the morning and 22.5 mg in the evening).
Participants in the placebo group followed an identical schedule with matching placebo capsules.
Cognitive assessments were administered at baseline and 24-week visit using a standardized neuropsychological battery. Clinical safety and tolerability were evaluated at each scheduled visit—baseline visit, 3-week visit, 6-week visit, 12-week visit, and 24-week visit—through physical examination, vital signs, hematological and biochemical monitoring (including serum Cp), and adverse event reporting. Ceruloplasmin concentrations below 10 mg/dL were prespecified as a safety threshold, but no such cases were observed. In line with the double-blind design, zinc dosage was not modified based on individual laboratory results.
2.6. Assessments and Outcome Measures
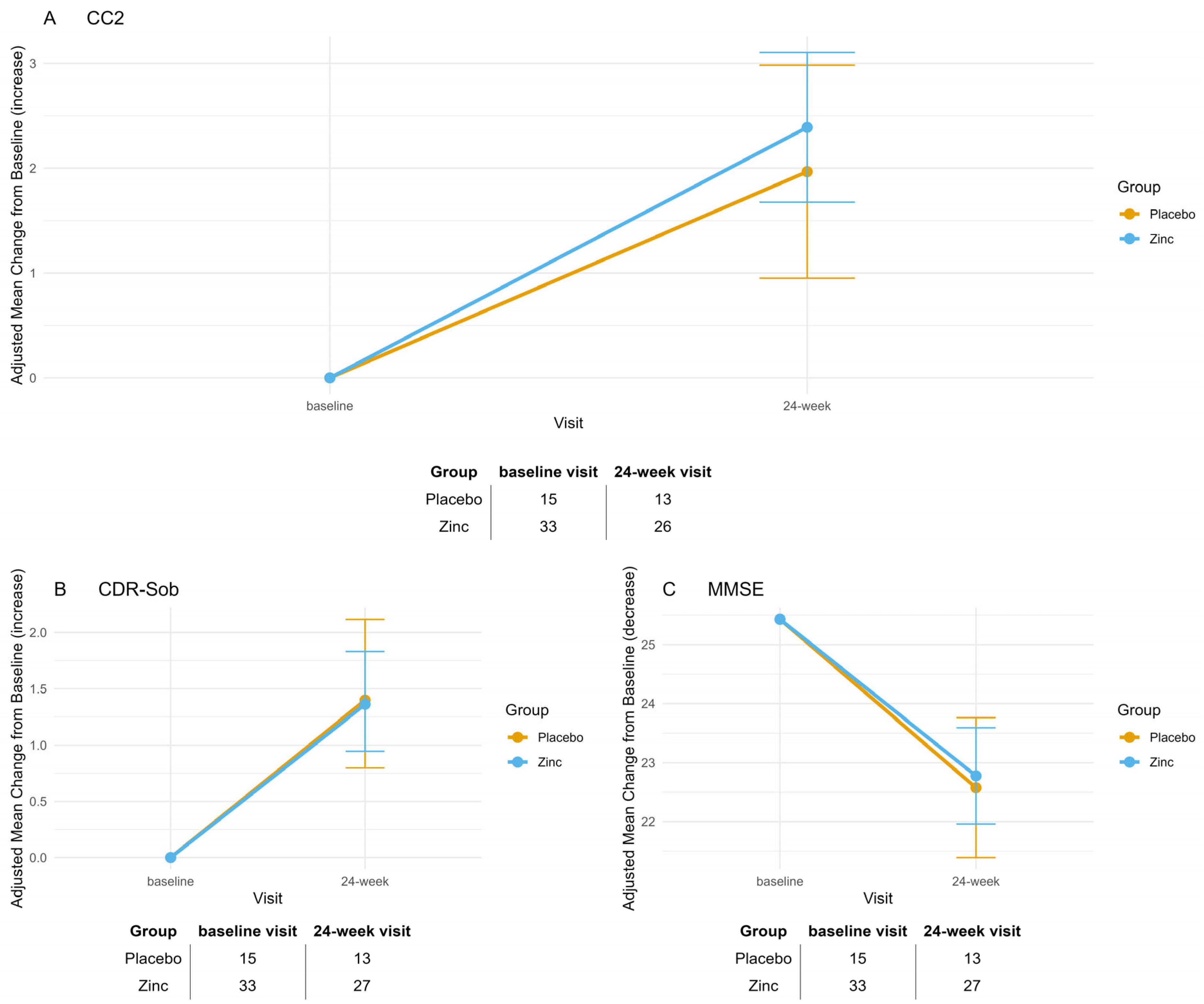
Cognitive functions were assessed using a standardized neuropsychological battery administered at baseline and 6 months. The battery includes, as a primary cognitive outcome, the Cognitive Composite 2 scale (CC2) [
14] (composed of the ADAS-3 and the cognitive portion of Clinical Dementia Rating–Sum of Boxes) [
12]. The secondary cognitive efficacy outcome measures were the Clinical Dementia Rating scale sum of Boxes (CDR-SoB) [
12]; the Mini-Mental State Examination (MMSE) [
15]. For safety, vital signs, Cp, and blood cell count (with formula) to monitor possible anemia were administered at each visit. Physical and neurological exams and an electrocardiogram were administered at BL. Safety was also assessed by monitoring and recording adverse events (AEs) and serious adverse events (SAEs).
Serum Cp concentration was measured using a validated immunoturbidimetric assay (Futura System S.r.l., Rome, Italy) on the Pentra 400 automated clinical chemistry analyzer (Horiba ABX, Montpellier, France). Calibration and quality control procedures used pooled healthy reference sera (IMCON and INCOMLOW; Futura System S.r.l.), following manufacturer guidelines.
