Valproate Damaging Effect on Erythrocyte Metabolism as a Decisive Factor in the Development of Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.2.1. Experimental Design
2.2.2. Animals
2.2.3. Preparative and Analytical Methods
2.2.4. Statistical Analysis
3. Results
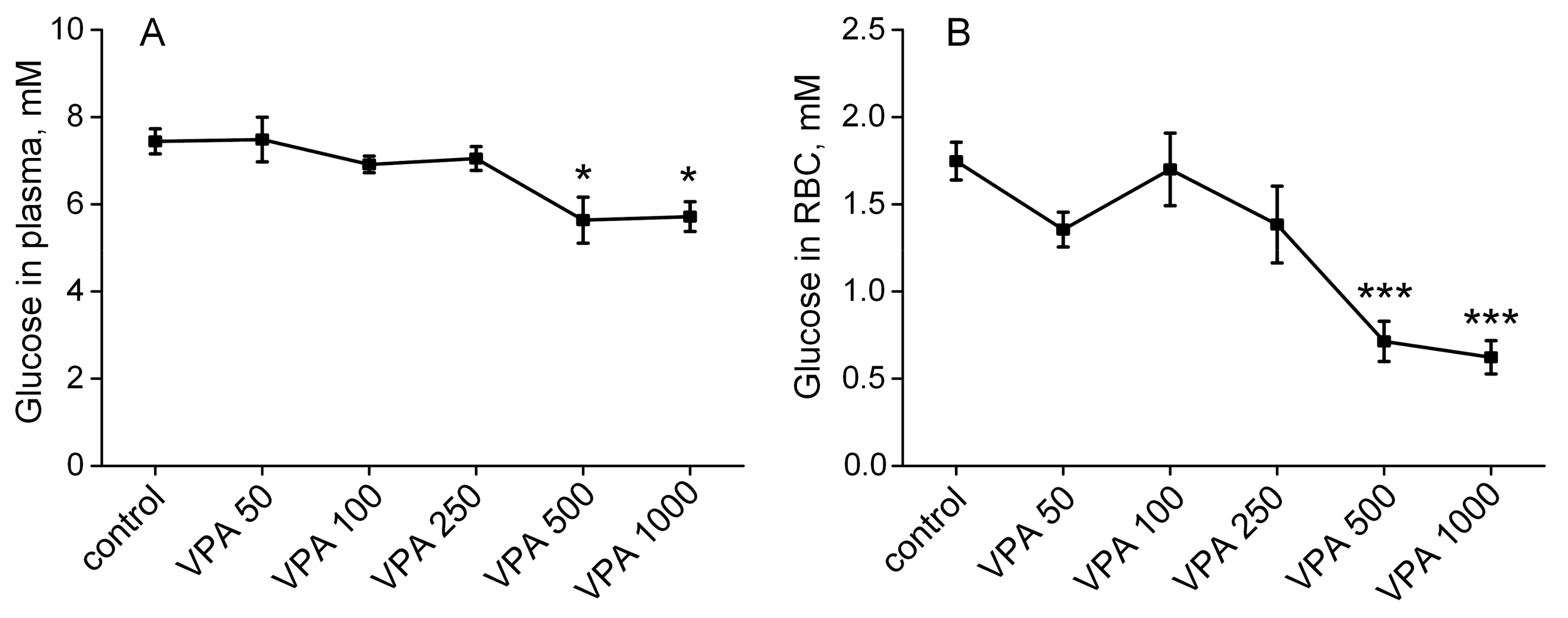
3.1. Effects of Different Doses of VPA on Glucose Concentration in Rat Plasma and Erythrocytes
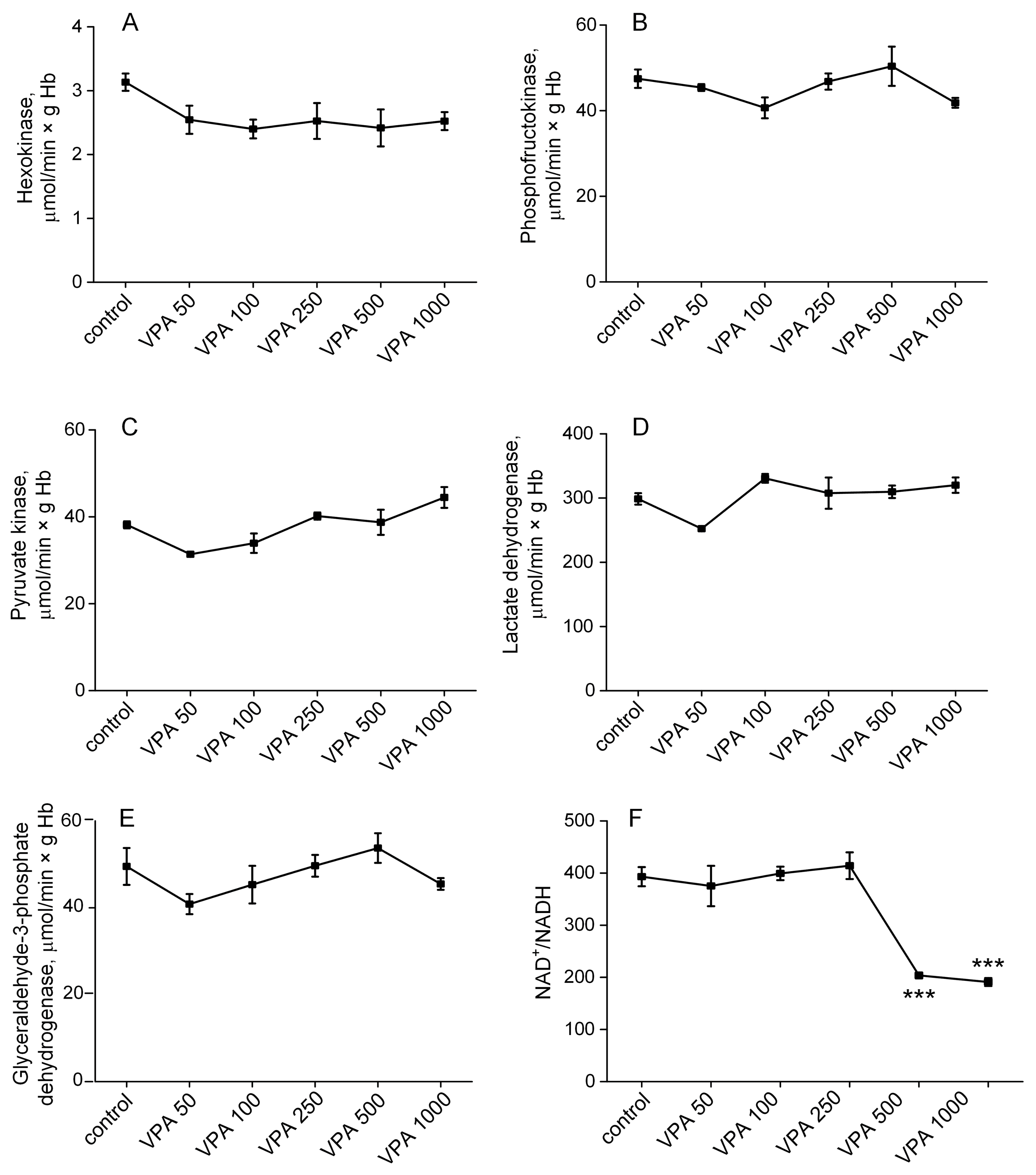
3.2. Effect of Different Doses of VPA on the Activity of the Regulatory Enzymes of Glycolysis in Rat Erythrocytes
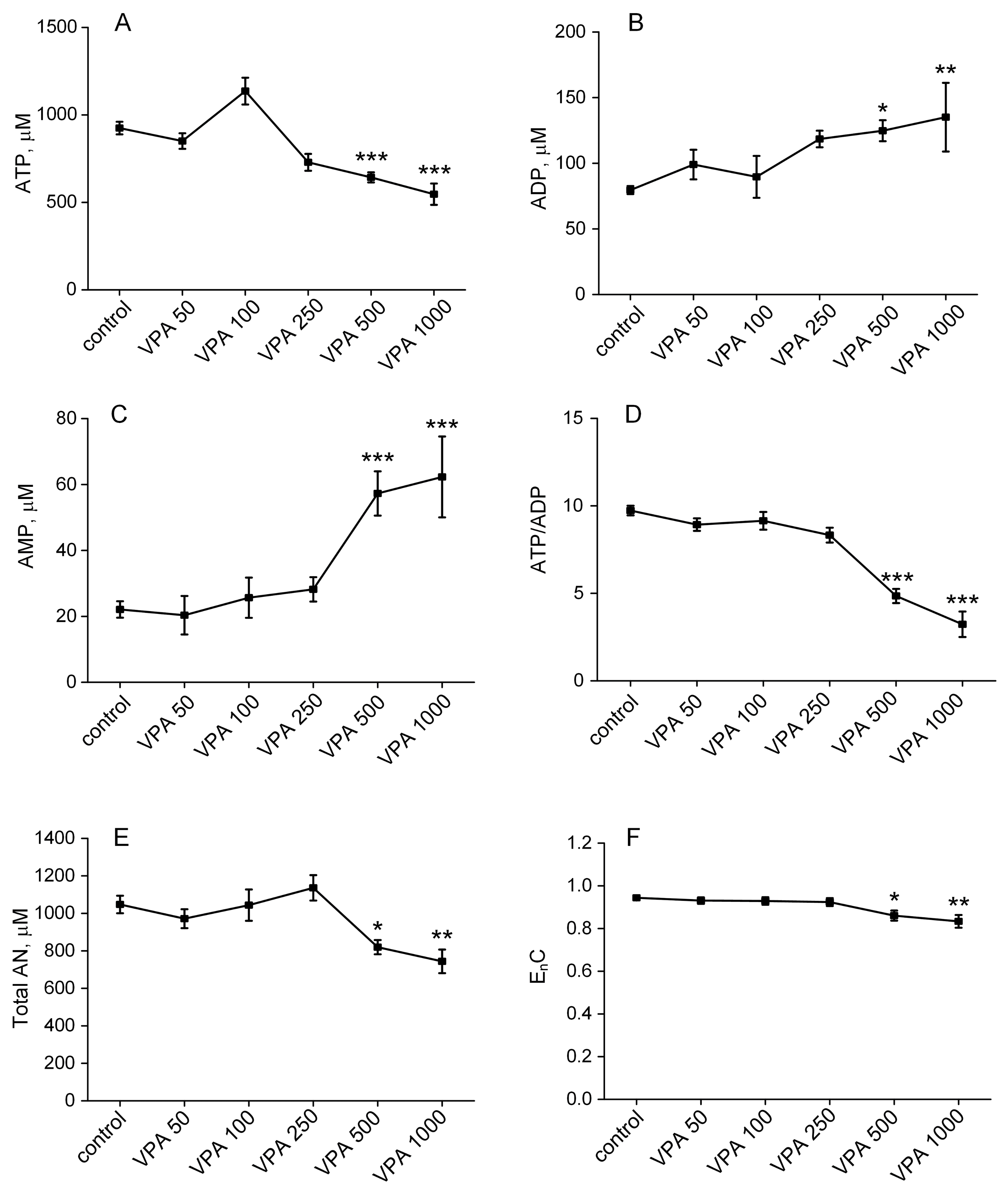
3.3. The Influence of Different Doses of VPA on the Content of Adenine Nucleotides in Rat Erythrocytes
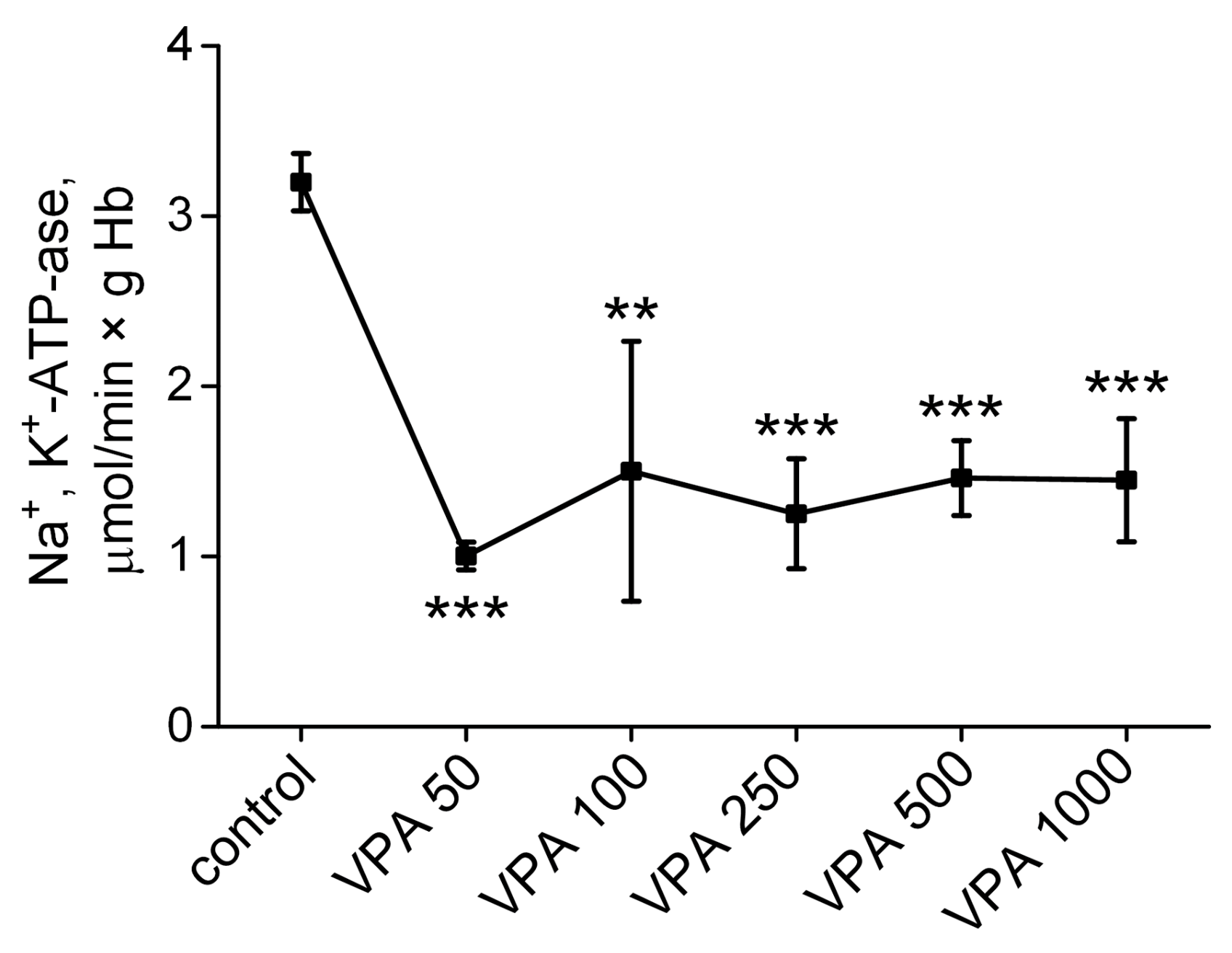
3.4. The Influence of Different Doses of VPA on the Activity of Na+, K+-ATPase in Rat Erythrocytes
3.5. Effect of VPA on the Activity of Antioxidant Defense Enzymes in Rat Erythrocytes
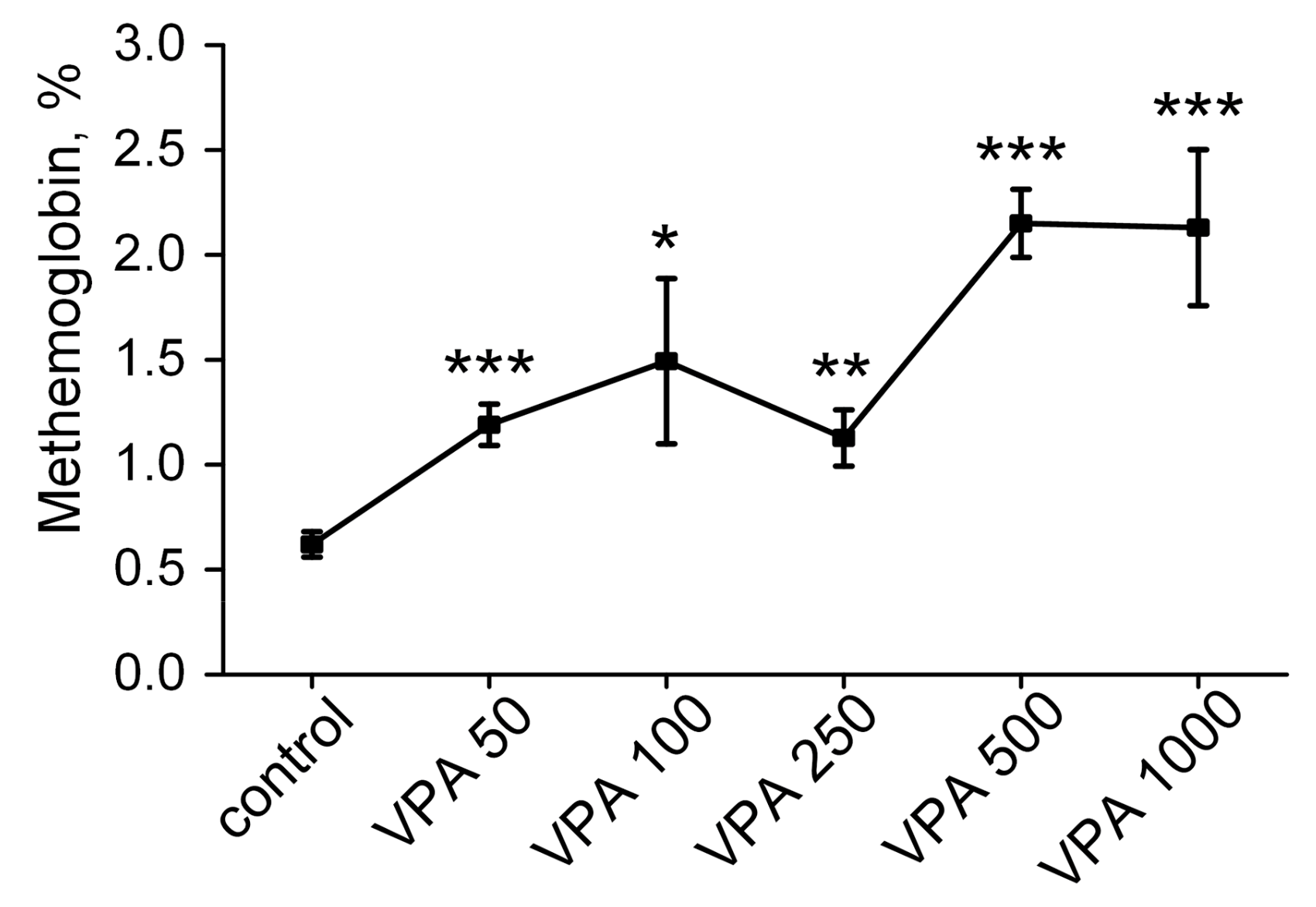
3.6. Methemoglobin Content in Rat Erythrocytes After Injection of VPA with Different Doses
3.7. The Effect of Different Doses of VPA on the Concentration of 2,3-DPG in Rat Erythrocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2,3-DPG | 2,3-diphosphoglycerate |
| AN | total adenine nucleotide content |
| CAT | catalase |
| CBF | cerebral blood flow |
| CPS-1 | carbamoylphosphate synthetase-1 |
| EnC | energy charge |
| G6PD | glucose-6-phosphate dehydrogenase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GP | glutathione peroxidase |
| GR | glutathione reductase |
| GT | glutathione transferase |
| HA | hyperammonemia |
| HK | hexokinase |
| LDH | lactate dehydrogenase |
| MetHb | methemoglobin |
| NTB | nitrotetrazolium blue dye |
| PK | pyruvate kinase |
| PFK | phosphofructokinase |
| SOD | superoxide dismutase |
| VIE | valproate-induced progressive encephalopathy |
| VPA | Valproic acid |
References
- Löscher, W. Basic Pharmacology of Valproate: A Review after 35 Years of Clinical Use for the Treatment of Epilepsy. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef] [PubMed]
- Keränen, T.; Sivenius, J. Side Effects of Carbamazepine, Valproate and Clonazepam during Long-Term Treatment of Epilepsy. Acta Neurol. Scand. Suppl. 1983, 97, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Mintzer, S.; Wheless, J.; Mattson, R.H. Adverse Effects of Antiepileptic Drugs: A Brief Overview of Important Issues. Expert. Rev. Neurother. 2010, 10, 885–891. [Google Scholar] [CrossRef]
- Powell-Jackson, P.R.; Tredger, J.M.; Williams, R. Hepatotoxicity to Sodium Valproate: A Review. Gut 1984, 25, 673–681. [Google Scholar] [CrossRef]
- Kennedy, G.M.; Lhatoo, S.D. CNS Adverse Events Associated with Antiepileptic Drugs. CNS Drugs 2008, 22, 739–760. [Google Scholar] [CrossRef]
- Nanau, R.M.; Neuman, M.G. Adverse Drug Reactions Induced by Valproic Acid. Clin. Biochem. 2013, 46, 1323–1338. [Google Scholar] [CrossRef]
- Pegg, E.J.; Zaman, F. Sodium Valproate-Related Hyperammonaemic Encephalopathy. BMJ Case Rep. 2014, 2014, bcr2014203899. [Google Scholar] [CrossRef]
- Chopra, A.; Kolla, B.P.; Mansukhani, M.P.; Netzel, P.; Frye, M.A. Valproate-Induced Hyperammonemic Encephalopathy: An Update on Risk Factors, Clinical Correlates and Management. Gen. Hosp. Psychiatry 2012, 34, 290–298. [Google Scholar] [CrossRef]
- Dealberto, M.-J.C.C. Valproate-Induced Hyperammonaemic Encephalopathy: Review of 14 Cases in the Psychiatric Setting. Int. Clin. Psychopharmacol. 2007, 22, 330–337. [Google Scholar] [CrossRef]
- König, S.A.; Siemes, H.; Bläker, F.; Boenigk, E.; Gross-Selbeck, G.; Hanefeld, F.; Haas, N.; Köhler, B.; Koelfen, W.; Korinthenberg, R. Severe Hepatotoxicity during Valproate Therapy: An Update and Report of Eight New Fatalities. Epilepsia 1994, 35, 1005–1015. [Google Scholar] [CrossRef]
