From Obscurity to Prominence: IPMK’s Expanding Role in Cellular Signaling, Physiology, and Disease
Abstract
1. Introduction
2. IPMK Exhibits Both Inositol Kinase and Lipid Kinase Enzymatic Activity
2.1. Lipid Kinase Enzymatic Activity
2.2. Inositol Kinase Enzymatic Activity
3. IPMK Structure and Function
Protein Architecture
| Protein | IPMK Interaction Regions | Verification Assay | Comments |
|---|---|---|---|
| AMPK | 125–182 and 209–416 | Dominant-negative | Exons 4 and 6; Phosphorylation of IPMK at Y174 facilitates IPMK-AMPK binding [44] |
| CK2 | 284–343 | In vitro phosphorylation | CK2 phosphorylates IPMK at S284. An IPMK putative binding site (283–287) for Ck2 is predicted [45]. |
| CREB-binding protein (CBP) | 1–75 | Dominant-negative | Mouse IPMK [22] |
| Chromatin remodeling complex SWI/SNF | 93–124, 125–182, and 208–416 | In vitro binding | The full-length IPMK was verified to bind the core subunits of SWI/SNF complex- SMARCB1/BRG1/BAF155 proteins, and IPMK exons 3, 4, and 6 interact with SMARCB1 Rpt1 and Rpt2 domains [20] |
| Disheveled-3 (DVL3) | 1–77 | In vitro binding | The binding of IPMK involves the PDZ (247–334) and the C-terminal tail (496–716) of Dvl3 [46] |
| mTOR | 1–60 | Dominant-negative | Exon 1 [23] |
| raptor | 61–416 | Dominant-negative | Interaction seen using 1–182 and 182–416 IPMK fragments but not 1–60 [23] |
| Nrf2 | 336–416 | Pulldown analysis | Interactions were evaluated endogenously in liver and brain (cortex) lysates [47]. |
| p53 | 125–184 | Dominant-negative | Exon 4 [48] |
| nuclear receptor SF-1 (NR5A1)/PIP2 | Full length | In vitro binding | IPMK binds SF-1/PIP2 only when PIP2 is incorporated into the hydrophobic ligand binding domain of SF-1 [21] |
| Ulk1 | 182–252 | Dominant-negative | IPMK may act as a scaffold to link AMPK with ULK1 [14] |
| SRF | 93–124, 125–182, and 208–416 | Dominant-negative | Exons 3, 4, and 6 are involved in binding with the SRF DNA binding domain (MADS); exons 3 and 4 are verified as the dominant-negative structure [46] |
| TRAF6 | 93–124 and 208–416 | Dominant-negative | Exons 4 &6. IPMK binds the N-terminal RING domain of TRAF6 (1–132) [29] |
| TFEB | 262–377 | In vitro binding | Positively charged residues in the IPMK NLS-320, 322, 323, 327, and 328 were mutated to verify TFEB interactions [49] |
| Residue | Mutational Effect on 3- or 6- Kinase Activity | Structural Element Involved | Mutants |
|---|---|---|---|
| Q78 | Both reduced | QPPPR Proline loop, sidechain proximity to Mg2+ ion, and b phosphate of ATP | Q78A [42] |
| R82 | Both reduced | QPPPR Proline loop, sidechain proximity to 4 and 5 position phosphates of IP3. | R82A [42] |
| E131 Ψ | - | Hinge, backbone proximity to ATP base | No mutational analysis performed for HsIPMK [42] |
| V133 Ψ | - | Hinge, backbone proximity to ATP base | No mutational analysis performed for HsIPMK |
| D144 | Only 6-kinase abrogated, 3- kinase activity intact | Proximity to the 3’ hydroxyl of ATP ribose | D144N/K146A double mutant [50] |
| K146 | Only 6-kinase abrogated, 3- kinase activity intact | Proximity to IP3 hydroxyl at position 3 | D144N/K146A double mutant [50] |
| K160 | Both reduced | IP Helices, sidechain proximity to IP3 phosphates at positions 4 and 5 | K160 [42] |
| Q163 | Both reduced | IP Helices, sidechain proximity to IP3 phosphate at positions 5 | Q163A, Q163K, Q163R [42] |
| Q164 | Both reduced | IP Helices, sidechain proximity to IP3 hydroxyl at positions 2 | Q164A, Q164K, Q164R [42] |
| K167 | IP Helices, sidechain proximity to IP3 phosphate at positions 1 | K167A [42] | |
| Q196 | sidechain proximity to IP3 phosphate at positions 1 | Q196A, Q196K, Q196R [42] | |
| D385 ℵ | - | Catalytic site, sidechain proximity to both Mg2+ ions and the a and b phosphates of ATP | No mutational analysis performed for HsIPMK [52] |
| H388 * | Both reduced | Catalytic site, sidechain proximity to a Mg2+ ion, and the IP3 phosphate at positions 4 | H388A [42,52] |