2.7. Statistical Methods
2.7.1. Sample Size Calculation
To determine the necessary sample size, we based our estimation on CC2, a composite outcome combining both cognitive (ADAS-Cog) and functional (CDR-SOB) measures [
14]. This composite scale captures domains most vulnerable to early neurodegeneration and is, therefore, a sensitive marker for treatment effects in patients with MCI [
14]. We referred to effect sizes reported by George G Brewer [
9], who observed a Cohen’s d of 0.69 for ADAS-Cog and 0.72 for CDR-SOB in a 6-month randomized trial comparing zinc therapy to placebo in AD patients. The ZINCAiD trial differed from Brewer’s study in two clinically meaningful ways that could plausibly enhance treatment responsiveness: (i) It had stricter inclusion criteria. Brewer’s sample included general AD patients without biomarker selection. In contrast, ZINCAiD recruited individuals with both elevated nonceruloplasmin copper (>1.6 μmol/L) and β-amyloid positivity—biomarkers identifying a subgroup more likely to benefit from zinc modulation. (ii) It targeted an earlier disease stage. While Brewer enrolled patients with established AD, ZINCAiD targeted the prodromal stage (MCI), where intervention is more likely to preserve function before irreversible neurodegeneration occurs. Despite these potentially favorable differences, we adopted a conservative approach using the lower of Brewer’s reported effect sizes (d = 0.69) for power estimation. Based on α = 0.05, 80% power, and a 2:1 treatment-to-placebo allocation, the required sample size was calculated at 60 participants (40 in the treatment group, 20 in the placebo group) using G*Power (version 3.1). This ensured sufficient power to detect a medium-to-large effect, while recognizing that, given the specific characteristics of our sample, a somewhat enhanced treatment effect was considered plausible.