- Duarte, J.; Macias, S.; Coria, F.; Fernandez, E.; Clavería, L.E. Valproate-Induced Coma: Case Report and Literature Review. Ann. Pharmacother. 1993, 27, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.D.; Oleinik, O.E.; Glauser, T.A.; Maertens, P.; Eder, D.N.; Pippenger, C.E. Altered Antioxidant Enzyme Activities in Children with a Serious Adverse Experience Related to Valproic Acid Therapy. Neuropediatrics 1998, 29, 195–201. [Google Scholar] [CrossRef]
- Coulter, D.L.; Allen, R.J. Secondary Hyperammonaemia: A Possible Mechanism for Valproate Encephalopathy. Lancet 1980, 1, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Sugawara, N.; Maruo, K.; Yasui-Furukori, N.; Shimoda, K. Blood Levels of Ammonia and Carnitine in Patients Treated with Valproic Acid: A Meta-Analysis. Clin. Psychopharmacol. Neurosci. 2022, 20, 536–547. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Mani, U. Valproic Acid Induced Liver Injury: An Insight into Molecular Toxicological Mechanism. Environ. Toxicol. Pharmacol. 2022, 95, 103967. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminski, Y.; Lopata, O.; Muravyov, N.; Felipo, V. Blocking NMDA Receptors Prevents the Oxidative Stress Induced by Acute Ammonia Intoxication. Free Radic. Biol. Med. 1999, 26, 1369–1374. [Google Scholar] [CrossRef]
- Bjerring, P.N.; Bjerrum, E.J.; Larsen, F.S. Impaired Cerebral Microcirculation Induced by Ammonium Chloride in Rats Is Due to Cortical Adenosine Release. J. Hepatol. 2018, 68, 1137–1143. [Google Scholar] [CrossRef]
- Cooper, A.J.; Plum, F. Biochemistry and Physiology of Brain Ammonia. Physiol. Rev. 1987, 67, 440–519. [Google Scholar] [CrossRef]
- Kosenko, E.; Felipo, V.; Montoliu, C.; Grisolía, S.; Kaminsky, Y. Effects of Acute Hyperammonemia in Vivo on Oxidative Metabolism in Nonsynaptic Rat Brain Mitochondria. Metab. Brain Dis. 1997, 12, 69–82. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.G.; Felipo, V.; Miñana, M.D.; Grisolía, S. Chronic Hyperammonemia Prevents Changes in Brain Energy and Ammonia Metabolites Induced by Acute Ammonium Intoxication. Biochim. Biophys. Acta 1993, 1180, 321–326. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Tikhonova, L.A.; Alilova, G.A.; Montoliu, C.; Barreto, G.E.; Aliev, G.; Kaminsky, Y.G. Portacaval Shunting Causes Differential Mitochondrial Superoxide Production in Brain Regions. Free Radic. Biol. Med. 2017, 113, 109–118. [Google Scholar] [CrossRef]
- Kosenko, E.; Kaminsky, Y.; Kaminsky, A.; Valencia, M.; Lee, L.; Hermenegildo, C.; Felipo, V. Superoxide Production and Antioxidant Enzymes in Ammonia Intoxication in Rats. Free Radic. Res. 1997, 27, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D. Oxidative and Nitrosative Stress in Ammonia Neurotoxicity. Hepatology 2003, 37, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, M.; Albrecht, J. Oxidative and Nitrosative Stress in Ammonia Neurotoxicity. Neurochem. Int. 2013, 62, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Tikhonova, L.; Alilova, G.; Montoliu, C. Is NMDA-Receptor-Mediated Oxidative Stress in Mitochondria of Peripheral Tissues the Essential Factor in the Pathogenesis of Hepatic Encephalopathy? J. Clin. Med. 2022, 11, 827. [Google Scholar] [CrossRef]
- Kosenko, E.; Llansola, M.; Montoliu, C.; Monfort, P.; Rodrigo, R.; Hernandez-Viadel, M.; Erceg, S.; Sánchez-Perez, A.M.; Felipo, V. Glutamine Synthetase Activity and Glutamine Content in Brain: Modulation by NMDA Receptors and Nitric Oxide. Neurochem. Int. 2003, 43, 493–499. [Google Scholar] [CrossRef]
- Monfort, P.; Kosenko, E.; Erceg, S.; Canales, J.-J.; Felipo, V. Molecular Mechanism of Acute Ammonia Toxicity: Role of NMDA Receptors. Neurochem. Int. 2002, 41, 95–102. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Giguère, J.F.; Michaud, J.; Lavoie, J.; Layrargues, G.P. Ammonia: Key Factor in the Pathogenesis of Hepatic Encephalopathy. Neurochem. Pathol. 1987, 6, 1–12. [Google Scholar] [CrossRef]
