Unraveling the Mystery of Hemoglobin in Hypoxia-Accelerated Neurodegenerative Diseases
Abstract
1. Introduction
2. Defining the Role of Hypoxia in Acute and Chronic NDDs
2.1. Aging as the Primary Risk Factor for Both Acute and Chronic NDDs
2.2. Hypoxia as a Common Pathological Feature in Acute and Chronic NDDs
| Classification | Disease | Proportion of Age Groups Among Patients | Main Pathological Mechanism |
|---|---|---|---|
| Acute NDDs | Stroke | ~10% prevalence in general population; ~90% among individuals aged ≥70 years [30]. | Ischemic stroke: ischemia; glucose deprivation; hypoxia [36]. Hemorrhagic stroke: cerebral hemorrhage; ischemia and hypoxia secondary to cerebral hemorrhage [25]. |
| Traumatic brain injury | / | Primary brain injury caused by external force; secondary brain hemorrhage, ischemia, and hypoxia [26]. | |
| Chronic NDDs | Parkinson’s disease | ~20% prevalence among individuals aged <70 years; ~80% among those aged ≥70 years [31,32]. | Progressive loss of dopaminergic neurons; abnormal protein degradation system; abnormal aggregation of α-syn; mitochondrial dysfunction-induced hypoxia; genetic factors [27,37]. |
| Alzheimer’s disease | ~26% prevalence among individuals aged <75 years; ~74% among those aged ≥75 years [33]. | Abnormal accumulation of amyloid-β plaques and tau neurofibrillary tangles; cerebrovascular disease; mitochondrial dysfunction-induced hypoxia; genetic factors [38,39]. | |
| Amyotrophic lateral sclerosis | Average age of onset ~65 years [35], peak prevalence at ~75 years [34]. | Abnormal accumulation of TDP-43 protein; genetic factors [28,29]. |
3. Hb: An Overlooked Research Target in Hypoxia–Aging Diseases
3.1. Unexpected Expression and Functionality of Non-Erythroid Hemoglobin
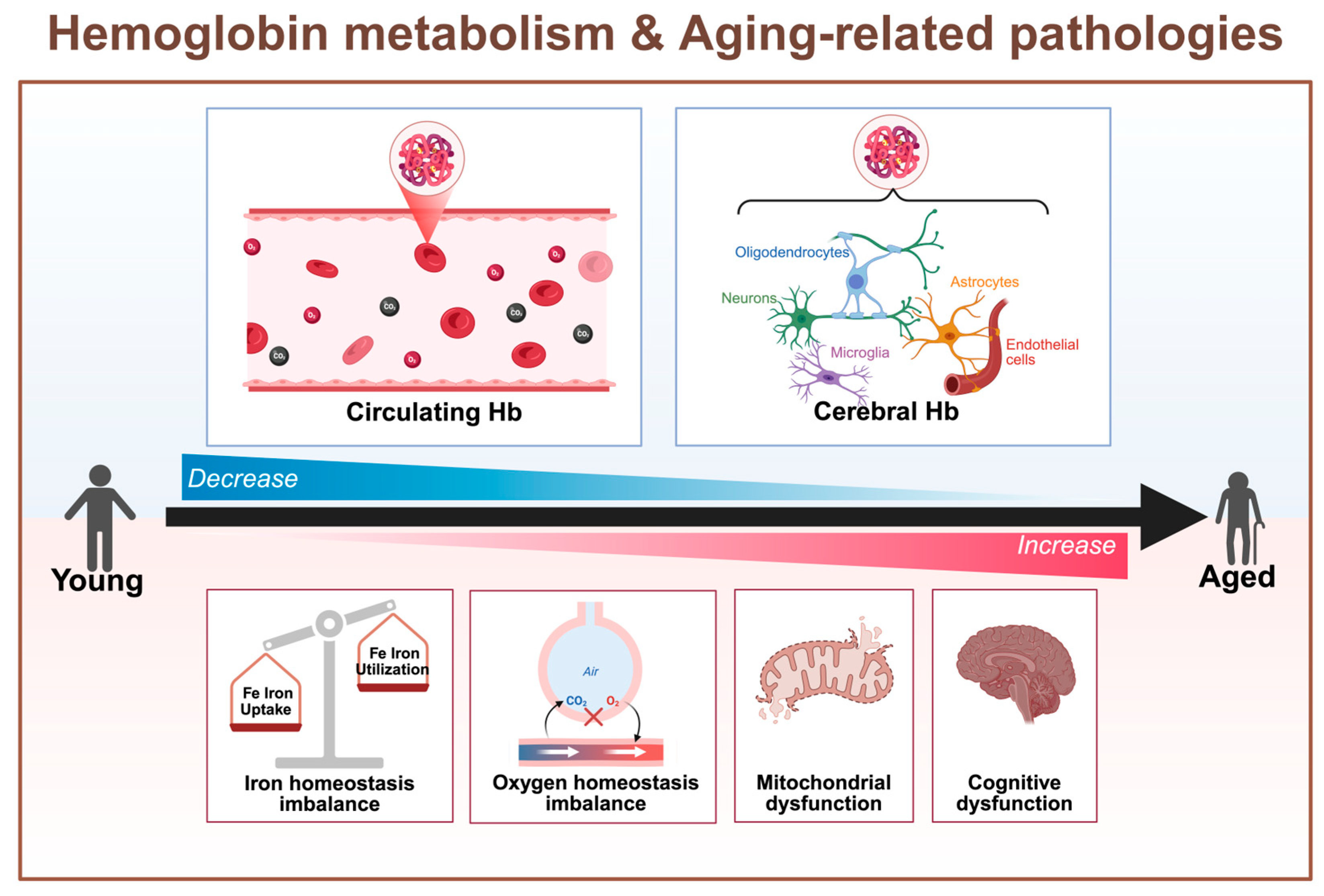
3.2. The Close Relationship Between Aging and Hb
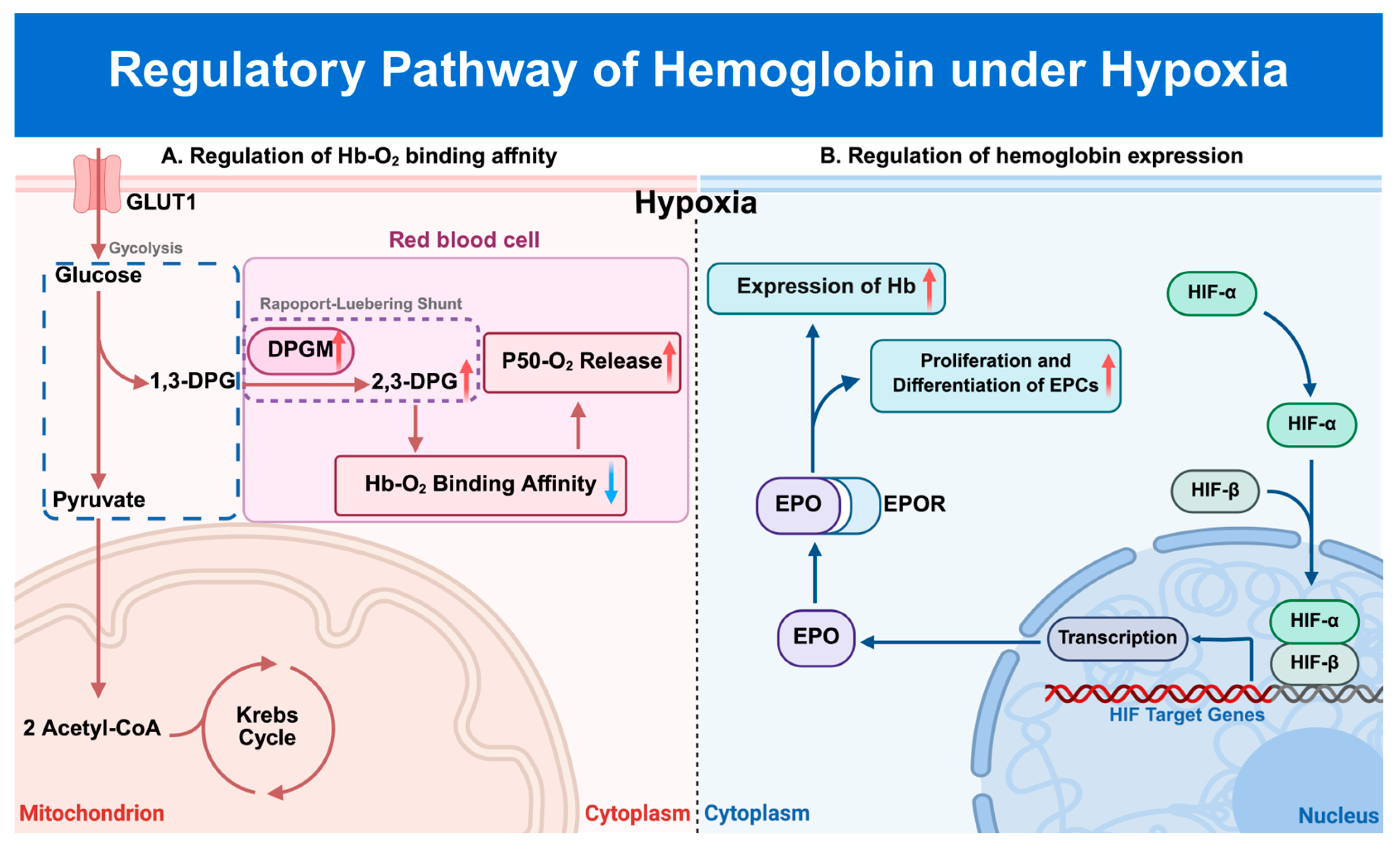
3.3. Regulation of Hb by Hypoxic Stress
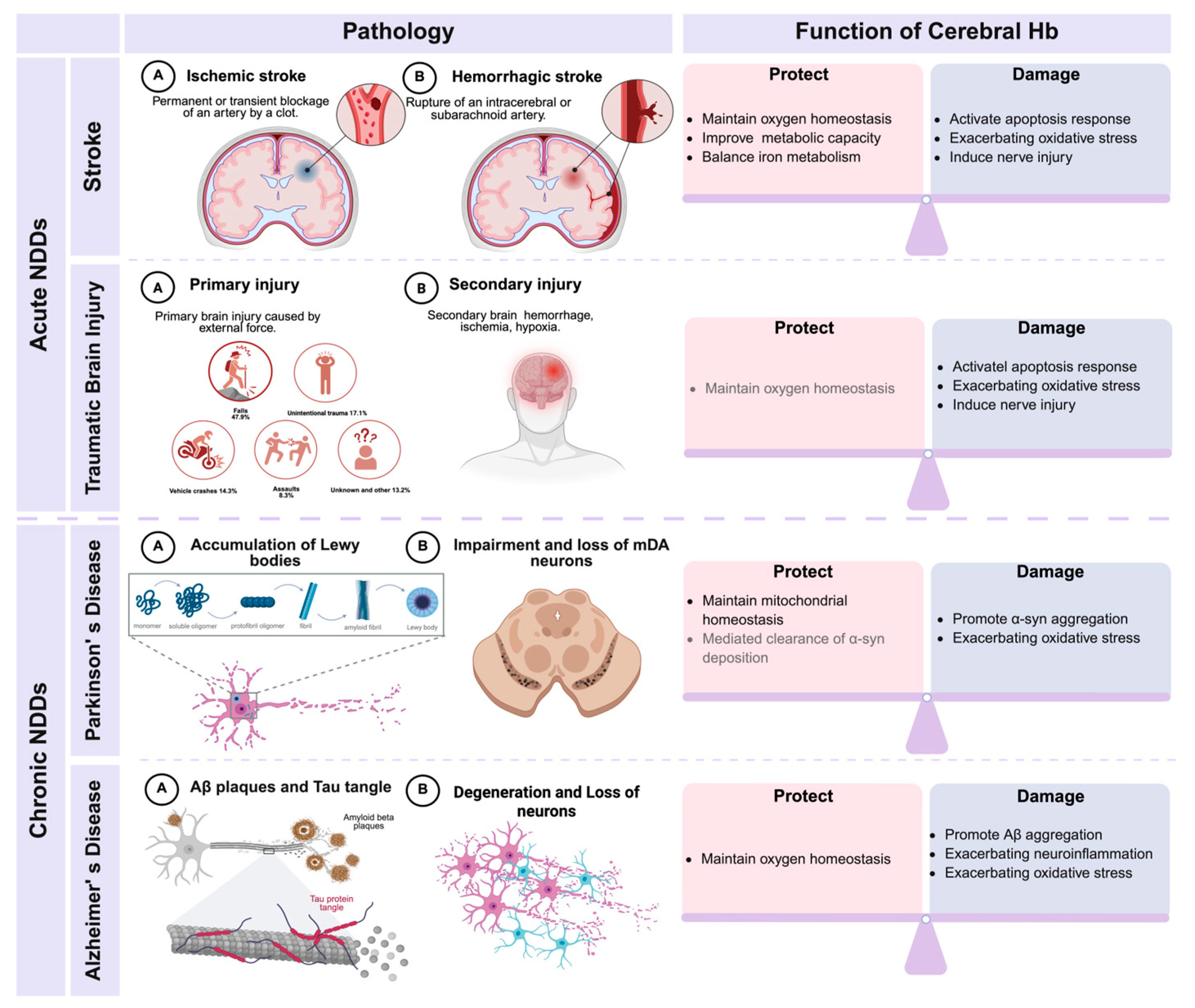
4. Current and Potential Research on the Relationship Between Hb and Hypoxia-Related NDDs
4.1. Hb and Acute NDDs
4.1.1. Stroke
4.1.2. TBI
4.2. Hb and Chronic NDDs
4.2.1. Parkinson’s Disease
4.2.2. Alzheimer’s Disease
4.2.3. Amyotrophic Lateral Sclerosis
5. Discussion and Future Directions
| Stress/Disease | Species | Age/Disease Model/Disease Stage | Cell Type | Hb Expression | Functional Implication | Reference |
|---|---|---|---|---|---|---|
| Aging | Mouse | 6/12/18 months | / | Hbα ↓, Hbβ ↓ | Protective | [10] |
| Cynomolgus | 3–4/10–12/15 years | Neuron (Non-marker) | Hbα ↓, Hbβ ↓ | Protective | [61] | |
| Hypoxia | Mouse | 7% O2 treatment for 28 days | Neuron (Map2+) | Hbα ↑ | Protective | [155] |
| Stroke | Rat | dMCAO | Neuron (NeuN+) | Hbα ↑, Hbβ ↑ | Protective | [97] |
| Rat | OGD | Neuron (Primary cell) | Hbα ↑, Hbβ ↑ | Protective | [97] | |
| Mouse | BCAO | Neuron (Non-marker) | Hbα ↑ | Protective | [10] | |
| Parkinson’s disease | Patient | Braak stage 0, I, II | Neuron (Non-marker) | Hbα -, Hbβ - | Unclear | [82] |
| Patient | Braak stage III, IV, V, VI | / | Hbα -, Hbβ - | Unclear | [165] | |
| Patient | UPDRS = 54, 70, 45.5 | Neuron (Non-marker) | Hbα -, Hbβ - | Unclear | [130] | |
| Alzheimer’s disease | Patient | Braak stage III | Neuron (Non-marker) | Hbα -, Hbβ - | Unclear | [82] |
| Patient | Braak stage V, VI | Neuron (Non-marker) | Hbα -, Hbβ - | Pathogenic | [140] | |
| Mouse | APP/PS1transgenic | Neuron (NeuN+) Oligodendrocyte (OSP+) Astrocyte (GFAP+) Microglia (Iba1+) | Hbα ↑ | Pathogenic | [141] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,3-DPG | 2,3-diphosphoglycerate |
| Aβ | Amyloid β-protein |
| AD | Alzheimer’s disease |
| ALS | Amyotrophic lateral sclerosis |
| α-syn | Alpha-synuclein |
| CNS | Central nervous system |
| DPGM | Phosphoglycerate mutase |
| EPO | Erythropoietin |
| EPOR | Erythropoietin receptor |
| EPC | Erythroid precursor cell |
| Hb | Hemoglobin |
| Hbα/β | Hemoglobin alpha/beta subunit |
| HIF | Hypoxia-inducible factor |
| HIF-α/β | Hypoxia-inducible factor subunit α/β |
| HRE | Hypoxia response element |
| HS | Hemorrhagic stroke |
| H2O2 | Hydrogen peroxide |
| IS | Ischemic stroke |
| mDA neurons | Midbrain dopaminergic neurons |
| NDDs | Neurodegenerative diseases |
| Ngb | Neuroglobin |
| NO | Nitric oxide |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| TBI | Traumatic brain injury |
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2024: Summary of Results (UN DESA/POP/2024/TR/NO.9); UN: Geneva, Switzerland, 2024. [Google Scholar]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Neurodegeneration: What is it and where are we? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.Y.; Qu, Y.; Yang, Y.Q.; Liu, J.C.; Zhang, Y.F.; Zhou, S.Y.; He, Q.Y.; Jin, H.; Yang, Y.; Guo, Z.N. Cellular senescence: A novel therapeutic target for central nervous system diseases. Biomed. Pharmacother. 2024, 179, 117311. [Google Scholar] [CrossRef]