4. IPMK-Regulated Cellular Signaling Pathways Influence Various Cell Functions
4.1. Cell Functions Require IPMK’s Lipid Kinase Activity
4.1.1. Cell Migration
4.1.2. Cell Proliferation and Tissue Regeneration
4.1.3. IPMK’s Lipid Kinase Activity Influences DNA Repair
4.1.4. PI3-Kinase Activity of IPMK Regulates Genotoxic Stress-Mediated Cell Death
4.1.5. IPMK’s PI3-Kinase Activity Influences Transcription
4.2. Cell Function That Requires IPMK’s Inositol Kinase Activity
4.2.1. Chromatin Organization
4.2.2. mRNA Transcription in Yeast
4.2.3. mRNA Export and Translation
4.2.4. Necroptosis
4.2.5. IPMK’s Inositol Kinase Activity Regulates Histone Acetylation in Mammalian Cells
4.3. IPMK Influences Cell Function in a Kinase-Independent Manner
4.3.1. Autophagy
4.3.2. mTORC1 Activation and Nutrient Sensing
4.3.3. AMPK Activation
4.3.4. p53 Activation
4.3.5. NF-kB Mediated Transcription
4.3.6. Role of IPMK in YAP Signaling
4.3.7. Role of IPMK in DNA Methylation
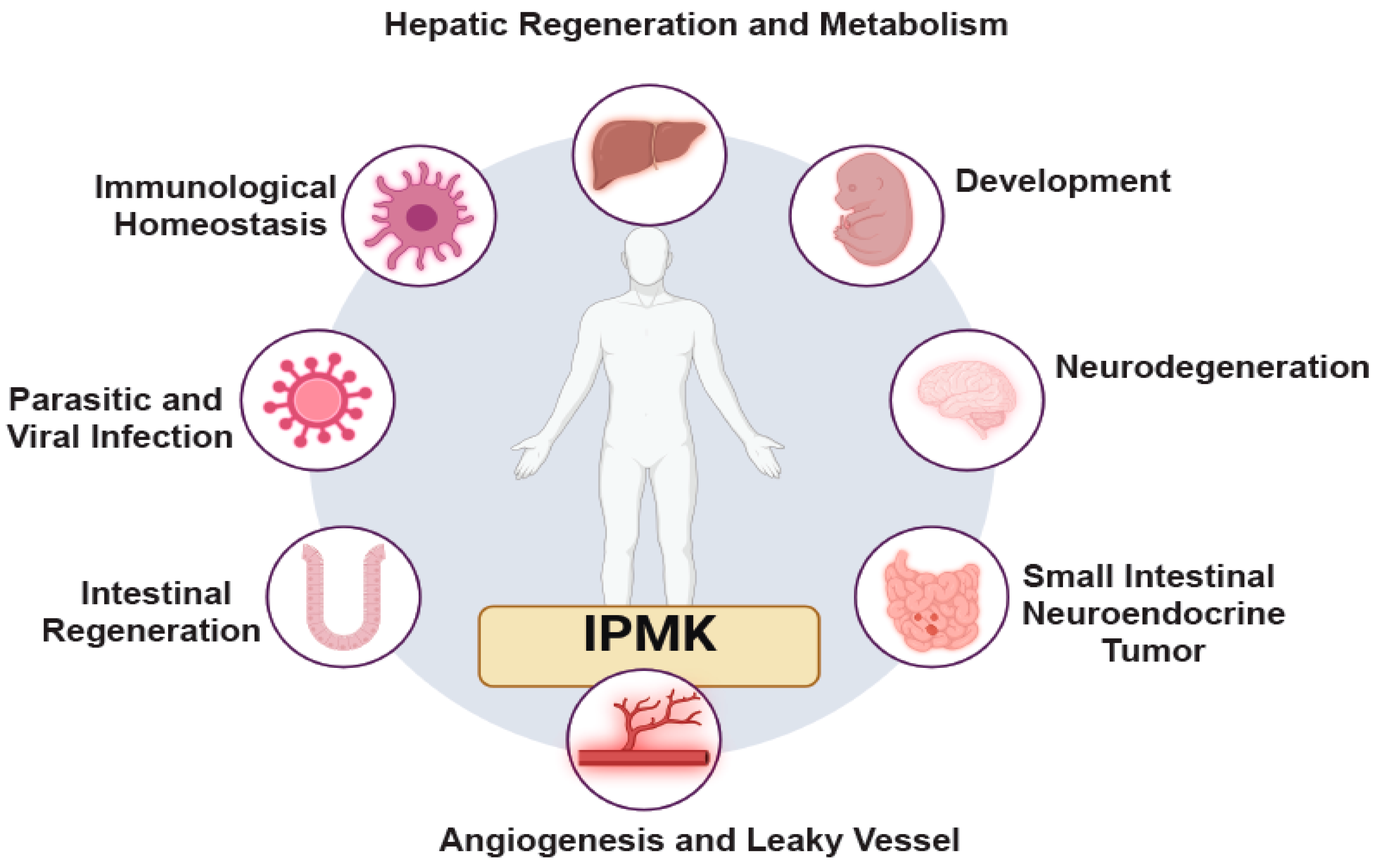
5. Importance of IPMK in Physiology and Diseases
5.1. Developmental Abnormalities and Embryonic Lethality
5.1.1. Mice
5.1.2. Drosophila Melanogaster (Fruit Fly)
5.2. Intestinal Diseases
5.3. Intestinal Neuroendocrine Tumor
5.4. Liver Regeneration and Insulin Signaling
5.5. Immune Signaling
5.5.1. T-Cell
5.5.2. T Helper Cells
5.5.3. B-Cell
5.5.4. Macrophage-Mediated Immune Response
5.6. Leaky Vessel
5.7. Parasitic and Viral Infections
5.8. Neurodegeneration
5.8.1. Huntington’s Disease
5.8.2. Alzheimer’s Disease
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berridge, M.J.; Irvine, R.F. Inositol Trisphosphate, a Novel Second Messenger in Cellular Signal Transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef]
- Berridge, M.J.; Dawson, R.M.; Downes, C.P.; Heslop, J.P.; Irvine, R.F. Changes in the Levels of Inositol Phosphates after Agonist-Dependent Hydrolysis of Membrane Phosphoinositides. Biochem. J. 1983, 212, 473–482. [Google Scholar] [CrossRef]
- Burgess, G.M.; Godfrey, P.P.; McKinney, J.S.; Berridge, M.J.; Irvine, R.F.; Putney, J.W. The Second Messenger Linking Receptor Activation to Internal Ca Release in Liver. Nature 1984, 309, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Cooper, E. PI-3 Kinase and IP3: Partners in NT3-Induced Synaptic Transmission. Nat. Neurosci. 2001, 4, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, L.; Tso, A.; Wang, H.; Cui, L.; Lin, L.; Wang, X.; Ren, M.; Fang, X.; Liu, J.; et al. Inositol 1,4,5-Trisphosphate Receptors Are Essential for Fetal-Maternal Connection and Embryo Viability. PLoS Genet. 2020, 16, e1008739. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Galluzzi, L.; Pinton, P. Ca2+ Fluxes and Cancer. Mol. Cell 2020, 78, 1055–1069. [Google Scholar] [CrossRef]
- Kuchay, S.; Giorgi, C.; Simoneschi, D.; Pagan, J.; Missiroli, S.; Saraf, A.; Florens, L.; Washburn, M.P.; Collazo-Lorduy, A.; Castillo-Martin, M.; et al. PTEN Counteracts FBXL2 to Promote IP3R3- and Ca2+-Mediated Apoptosis Limiting Tumour Growth. Nature 2017, 546, 554–558. [Google Scholar] [CrossRef]
- Metwally, E.; Zhao, G.; Zhang, Y.Q. The Calcium-Dependent Protease Calpain in Neuronal Remodeling and Neurodegeneration. Trends Neurosci. 2021, 44, 741–752. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kim, S.; Snyder, S.H. Inositol Pyrophosphates as Mammalian Cell Signals. Sci. Signal. 2011, 4, re1. [Google Scholar] [CrossRef]
- Kim, E.; Beon, J.; Lee, S.; Park, J.; Kim, S. IPMK: A Versatile Regulator of Nuclear Signaling Events. Adv. Biol. Regul. 2016, 61, 25–32. [Google Scholar] [CrossRef]
- Saiardi, A.; Erdjument-Bromage, H.; Snowman, A.M.; Tempst, P.; Snyder, S.H. Synthesis of Diphosphoinositol Pentakisphosphate by a Newly Identified Family of Higher Inositol Polyphosphate Kinases. Curr. Biol. 1999, 9, 1323–1326. [Google Scholar] [CrossRef]