- Aires, C.C.P.; van Cruchten, A.; Ijlst, L.; de Almeida, I.T.; Duran, M.; Wanders, R.J.A.; Silva, M.F.B. New Insights on the Mechanisms of Valproate-Induced Hyperammonemia: Inhibition of Hepatic N-Acetylglutamate Synthase Activity by Valproyl-CoA. J. Hepatol. 2011, 55, 426–434. [Google Scholar] [CrossRef]
- Rumbach, L.; Mutet, C.; Cremel, G.; Marescaux, C.A.; Micheletti, G.; Warter, J.M.; Waksman, A. Effects of Sodium Valproate on Mitochondrial Membranes: Electron Paramagnetic Resonance and Transmembrane Protein Movement Studies. Mol. Pharmacol. 1986, 30, 270–273. [Google Scholar] [CrossRef]
- Rumbach, L.; Warter, J.M.; Rendon, A.; Marescaux, C.; Micheletti, G.; Waksman, A. Inhibition of Oxidative Phosphorylation in Hepatic and Cerebral Mitochondria of Sodium Valproate-Treated Rats. J. Neurol. Sci. 1983, 61, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.; Martin, A.; Ugarte, M.; Valdivieso, F. Inhibition by Valproic Acid of Pyruvate Uptake by Brain Mitochondria. Biochem. Pharmacol. 1982, 31, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, P.E.R.; Penaloza, A.; Zahir, S.; Gris, M. Science Review: Carnitine in the Treatment of Valproic Acid-Induced Toxicity—What Is the Evidence? Crit. Care 2005, 9, 431–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coude, F.X.; Rabier, D.; Cathelineau, L.; Grimber, G.; Parvy, P.; Kamoun, P.P. A Mechanism for Valproate-Induced Hyperammonemia. Pediatr. Res. 1981, 15, 974–975. [Google Scholar] [CrossRef]
- Qureshi, I.A.; Letarte, J.; Tuchweber, B.; Yousef, I.; Qureshi, S.R. Hepatotoxicity of Sodium Valproate in Ornithine Transcarbamylase-Deficient Mice. Toxicol. Lett. 1985, 25, 297–306. [Google Scholar] [CrossRef]
- Horiuchi, M.; Imamura, Y.; Nakamura, N.; Maruyama, I.; Saheki, T. Carbamoylphosphate Synthetase Deficiency in an Adult: Deterioration Due to Administration of Valproic Acid. J. Inherit. Metab. Dis. 1993, 16, 39–45. [Google Scholar] [CrossRef]
- Bachmann, C.; Colombo, J.P. Increased Tryptophan Uptake into the Brain in Hyperammonemia. Life Sci. 1983, 33, 2417–2424. [Google Scholar] [CrossRef]
- Call, G.; Seay, A.R.; Sherry, R.; Qureshi, I.A. Clinical Features of Carbamyl Phosphate Synthetase-I Deficiency in an Adult. Ann. Neurol. 1984, 16, 90–93. [Google Scholar] [CrossRef]
- Chou, H.-F.; Yang, R.-C.; Chen, C.Y.; Jong, Y.-J. Valproate-Induced Hyperammonemic Encephalopathy. Pediatr. Neonatol. 2008, 49, 201–204. [Google Scholar] [CrossRef]
- Dixit, S.; Namdeo, M.; Azad, S. Valproate Induced Delirium Due to Hyperammonemia in a Case of Acute Mania: A Diagnostic Dilemma. J. Clin. Diagn. Res. 2015, 9, VD01–VD02. [Google Scholar] [CrossRef]
- Surendran, I.; Sahoo, S.; Gupta, G.; Chauhan, N.; Grover, S. Valproate-Induced Hyperammonemic Encephalopathy in an Elderly Patient with Bipolar Disorder. J. Geriatr. Ment. Health 2016, 3, 172–175. [Google Scholar] [CrossRef]
- Zaret, B.S.; Beckner, R.R.; Marini, A.M.; Wagle, W.; Passarelli, C. Sodium Valproate-Induced Hyperammonemia without Clinical Hepatic Dysfunction. Neurology 1982, 32, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Sammar, A.; Tawfik, M.; Fatima, F.; Butler, A.; Aylor-Lee, K. Valproate-Induced Hyperammonemic Encephalopathy Causing New-Onset Seizures. Cureus 2023, 15, e47288. [Google Scholar] [CrossRef] [PubMed]
- Mehndiratta, M.M.; Mehndiratta, P.; Phul, P.; Garg, S. Valproate Induced Non Hepatic Hyperammonaemic Encephalopathy (VNHE)—A Study from Tertiary Care Referral University Hospital, North India. J. Pak. Med. Assoc. 2008, 58, 627–631. [Google Scholar]
- Farooq, O.; Zunga, P.M.; Dar, M.I.; Rather, A.Q.; Rashid, S.; Basu, J.; Dar, I.H.; Ashraf, M. Non-Hyperammonemic Valproate Encephalopathy. Ann. Neurosci. 2014, 21, 76–79. [Google Scholar] [CrossRef]
- Trojak, B.; de la Gastine, B.; Dollfus, S. Valproate-Induced Encephalopathy Related to Concurrent Antimanic Medications. J. Neuropsychiatry Clin. Neurosci. 2011, 23, E22–E23. [Google Scholar] [CrossRef]