- Barbieri, M.; Chiodini, P.; Di Gennaro, P.; Hafez, G.; Liabeuf, S.; Malyszko, J.; Mani, L.Y.; Mattace-Raso, F.; Pepin, M.; Perico, N.; et al. Efficacy of erythropoietin as a neuroprotective agent in CKD-associated cognitive dysfunction: A literature systematic review. Pharmacol. Res. 2024, 203, 107146. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and brain aging: Neurodegeneration or neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Tang, F.; Pratap, U.P.; Sareddy, G.R.; Dhandapani, K.M.; Capuano, A.; Arvanitakis, Z.; Vadlamudi, R.K.; Brann, D.W. Regulation and Role of Neuron-Derived Hemoglobin in the Mouse Hippocampus. Int. J. Mol. Sci. 2022, 23, 5360. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Huang, Z.T.; Yuan, M.H.; Jing, F.; Cai, R.L.; Zou, Q.; Pu, Y.S.; Wang, S.Y.; Chen, F.; Yi, W.M.; et al. Role of Hypoxia Inducible Factor-1α in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 80, 949–961. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Savyuk, M.O.; Ponimaskin, E.; Vedunova, M.V. Hypoxia-Inducible Factor (HIF) in Ischemic Stroke and Neurodegenerative Disease. Front. Cell Dev. Biol. 2021, 9, 703084. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Syed, M.M.K.; Lashuel, H.A.; Millet, G.P. Hypoxia Conditioning as a Promising Therapeutic Target in Parkinson’s Disease? Mov. Disord. 2021, 36, 857–861. [Google Scholar] [CrossRef]
- Schechter, A.N.; Gladwin, M.T. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N. Engl. J. Med. 2003, 348, 1483–1485. [Google Scholar] [CrossRef]
- Tezel, T.H.; Geng, L.; Lato, E.B.; Schaal, S.; Liu, Y.; Dean, D.; Klein, J.B.; Kaplan, H.J. Synthesis and secretion of hemoglobin by retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1911–1919. [Google Scholar] [CrossRef]
- Saha, D.; Koli, S.; Patgaonkar, M.; Reddy, K.V. Expression of hemoglobin-α and β subunits in human vaginal epithelial cells and their functional significance. PLoS ONE 2017, 12, e0171084. [Google Scholar] [CrossRef]
- Setton-Avruj, C.P.; Musolino, P.L.; Salis, C.; Alló, M.; Bizzozero, O.; Villar, M.J.; Soto, E.F.; Pasquini, J.M. Presence of alpha-globin mRNA and migration of bone marrow cells after sciatic nerve injury suggests their participation in the degeneration/regeneration process. Exp. Neurol. 2007, 203, 568–578. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.S.; Baker, R.D.; Nowak, N.J.; Zhu, L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS ONE 2011, 6, e24363. [Google Scholar] [CrossRef]
- Nishi, H.; Inagi, R.; Kato, H.; Tanemoto, M.; Kojima, I.; Son, D.; Fujita, T.; Nangaku, M. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. J. Am. Soc. Nephrol. 2008, 19, 1500–1508. [Google Scholar] [CrossRef]
- Biagioli, M.; Pinto, M.; Cesselli, D.; Zaninello, M.; Lazarevic, D.; Roncaglia, P.; Simone, R.; Vlachouli, C.; Plessy, C.; Bertin, N.; et al. Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15454–15459. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, J.; Li, S.; Liu, C.; Liu, Y.; Chen, J.; Liu, N.; Liu, S.; Huang, H. Sex difference in human diseases: Mechanistic insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 238. [Google Scholar] [CrossRef]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Secondo, A.; Bagetta, G.; Amantea, D. On the Role of Store-Operated Calcium Entry in Acute and Chronic Neurodegenerative Diseases. Front. Mol. Neurosci. 2018, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Xu, Y.; Li, Y.; Feng, H.; Chen, Y. Targeting brain-peripheral immune responses for secondary brain injury after ischemic and hemorrhagic stroke. J. Neuroinflamm. 2024, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- van Hameren, G.; Aboghazleh, R.; Parker, E.; Dreier, J.P.; Kaufer, D.; Friedman, A. From spreading depolarization to blood-brain barrier dysfunction: Navigating traumatic brain injury for novel diagnosis and therapy. Nat. Rev. Neurol. 2024, 20, 408–425. [Google Scholar] [CrossRef]
- Akbar, M.; Toppo, P.; Nazir, A. Ageing, proteostasis, and the gut: Insights into neurological health and disease. Ageing Res. Rev. 2024, 101, 102504. [Google Scholar] [CrossRef]
- Ilieva, H.; Vullaganti, M.; Kwan, J. Advances in molecular pathology, diagnosis, and treatment of amyotrophic lateral sclerosis. BMJ 2023, 383, e075037. [Google Scholar] [CrossRef]
- Tziortzouda, P.; Van Den Bosch, L.; Hirth, F. Triad of TDP43 control in neurodegeneration: Autoregulation, localization and aggregation. Nat. Rev. Neurosci. 2021, 22, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; ElHafeez, S.A.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef]