- Fu, C.; Tyagi, R.; Chin, A.C.; Rojas, T.; Li, R.-J.; Guha, P.; Bernstein, I.A.; Rao, F.; Xu, R.; Cha, J.Y.; et al. Inositol Polyphosphate Multikinase Inhibits Angiogenesis via Inositol Pentakisphosphate-Induced HIF-1α Degradation. Circ. Res. 2018, 122, 457–472. [Google Scholar] [CrossRef]
- Guha, P.; Tyagi, R.; Chowdhury, S.; Reilly, L.; Fu, C.; Xu, R.; Resnick, A.C.; Snyder, S.H. IPMK Mediates Activation of ULK Signaling and Transcriptional Regulation of Autophagy Linked to Liver Inflammation and Regeneration. Cell Rep. 2019, 26, 2692–2703.e7. [Google Scholar] [CrossRef]
- Maag, D.; Maxwell, M.J.; Hardesty, D.A.; Boucher, K.L.; Choudhari, N.; Hanno, A.G.; Ma, J.F.; Snowman, A.S.; Pietropaoli, J.W.; Xu, R.; et al. Inositol Polyphosphate Multikinase Is a Physiologic PI3-Kinase That Activates Akt/PKB. Proc. Natl. Acad. Sci. USA 2011, 108, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bhandari, R.; Brearley, C.A.; Saiardi, A. The Inositol Phosphate Signalling Network in Physiology and Disease. Trends Biochem. Sci. 2024, 49, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, V.O.; Savill, J.M.; Chavali, S.; Jonsdottir, A.B.; Rajendra, E.; Grüner, T.; Laskey, R.A.; Babu, M.M.; Venkitaraman, A.R. Human Inositol Polyphosphate Multikinase Regulates Transcript-Selective Nuclear MRNA Export to Preserve Genome Integrity. Mol. Cell 2013, 51, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Saiardi, A.; Caffrey, J.J.; Snyder, S.H.; Shears, S.B. Inositol Polyphosphate Multikinase (ArgRIII) Determines Nuclear MRNA Export in Saccharomyces Cerevisiae. FEBS Lett. 2000, 468, 28–32. [Google Scholar] [CrossRef]
- Steger, D.J.; Haswell, E.S.; Miller, A.L.; Wente, S.R.; O’Shea, E.K. Regulation of Chromatin Remodeling by Inositol Polyphosphates. Science 2003, 299, 114–116. [Google Scholar] [CrossRef]
- Beon, J.; Han, S.; Yang, H.; Park, S.E.; Hyun, K.; Lee, S.-Y.; Rhee, H.-W.; Seo, J.K.; Kim, J.; Kim, S.; et al. Inositol Polyphosphate Multikinase Physically Binds to the SWI/SNF Complex and Modulates BRG1 Occupancy in Mouse Embryonic Stem Cells. Elife 2022, 11, e73523. [Google Scholar] [CrossRef]
- Blind, R.D.; Suzawa, M.; Ingraham, H.A. Direct Modification and Activation of a Nuclear Receptor-PIP2 Complex by the Inositol Lipid Kinase IPMK. Sci. Signal. 2012, 5, ra44. [Google Scholar] [CrossRef]
- Xu, R.; Paul, B.D.; Smith, D.R.; Tyagi, R.; Rao, F.; Khan, A.B.; Blech, D.J.; Vandiver, M.S.; Harraz, M.M.; Guha, P.; et al. Inositol Polyphosphate Multikinase Is a Transcriptional Coactivator Required for Immediate Early Gene Induction. Proc. Natl. Acad. Sci. USA 2013, 110, 16181–16186. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.F.; Maag, D.; Maxwell, M.J.; Resnick, A.C.; Juluri, K.R.; Chakraborty, A.; Koldobskiy, M.A.; Cha, S.H.; Barrow, R.; et al. Amino Acid Signaling to MTOR Mediated by Inositol Polyphosphate Multikinase. Cell Metab. 2011, 13, 215–221. [Google Scholar] [CrossRef]
- Seeds, A.M.; Tsui, M.M.; Sunu, C.; Spana, E.P.; York, J.D. Inositol Phosphate Kinase 2 Is Required for Imaginal Disc Development in Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, 15660–15665. [Google Scholar] [CrossRef]
- Frederick, J.P.; Mattiske, D.; Wofford, J.A.; Megosh, L.C.; Drake, L.Y.; Chiou, S.-T.; Hogan, B.L.M.; York, J.D. An Essential Role for an Inositol Polyphosphate Multikinase, Ipk2, in Mouse Embryogenesis and Second Messenger Production. Proc. Natl. Acad. Sci. USA 2005, 102, 8454–8459. [Google Scholar] [CrossRef] [PubMed]
- Resnick, A.C.; Snowman, A.M.; Kang, B.N.; Hurt, K.J.; Snyder, S.H.; Saiardi, A. Inositol Polyphosphate Multikinase Is a Nuclear PI3-Kinase with Transcriptional Regulatory Activity. Proc. Natl. Acad. Sci. USA 2005, 102, 12783–12788. [Google Scholar] [CrossRef] [PubMed]
- Resnick, A.C.; Saiardi, A. Inositol Polyphosphate Multikinase: Metabolic Architect of Nuclear Inositides. Front. Biosci. 2008, 13, 856–866. [Google Scholar] [CrossRef]