- Guin, D.S.; Biswas, S.; Saha, K. A Case of Non-Hyperammonemic Valproate-Induced Encephalopathy. Neurol. Asia 2006, 11, 135–137. [Google Scholar]
- Agrawal, A.; Agrawal, R. Valproate-Induced Non-Hepatic Hyperammonaemic Encephalopathy: A Rare Complication of Chronic Valproate Therapy. Int. J. Clin. Pediatr. 2012, 1, 85–87. [Google Scholar] [CrossRef][Green Version]
- Khoo, C.L.; Naik, S.; Lua, R.; Chai, S.B.; Liew, A.; Sim, K. Valproate-Induced Hyperammonemia in Mental Retardation: A Case Report and Review of the Literature. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 561–562. [Google Scholar] [CrossRef]
- Lowenstein, J.M. Ammonia Production in Muscle and Other Tissues: The Purine Nucleotide Cycle. Physiol. Rev. 1972, 52, 382–414. [Google Scholar] [CrossRef]
- Cooper, A.J.L. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Cerebral Ammonia Homeostasis. Neurochem. Res. 2012, 37, 2439–2455. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.C.; Lemasters, J.J. The Mitochondrial Permeability Transition: A New Pathophysiological Mechanism for Reye’s Syndrome and Toxic Liver Injury. J. Pharmacol. Exp. Ther. 1996, 278, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Lemberg, A.; Fernández, M.A.; Coll, C.; Rosello, D.O.; Romay, S.; Perazzo, J.C.; Filinger, E.J. Reyes’s Syndrome, Encephalopathy, Hyperammonemia and Acetyl Salicylic Acid Ingestion in a City Hospital of Buenos Aires, Argentina. Curr. Drug Saf. 2009, 4, 17–21. [Google Scholar] [CrossRef]
- Rosier, F.; Lambert, D.; Mertens-Strijthagen, M. Effect of Glucose Deprivation on Rat Glutamine Synthetase in Cultured Astrocytes. Biochem. J. 1996, 315 Pt 2, 607–612. [Google Scholar] [CrossRef]
- Hoyer, S.; Oesterreich, K.; Wagner, O. Glucose Metabolism as the Site of the Primary Abnormality in Early-Onset Dementia of Alzheimer Type? J. Neurol. 1988, 235, 143–148. [Google Scholar] [CrossRef]
- Hoyer, S.; Nitsch, R.; Oesterreich, K. Ammonia Is Endogenously Generated in the Brain in the Presence of Presumed and Verified Dementia of Alzheimer Type. Neurosci. Lett. 1990, 117, 358–362. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Tikhonova, L.A.; Kaminsky, Y.G. Ammonia and Enzymes of Ammonia Metabolism in Different Brain Regions in Hyperammonemia. Neurochem. J. 2015, 9, 133–140. [Google Scholar] [CrossRef]
- Leiderman, D.B.; Balish, M.; Bromfield, E.B.; Theodore, W.H. Effect of Valproate on Human Cerebral Glucose Metabolism. Epilepsia 1991, 32, 417–422. [Google Scholar] [CrossRef]
- Alilova, G.; Tikhonova, L.; Montoliu, C.; Kosenko, E. Ammoniagenic Action of Valproate without Signs of Hepatic Dysfunction in Rats: Possible Causes and Supporting Evidence. Biomolecules 2024, 14, 370. [Google Scholar] [CrossRef]
- Blass, J.P. Glucose/Mitochondria in Neurological Conditions. Int. Rev. Neurobiol. 2002, 51, 325–376. [Google Scholar] [CrossRef]
- Bailey, D.M.; Willie, C.K.; Hoiland, R.L.; Bain, A.R.; MacLeod, D.B.; Santoro, M.A.; DeMasi, D.K.; Andrijanic, A.; Mijacika, T.; Barak, O.F.; et al. Surviving Without Oxygen: How Low Can the Human Brain Go? High. Alt. Med. Biol. 2017, 18, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Leithner, C.; Royl, G. The Oxygen Paradox of Neurovascular Coupling. J. Cereb. Blood Flow. Metab. 2014, 34, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Siesjö, B.K. Cerebral Circulation and Metabolism. J. Neurosurg. 1984, 60, 883–908. [Google Scholar] [CrossRef]
- De la Torre, J.C. Critically Attained Threshold of Cerebral Hypoperfusion: The CATCH Hypothesis of Alzheimer’s Pathogenesis. Neurobiol. Aging 2000, 21, 331–342. [Google Scholar] [CrossRef]
- Sanap, M.N.; Worthley, L.I.G. Neurologic Complications of Critical Illness: Part I. Altered States of Consciousness and Metabolic Encephalopathies. Crit. Care Resusc. 2002, 4, 119–132. [Google Scholar] [CrossRef]