- Al-Chalabi, A.; Hardiman, O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013, 9, 617–628. [Google Scholar] [CrossRef]
- Chiò, A.; Mora, G.; Moglia, C.; Manera, U.; Canosa, A.; Cammarosano, S.; Ilardi, A.; Bertuzzo, D.; Bersano, E.; Cugnasco, P.; et al. Secular Trends of Amyotrophic Lateral Sclerosis: The Piemonte and Valle d’Aosta Register. JAMA Neurol. 2017, 74, 1097–1104. [Google Scholar] [CrossRef]
- Qin, C.; Yang, S.; Chu, Y.H.; Zhang, H.; Pang, X.W.; Chen, L.; Zhou, L.Q.; Chen, M.; Tian, D.S.; Wang, W. Signaling pathways involved in ischemic stroke: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 215. [Google Scholar] [CrossRef]
- Seike, N.; Yokoseki, A.; Takeuchi, R.; Saito, K.; Miyahara, H.; Miyashita, A.; Ikeda, T.; Aida, I.; Nakajima, T.; Kanazawa, M.; et al. Genetic Variations and Neuropathologic Features of Patients with PRKN Mutations. Mov. Disord. 2021, 36, 1634–1643. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, Y.; Wang, J.Z. Updates in Alzheimer’s disease: From basic research to diagnosis and therapies. Transl. Neurodegener. 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Zheng, R.; Yan, Y.; Pu, J.; Zhang, B. Physiological and Pathological Functions of Neuronal Hemoglobin: A Key Underappreciated Protein in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 9088. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A.F.; Gorr, T.A. A globin in every cell? Proc. Natl. Acad. Sci. USA 2006, 103, 2469–2470. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.E.; Vinogradov, S.N. Nonvertebrate hemoglobins: Functions and molecular adaptations. Physiol. Rev. 2001, 81, 569–628. [Google Scholar] [CrossRef]
- Scholander, P.F. Oxygen transport through hemoglobin solutions. Science 1960, 131, 585–590. [Google Scholar] [CrossRef]
- Reeder, B.J. The redox activity of hemoglobins: From physiologic functions to pathologic mechanisms. Antioxid. Redox Signal 2010, 13, 1087–1123. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Crawford, J.H.; Patel, R.P. The biochemistry of nitric oxide, nitrite, and hemoglobin: Role in blood flow regulation. Free Radic. Biol. Med. 2004, 36, 707–717. [Google Scholar] [CrossRef]
- Codrich, M.; Bertuzzi, M.; Russo, R.; Francescatto, M.; Espinoza, S.; Zentilin, L.; Giacca, M.; Cesselli, D.; Beltrami, A.P.; Ascenzi, P.; et al. Neuronal hemoglobin affects dopaminergic cells’ response to stress. Cell Death Dis. 2017, 8, e2538. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.K.; Alkhayer, K.; Shelestak, J.; Clements, R.; Freeman, E.; McDonough, J. Erythropoietin Upregulates Brain Hemoglobin Expression and Supports Neuronal Mitochondrial Activity. Mol. Neurobiol. 2018, 55, 8051–8058. [Google Scholar] [CrossRef]
- He, Y.; Hua, Y.; Lee, J.Y.; Liu, W.; Keep, R.F.; Wang, M.M.; Xi, G. Brain alpha- and beta-globin expression after intracerebral hemorrhage. Transl. Stroke Res. 2010, 1, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Orre, M.; Kamphuis, W.; Osborn, L.M.; Melief, J.; Kooijman, L.; Huitinga, I.; Klooster, J.; Bossers, K.; Hol, E.M. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol. Aging 2014, 35, 1–14. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Straub, A.C. The emerging roles of somatic globins in cardiovascular redox biology and beyond. Redox Biol. 2013, 1, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.D.; Hamza, I. Mitochondrial heme: An exit strategy at last. J. Clin. Investig. 2012, 122, 4328–4330. [Google Scholar] [CrossRef]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function, and assembly of heme centers in mitochondrial respiratory complexes. Biochim. Biophys. Acta 2012, 1823, 1604–1616. [Google Scholar] [CrossRef]
- Shephard, F.; Greville-Heygate, O.; Marsh, O.; Anderson, S.; Chakrabarti, L. A mitochondrial location for haemoglobins--dynamic distribution in ageing and Parkinson’s disease. Mitochondrion 2014, 14, 64–72. [Google Scholar] [CrossRef]
- Schelshorn, D.W.; Schneider, A.; Kuschinsky, W.; Weber, D.; Krüger, C.; Dittgen, T.; Bürgers, H.F.; Sabouri, F.; Gassler, N.; Bach, A.; et al. Expression of hemoglobin in rodent neurons. J. Cereb. Blood Flow Metab. 2009, 29, 585–595. [Google Scholar] [CrossRef]
- Brown, N.; Alkhayer, K.; Clements, R.; Singhal, N.; Gregory, R.; Azzam, S.; Li, S.; Freeman, E.; McDonough, J. Neuronal Hemoglobin Expression and Its Relevance to Multiple Sclerosis Neuropathology. J. Mol. Neurosci. 2016, 59, 1–17. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change with Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, W.W.; Speck, E.; Leonard, V.G. Variation of the hemoglobin level with age and sex. Blood 1954, 9, 999–1007. [Google Scholar] [CrossRef]
