The Upregulation of L1CAM by SVHRSP Mitigates Neuron Damage, Spontaneous Seizures, and Cognitive Dysfunction in a Kainic Acid-Induced Rat Model of Epilepsy
Abstract
1. Introduction
2. Material and Methods
2.1. Peptide Synthesis
2.2. Animals
2.3. Electrode Implantation and EEG Recording
2.4. KA Model of Epilepsy
2.5. Open-Field Test (OFT)
2.6. Object Recognition Test (ORT)
2.7. T-Maze Spontaneous Alternation Test
2.8. Object Location Test (OLT)
2.9. RNA Sequencing, Differential Gene Analysis, and Pathway Enrichment
2.10. Immunohistochemistry
2.11. Western Blot Analysis
2.12. Immunofluorescence Staining
2.13. Cell Culture and Kainic Acid Treatment
2.14. Statistical Analysis
3. Results
3.1. SVHRSP Reduces Spontaneous Seizure Activity in Epileptic Rats
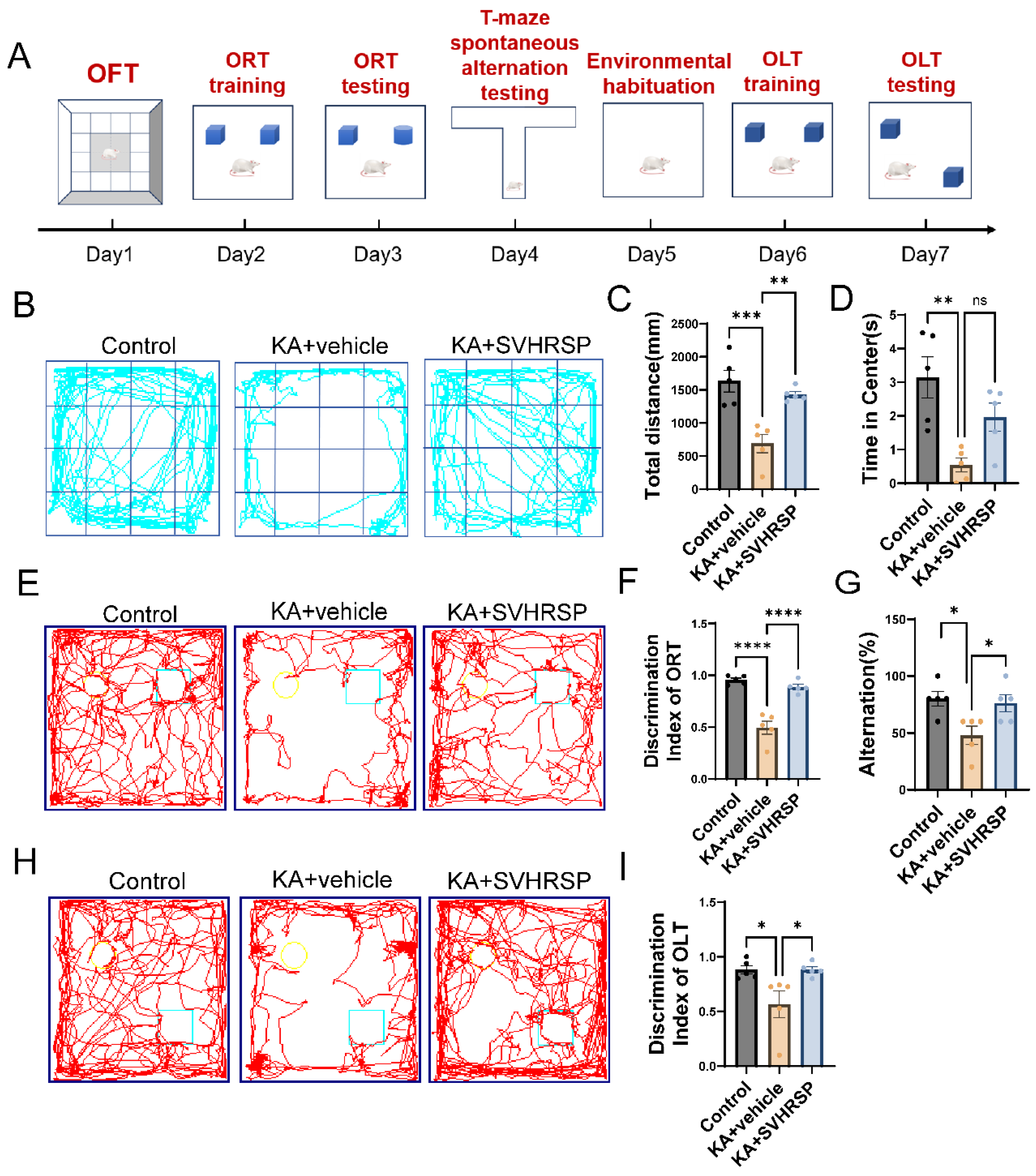
3.2. SVHRSP Alleviates Anxiety-like Behaviors and Cognitive Impairments in Epileptic Rats
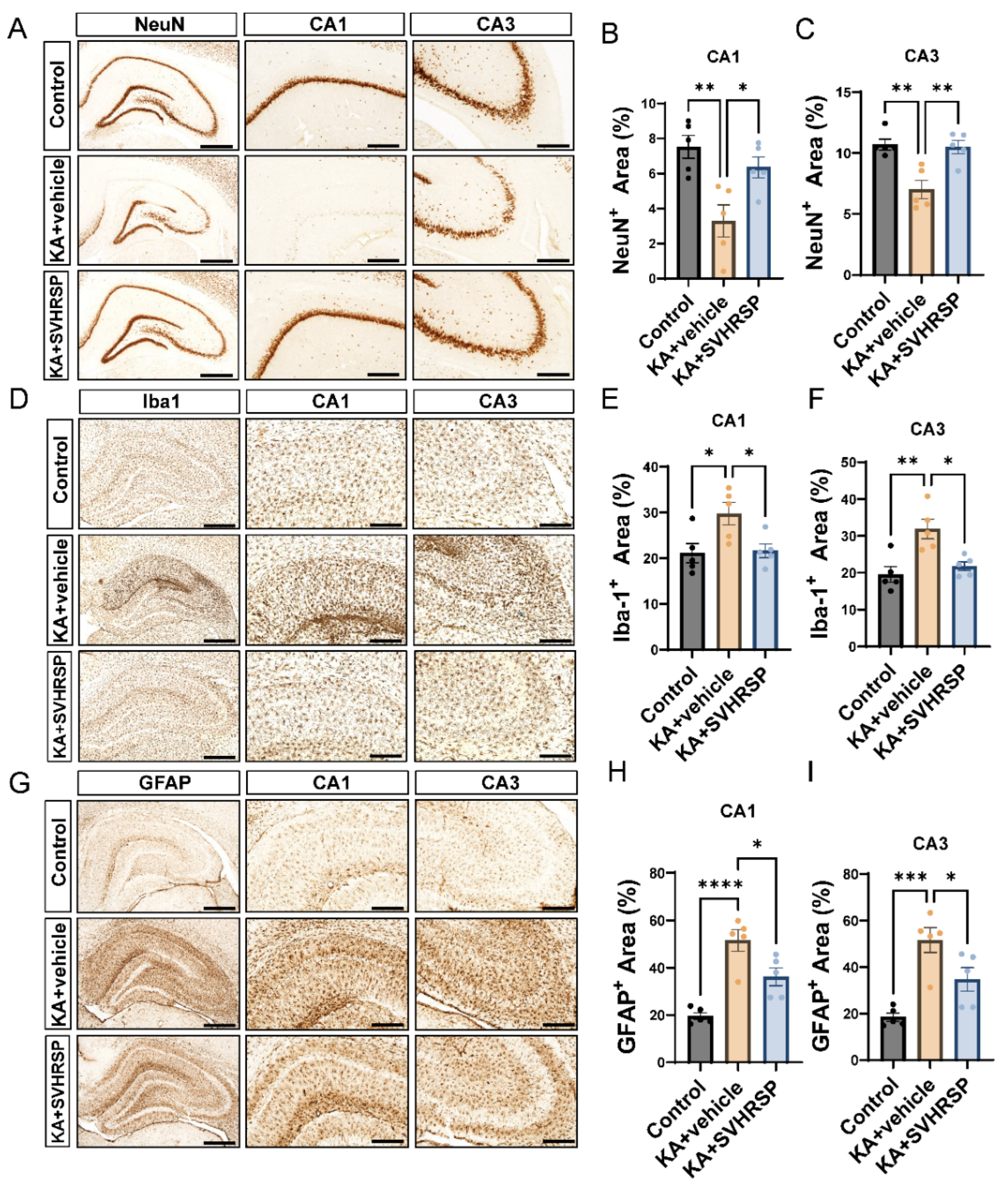
3.3. SVHRSP Alleviates KA-Induced Hippocampal Sclerosis in Epileptic Rats
3.4. Transcriptomic Analysis Reveals That SVHRSP Enhances Neuroplasticity-Related Pathways Including Cell Adhesion Markers and Axon Guidance Cues
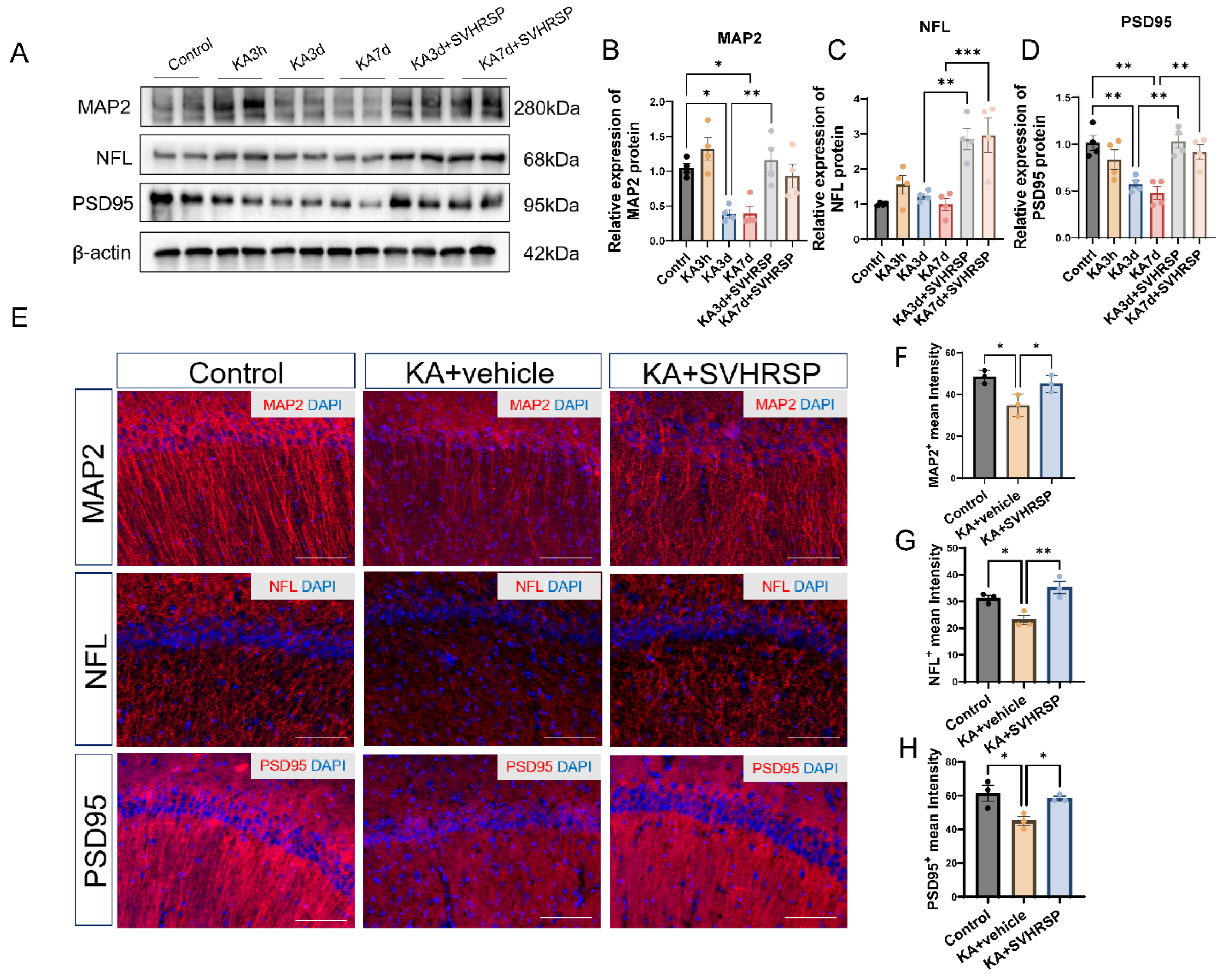
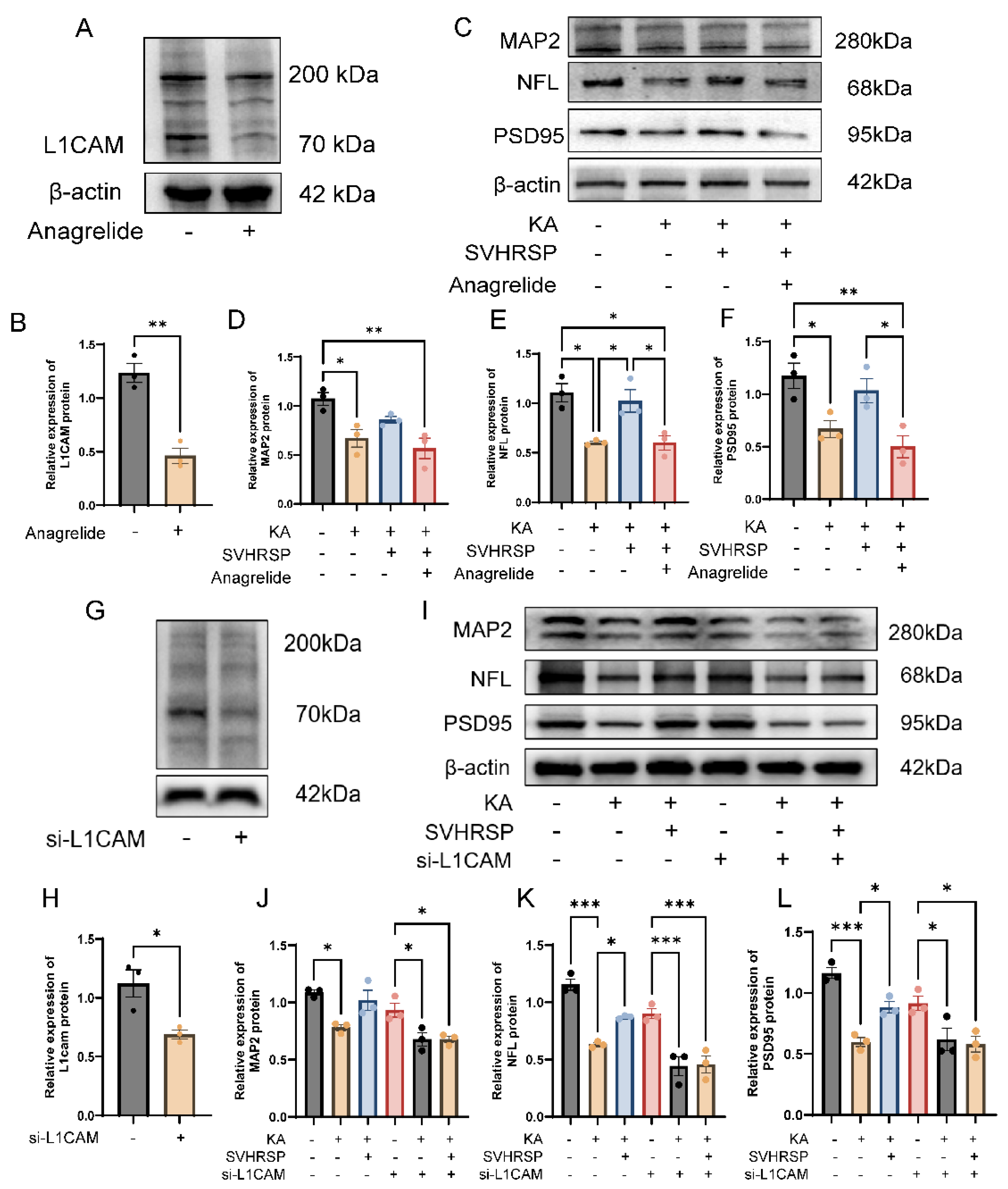
3.5. SVHRSP Attenuates Abnormalities of Neuronal Markers in Epileptic Rats
3.6. SVHRSP Increases the Expression of a L1CAM Fragment in Epileptic Rats
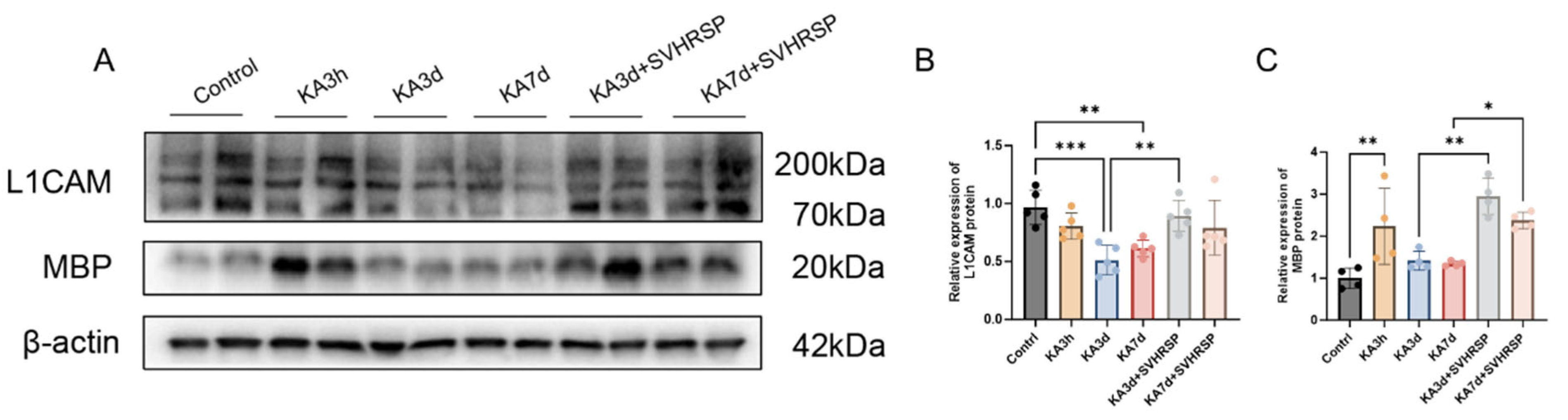
3.7. L1CAM Mediates the Protective Effects of SVHRSP Against Damage of Cultured Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AED | Anti-epileptic Drug |
| ANOVA | Analysis of Variance |
| BSA | Bovine Serum Albumin |
| CAMs | Cell Adhesion Molecules |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DBP | Disulfide-Bridged Peptides |
| DEG | Differentially Expressed Gene |
| DI | Discrimination Index |
| EEG | Electroencephalogram |
| FPKM | Fragments Per Kilobase of Transcript Per Million Mapped Reads |