- Berisavac, I.I.; Jovanović, D.R.; Padjen, V.V.; Ercegovac, M.D.; Stanarčević, P.D.J.; Budimkić-Stefanović, M.S.; Radović, M.M.; Beslać-Bumbaširević, L.G. How to Recognize and Treat Metabolic Encephalopathy in Neurology Intensive Care Unit. Neurol. India 2017, 65, 123–128. [Google Scholar] [CrossRef]
- Gaillard, W.D.; Zeffiro, T.; Fazilat, S.; DeCarli, C.; Theodore, W.H. Effect of Valproate on Cerebral Metabolism and Blood Flow: An 18F-2-Deoxyglucose and 15O Water Positron Emission Tomography Study. Epilepsia 1996, 37, 515–521. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M.J. Effectiveness of First Antiepileptic Drug. Epilepsia 2001, 42, 1255–1260. [Google Scholar] [CrossRef]
- Goldstein, L.B. Common Drugs May Influence Motor Recovery after Stroke. The Sygen In Acute Stroke Study Investigators. Neurology 1995, 45, 865–871. [Google Scholar] [CrossRef]
- Sözüer, D.T.; Onsel, C.; Altiok, E.; Uslu, I.; Yalçin, E. Cerebellar and Subcortical Blood Flow Abnormalities in Children with Partial Epilepsy. Brain Dev. 1996, 18, 95–98. [Google Scholar] [CrossRef]
- Rhodes, C.E.; Denault, D.; Varacallo, M.A. Physiology, Oxygen Transport. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Abdelsalam, M. Permissive Hypoxemia: Is It Time to Change Our Approach? Chest 2006, 129, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Eaton, J.W. Erythrocyte Metabolism: Interaction with Oxygen Transport. Science 1971, 171, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Oelshlegel, F.J.; Moore, L.G.; Noble, N.A. In Vivo Red Cell Glycolytic Control and DPG-ATP Levels. Ann. N. Y. Acad. Sci. 1974, 241, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, R.; van Solinge, W.W. The Energy-Less Red Blood Cell Is Lost: Erythrocyte Enzyme Abnormalities of Glycolysis. Blood 2005, 106, 4034–4042. [Google Scholar] [CrossRef]
- Robinson, J.M.; Lancaster, J.R. Hemoglobin-Mediated, Hypoxia-Induced Vasodilation via Nitric Oxide: Mechanism(s) and Physiologic versus Pathophysiologic Relevance. Am. J. Respir. Cell Mol. Biol. 2005, 32, 257–261. [Google Scholar] [CrossRef]
- Richardson, K.J.; Kuck, L.; Simmonds, M.J. Beyond Oxygen Transport: Active Role of Erythrocytes in the Regulation of Blood Flow. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H866–H872. [Google Scholar] [CrossRef]
- Highley, M.S.; De Bruijn, E.A. Erythrocytes and the Transport of Drugs and Endogenous Compounds. Pharm. Res. 1996, 13, 186–195. [Google Scholar] [CrossRef]
- Wong, H.Y.; Chu, T.S.; Lai, J.C.; Fung, K.P.; Fok, T.F.; Fujii, T.; Ho, Y.Y. Sodium Valproate Inhibits Glucose Transport and Exacerbates Glut1-Deficiency in Vitro. J. Cell Biochem. 2005, 96, 775–785. [Google Scholar] [CrossRef]
- Lynch, A.; Tobias, J.D. Acute Valproate Ingestion Induces Symptomatic Methemoglobinemia. Pediatr. Emerg. Care 1998, 14, 205–207. [Google Scholar] [CrossRef]
- Oprisan, B.; Stoica, I.; Avadanei, M.I. Morphological Changes Induced in Erythrocyte Membrane by the Antiepileptic Treatment: An Atomic Force Microscopy Study. Microsc. Res. Tech. 2017, 80, 364–373. [Google Scholar] [CrossRef]
- Ozkara, C.; Dreifuss, F.E.; Apperson Hansen, C. Changes in Red Blood Cells with Valproate Therapy. Acta Neurol. Scand. 1993, 88, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Bussel, J.B. Hematologic Toxicity of Sodium Valproate. J. Pediatr. Hematol. Oncol. 2000, 22, 62–65. [Google Scholar] [CrossRef] [PubMed]
- McGuire, G.M.; Macphee, G.J.; Thompson, G.G.; Moore, M.R.; Brodie, M.J. Effects of Sodium Valproate on Haem Biosynthesis in Man: Implications for Seizure Management in the Porphyric Patient. Eur. J. Clin. Investig. 1988, 18, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Nau, H. Valproic Acid: Metabolite Concentrations in Plasma and Brain, Anticonvulsant Activity, and Effects on GABA Metabolism during Subacute Treatment in Mice. Arch. Int. Pharmacodyn. Ther. 1982, 257, 20–31. [Google Scholar]