- Shah, R.C.; Schneider, J.A.; Leurgans, S.; Bennett, D.A. Association of lower hemoglobin level and neuropathology in community-dwelling older persons. J. Alzheimers Dis. 2012, 32, 579–586. [Google Scholar] [CrossRef]
- Deng, Q.; Zhou, X.; Chen, J.; Pan, M.; Gao, H.; Zhou, J.; Wang, D.; Chen, Q.; Zhang, X.; Wang, Q.; et al. Lower hemoglobin levels in patients with parkinson’s disease are associated with disease severity and iron metabolism. Brain Res. 2017, 1655, 145–151. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Li, X.; Yu, S. Hemoglobin-α-synuclein complex exhibited age-dependent alterations in the human striatum and peripheral RBCs. Neurosci. Lett. 2020, 736, 135274. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; Li, X.; Li, X.; Yu, S. Neuronal hemoglobin in mitochondria is reduced by forming a complex with α-synuclein in aging monkey brains. Oncotarget 2016, 7, 7441–7454. [Google Scholar] [CrossRef] [PubMed]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef]
- Nyakas, C.; Buwalda, B.; Luiten, P.G. Hypoxia and brain development. Prog. Neurobiol. 1996, 49, 1–51. [Google Scholar] [CrossRef]
- Negrete, B., Jr.; Ackerly, K.L.; Dichiera, A.M.; Esbaugh, A.J. Respiratory plasticity improves aerobic performance in hypoxia in a marine teleost. Sci. Total Environ. 2022, 849, 157880. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, B.; Katyal, G.; Taylor, C.; Dowle, A.; Papetti, C.; Lucassen, M.; Moisoi, N.; Chakrabarti, L. Mitochondrial Haemoglobin Is Upregulated with Hypoxia in Skeletal Muscle and Has a Conserved Interaction with ATP Synthase and Inhibitory Factor 1. Cells 2023, 12, 912. [Google Scholar] [CrossRef]
- Emara, M.; Turner, A.R.; Allalunis-Turner, J. Hypoxia differentially upregulates the expression of embryonic, fetal and adult hemoglobin in human glioblastoma cells. Int. J. Oncol. 2014, 44, 950–958. [Google Scholar] [CrossRef]
- Tezel, G.; Yang, X.; Luo, C.; Cai, J.; Kain, A.D.; Powell, D.W.; Kuehn, M.H.; Pierce, W.M. Hemoglobin expression and regulation in glaucoma: Insights into retinal ganglion cell oxygenation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Bautista, N.M. Altitude acclimatization, hemoglobin-oxygen affinity, and circulatory oxygen transport in hypoxia. Mol. Aspects Med. 2022, 84, 101052. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Scott, G.R. Life Ascending: Mechanism and Process in Physiological Adaptation to High-Altitude Hypoxia. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 503–526. [Google Scholar] [CrossRef]
- Storz, J.F.; Scott, G.R.; Cheviron, Z.A. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 2010, 213, 4125–4136. [Google Scholar] [CrossRef]
- Frisancho, A.R. Functional adaptation to high altitude hypoxia. Science 1975, 187, 313–319. [Google Scholar] [CrossRef]
- Lenfant, C.; Torrance, J.D.; Reynafarje, C. Shift of the O2-Hb dissociation curve at altitude: Mechanism and effect. J. Appl. Physiol. 1971, 30, 625–631. [Google Scholar] [CrossRef]
- Bellelli, A.; Tame, J.R.H. Hemoglobin allostery and pharmacology. Mol. Aspects Med. 2022, 84, 101037. [Google Scholar] [CrossRef] [PubMed]
- Simonson, T.S.; Wei, G.; Wagner, H.E.; Wuren, T.; Qin, G.; Yan, M.; Wagner, P.D.; Ge, R.L. Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J. Physiol. 2015, 593, 3207–3218. [Google Scholar] [CrossRef]
- Signore, A.V.; Yang, Y.Z.; Yang, Q.Y.; Qin, G.; Moriyama, H.; Ge, R.L.; Storz, J.F. Adaptive Changes in Hemoglobin Function in High-Altitude Tibetan Canids Were Derived via Gene Conversion and Introgression. Mol. Biol. Evol. 2019, 36, 2227–2237. [Google Scholar] [CrossRef]
- Signore, A.V.; Storz, J.F. Biochemical pedomorphosis and genetic assimilation in the hypoxia adaptation of Tibetan antelope. Sci. Adv. 2020, 6, eabb5447. [Google Scholar] [CrossRef]
- Wearing, O.H.; Ivy, C.M.; Gutiérrez-Pinto, N.; Velotta, J.P.; Campbell-Staton, S.C.; Natarajan, C.; Cheviron, Z.A.; Storz, J.F.; Scott, G.R. The adaptive benefit of evolved increases in hemoglobin-O2 affinity is contingent on tissue O2 diffusing capacity in high-altitude deer mice. BMC Biol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.S.; Otecko, N.O.; Wang, W.; Shi, P.; Wu, D.D.; Zhang, Y.P. Hypoxia potentially promotes Tibetan longevity. Cell Res. 2017, 27, 302–305. [Google Scholar] [CrossRef]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Prabhakar, N.R.; Semenza, G.L. Systems biology of oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1382. [Google Scholar] [CrossRef]