2.7.2. Statistical Analysis
Normality of continuous variables was assessed through both statistical tests and visual inspection of distribution plots. Descriptive statistics were calculated to summarize baseline characteristics. Between-group comparisons of continuous variables were conducted using independent samples
t-tests or their non-parametric alternatives, as appropriate. Categorical variables were compared using chi-squared tests or Fisher’s Exact test, depending on expected cell counts. Longitudinal changes from baseline visit to 24-week visit were analyzed using mixed-effects models for repeated measures, including group-by-visit interaction terms to evaluate differential treatment effects over time. All models were adjusted for baseline values. Model diagnostics included assessments for outliers, residual normality, and heteroskedasticity. Under the missing-at-random (MAR) assumption, mixed-effects models implicitly handle missing outcome data, accommodating incomplete follow-up without imputation. Two analyses were performed. The primary analysis compared the original randomized groups: zinc therapy vs. placebo. The other was a post hoc exploratory analysis in which participants were grouped according to their biological response: (A) participants receiving zinc who exhibited a ≥20% reduction in ceruloplasmin levels between baseline and 12-week visit, high-dose phase; (B) all other participants, including non-responders and those in the placebo group. The decision to stratify participants based on pharmacodynamic response was grounded in previous evidence showing that a ≥20% reduction in serum ceruloplasmin reflects a biologically meaningful threshold for zinc-induced copper depletion [
16,
17,
18]. This classification follows the rationale outlined in Section 5.3 of the EMA guidelines [
19], which supports the identification of biologically plausible subgroups in exploratory analysis when underpinned by a sound mechanistic rationale and external evidence. Statistical findings reinforced this approach: A one-way ANOVA on mean percent change in Cp showed a strong group effect [F(2,36) = 50.42,
p < 0.0001)], with post hoc Tukey HSD tests revealing no significant difference between Non-Responders and Placebo (mean difference = 0.25%,
p = 0.998), but large, highly significant differences between Responders and Placebo (mean difference = 35.74%,
p < 0.0001) and Responders and Non-Responders (mean difference = 35.49%,
p < 0.0001). These results indicate that Non-Responders and Placebo participants share similar pharmacodynamic profiles, and justify their aggregation into a unified comparator group. This classification strategy is consistent with the exploratory nature of the study, aimed at estimating treatment effects specifically in biologically engaged participants [