- Ahn, H.; Roh, J.S.; Lee, S.; Beon, J.; Lee, B.; Sohn, D.H.; Kim, S. Myeloid IPMK Promotes the Resolution of Serum Transfer-Induced Arthritis in Mice. Anim. Cells Syst. 2021, 25, 219–226. [Google Scholar] [CrossRef]
- Kim, E.; Beon, J.; Lee, S.; Park, S.J.; Ahn, H.; Kim, M.G.; Park, J.E.; Kim, W.; Yuk, J.-M.; Kang, S.-J.; et al. Inositol Polyphosphate Multikinase Promotes Toll-like Receptor-Induced Inflammation by Stabilizing TRAF6. Sci. Adv. 2017, 3, e1602296. [Google Scholar] [CrossRef]
- Bang, S.; Chen, Y.; Ahima, R.S.; Kim, S.F. Convergence of IPMK and LKB1-AMPK Signaling Pathways on Metformin Action. Mol. Endocrinol. 2014, 28, 1186–1193. [Google Scholar] [CrossRef]
- Lee, S.; Beon, J.; Kim, M.-G.; Kim, S. Inositol Polyphosphate Multikinase in Adipocytes Is Dispensable for Regulating Energy Metabolism and Whole Body Metabolic Homeostasis. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E401–E409. [Google Scholar] [CrossRef]
- Yokoyama, J.S.; Wang, Y.; Schork, A.J.; Thompson, W.K.; Karch, C.M.; Cruchaga, C.; McEvoy, L.K.; Witoelar, A.; Chen, C.-H.; Holland, D.; et al. Association Between Genetic Traits for Immune-Mediated Diseases and Alzheimer Disease. JAMA Neurol. 2016, 73, 691–697. [Google Scholar] [CrossRef]
- Park, S.E.; Lee, D.; Jeong, J.W.; Lee, S.-H.; Park, S.J.; Ryu, J.; Oh, S.K.; Yang, H.; Fang, S.; Kim, S. Gut Epithelial Inositol Polyphosphate Multikinase Alleviates Experimental Colitis via Governing Tuft Cell Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 1235–1256. [Google Scholar] [CrossRef]
- Reilly, L.; Semenza, E.R.; Koshkaryan, G.; Mishra, S.; Chatterjee, S.; Abramson, E.; Mishra, P.; Sei, Y.; Wank, S.A.; Donowitz, M.; et al. Loss of PI3k Activity of Inositol Polyphosphate Multikinase Impairs PDK1-Mediated AKT Activation, Cell Migration, and Intestinal Homeostasis. iScience 2023, 26, 106623. [Google Scholar] [CrossRef] [PubMed]
- Sei, Y.; Zhao, X.; Forbes, J.; Szymczak, S.; Li, Q.; Trivedi, A.; Voellinger, M.; Joy, G.; Feng, J.; Whatley, M.; et al. A Hereditary Form of Small Intestinal Carcinoid Associated With a Germline Mutation in Inositol Polyphosphate Multikinase. Gastroenterology 2015, 149, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Leevers, S.J.; Ahmadi, K.; Timms, J.; Katso, R.; Driscoll, P.C.; Woscholski, R.; Parker, P.J.; Waterfield, M.D. Synthesis and Function of 3-Phosphorylated Inositol Lipids. Annu. Rev. Biochem. 2001, 70, 535–602. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.F.; Schell, M.J. Back in the Water: The Return of the Inositol Phosphates. Nat. Rev. Mol. Cell Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef]
- Desfougères, Y.; Wilson, M.S.C.; Laha, D.; Miller, G.J.; Saiardi, A. ITPK1 Mediates the Lipid-Independent Synthesis of Inositol Phosphates Controlled by Metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 24551–24561. [Google Scholar] [CrossRef]
- Letcher, A.J.; Schell, M.J.; Irvine, R.F. Do Mammals Make All Their Own Inositol Hexakisphosphate? Biochem. J. 2008, 416, 263–270. [Google Scholar] [CrossRef]
- Stuart, J.A.; Anderson, K.L.; French, P.J.; Kirk, C.J.; Michell, R.H. The Intracellular Distribution of Inositol Polyphosphates in HL60 Promyeloid Cells. Biochem. J. 1994, 303 Pt 2, 517–525. [Google Scholar] [CrossRef]
- Seacrist, C.D.; Blind, R.D. Crystallographic and Kinetic Analyses of Human IPMK Reveal Disordered Domains Modulate ATP Binding and Kinase Activity. Sci. Rep. 2018, 8, 16672. [Google Scholar] [CrossRef]
- Wang, H.; Shears, S.B. Structural Features of Human Inositol Phosphate Multikinase Rationalize Its Inositol Phosphate Kinase and Phosphoinositide 3-Kinase Activities. J. Biol. Chem. 2017, 292, 18192–18202. [Google Scholar] [CrossRef]
- Gu, C.; Stashko, M.A.; Puhl-Rubio, A.C.; Chakraborty, M.; Chakraborty, A.; Frye, S.V.; Pearce, K.H.; Wang, X.; Shears, S.B.; Wang, H. Inhibition of Inositol Polyphosphate Kinases by Quercetin and Related Flavonoids: A Structure-Activity Analysis. J. Med. Chem. 2019, 62, 1443–1454. [Google Scholar] [CrossRef]