- Ferrer, I.; Gómez, A.; Carmona, M.; Huesa, G.; Porta, S.; Riera-Codina, M.; Biagioli, M.; Gustincich, S.; Aso, E. Neuronal hemoglobin is reduced in Alzheimer’s disease, argyrophilic grain disease, Parkinson’s disease, and dementia with Lewy bodies. J. Alzheimers Dis. 2011, 23, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Altinoz, M.A.; Guloksuz, S.; Schmidt-Kastner, R.; Kenis, G.; Ince, B.; Rutten, B.P.F. Involvement of hemoglobins in the pathophysiology of Alzheimer’s disease. Exp. Gerontol. 2019, 126, 110680. [Google Scholar] [CrossRef]
- Lenart, J. Mitochondria in brain hypoxia. Postepy Hig. Med. Dosw. 2017, 71, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Peers, C.; Pearson, H.A.; Boyle, J.P. Hypoxia and Alzheimer’s disease. Essays Biochem. 2007, 43, 153–164. [Google Scholar] [CrossRef]
- Peers, C.; Dallas, M.L.; Boycott, H.E.; Scragg, J.L.; Pearson, H.A.; Boyle, J.P. Hypoxia and neurodegeneration. Ann. N. Y. Acad. Sci. 2009, 1177, 169–177. [Google Scholar] [CrossRef]
- Quan, H.; Koltai, E.; Suzuki, K.; Aguiar, A.S., Jr.; Pinho, R.; Boldogh, I.; Berkes, I.; Radak, Z. Exercise, redox system and neurodegenerative diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165778. [Google Scholar] [CrossRef]
- Auvinen, J.; Tapio, J.; Karhunen, V.; Kettunen, J.; Serpi, R.; Dimova, E.Y.; Gill, D.; Soininen, P.; Tammelin, T.; Mykkänen, J.; et al. Systematic evaluation of the association between hemoglobin levels and metabolic profile implicates beneficial effects of hypoxia. Sci. Adv. 2021, 7, eabi4822. [Google Scholar] [CrossRef]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef]
- Duan, H.; Geng, X.; Ding, Y. Hepatic responses following acute ischemic stroke: A clinical research update. Brain Circ. 2023, 9, 57–60. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Yao, Y.; Chen, L.; Liu, G.; Zhang, R.; Liu, Q.; Shi, F.D.; Hao, J. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. Faseb. J. 2016, 30, 3388–3399. [Google Scholar] [CrossRef] [PubMed]
- Eren, F.; Yilmaz, S.E. Neuroprotective approach in acute ischemic stroke: A systematic review of clinical and experimental studies. Brain Circ. 2022, 8, 172–179. [Google Scholar] [CrossRef]
- Sperring, C.P.; Savage, W.M.; Argenziano, M.G.; Leifer, V.P.; Alexander, J.; Echlov, N.; Spinazzi, E.F.; Connolly, E.S., Jr. No-Reflow Post-Recanalization in Acute Ischemic Stroke: Mechanisms, Measurements, and Molecular Markers. Stroke 2023, 54, 2472–2480. [Google Scholar] [CrossRef]
- Zhao, M.; Qiao, Y.; Weiss, A.; Zhao, W. Neuroprotective strategies in acute ischemic stroke: A narrative review of recent advances and clinical outcomes. Brain Circ. 2024, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kellert, L.; Martin, E.; Sykora, M.; Bauer, H.; Gussmann, P.; Diedler, J.; Herweh, C.; Ringleb, P.A.; Hacke, W.; Steiner, T.; et al. Cerebral oxygen transport failure?: Decreasing hemoglobin and hematocrit levels after ischemic stroke predict poor outcome and mortality: STroke: RelevAnt Impact of hemoGlobin, Hematocrit and Transfusion (STRAIGHT)—An observational study. Stroke 2011, 42, 2832–2837. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; del Zoppo, G.; Alberts, M.J.; Bhatt, D.L.; Brass, L.; Furlan, A.; Grubb, R.L.; Higashida, R.T.; Jauch, E.C.; Kidwell, C.; et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007, 38, 1655–1711. [Google Scholar] [CrossRef]
- He, Y.; Hua, Y.; Liu, W.; Hu, H.; Keep, R.F.; Xi, G. Effects of cerebral ischemia on neuronal hemoglobin. J. Cereb. Blood Flow Metab. 2009, 29, 596–605. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.N.; Nguyen, T.N.; Chen, H.S. Time from Onset to Remote Ischemic Conditioning and Clinical Outcome After Acute Moderate Ischemic Stroke. Ann. Neurol. 2023, 94, 561–571. [Google Scholar] [CrossRef]
- Caughey, M.C.; Loehr, L.R.; Key, N.S.; Derebail, V.K.; Gottesman, R.F.; Kshirsagar, A.V.; Grove, M.L.; Heiss, G. Sickle cell trait and incident ischemic stroke in the Atherosclerosis Risk in Communities study. Stroke 2014, 45, 2863–2867. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Yu, J.; Chen, Y.; Luo, L.; He, F.; Wei, R.; Yuan, H.; Ji, R.; Chen, H.; et al. Low Hemoglobin Levels at Admission Are Independently Associated with Cognitive Impairment after Ischemic Stroke: A Multicenter, Population-Based Study. Transl. Stroke Res. 2020, 11, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Lee, J.S.; Kim, B.J.; Kim, J.T.; Lee, J.; Cha, J.K.; Kim, D.H.; Cho, Y.J.; Hong, K.S.; Lee, S.J.; et al. Influence of Hemoglobin Concentration on Stroke Recurrence and Composite Vascular Events. Stroke 2020, 51, 1309–1312. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Walser, M.; Svensson, J.; Karlsson, L.; Motalleb, R.; Åberg, M.; Kuhn, H.G.; Isgaard, J.; Åberg, N.D. Growth Hormone and Neuronal Hemoglobin in the Brain-Roles in Neuroprotection and Neurodegenerative Diseases. Front. Endocrinol. 2020, 11, 606089. [Google Scholar] [CrossRef] [PubMed]