19]. Where appropriate,
p-values were adjusted for multiple comparisons using the Bonferroni method. All statistical analyses were conducted in R (version 4.3.2). A two-sided
p-value < 0.05 was considered statistically significant.
2.8. Role of the Funding Source
The study was funded by the Alzheimer’s Association Part the Cloud: Translational Research Funding for Alzheimer’s Disease (PTC) PTC-19-602325, EudraCT 2019-000604-15, through a competitive peer-reviewed grant. The sponsor had no role in the design, conduct, analysis, interpretation, or reporting of the study.
4. Discussion
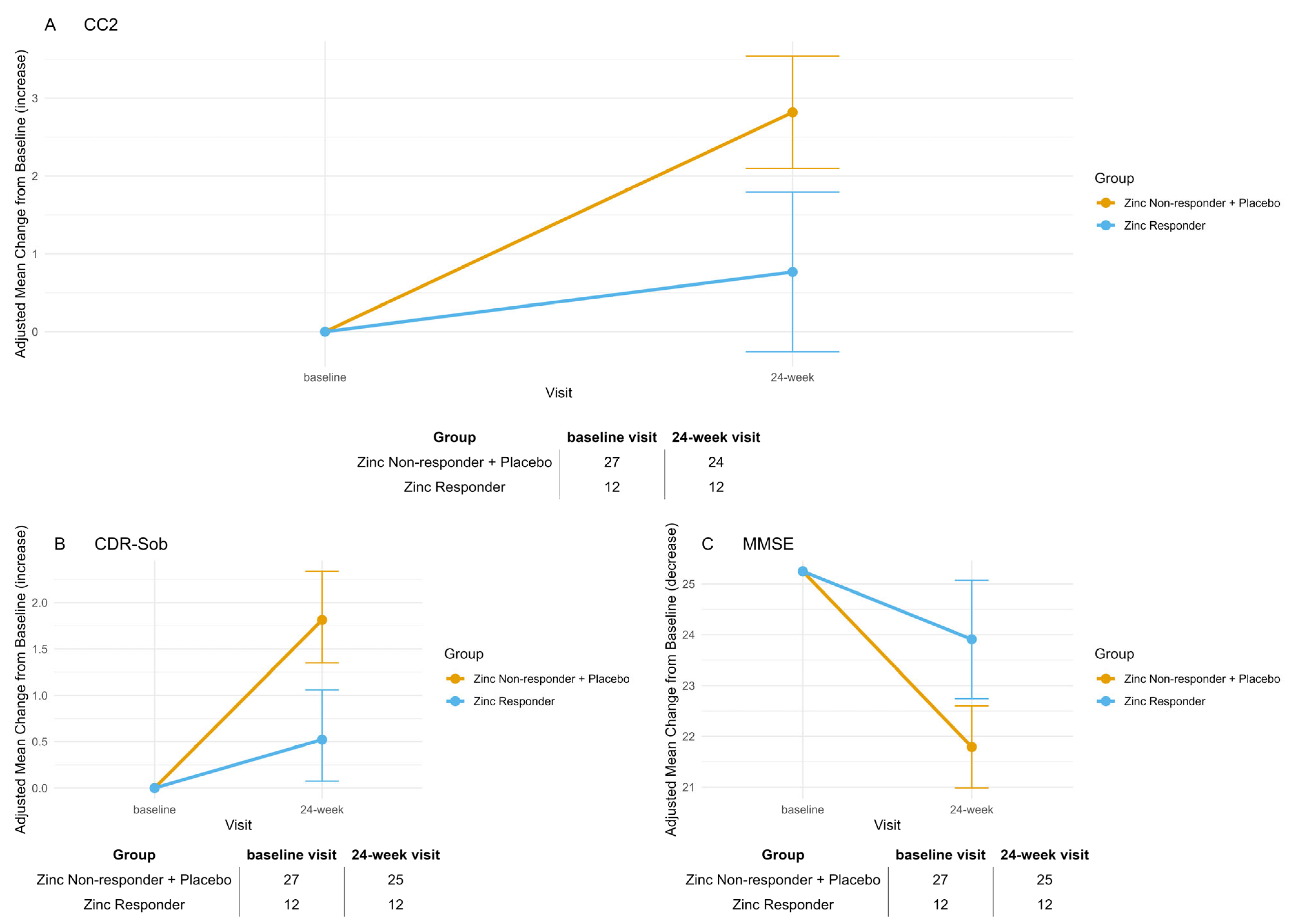
The randomized, placebo-controlled clinical trial ZINCAiD provides preliminary, biologically anchored evidence that zinc therapy may stabilize cognitive function in a pharmacodynamically defined subset of MCI patients, specifically, those having a ≥20% reduction in serum ceruloplasmin [
16,
17,
18,
20]. Despite the limited sample size and lack of efficacy in the primary zinc vs. placebo comparison, a consistent pattern emerged across three clinical scales (MMSE, CC2, and CDR-SOB): Only patients on zinc who reached a pharmacodynamically relevant copper-deficient state maintained cognitive stability, while all others declined significantly. The rationale for targeting zinc therapy to a copper-related metabolic phenotype derives from a long-standing hypothesis that implicates elevated non-ceruloplasmin copper (also called “free copper” or exchangeable copper [
13]) as a contributor to neurotoxicity in AD [
9,
21]. Although only exchangeable copper was measured in this study, we acknowledge the terminological distinctions commonly used in the literature. “Non-ceruloplasmin copper” (non-Cp Cu) is an indirect estimate derived by subtracting ceruloplasmin-bound copper—calculated from serum ceruloplasmin concentrations using the Walshe formula—from total copper. “Exchangeable copper,” by contrast, is measured directly through ultrafiltration and reflects copper loosely bound to low-affinity carriers such as albumin and amino acids. The term “free copper” is often used synonymously with non-Cp Cu, although truly unbound ionic copper likely exists only at attomolar levels. Despite their differing methodologies, our previous work has shown that non-Cp Cu and exchangeable copper provide functionally equivalent information in characterizing copper dysregulation in AD [
13].
Zinc acts by inducing metallothioneins in enterocytes, thereby blocking intestinal copper absorption and gradually depleting systemic excess copper levels [
22,
23,
24].