| GFAP | Glial Fibrillary Acidic Protein |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| HRP | Horseradish Peroxidase |
| KA | Kainic Acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| L1-70 | 70 kDa Functional Fragment of L1CAM |
| L1CAM | L1 Cell Adhesion Molecule |
| MAP2 | Microtubule-Associated Protein 2 |
| MBP | Myelin Basic Protein |
| NDBP | Non-Disulfide-Bridged Peptides |
| NFL | Neurofilament Light Chain |
| OLT | Object Location Test |
| OFT | Open-Field Test |
| ORT | Object Recognition Test |
| PBS | Phosphate-Buffered Saline |
| PSD95 | Postsynaptic Density Protein 95 |
| qPCR | Quantitative Polymerase Chain Reaction |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| SE | Status Epilepticus |
| SEM | Standard Error of the Mean |
| SVHRSP | Scorpion Venom Heat-Resistant Synthetic Peptide |
| SRS | Spontaneous Recurrent Seizures |
| TBST | Tris-Buffered Saline with Tween 20 |
| TLE | Temporal Lobe Epilepsy |
References
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef]
- Blumcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Bartolomei, F.; Bernasconi, A.; Bernasconi, N.; Bien, C.G.; Cendes, F.; Coras, R.; et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2013, 54, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Eid, T.; Lothman, E.W.; Kohler, C.; Schwarcz, R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J. Neurosci. 1995, 15, 6301–6313. [Google Scholar] [CrossRef]
- de Lanerolle, N.C.; Kim, J.H.; Williamson, A.; Spencer, S.S.; Zaveri, H.P.; Eid, T.; Spencer, D.D. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: Evidence for distinctive patient subcategories. Epilepsia 2003, 44, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Cid, E.; Marquez-Galera, A.; Valero, M.; Gal, B.; Medeiros, D.C.; Navarron, C.M.; Ballesteros-Esteban, L.; Reig-Viader, R.; Morales, A.V.; Fernandez-Lamo, I.; et al. Sublayer- and cell-type-specific neurodegenerative transcriptional trajectories in hippocampal sclerosis. Cell Rep. 2021, 35, 109229. [Google Scholar] [CrossRef]