- Bang, S.; Kim, S.; Dailey, M.J.; Chen, Y.; Moran, T.H.; Snyder, S.H.; Kim, S.F. AMP-Activated Protein Kinase Is Physiologically Regulated by Inositol Polyphosphate Multikinase. Proc. Natl. Acad. Sci. USA 2012, 109, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Nalaskowski, M.M.; Ehm, P.; Schröder, C.; Naj, X.; Brehm, M.A.; Mayr, G.W. Nucleocytoplasmic Shuttling of Human Inositol Phosphate Multikinase Is Influenced by CK2 Phosphorylation. Biol. Chem. 2012, 393, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tyagi, R.; Lee, J.-Y.; Park, J.; Kim, Y.-R.; Beon, J.; Chen, P.Y.; Cha, J.Y.; Snyder, S.H.; Kim, S. Inositol Polyphosphate Multikinase Is a Coactivator for Serum Response Factor-Dependent Induction of Immediate Early Genes. Proc. Natl. Acad. Sci. USA 2013, 110, 19938–19943. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Chakraborty, S.; Tripathi, S.J.; Jung, I.-R.; Kim, S.F.; Snyder, S.H.; Paul, B.D. Inositol Polyphosphate Multikinase Modulates Redox Signaling through Nuclear Factor Erythroid 2-Related Factor 2 and Glutathione Metabolism. iScience 2023, 26, 107199. [Google Scholar] [CrossRef]
- Xu, R.; Sen, N.; Paul, B.D.; Snowman, A.M.; Rao, F.; Vandiver, M.S.; Xu, J.; Snyder, S.H. Inositol Polyphosphate Multikinase Is a Coactivator of P53-Mediated Transcription and Cell Death. Sci. Signal. 2013, 6, ra22. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Zhao, Y.G.; Zheng, H.; Zhao, H.; Liu, N.; Zhang, H. Inositol Polyphosphate Multikinase Inhibits Liquid-Liquid Phase Separation of TFEB to Negatively Regulate Autophagy Activity. Dev. Cell 2020, 55, 588–602.e7. [Google Scholar] [CrossRef]
- Dovey, C.M.; Diep, J.; Clarke, B.P.; Hale, A.T.; McNamara, D.E.; Guo, H.; Brown, N.W.; Cao, J.Y.; Grace, C.R.; Gough, P.J.; et al. MLKL Requires the Inositol Phosphate Code to Execute Necroptosis. Mol. Cell 2018, 70, 936–948.e7. [Google Scholar] [CrossRef]
- Wang, H.; DeRose, E.F.; London, R.E.; Shears, S.B. IP6K Structure and the Molecular Determinants of Catalytic Specificity in an Inositol Phosphate Kinase Family. Nat. Commun. 2014, 5, 4178. [Google Scholar] [CrossRef]
- González, B.; Schell, M.J.; Letcher, A.J.; Veprintsev, D.B.; Irvine, R.F.; Williams, R.L. Structure of a Human Inositol 1,4,5-Trisphosphate 3-Kinase: Substrate Binding Reveals Why It Is Not a Phosphoinositide 3-Kinase. Mol. Cell 2004, 15, 689–701. [Google Scholar] [CrossRef]
- Loovers, H.M.; Postma, M.; Keizer-Gunnink, I.; Huang, Y.E.; Devreotes, P.N.; van Haastert, P.J.M. Distinct Roles of PI(3,4,5)P3 during Chemoattractant Signaling in Dictyostelium: A Quantitative in Vivo Analysis by Inhibition of PI3-Kinase. Mol. Biol. Cell 2006, 17, 1503–1513. [Google Scholar] [CrossRef]
- Tu-Sekine, B.; Padhi, A.; Jin, S.; Kalyan, S.; Singh, K.; Apperson, M.; Kapania, R.; Hur, S.C.; Nain, A.; Kim, S.F. Inositol Polyphosphate Multikinase Is a Metformin Target That Regulates Cell Migration. FASEB J. 2019, 33, 14137–14146. [Google Scholar] [CrossRef]
- Pinner, S.; Sahai, E. PDK1 Regulates Cancer Cell Motility by Antagonising Inhibition of ROCK1 by RhoE. Nat. Cell Biol. 2008, 10, 127–137. [Google Scholar] [CrossRef]
- Insall, R.H.; Weiner, O.D. PIP3, PIP2, and Cell Movement--Similar Messages, Different Meanings? Dev. Cell 2001, 1, 743–747. [Google Scholar] [CrossRef]
- van Haastert, P.J.M.; Keizer-Gunnink, I.; Kortholt, A. Essential Role of PI3-Kinase and Phospholipase A2 in Dictyostelium Discoideum Chemotaxis. J. Cell Biol. 2007, 177, 809–816. [Google Scholar] [CrossRef]
- Jung, I.-R.; Ahima, R.S.; Kim, S.F. Inositol Polyphosphate Multikinase Modulates Free Fatty Acids-Induced Insulin Resistance in Primary Mouse Hepatocytes. J. Cell. Biochem. 2023, 124, 1695–1704. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Hariharan, A.; Bastianello, G.; Toyama, Y.; Shivashankar, G.V.; Foiani, M.; Sheetz, M.P. DNA Damage Causes Rapid Accumulation of Phosphoinositides for ATR Signaling. Nat. Commun. 2017, 8, 2118. [Google Scholar] [CrossRef]