The cognitive stabilization observed in those patients who reached a pharmacodynamic state of chemical copper deficiency supports the hypothesis that copper dysregulation may be a tractable contributor to disease progression in this subgroup—an insight deeply rooted in the clinical experience of Brewer and Hoogenraad, who treated hundreds of patients with copper metabolism disorders using zinc-based therapy [
9,
25]. Indeed, the associated reduction in serum ceruloplasmin levels reflects a pharmacodynamically meaningful decline in bioavailable copper—sufficient to affect biological pathways implicated in disease progression [
17]. This effect aligns with previous studies showing rapid ceruloplasmin decrease in response to high-zinc diets or anti-copper therapy [
16,
17]. Ceruloplasmin is widely recognized as a reliable pharmacodynamic marker of copper depletion in both Wilsonian and non-Wilsonian contexts. In such conditions, a ≥20% reduction in serum ceruloplasmin is used as a surrogate for systemic copper depletion and correlates with biological target engagement [
16,
17,
18]. We therefore classified as Zinc Responders those individuals showing a ≥20% reduction after 12 weeks of full-dose zinc. This threshold is biologically justified, as prior studies confirmed that such a reduction reflects effective hepatic copper depletion via metallothionein induction [
7,
16,
17]. Although zinc was administered at a fixed dose due to the double-blind design, a substantial proportion of participants still reached the pharmacodynamic threshold, indicating that 135 mg/day elemental zinc was sufficient to induce copper depletion in responsive individuals. However, some did not show this response, underscoring interindividual variability and suggesting that only a subset may reach the therapeutic window under fixed dosing. This supports the value of dose titration in future trials. Serum ceruloplasmin may also serve as a low-cost, minimally invasive biomarker to personalize treatment and facilitate broader implementation in phase II studies.
Our data suggest that pharmacodynamic copper depletion, as indicated by ≥20% reduction in ceruloplasmin, is a necessary condition for cognitive stabilization under zinc therapy. However, target engagement alone may not be sufficient in all cases to guarantee clinical benefit, as additional biological or clinical factors may influence the therapeutic response. These findings raise important questions regarding how best to monitor and interpret biological response in the context of a blinded, fixed-dose intervention. A key methodological limitation is the incompatibility between pharmacodynamic dose titration and double-blind design in our exploratory, non-commercial study. In open-label studies of anti-copper therapy, including those by Brewer and Hoogenraad [
7,
8], zinc doses were adjusted using real-time ceruloplasmin monitoring to achieve individualized copper depletion. By contrast, ZINCAiD used a fixed-dose, double-blind design to preserve internal validity, which precluded dose adjustment during the trial. Consequently, target engagement was assessed only retrospectively, identifying a biologically defined subgroup post hoc, as permitted by EMA guidelines Section 5.3 [
19]. To avoid unblinding through pharmacodynamic monitoring, a Cp-guided titration strategy would require an open-label or adaptive trial design—both more complex and resource-intensive than the current randomized controlled framework.
While our study was not powered to prospectively validate this biological stratification, the consistency between pharmacodynamic response and clinical trajectory provides a compelling rationale for future targeted trials.
The increase in ceruloplasmin levels observed at the 12-week visit among Zinc Responders is consistent with the protocol-defined halving of zinc dosage after Week 12, from 135 mg/day to 65 mg/day. This pharmacodynamic rebound likely reflects the dose-dependent induction of intestinal metallothionein, which is critical for zinc-mediated copper sequestration [
16,
17]. Lower zinc exposure may reduce metallothionein expression, leading to increased copper absorption and a subsequent rise in ceruloplasmin synthesis. These findings highlight the need to maintain adequate zinc dosing to sustain copper control, especially in individuals with biologically proven responsiveness.