- Perucca, E.; Brodie, M.J.; Kwan, P.; Tomson, T. 30 years of second-generation antiseizure medications: Impact and future perspectives. Lancet Neurol. 2020, 19, 544–556. [Google Scholar] [CrossRef]
- Carvill, G.L.; Dulla, C.G.; Lowenstein, D.H.; Brooks-Kayal, A.R. The path from scientific discovery to cures for epilepsy. Neuropharmacology 2020, 167, 107702. [Google Scholar] [CrossRef]
- Schaefer, N.; Rotermund, C.; Blumrich, E.M.; Lourenco, M.V.; Joshi, P.; Hegemann, R.U.; Jamwal, S.; Ali, N.; Garcia Romero, E.M.; Sharma, S.; et al. The malleable brain: Plasticity of neural circuits and behavior—A review from students to students. J. Neurochem. 2017, 142, 790–811. [Google Scholar] [CrossRef]
- Yamagata, M.; Sanes, J.R.; Weiner, J.A. Synaptic adhesion molecules. Curr. Opin. Cell Biol. 2003, 15, 621–632. [Google Scholar] [CrossRef]
- Duncan, B.W.; Murphy, K.E.; Maness, P.F. Molecular Mechanisms of L1 and NCAM Adhesion Molecules in Synaptic Pruning, Plasticity, and Stabilization. Front. Cell Dev. Biol. 2021, 9, 625340. [Google Scholar]
- Colombo, F.; Meldolesi, J. L1-CAM and N-CAM: From Adhesion Proteins to Pharmacological Targets. Trends Pharmacol. Sci. 2015, 36, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, I.I.; Lutz, D. Functional Diversity of Neuronal Cell Adhesion and Recognition Molecule L1CAM through Proteolytic Cleavage. Cells 2022, 11, 3085. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Miyai, K.; Ninomiya, A.; Yamasaki, H.; Tamura, H.; Nakamura, Y.; Shiosaka, S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J. Neurosci. 2003, 23, 7727–7736. [Google Scholar] [CrossRef] [PubMed]
- Patzke, C.; Acuna, C.; Giam, L.R.; Wernig, M.; Sudhof, T.C. Conditional deletion of L1CAM in human neurons impairs both axonal and dendritic arborization and action potential generation. J. Exp. Med. 2016, 213, 499–515. [Google Scholar] [CrossRef]