- Lamm, N.; Read, M.N.; Nobis, M.; Van Ly, D.; Page, S.G.; Masamsetti, V.P.; Timpson, P.; Biro, M.; Cesare, A.J. Nuclear F-Actin Counteracts Nuclear Deformation and Promotes Fork Repair during Replication Stress. Nat. Cell Biol. 2020, 22, 1460–1470. [Google Scholar] [CrossRef]
- Choi, S.; Chen, M.; Cryns, V.L.; Anderson, R.A. A Nuclear Phosphoinositide Kinase Complex Regulates P53. Nat. Cell Biol. 2019, 21, 462–475. [Google Scholar] [CrossRef]
- York, J.D.; Odom, A.R.; Murphy, R.; Ives, E.B.; Wente, S.R. A Phospholipase C-Dependent Inositol Polyphosphate Kinase Pathway Required for Efficient Messenger RNA Export. Science 1999, 285, 96–100. [Google Scholar] [CrossRef]
- Alcázar-Román, A.R.; Bolger, T.A.; Wente, S.R. Control of MRNA Export and Translation Termination by Inositol Hexakisphosphate Requires Specific Interaction with Gle1. J. Biol. Chem. 2010, 285, 16683–16692. [Google Scholar] [CrossRef]
- Taylor, R.; Chen, P.-H.; Chou, C.-C.; Patel, J.; Jin, S. V KCS1 Deletion in Saccharomyces Cerevisiae Leads to a Defect in Translocation of Autophagic Proteins and Reduces Autophagosome Formation. Autophagy 2012, 8, 1300–1311. [Google Scholar] [CrossRef]
- Kanki, T.; Wang, K.; Baba, M.; Bartholomew, C.R.; Lynch-Day, M.A.; Du, Z.; Geng, J.; Mao, K.; Yang, Z.; Yen, W.-L.; et al. A Genomic Screen for Yeast Mutants Defective in Selective Mitochondria Autophagy. Mol. Biol. Cell 2009, 20, 4730–4738. [Google Scholar] [CrossRef]
- Guha, P.; Harraz, M.M.; Snyder, S.H. Cocaine Elicits Autophagic Cytotoxicity via a Nitric Oxide-GAPDH Signaling Cascade. Proc. Natl. Acad. Sci. USA 2016, 113, 1417–1422. [Google Scholar] [CrossRef]
- Gat, Y.; Schuller, J.M.; Lingaraju, M.; Weyher, E.; Bonneau, F.; Strauss, M.; Murray, P.J.; Conti, E. InsP6 Binding to PIKK Kinases Revealed by the Cryo-EM Structure of an SMG1-SMG8-SMG9 Complex. Nat. Struct. Mol. Biol. 2019, 26, 1089–1093. [Google Scholar] [CrossRef]
- Scaiola, A.; Mangia, F.; Imseng, S.; Boehringer, D.; Berneiser, K.; Shimobayashi, M.; Stuttfeld, E.; Hall, M.N.; Ban, N.; Maier, T. The 3.2-Å Resolution Structure of Human MTORC2. Sci. Adv. 2020, 6, eabc1251. [Google Scholar] [CrossRef]
- Rameh, L.E.; York, J.D.; Blind, R.D. Inositol Phosphates Dynamically Enhance Stability, Solubility, and Catalytic Activity of MTOR. J. Biol. Chem. 2025, 301, 108095. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Chen, M.; Choi, S.; Wen, T.; Chen, C.; Thapa, N.; Lee, J.H.; Cryns, V.L.; Anderson, R.A. A P53-Phosphoinositide Signalosome Regulates Nuclear AKT Activation. Nat. Cell Biol. 2022, 24, 1099–1113. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, J.; Sia, Y.; Chen, Z. Mechanism of Action of the SWI/SNF Family Complexes. Nucleus 2023, 14, 2165604. [Google Scholar] [CrossRef]
- Odom, A.R.; Stahlberg, A.; Wente, S.R.; York, J.D. A Role for Nuclear Inositol 1,4,5-Trisphosphate Kinase in Transcriptional Control. Science 2000, 287, 2026–2029. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Misra, R.P. Expression of the Serum Response Factor Gene Is Regulated by Serum Response Factor Binding Sites. J. Biol. Chem. 1996, 271, 16535–16543. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Apaijai, N.; Chattipakorn, N.; Chattipakorn, S.C. The Possible Roles of Necroptosis during Cerebral Ischemia and Ischemia/Reperfusion Injury. Arch. Biochem. Biophys. 2020, 695, 108629. [Google Scholar] [CrossRef]
- Watson, P.J.; Fairall, L.; Santos, G.M.; Schwabe, J.W.R. Structure of HDAC3 Bound to Co-Repressor and Inositol Tetraphosphate. Nature 2012, 481, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Millard, C.J.; Watson, P.J.; Celardo, I.; Gordiyenko, Y.; Cowley, S.M.; Robinson, C.V.; Fairall, L.; Schwabe, J.W.R. Class I HDACs Share a Common Mechanism of Regulation by Inositol Phosphates. Mol. Cell 2013, 51, 57–67. [Google Scholar] [CrossRef]