- Galea, I.; Durnford, A.; Glazier, J.; Mitchell, S.; Kohli, S.; Foulkes, L.; Norman, J.; Darekar, A.; Love, S.; Bulters, D.O.; et al. Iron Deposition in the Brain After Aneurysmal Subarachnoid Hemorrhage. Stroke 2022, 53, 1633–1642. [Google Scholar] [CrossRef]
- Liu, R.; Cao, S.; Hua, Y.; Keep, R.F.; Huang, Y.; Xi, G. CD163 Expression in Neurons After Experimental Intracerebral Hemorrhage. Stroke 2017, 48, 1369–1375. [Google Scholar] [CrossRef]
- Wang, X.; Mori, T.; Sumii, T.; Lo, E.H. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: Caspase activation and oxidative stress. Stroke 2002, 33, 1882–1888. [Google Scholar] [CrossRef]
- Masel, B.E.; DeWitt, D.S. Traumatic brain injury: A disease process, not an event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef]
- Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004, 115, 4–18. [Google Scholar] [CrossRef]
- Robicsek, S.A.; Bhattacharya, A.; Rabai, F.; Shukla, K.; Doré, S. Blood-Related Toxicity after Traumatic Brain Injury: Potential Targets for Neuroprotection. Mol. Neurobiol. 2020, 57, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.F.; Loftspring, M.; Wurster, W.L.; Beiler, S.; Beiler, C.; Wagner, K.R.; Pyne-Geithman, G.J. Bilirubin oxidation products, oxidative stress, and intracerebral hemorrhage. Acta Neurochir. Suppl. 2008, 105, 7–12. [Google Scholar] [CrossRef]
- Qu, Y.; Chen-Roetling, J.; Benvenisti-Zarom, L.; Regan, R.F. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J. Neurosurg. 2007, 106, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Talving, P.; Lustenberger, T.; Inaba, K.; Lam, L.; Mohseni, S.; Chan, L.; Demetriades, D. Erythropoiesis-stimulating agent administration and survival after severe traumatic brain injury: A prospective study. Arch. Surg. 2012, 147, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Nirula, R.; Diaz-Arrastia, R.; Brasel, K.; Weigelt, J.A.; Waxman, K. Safety and efficacy of erythropoietin in traumatic brain injury patients: A pilot randomized trial. Crit. Care Res. Pract. 2010, 2010, 209848. [Google Scholar] [CrossRef]
- Robertson, C.S.; Hannay, H.J.; Yamal, J.M.; Gopinath, S.; Goodman, J.C.; Tilley, B.C.; Baldwin, A.; Rivera Lara, L.; Saucedo-Crespo, H.; Ahmed, O.; et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA 2014, 312, 36–47. [Google Scholar] [CrossRef]
- Napolitano, L.M.; Fabian, T.C.; Kelly, K.M.; Bailey, J.A.; Block, E.F.; Langholff, W.; Enny, C.; Corwin, H.L. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J. Trauma 2008, 65, 285–297. [Google Scholar] [CrossRef]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Skogar, Ö.; Nilsson, M.; Lökk, J. Gender differences in diagnostic tools, medication, time to medication, and nonmotor symptoms in Parkinsonian patients. Brain Circ. 2022, 8, 192–199. [Google Scholar] [CrossRef]
- Halliday, G.; Lees, A.; Stern, M. Milestones in Parkinson’s disease—Clinical and pathologic features. Mov. Disord. 2011, 26, 1015–1021. [Google Scholar] [CrossRef]
- Guo, M.; Ji, X.; Liu, J. Hypoxia and Alpha-Synuclein: Inextricable Link Underlying the Pathologic Progression of Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 919343. [Google Scholar] [CrossRef]
- Guo, M.; Liu, W.; Luo, H.; Shao, Q.; Li, Y.; Gu, Y.; Guan, Y.; Ma, W.; Chen, M.; Yang, H.; et al. Hypoxic stress accelerates the propagation of pathological alpha-synuclein and degeneration of dopaminergic neurons. CNS Neurosci. Ther. 2023, 29, 544–558. [Google Scholar] [CrossRef]
- Freed, J.; Chakrabarti, L. Defining a role for hemoglobin in Parkinson’s disease. NPJ Parkinsons Dis. 2016, 2, 16021. [Google Scholar] [CrossRef] [PubMed]
- Garritsen, O.; van Battum, E.Y.; Grossouw, L.M.; Pasterkamp, R.J. Development, wiring and function of dopamine neuron subtypes. Nat. Rev. Neurosci. 2023, 24, 134–152. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Yehuda, S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989, 50, 607–615. [Google Scholar] [CrossRef]
- Berg, D.; Hochstrasser, H. Iron metabolism in Parkinsonian syndromes. Mov. Disord. 2006, 21, 1299–1310. [Google Scholar] [CrossRef]