- Cattaneo, C.I.; Ressico, F.; Valsesia, R.; D’Innella, P.; Ballabio, M.; Fornaro, M. Sudden valproate-induced hyperammonemia managed with L-carnitine in a medically healthy bipolar patient: Essential review of the literature and case report. Medicine 2017, 96, e8117. [Google Scholar] [CrossRef]
- Eubanks, A.L.; Aguirre, B.; Bourgeois, J.A. Severe Acute Hyperammonemia after Brief Exposure to Valproate. Psychosomatics 2008, 49, 82–83. [Google Scholar] [CrossRef]
- Connacher, A.A.; Macnab, M.S.; Moody, J.P.; Jung, R.T. Fatality Due to Massive Overdose of Sodium Valproate. Scott. Med. J. 1987, 32, 85–86. [Google Scholar] [CrossRef]
- Iqbal, M.O.; Manzoor, M.; Mumtaz, A.; Riaz, R.; Arshad, S.; Khan, I.A.; Javaid, U.; Manzoor, Z.; Munawar, S.H.; Andleeb, S.; et al. Evaluation of the Hepatoprotective Activity of Hydroalcoholic Extract of Alhagi Camelorum against Valproic Acid-Induced Hepatotoxicity in Rats. Biomed. Pharmacother. 2022, 150, 112953. [Google Scholar] [CrossRef]
- Anderson, G.D.; Acheampong, A.A.; Wilensky, A.J.; Levy, R.H. Effect of Valproate Dose on Formation of Hepatotoxic Metabolites. Epilepsia 1992, 33, 736–742. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism: A Manual of Biochemical Methods; Grune & Stratton: New York, NY, USA, 1971. [Google Scholar]
- Kosenko, E.A.; Aliev, G.; Kaminsky, Y.G. Relationship between Chronic Disturbance of 2,3-Diphosphoglycerate Metabolism in Erythrocytes and Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2016, 15, 113–123. [Google Scholar] [CrossRef]
- Atkinson, D.E. The Energy Charge of the Adenylate Pool as a Regulatory Parameter. Interaction with Feedback Modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.H.; Lund, P.; Krebs, H.A. The Redox State of Free Nicotinamide-Adenine Dinucleotide in the Cytoplasm and Mitochondria of Rat Liver. Biochem. J. 1967, 103, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1984; Volume 3, pp. 273–286. [Google Scholar]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Spooner, R.J. Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Wiley: New York, NY, USA, 1984; Volume 3, pp. 259–265. [Google Scholar]
- Warholm, M.; Guthenberg, C.; von Bahr, C.; Mannervik, B. Glutathione Transferases from Human Liver. Methods Enzymol. 1985, 113, 499–504. [Google Scholar] [CrossRef]
- Standardization of Procedures for the Study of Glucose-6-Phosphate Dehydrogenase. Report of a WHO Scientific Group. World Health Organ. Tech. Rep. Ser. 1967, 366, 1–53.
- Beutler, E.; Blume, K.G.; Kaplan, J.C.; Löhr, G.W.; Ramot, B.; Valentine, W.N. International Committee for Standardization in Haematology: Recommended Methods for Red-Cell Enzyme Analysis. Br. J. Haematol. 1977, 35, 331–340. [Google Scholar] [CrossRef]
- Bergmeyer, H.-U. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Pylvänen, V.; Pakarinen, A.; Knip, M.; Isojärvi, J. Characterization of Insulin Secretion in Valproate-Treated Patients with Epilepsy. Epilepsia 2006, 47, 1460–1464. [Google Scholar] [CrossRef]
- Fang, J.; Chen, S.; Tong, N.; Chen, L.; An, D.; Mu, J.; Zhou, D. Metabolic Syndrome among Chinese Obese Patients with Epilepsy on Sodium Valproate. Seizure 2012, 21, 578–582. [Google Scholar] [CrossRef]
- Newsholme, E.A. Carbohydrate Metabolism in Vivo: Regulation of the Blood Glucose Level. Clin. Endocrinol. Metab. 1976, 5, 543–578. [Google Scholar] [CrossRef]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; D’Alessandro, A.; Nikolaidis, M.G. Erythrocyte Metabolism. Acta Physiol 2024, 240, e14081. [Google Scholar] [CrossRef] [PubMed]
- Tilton, W.M.; Seaman, C.; Carriero, D.; Piomelli, S. Regulation of Glycolysis in the Erythrocyte: Role of the Lactate/Pyruvate and NAD/NADH Ratios. J. Lab. Clin. Med. 1991, 118, 146–152. [Google Scholar] [PubMed]