- Chatterjee, S.; Preval, L.V.; Sin, Z.; Tran, N.; Ritter, K.; Su, X.B.; Centlivre, J.P.; Ragsac, S.J.; Van, R.; Park, S.; et al. Inositol Hexaphosphate (InsP6) Activates the HDAC1/3 Epigenetic Axis to Maintain Intestinal Barrier Function. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sowd, G.A.; Stivison, E.A.; Chapagain, P.; Hale, A.T.; Poland, J.C.; Rameh, L.E.; Blind, R.D. IPMK Regulates HDAC3 Activity and Histone H4 Acetylation in Human Cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ghosh, S.; Sin, Z.; Babu, V.S.; Preval, L.V.; Davis, E.; Tran, N.; Bammidi, S.; Gautam, P.; Hose, S.; et al. Age-Dependent Histone Deacetylase 3 Regulation by ΒA3/A1-Crystallin and Inositol Hexaphosphate in Retinal Pigmented Epithelial Cells Reveals a Novel Pathway in Age-Related Macular Degeneration. Aging Cell 2025, e70163. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Guha, P.; Snyder, S.H. Noncatalytic Functions of IPMK Are Essential for Activation of Autophagy and Liver Regeneration. Autophagy 2019, 15, 1473–1474. [Google Scholar] [CrossRef]
- Pan, Z.; Li, S.-J.; Guo, H.; Li, Z.-H.; Fei, X.; Chang, S.-M.; Yang, Q.-C.; Cheng, D.-D. Ebastine Exerts Antitumor Activity and Induces Autophagy by Activating AMPK/ULK1 Signaling in an IPMK-Dependent Manner in Osteosarcoma. Int. J. Biol. Sci. 2023, 19, 537–551. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, H.; Wang, B.; Ao, Q.; Shi, J.; He, Z. MicroRNA-23b Alleviates Neuroinflammation and Brain Injury in Intracerebral Hemorrhage by Targeting Inositol Polyphosphate Multikinase. Int. Immunopharmacol. 2019, 76, 105887. [Google Scholar] [CrossRef]
- Schwartz, D.; Rotter, V. P53-Dependent Cell Cycle Control: Response to Genotoxic Stress. Semin. Cancer Biol. 1998, 8, 325–336. [Google Scholar] [CrossRef]
- Jung, O.; Baek, M.-J.; Wooldrik, C.; Johnson, K.R.; Fisher, K.W.; Lou, J.; Ricks, T.J.; Wen, T.; Best, M.D.; Cryns, V.L.; et al. Nuclear Phosphoinositide Signaling Promotes YAP/TAZ-TEAD Transcriptional Activity in Breast Cancer. EMBO J. 2024, 43, 1740–1769. [Google Scholar] [CrossRef] [PubMed]
- Sin, Z.; Kinnear, E.; Doshi, R.; Chatterjee, S.; Derbel, H.; Guha, P.; Liu, Q. IPMK Depletion Influences Genome-Wide DNA Methylation. Biochem. Biophys. Res. Commun. 2025, 766, 151874. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.-R.; Anokye-Danso, F.; Jin, S.; Ahima, R.S.; Kim, S.F. IPMK Modulates Hepatic Glucose Production and Insulin Signaling. J. Cell. Physiol. 2022, 237, 3421–3432. [Google Scholar] [CrossRef]
- Verbsky, J.; Lavine, K.; Majerus, P.W. Disruption of the Mouse Inositol 1,3,4,5,6-Pentakisphosphate 2-Kinase Gene, Associated Lethality, and Tissue Distribution of 2-Kinase Expression. Proc. Natl. Acad. Sci. USA 2005, 102, 8448–8453. [Google Scholar] [CrossRef]
- Ahmed, I.; Sbodio, J.I.; Harraz, M.M.; Tyagi, R.; Grima, J.C.; Albacarys, L.K.; Hubbi, M.E.; Xu, R.; Kim, S.; Paul, B.D.; et al. Huntington’s Disease: Neural Dysfunction Linked to Inositol Polyphosphate Multikinase. Proc. Natl. Acad. Sci. USA 2015, 112, 9751–9756. [Google Scholar] [CrossRef]
- Dato, S.; Crocco, P.; De Rango, F.; Iannone, F.; Maletta, R.; Bruni, A.C.; Saiardi, A.; Rose, G.; Passarino, G. IP6K3 and IPMK Variations in LOAD and Longevity: Evidence for a Multifaceted Signaling Network at the Crossroad between Neurodegeneration and Survival. Mech. Ageing Dev. 2021, 195, 111439. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.; Haas, P.; Moretti, N.S.; Schenkman, S.; Stuart, K. Chemogenetic Characterization of Inositol Phosphate Metabolic Pathway Reveals Druggable Enzymes for Targeting Kinetoplastid Parasites. Cell Chem. Biol. 2016, 23, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Cestari, I.; Anupama, A.; Stuart, K. Inositol Polyphosphate Multikinase Regulation of Trypanosoma Brucei Life Stage Development. Mol. Biol. Cell 2018, 29, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Pak, A.J.; Voth, G.A. Critical Mechanistic Features of HIV-1 Viral Capsid Assembly. Sci. Adv. 2023, 9, eadd7434. [Google Scholar] [CrossRef]