- Santulli, C.; Bon, C.; De Cecco, E.; Codrich, M.; Narkiewicz, J.; Parisse, P.; Perissinotto, F.; Santoro, C.; Persichetti, F.; Legname, G.; et al. Neuronal haemoglobin induces loss of dopaminergic neurons in mouse Substantia nigra, cognitive deficits and cleavage of endogenous α-synuclein. Cell Death Dis. 2022, 13, 1048. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Saito, Y.; Kawashima, A.; Ruberu, N.N.; Fujiwara, H.; Koyama, S.; Sawabe, M.; Arai, T.; Nagura, H.; Yamanouchi, H.; Hasegawa, M.; et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J. Neuropathol. Exp. Neurol. 2003, 62, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Tarutani, A.; Arai, T.; Murayama, S.; Hisanaga, S.I.; Hasegawa, M. Potent prion-like behaviors of pathogenic α-synuclein and evaluation of inactivation methods. Acta Neuropathol. Commun. 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Killinger, B.A.; Marshall, L.L.; Chatterjee, D.; Chu, Y.; Bras, J.; Guerreiro, R.; Kordower, J.H. In situ proximity labeling identifies Lewy pathology molecular interactions in the human brain. Proc. Natl. Acad. Sci. USA 2022, 119, e2114405119. [Google Scholar] [CrossRef]
- Savica, R.; Grossardt, B.R.; Carlin, J.M.; Icen, M.; Bower, J.H.; Ahlskog, J.E.; Maraganore, D.M.; Steensma, D.P.; Rocca, W.A. Anemia or low hemoglobin levels preceding Parkinson disease: A case-control study. Neurology 2009, 73, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Huang, Y.H.; Liu, H.Y.; Chiou, H.Y.; Chan, L.; Chien, L.N. Newly Diagnosed Anemia Increases Risk of Parkinson’s disease: A Population-Based Cohort Study. Sci. Rep. 2016, 6, 29651. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Mangal, R.; Ding, Y. Mini review: Prospective therapeutic targets of Alzheimer’s disease. Brain Circ. 2022, 8, 1–5. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef]
- Vickers, J.C.; Mitew, S.; Woodhouse, A.; Fernandez-Martos, C.M.; Kirkcaldie, M.T.; Canty, A.J.; McCormack, G.H.; King, A.E. Defining the earliest pathological changes of Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 281–287. [Google Scholar] [CrossRef]
- Qiang, Y.X.; Deng, Y.T.; Zhang, Y.R.; Wang, H.F.; Zhang, W.; Dong, Q.; Feng, J.F.; Cheng, W.; Yu, J.T. Associations of blood cell indices and anemia with risk of incident dementia: A prospective cohort study of 313,448 participants. Alzheimers Dement. 2023, 19, 3965–3976. [Google Scholar] [CrossRef]
- Faux, N.G.; Rembach, A.; Wiley, J.; Ellis, K.A.; Ames, D.; Fowler, C.J.; Martins, R.N.; Pertile, K.K.; Rumble, R.L.; Trounson, B.; et al. An anemia of Alzheimer’s disease. Mol. Psychiatry 2014, 19, 1227–1234. [Google Scholar] [CrossRef]
- Wu, C.W.; Liao, P.C.; Yu, L.; Wang, S.T.; Chen, S.T.; Wu, C.M.; Kuo, Y.M. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol. Dis. 2004, 17, 367–377. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Lee, C.W.; Shih, Y.H.; Yang, T.; Yu, L.; Kuo, Y.M. Interactions between amyloid-β and hemoglobin: Implications for amyloid plaque formation in Alzheimer’s disease. PLoS ONE 2012, 7, e33120. [Google Scholar] [CrossRef] [PubMed]
- Raymackers, J.; Daniels, A.; De Brabandere, V.; Missiaen, C.; Dauwe, M.; Verhaert, P.; Vanmechelen, E.; Meheus, L. Identification of two-dimensionally separated human cerebrospinal fluid proteins by N-terminal sequencing, matrix-assisted laser desorption/ionization--mass spectrometry, nanoliquid chromatography-electrospray ionization-time of flight-mass spectrometry, and tandem mass spectrometry. Electrophoresis 2000, 21, 2266–2283. [Google Scholar] [CrossRef]
- Shah, R.C.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Hemoglobin level in older persons and incident Alzheimer disease: Prospective cohort analysis. Neurology 2011, 77, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Falvey, C.; Harris, T.B.; Simonsick, E.M.; Satterfield, S.; Ferrucci, L.; Metti, A.L.; Patel, K.V.; Yaffe, K. Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology 2013, 81, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zinman, L.; Cudkowicz, M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol. 2011, 10, 481–490. [Google Scholar] [CrossRef]
- Sebastià, J.; Kieran, D.; Breen, B.; King, M.A.; Netteland, D.F.; Joyce, D.; Fitzpatrick, S.F.; Taylor, C.T.; Prehn, J.H. Angiogenin protects motoneurons against hypoxic injury. Cell Death Differ. 2009, 16, 1238–1247. [Google Scholar] [CrossRef]
- Sun, J.; Carrero, J.J.; Zagai, U.; Evans, M.; Ingre, C.; Pawitan, Y.; Fang, F. Blood biomarkers and prognosis of amyotrophic lateral sclerosis. Eur. J. Neurol. 2020, 27, 2125–2133. [Google Scholar] [CrossRef]
- Horng, L.Y.; Hsu, P.L.; Chen, L.W.; Tseng, W.Z.; Hsu, K.T.; Wu, C.L.; Wu, R.T. Activating mitochondrial function and haemoglobin expression with EH-201, an inducer of erythropoietin in neuronal cells, reverses memory impairment. Br. J. Pharmacol. 2015, 172, 4741–4756. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, A.A.; Kvist, S.V.; Brinkman, N.; Gentinetta, T.; Illa, M.; Ortenlöf, N.; Holmqvist, B.; Ley, D.; Gram, M. Cell-free oxidized hemoglobin drives reactive oxygen species production and pro-inflammation in an immature primary rat mixed glial cell culture. J. Neuroinflamm. 2021, 18, 42. [Google Scholar] [CrossRef]