- Venturini, G.; Silei, O.; Palladini, G.; Carolei, A.; Margotta, V. Aminergic Neurotransmitters and Adenylate Cyclase in Hydra. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1984, 78, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Garby, L.; De Verdierch, C.H. Glucose Metabolism in Normal Erythrocytes. I. Kinetics of the Hexokinase Reaction in Intact Cells. Scand. J. Haematol. 1964, 1, 150–167. [Google Scholar] [CrossRef]
- Sprague, R.S.; Stephenson, A.H.; Ellsworth, M.L. Red Not Dead: Signaling in and from Erythrocytes. Trends Endocrinol. Metab. 2007, 18, 350–355. [Google Scholar] [CrossRef]
- Clausen, T.; Van Hardeveld, C.; Everts, M.E. Significance of Cation Transport in Control of Energy Metabolism and Thermogenesis. Physiol. Rev. 1991, 71, 733–774. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. The Role of Red Blood Cell Deformability and Na,K-ATPase Function in Selected Risk Factors of Cardiovascular Diseases in Humans: Focus on Hypertension, Diabetes Mellitus and Hypercholesterolemia. Physiol. Res. 2016, 65 (Suppl. S1), S43–S54. [Google Scholar] [CrossRef]
- Perlman, B.J.; Goldstein, D.B. Membrane-Disordering Potency and Anticonvulsant Action of Valproic Acid and Other Short-Chain Fatty Acids. Mol. Pharmacol. 1984, 26, 83–89. [Google Scholar] [CrossRef]
- Jamme, I.; Petit, E.; Divoux, D.; Gerbi, A.; Maixent, J.M.; Nouvelot, A. Modulation of Mouse Cerebral Na+,K(+)-ATPase Activity by Oxygen Free Radicals. Neuroreport 1995, 7, 333–337. [Google Scholar]
- Kourie, J.I. Interaction of Reactive Oxygen Species with Ion Transport Mechanisms. Am. J. Physiol. 1998, 275, C1–C24. [Google Scholar] [CrossRef]
- Taleb, A.; Lin, W.; Xu, X.; Zhang, G.; Zhou, Q.-G.; Naveed, M.; Meng, F.; Fukunaga, K.; Han, F. Emerging Mechanisms of Valproic Acid-Induced Neurotoxic Events in Autism and Its Implications for Pharmacological Treatment. Biomed. Pharmacother. 2021, 137, 111322. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Lucas, S.M.; Ramos, C.D.; Green, E.J.; Nuttall, D.J.; Clark, D.S.; Marchant, E.D.; Hancock, C.R.; Piorczynski, T.B. Valproic Acid Promotes SOD2 Acetylation: A Potential Mechanism of Valproic Acid-Induced Oxidative Stress in Developing Systems. Free Radic. Res. 2021, 55, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Pippenger, C.E. Valproate Therapy Depresses GSH-Px and SOD Enzyme Activity. A Possible Mechanism for VPA Induced Idiosyncratic Drug Toxicity. Clin. Chem. 1989, 35, 1173. [Google Scholar]
- Cengiz, M.; Yüksel, A.; Seven, M. The Effects of Carbamazepine and Valproic Acid on the Erythrocyte Glutathione, Glutathione Peroxidase, Superoxide Dismutase and Serum Lipid Peroxidation in Epileptic Children. Pharmacol. Res. 2000, 41, 423–425. [Google Scholar] [CrossRef]
- Muriel, P.; Castañeda, G.; Ortega, M.; Noël, F. Insights into the Mechanism of Erythrocyte Na+/K+-ATPase Inhibition by Nitric Oxide and Peroxynitrite Anion. J. Appl. Toxicol. 2003, 23, 275–278. [Google Scholar] [CrossRef]
- Fridovich, I. ROS-Dependent Enzymes. In Redox Biochemistry; Banerjee, R., Becker, D.F., Dickman, M.B., Gladyshev, V.N., Ragsdale, S.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Veech, R.L.; Eggleston, L.V.; Krebs, H.A. The Redox State of Free Nicotinamide-Adenine Dinucleotide Phosphate in the Cytoplasm of Rat Liver. Biochem. J. 1969, 115, 609–619. [Google Scholar] [CrossRef]
- Wurster, B.; Hess, B. Kinetic Analysis of He Glucosephosphate Isomerase-Glucose-6-Phosphate Dehydrogenase System from Yeast in Vitro. Hoppe Seylers Z Physiol. Chem. 1970, 351, 1537–1544. [Google Scholar] [CrossRef]
- Scott, M.D.; Zuo, L.; Lubin, B.H.; Chiu, D.T. NADPH, Not Glutathione, Status Modulates Oxidant Sensitivity in Normal and Glucose-6-Phosphate Dehydrogenase-Deficient Erythrocytes. Blood 1991, 77, 2059–2064. [Google Scholar] [CrossRef]
- Scott, M.D.; Wagner, T.C.; Chiu, D.T. Decreased Catalase Activity Is the Underlying Mechanism of Oxidant Susceptibility in Glucose-6-Phosphate Dehydrogenase-Deficient Erythrocytes. Biochim. Biophys. Acta 1993, 1181, 163–168. [Google Scholar] [CrossRef]