- Mallery, D.L.; Kleinpeter, A.B.; Renner, N.; Faysal, K.M.R.; Novikova, M.; Kiss, L.; Wilson, M.S.C.; Ahsan, B.; Ke, Z.; Briggs, J.A.G.; et al. A Stable Immature Lattice Packages IP6 for HIV Capsid Maturation. Sci. Adv. 2021, 7, eabe4716. [Google Scholar] [CrossRef]
- Kim, W.; Kim, E.; Min, H.; Kim, M.G.; Eisenbeis, V.B.; Dutta, A.K.; Pavlovic, I.; Jessen, H.J.; Kim, S.; Seong, R.H. Inositol Polyphosphates Promote T Cell-Independent Humoral Immunity via the Regulation of Bruton’s Tyrosine Kinase. Proc. Natl. Acad. Sci. USA 2019, 116, 12952–12957. [Google Scholar] [CrossRef]
- Zou, X.; Wang, K.; Deng, Y.; Guan, P.; Pu, Q.; Wang, Y.; Mou, J.; Du, Y.; Lou, X.; Wang, S.; et al. Hypoxia-Inducible Factor 2α Promotes Pathogenic Polarization of Stem-like Th2 Cells via Modulation of Phospholipid Metabolism. Immunity 2024, 57, 2808–2826.e8. [Google Scholar] [CrossRef]
- Hay, A.M.; Howie, H.L.; Gorham, J.D.; D’Alessandro, A.; Spitalnik, S.L.; Hudson, K.E.; Zimring, J.C. Mouse Background Genetics in Biomedical Research: The Devil’s in the Details. Transfusion 2021, 61, 3017–3025. [Google Scholar] [CrossRef]
- de Mestier, L.; Pasmant, E.; Fleury, C.; Brixi, H.; Sohier, P.; Féron, T.; Diebold, M.-D.; Clauser, E.; Cadiot, G.; Groupe d’Étude des Tumeurs Endocrines. Familial Small-Intestine Carcinoids: Chromosomal Alterations and Germline Inositol Polyphosphate Multikinase Sequencing. Dig. Liver Dis. 2017, 49, 98–102. [Google Scholar] [CrossRef]
- Min, H.; Kim, W.; Hong, S.; Lee, S.; Jeong, J.; Kim, S.; Seong, R.H. Differentiation and Homeostasis of Effector Treg Cells Are Regulated by Inositol Polyphosphates Modulating Ca2+ Influx. Proc. Natl. Acad. Sci. USA 2022, 119, e2121520119. [Google Scholar] [CrossRef]
- Hong, S.; Kim, K.; Shim, Y.-R.; Park, J.; Choi, S.E.; Min, H.; Lee, S.; Song, J.-J.; Kang, S.-J.; Jeong, W.-I.; et al. A Non-Catalytic Role of IPMK Is Required for PLCγ1 Activation in T Cell Receptor Signaling by Stabilizing the PLCγ1-Sam68 Complex. Cell Commun. Signal. 2024, 22, 526. [Google Scholar] [CrossRef]
- Yuk, C.M.; Hong, S.; Kim, D.; Kim, M.; Jeong, H.-W.; Park, S.J.; Min, H.; Kim, W.; Lim, J.; Kim, H.D.; et al. Inositol Polyphosphate Multikinase Regulates Th1 and Th17 Cell Differentiation by Controlling Akt-MTOR Signaling. Cell Rep. 2025, 44, 115281. [Google Scholar] [CrossRef]
- Kozakiewicz, L.; Phuah, J.; Flynn, J.; Chan, J. The Role of B Cells and Humoral Immunity in Mycobacterium tuberculosis Infection. In The New Paradigm of Immunity to Tuberculosis; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; Volume 783, pp. 225–250. [Google Scholar] [CrossRef]
- Suchting, S.; Freitas, C.; le Noble, F.; Benedito, R.; Bréant, C.; Duarte, A.; Eichmann, A. The Notch Ligand Delta-like 4 Negatively Regulates Endothelial Tip Cell Formation and Vessel Branching. Proc. Natl. Acad. Sci. USA 2007, 104, 3225–3230. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.H.; Chatterjee, S.; Viera-Preval, L.; Guha, P. From Obscurity to Prominence: IPMK’s Expanding Role in Cellular Signaling, Physiology, and Disease. Biomolecules 2025, 15, 1266. https://doi.org/10.3390/biom15091266
Mishra SH, Chatterjee S, Viera-Preval L, Guha P. From Obscurity to Prominence: IPMK’s Expanding Role in Cellular Signaling, Physiology, and Disease. Biomolecules. 2025; 15(9):1266. https://doi.org/10.3390/biom15091266
Chicago/Turabian StyleMishra, Subrata H., Sujan Chatterjee, Loretta Viera-Preval, and Prasun Guha. 2025. "From Obscurity to Prominence: IPMK’s Expanding Role in Cellular Signaling, Physiology, and Disease" Biomolecules 15, no. 9: 1266. https://doi.org/10.3390/biom15091266
APA StyleMishra, S. H., Chatterjee, S., Viera-Preval, L., & Guha, P. (2025). From Obscurity to Prominence: IPMK’s Expanding Role in Cellular Signaling, Physiology, and Disease. Biomolecules, 15(9), 1266. https://doi.org/10.3390/biom15091266