- Collister, D.; Komenda, P.; Hiebert, B.; Gunasekara, R.; Xu, Y.; Eng, F.; Lerner, B.; Macdonald, K.; Rigatto, C.; Tangri, N. The Effect of Erythropoietin-Stimulating Agents on Health-Related Quality of Life in Anemia of Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 472–478. [Google Scholar] [CrossRef]
- Eaton, W.A. Impact of hemoglobin biophysical studies on molecular pathogenesis and drug therapy for sickle cell disease. Mol. Aspects Med. 2022, 84, 100971. [Google Scholar] [CrossRef]
- Bauer, D.E.; Kamran, S.C.; Orkin, S.H. Reawakening fetal hemoglobin: Prospects for new therapies for the β-globin disorders. Blood 2012, 120, 2945–2953. [Google Scholar] [CrossRef]
- Erbayraktar, S.; Grasso, G.; Sfacteria, A.; Xie, Q.W.; Coleman, T.; Kreilgaard, M.; Torup, L.; Sager, T.; Erbayraktar, Z.; Gokmen, N.; et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 6741–6746. [Google Scholar] [CrossRef]
- Simon, F.; Floros, N.; Ibing, W.; Schelzig, H.; Knapsis, A. Neurotherapeutic potential of erythropoietin after ischemic injury of the central nervous system. Neural Regen. Res. 2019, 14, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, Y.; Jin, F.; Xu, Z.; Gu, Y.; Guo, M.; Shao, Q.; Liu, Y.; Luo, H.; Wang, Y.; et al. Brain-derived exosomal hemoglobin transfer contributes to neuronal mitochondrial homeostasis under hypoxia. Elife 2025, 13, RP99986. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qiu, Y.; Yang, Q.; Tang, S.; Gong, J.; Fan, H.; Wu, Y.; Lu, X. Repetitive transcranial magnetic stimulation combined with cognitive training for cognitive function and activities of daily living in patients with post-stroke cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 87, 101919. [Google Scholar] [CrossRef]
- Aleyasin, H.; Rousseaux, M.W.; Phillips, M.; Kim, R.H.; Bland, R.J.; Callaghan, S.; Slack, R.S.; During, M.J.; Mak, T.W.; Park, D.S. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA 2007, 104, 18748–18753. [Google Scholar] [CrossRef]
- Lohmann, S.; Grigoletto, J.; Bernis, M.E.; Pesch, V.; Ma, L.; Reithofer, S.; Tamgüney, G. Ischemic stroke causes Parkinson’s disease-like pathology and symptoms in transgenic mice overexpressing alpha-synuclein. Acta Neuropathol. Commun. 2022, 10, 26. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Peel, A.; Mao, X.O.; Xie, L.; Greenberg, D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 3497–3500. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Aslani, S.; Barreto, G.E.; Báez-Jurado, E.; Kiaie, N.; Jamialahmadi, T.; Sahebkar, A. The potential of mitochondrial modulation by neuroglobin in treatment of neurological disorders. Free Radic. Biol. Med. 2021, 162, 471–477. [Google Scholar] [CrossRef]
- Jin, K.; Mao, Y.; Mao, X.; Xie, L.; Greenberg, D.A. Neuroglobin expression in ischemic stroke. Stroke 2010, 41, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chang, M.; Wang, J.; Liu, Y. Neuroglobin functions as a prognostic marker and promotes the tumor growth of glioma via suppressing apoptosis. Biomed. Pharmacother. 2017, 88, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Jiang, H.F.; Li, L.; Lai, X.J.; Liu, Q.R.; Yu, S.B.; Yi, C.L.; Chen, X.Q. Neuroglobin Facilitates Neuronal Oxygenation through Tropic Migration under Hypoxia or Anemia in Rat: How Does the Brain Breathe? Neurosci. Bull. 2023, 39, 1481–1496. [Google Scholar] [CrossRef]
- Shephard, F.; Greville-Heygate, O.; Liddell, S.; Emes, R.; Chakrabarti, L. Analysis of Mitochondrial haemoglobin in Parkinson’s disease brain. Mitochondrion 2016, 29, 45–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Jin, F.; Geng, Z.; Xu, Z.; Shao, Q.; Liu, G.; Ji, X.; Liu, J. Unraveling the Mystery of Hemoglobin in Hypoxia-Accelerated Neurodegenerative Diseases. Biomolecules 2025, 15, 1221. https://doi.org/10.3390/biom15091221
Tian Z, Jin F, Geng Z, Xu Z, Shao Q, Liu G, Ji X, Liu J. Unraveling the Mystery of Hemoglobin in Hypoxia-Accelerated Neurodegenerative Diseases. Biomolecules. 2025; 15(9):1221. https://doi.org/10.3390/biom15091221
Chicago/Turabian StyleTian, Zhengming, Feiyang Jin, Zhuowen Geng, Zirui Xu, Qianqian Shao, Guiyou Liu, Xunming Ji, and Jia Liu. 2025. "Unraveling the Mystery of Hemoglobin in Hypoxia-Accelerated Neurodegenerative Diseases" Biomolecules 15, no. 9: 1221. https://doi.org/10.3390/biom15091221
APA StyleTian, Z., Jin, F., Geng, Z., Xu, Z., Shao, Q., Liu, G., Ji, X., & Liu, J. (2025). Unraveling the Mystery of Hemoglobin in Hypoxia-Accelerated Neurodegenerative Diseases. Biomolecules, 15(9), 1221. https://doi.org/10.3390/biom15091221