The correlation between the biochemical engagement and cognitive stabilization in Zinc Responders reinforces the hypothesis that modulating copper metabolism may influence trajectories in biologically selected MCI patients. This association was further supported by converging results across two validated clinical endpoints—CDR-SOB and CC2—allowing comparison between a widely adopted, semi-structured staging tool and a neuropsychological composite designed for sensitivity to early cognitive changes [
14,
26]. CDR-SOB is widely used in MCI trials for disease staging [
1,
27], but its semi-structured format may limit sensitivity to subtle pharmacological effects [
26]. By contrast, our primary outcome, CC2 [
14], combines standardized neuropsychological tests into a quantitative index tailored to detect small yet clinically meaningful changes. In our study, CC2 showed significant cognitive stability in Zinc Responders over 24 weeks, with effect sizes exceeding those typically seen with CDR-SOB. For instance, the Lecanemab phase 3 trial reported a ~0.45 CDR-SOB difference at 18 months [
1], while Koch et al. observed a ~0.7 reduction after 6 months of stimulation in Aβ-positive MCI [
27]. These effects were modest and based on global measures [
1,
9,
27]. ZINCAiD prioritized a cognitive composite increasingly adopted in early-phase AD trials [
14], and the concordant improvements in CC2 and CDR-SOB among responders reinforce the observed treatment effect.
The emerging concept of cuproptosis—a copper-driven, regulated cell death pathway—provides a mechanistic link between systemic copper dysregulation and neurodegeneration. Triggered by excess copper binding to lipoylated tricarboxylic acid cycle enzymes, it induces proteotoxic stress, mitochondrial dysfunction, and cell death [
6]. Initially described in cancer, cuproptosis is now being explored in neurodegenerative disorders, including AD, where mitochondrial vulnerability is a hallmark. In this light, the cognitive stabilization seen in Zinc Responders may stem from zinc-induced metallothioneins sequestering copper and limiting mitochondrial accumulation. By reducing cuproptosis susceptibility, zinc may confer neuroprotection in copper-related phenotypes. Though direct evidence in the human brain is lacking, the association between biochemical engagement (≥20% Cp reduction) and clinical benefit in our study supports its relevance. Future trials with mechanistic biomarkers (e.g., mitochondrial stress, lipoylated aggregates) may clarify cuproptosis’ role in MCI and treatment response.
Our findings are consistent with previous trials suggesting zinc may attenuate cognitive decline in AD and MCI. Studies have reported cognitive stabilization—especially in older adults or individuals with altered copper metabolism [
9,
28]. In a 6-month study of patients over 70 with probable AD, Brewer showed that zinc acetate slowed decline on ADAS-Cog and CDR-SOB, with similar trends in MMSE [
9]. ZINCAiD built on this by applying biomarker-based stratification and enrolling participants at the prodromal stage, enabling a more targeted assessment of zinc’s pharmacodynamic and cognitive effects.
Zinc’s neuroprotective effects stem not only from correcting copper imbalance but also from direct actions on synaptic and cellular processes, including N-methyl-D-aspartate (NMDA) receptor modulation and oxidative stress reduction [
29,
30]. In traumatic brain injury, zinc therapy has been associated with reduced mortality and improved Glasgow Coma Scale scores in a 1-month trial, while a 2018 phase II randomized controlled trial reported superior clinical and inflammatory outcomes in zinc-treated patients compared to placebo. These findings underpin the 2022 Canadian Network for Mood and Anxiety Treatments (CANMAT) guideline, which recommends zinc—with Level 2 evidence—for cognitive and psychiatric support [
31].