- Lin, J.; Chooi, W.H.; Ong, W.; Zhang, N.; Bechler, M.E.; Ffrench-Constant, C.; Chew, S.Y. Oriented and sustained protein expression on biomimicking electrospun fibers for evaluating functionality of cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111407. [Google Scholar] [CrossRef]
- Tai, Y.; Gallo, N.B.; Wang, M.; Yu, J.R.; Van Aelst, L. Axo-axonic Innervation of Neocortical Pyramidal Neurons by GABAergic Chandelier Cells Requires AnkyrinG-Associated L1CAM. Neuron 2019, 102, 358–372 e9. [Google Scholar] [CrossRef]
- Yan, Z.; Chu, L.; Jia, X.; Lin, L.; Cheng, S. Myelin basic protein enhances axonal regeneration from neural progenitor cells. Cell Biosci. 2021, 11, 80. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Wu, W.; Wang, Y. Peptides with therapeutic potential in the venom of the scorpion Buthus martensii Karsch. Peptides 2019, 115, 43–50. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Caliskan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef]
- Batool, S.; Noureen, N.; Kamal, M.A. Structure Activity Relationship of Venom Toxins Targeting Potassium Channels. Curr. Drug Metab. 2018, 19, 714–720. [Google Scholar] [CrossRef]
- Zeng, X.C.; Corzo, G.; Hahin, R. Scorpion venom peptides without disulfide bridges. IUBMB Life 2005, 57, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sui, A.R.; Piao, H.; Xiong, S.T.; Zhang, P.; Guo, S.Y.; Kong, Y.; Gao, C.Q.; Wang, Z.X.; Yang, J.; Ge, B.Y.; et al. Scorpion venom heat-resistant synthesized peptide ameliorates epileptic seizures and imparts neuroprotection in rats mediated by NMDA receptors. Eur. J. Pharmacol. 2024, 978, 176704. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Li, N.; Li, D.; Sui, A.; Khan, K.; Ge, B.; Li, S.; Li, S.; Zhao, J. Scorpion venom heat-resistant synthesized peptide ameliorates 6-OHDA-induced neurotoxicity and neuroinflammation: Likely role of Na(v) 1.6 inhibition in microglia. Br. J. Pharmacol. 2021, 178, 3553–3569. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- Sharma, S.; Puttachary, S.; Thippeswamy, A.; Kanthasamy, A.G.; Thippeswamy, T. Status Epilepticus: Behavioral and Electroencephalography Seizure Correlates in Kainate Experimental Models. Front. Neurol. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Hakataya, S.; Katsu, N.; Okanoya, K.; Toya, G. An exploratory study of behavioral traits and the establishment of social relationships in female laboratory rats. PLoS ONE 2023, 18, e0295280. [Google Scholar] [CrossRef]
- Deacon, R.M.; Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef]
- d’Isa, R.; Comi, G.; Leocani, L. Apparatus design and behavioural testing protocol for the evaluation of spatial working memory in mice through the spontaneous alternation T-maze. Sci. Rep. 2021, 11, 21177. [Google Scholar] [CrossRef]
- Nagaraj, V.; Mikhail, M.; Baronio, M.; Gatto, A.; Nayak, A.; Theis, T.; Cavallaro, U.; Schachner, M. Antagonistic L1 Adhesion Molecule Mimetic Compounds Inhibit Glioblastoma Cell Migration In Vitro. Biomolecules 2022, 12, 439. [Google Scholar] [CrossRef]
- Matsubara, T.; Ayuzawa, S.; Aoki, T.; Fujiomto, A.; Osuka, S.; Matsumura, A. The patient had a normal magnetic resonance imaging and temporal lobe epilepsy secondary to a porencephalic cyst but showed structural lesions (hippocampal sclerosis). Epilepsy Behav. Case Rep. 2013, 1, 153–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.R.; Cai, M. Antiseizure effects of Lilii Bulbus on pentylenetetrazol kindling-induced seizures in mice: Involvement of Reelin, Netrin-1, and semaphorin. Biomed. Pharmacother. 2024, 173, 116385. [Google Scholar] [CrossRef]
- Stoeckli, E.T. Understanding axon guidance: Are we nearly there yet? Development 2018, 145, dev151415. [Google Scholar] [CrossRef]
- Sahay, A.; Kim, C.H.; Sepkuty, J.P.; Cho, E.; Huganir, R.L.; Ginty, D.D.; Kolodkin, A.L. Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J. Neurosci. 2005, 25, 3613–3620. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, C.; Huang, H. The role of axon guidance molecules in the pathogenesis of epilepsy. Neural Regen. Res. 2025, 20, 1244–1257. [Google Scholar] [CrossRef]