The ZINCAiD protocol was designed under the scientific mentorship of George Brewer, a pioneer in copper biology and zinc therapy [
7,
9,
16,
17,
21,
24], and builds on decades of laboratory and clinical data suggesting a role for metal homeostasis in AD [
9,
21]. The philosophical and mechanistic foundation of this work is also closely aligned with the vision of T.U. Hoogenraad, whose contributions to the field have been recognized [
32]. This study, in many ways, represents a clinical continuation of that therapeutic perspective [
32]. In line with the hypothesis first proposed by T.U. Hoogenraad, some forms of AD may represent a senile variant of copper toxicosis, akin to a late-onset or heterozygous form of WD [
25], and reflect overlapping mechanisms with WD [
5]. This conceptual shift invites reconsideration of AD not only as a neurodegenerative disorder but also as a condition in which trace metal imbalance contributes to disease progression in a susceptible subset of individuals. From this perspective, the clinical stabilization observed in zinc “Responders” in the ZINCAiD trial—despite the limited sample size—may reflect a therapeutic benefit similar to that observed in WD under long-term zinc therapy. While such a proposition remains speculative, it raises the possibility that treatments validated in copper toxicosis could be repurposed for use in selected AD, guided by pharmacodynamic markers such as ceruloplasmin. This approach, if confirmed, may complement traditional trial frameworks with a precision medicine model.
While our study focused on zinc sulfate due to its favorable safety profile and clinical use in WD, alternative copper-lowering agents such as tetrathiomolybdate (TM) also warrant consideration. TM exerts dual action as a potent copper chelator and anti-angiogenic compound, and it has shown promise in oncology and neurodegeneration [
17,
33]. Comparative studies between zinc and TM could help elucidate differences in mechanisms of action, biomarker modulation, and cognitive outcomes in AD. Future head-to-head or combinatorial trials may provide valuable insights for optimizing patient-specific therapeutic strategies. In addition, the pharmacodynamic responsiveness observed in the ZINCAiD study may define a therapeutically relevant biological patient subgroup. Building on this pharmacodynamic distinction, emerging therapeutic strategies are now being explored to restore metal homeostasis with increased selectivity and mechanistic precision. Emerging strategies to correct brain copper and zinc dyshomeostasis in AD include peptide-mediated metal shuttles, nanoparticle-based delivery systems, and nutraceuticals with redox-active or metal-modulating properties. A recent study introduced a Cu(II) shuttle based on a cell-penetrating peptide that delivers bioavailable copper via Rab5- and Rab14-mediated endocytosis, reducing Cu–Aβ-induced oxidative stress [
34]. Zinc-based compounds such as zeolite–zinc improved memory and hippocampal integrity in Aβ-treated animals [
35], while hydroxytyrosol altered zinc and copper distribution in a transgenic AD mouse model [
36]. Furthermore, a clinical study combining olive polyphenols and S-acetyl-glutathione showed stabilization or improvement in cognitive function in patients with mild AD [
37]. Collectively, these findings support the development of targeted, mechanism-driven interventions acting on metal homeostasis and oxidative stress in early AD.
The primary limitation of the ZINCAiD trial is the modest sample size, which—while sufficient to detect a medium-to-large treatment effect—may limit the generalizability of the findings and the ability to explore subgroup effects. Although the a priori power analysis supported the planned sample size, the final enrolled cohort was smaller than expected. As such, the possibility of a type II error—failing to detect a true effect due to insufficient power—cannot be excluded, particularly in the primary comparison. A post hoc estimate based on the expected effect size (Cohen’s d = 0.69) and observed group sizes yielded a power of approximately 70.5%. While this is moderately acceptable, the results should be interpreted with caution, and future studies with larger samples will be essential to confirm these findings and enable more granular subgroup analyses. Additional limitations include the fixed-dose design, which did not allow for personalized zinc titration to ensure that all participants reached the targeted ceruloplasmin reduction, and the binary classification of “responders” based on a post hoc threshold—even though this threshold was pharmacodynamically justified and grounded in prior anti-copper therapy research. Furthermore, we did not incorporate longitudinal imaging or CSF biomarkers beyond the screening phase. Nevertheless, the consistency of clinical stabilization across multiple independent cognitive outcomes, coupled with a biologically coherent pharmacodynamic signal, strengthens the internal validity of the observed effects. This work suggests that biologically stratified therapy targeting copper metabolism may offer a low-cost, mechanism-driven approach to slowing cognitive decline in MCI. Future trials should aim to replicate these findings in larger, multicenter cohorts, using early ceruloplasmin reduction as both a marker of target engagement and a potential enrichment strategy for identifying treatment responders.