- Li, Y.; Tong, F.; Zhang, Y.; Cai, Y.; Ding, J.; Wang, Q.; Wang, X. Neuropilin-2 Signaling Modulates Mossy Fiber Sprouting by Regulating Axon Collateral Formation Through CRMP2 in a Rat Model of Epilepsy. Mol. Neurobiol. 2022, 59, 6817–6833. [Google Scholar] [CrossRef]
- Gorlewicz, A.; Kaczmarek, L. Pathophysiology of Trans-Synaptic Adhesion Molecules: Implications for Epilepsy. Front. Cell Dev. Biol. 2018, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Lukawski, K.; Czuczwar, S.J. Understanding mechanisms of drug resistance in epilepsy and strategies for overcoming it. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Hartz, A.M.S.; Bauer, B. Drug-Resistant Epilepsy: Multiple Hypotheses, Few Answers. Front. Neurol. 2017, 8, 301. [Google Scholar] [CrossRef]
- Fang, M.; Wei, J.L.; Tang, B.; Liu, J.; Chen, L.; Tang, Z.H.; Luo, J.; Chen, G.J.; Wang, X.F. Neuroligin-1 Knockdown Suppresses Seizure Activity by Regulating Neuronal Hyperexcitability. Mol. Neurobiol. 2016, 53, 270–284. [Google Scholar] [CrossRef]
- Saglietti, L.; Dequidt, C.; Kamieniarz, K.; Rousset, M.C.; Valnegri, P.; Thoumine, O.; Beretta, F.; Fagni, L.; Choquet, D.; Sala, C.; et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron 2007, 54, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Contractor, A.; Rogers, C.; Maron, C.; Henkemeyer, M.; Swanson, G.T.; Heinemann, S.F. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 2002, 296, 1864–1869. [Google Scholar] [CrossRef]
- Dong, X.; Liu, O.W.; Howell, A.S.; Shen, K. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell 2013, 155, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.L.; Schachner, M. A fragment of cell adhesion molecule L1 reduces amyloid-beta plaques in a mouse model of Alzheimer’s disease. Cell Death Dis. 2022, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Kraus, K.; Kleene, R.; Braren, I.; Loers, G.; Lutz, D.; Schachner, M. A fragment of adhesion molecule L1 is imported into mitochondria, and regulates mitochondrial metabolism and trafficking. J. Cell Sci. 2018, 131, jcs210500. [Google Scholar] [CrossRef]
- Kakad, P.P.; Penserga, T.; Davis, B.P.; Henry, B.; Boerner, J.; Riso, A.; Pielage, J.; Godenschwege, T.A. An ankyrin-binding motif regulates nuclear levels of L1-type neuroglian and expression of the oncogene Myc in Drosophila neurons. J. Biol. Chem. 2018, 293, 17442–17453. [Google Scholar] [CrossRef]
- Demyanenko, G.P.; Mohan, V.; Zhang, X.; Brennaman, L.H.; Dharbal, K.E.; Tran, T.S.; Manis, P.B.; Maness, P.F. Neural cell adhesion molecule NrCAM regulates Semaphorin 3F-induced dendritic spine remodeling. J. Neurosci. 2014, 34, 11274–11287. [Google Scholar] [CrossRef]
- Singh, R.; Rai, S.; Bharti, P.S.; Zehra, S.; Gorai, P.K.; Modi, G.P.; Rani, N.; Dev, K.; Inampudi, K.K.; Y, V.V.; et al. Circulating small extracellular vesicles in Alzheimer’s disease: A case-control study of neuro-inflammation and synaptic dysfunction. BMC Med. 2024, 22, 254. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.G.; Kim, M.H.; Kwack, K.B.; Park, J.K.; Kim, T.; Kim, J.W.; Ban, J.Y.; Chung, J.H. Association between neuronal cell adhesion molecule (NRCAM) single nucleotide polymorphisms and schizophrenia in a Korean population. Psychiatry Clin. Neurosci. 2009, 63, 123–124. [Google Scholar] [CrossRef]
- Ayalew, M.; Le-Niculescu, H.; Levey, D.F.; Jain, N.; Changala, B.; Patel, S.D.; Winiger, E.; Breier, A.; Shekhar, A.; Amdur, R.; et al. Convergent functional genomics of schizophrenia: From comprehensive understanding to genetic risk prediction. Mol. Psychiatry 2012, 17, 887–905. [Google Scholar]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.P.; Parnell, E.; Penzes, P. Dendritic structural plasticity and neuropsychiatric disease. Nat. Rev. Neurosci. 2018, 19, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Lukawski, K.; Czuczwar, S.J. Oxidative Stress and Neurodegeneration in Animal Models of Seizures and Epilepsy. Antioxidants 2023, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimers Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef]
- Chand Dakal, T.; Choudhary, K.; Tiwari, I.; Yadav, V.; Kumar Maurya, P.; Kumar Sharma, N. Unraveling the Triad: Hypoxia, Oxidative Stress and Inflammation in Neurodegenerative Disorders. Neuroscience 2024, 552, 126–141. [Google Scholar] [CrossRef]
- Kushwah, N.; Woeppel, K.; Dhawan, V.; Shi, D.; Cui, X.T. Effects of neuronal cell adhesion molecule L1 and nanoparticle surface modification on microglia. Acta Biomater. 2022, 149, 273–286. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Ge, B.; Li, H.; Huang, C.; Ji, Y.; Schachner, M.; Yin, S.; Li, S.; Zhao, J. The Upregulation of L1CAM by SVHRSP Mitigates Neuron Damage, Spontaneous Seizures, and Cognitive Dysfunction in a Kainic Acid-Induced Rat Model of Epilepsy. Biomolecules 2025, 15, 1032. https://doi.org/10.3390/biom15071032
Li Z, Ge B, Li H, Huang C, Ji Y, Schachner M, Yin S, Li S, Zhao J. The Upregulation of L1CAM by SVHRSP Mitigates Neuron Damage, Spontaneous Seizures, and Cognitive Dysfunction in a Kainic Acid-Induced Rat Model of Epilepsy. Biomolecules. 2025; 15(7):1032. https://doi.org/10.3390/biom15071032
Chicago/Turabian StyleLi, Zhen, Biying Ge, Haoqi Li, Chunyao Huang, Yunhan Ji, Melitta Schachner, Shengming Yin, Sheng Li, and Jie Zhao. 2025. "The Upregulation of L1CAM by SVHRSP Mitigates Neuron Damage, Spontaneous Seizures, and Cognitive Dysfunction in a Kainic Acid-Induced Rat Model of Epilepsy" Biomolecules 15, no. 7: 1032. https://doi.org/10.3390/biom15071032
APA StyleLi, Z., Ge, B., Li, H., Huang, C., Ji, Y., Schachner, M., Yin, S., Li, S., & Zhao, J. (2025). The Upregulation of L1CAM by SVHRSP Mitigates Neuron Damage, Spontaneous Seizures, and Cognitive Dysfunction in a Kainic Acid-Induced Rat Model of Epilepsy. Biomolecules, 15(7), 1032. https://doi.org/10.3390/biom15071032