- Tung, E.W.Y.; Winn, L.M. Valproic Acid Increases Formation of Reactive Oxygen Species and Induces Apoptosis in Postimplantation Embryos: A Role for Oxidative Stress in Valproic Acid-Induced Neural Tube Defects. Mol. Pharmacol. 2011, 80, 979–987. [Google Scholar] [CrossRef]
- Cho, D.-H.; Park, J.-H.; Joo Lee, E.; Jong Won, K.; Lee, S.-H.; Kim, Y.-H.; Hwang, S.; Ja Kwon, K.; Young Shin, C.; Song, K.-H.; et al. Valproic Acid Increases NO Production via the SH-PTP1-CDK5-eNOS-Ser(116) Signaling Cascade in Endothelial Cells and Mice. Free Radic. Biol. Med. 2014, 76, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Badar, A.; Bamosa, A.O.; Salahuddin, M.; Al Meheithif, A. Effect of Zamzam Water on Blood Methemoglobin Level in Young Rats. J. Family Community Med. 2019, 26, 30–35. [Google Scholar] [CrossRef]
- Brewer, G.J. 2,3-DPG and Erythrocyte Oxygen Affinity. Annu. Rev. Med. 1974, 25, 29–38. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Zhao, L.; Zhao, J.; You, G.; Yang, J.; Wang, Z.; Wang, Y.; Zhou, H. Sodium Pyruvate Ameliorates in Vitro Oxidative Damage Resulting from Lethal Methemoglobinemia. Int. J. Clin. Exp. Med. 2018, 11, 13055–13064. [Google Scholar]
- Juel, R. 2,3-Diphosphoglycerate: Its Role in Health and Disease. CRC Crit. Rev. Clin. Lab. Sci. 1979, 10, 113–146. [Google Scholar] [CrossRef]
- Kanner, A.M.; Balabanov, A. Valproate: A Practical Review of Its Uses in Neurological and Psychiatric Disorders. Expert. Rev. Neurother. 2002, 2, 151–165. [Google Scholar] [CrossRef]
- Davis, L.L.; Ryan, W.; Adinoff, B.; Petty, F. Comprehensive Review of the Psychiatric Uses of Valproate. J. Clin. Psychopharmacol. 2000, 20, 1S–17S. [Google Scholar] [CrossRef]
- Khoo, S.H.; Leyland, M.J. Cerebral Edema Following Acute Sodium Valproate Overdose. J. Toxicol. Clin. Toxicol. 1992, 30, 209–214. [Google Scholar] [CrossRef]
- Pavar, G.; Xu, N.; Sawar, K.; Trivedi, V.; Levine, D.L. Valproate-Induced Encephalopathy Presenting at Therapeutic Blood Concentrations: A Case Report and Literature Review. Cureus 2023, 15, e33559. [Google Scholar] [CrossRef]
- Bigler, D. Neurological Sequelae after Intoxication with Sodium Valproate. Acta Neurol. Scand. 1985, 72, 351–352. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C. Are Major Dementias Triggered by Poor Blood Flow to the Brain? Theoretical Considerations. J. Alzheimers Dis. 2017, 57, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Blass, J.P.; Gibson, G.E. Cerebrometabolic Aspects of Delirium in Relationship to Dementia. Dement. Geriatr. Cogn. Disord. 1999, 10, 335–338. [Google Scholar] [CrossRef]
- Rangan, A.; Herrick, J.L. Morphologic and Clinical Features of Acute on Chronic Valproate Toxicity. Mayo Clin. Proc. 2022, 97, 1692–1693. [Google Scholar] [CrossRef]
- Mazzulla, S.; Schella, A.; Gabriele, D.; Baldino, N.; Sesti, S.; Perrotta, E.; Costabile, A.; de Cindio, B. Oxidation of Human Red Blood Cells by a Free Radical Initiator: Effects on Rheological Properties. Clin. Hemorheol. Microcirc. 2015, 60, 375–388. [Google Scholar] [CrossRef]
- Serafini, G.; Magnani, M.; Stocchi, V.; Dachà, M.; Forniani, G. Rat Red Blood Cell Hexokinase Purification, Properties and Age-Dependence. Mol. Cell Biochem. 1986, 69, 179–185. [Google Scholar] [CrossRef]
- Nemkov, T.; Stephenson, D.; Erickson, C.; Dzieciatkowska, M.; Key, A.; Moore, A.; Earley, E.J.; Page, G.P.; Lacroix, I.S.; Stone, M.; et al. Regulation of Kynurenine Metabolism by Blood Donor Genetics and Biology Impacts Red Cell Hemolysis in Vitro and in Vivo. Blood 2024, 143, 456–472. [Google Scholar] [CrossRef]
- Kaminsky, Y.; Kosenko, E. AMP Deaminase and Adenosine Deaminase Activities in Liver and Brain Regions in Acute Ammonia Intoxication and Subacute Toxic Hepatitis. Brain Res. 2010, 1311, 175–181. [Google Scholar] [CrossRef]
- Kosenko, E.A.; Alilova, G.A.; Tikhonova, L.A. Impaired Enzymatic Antioxidant Defense in Erythrocytes of Rats with Ammonia-Induced Encephalopathy: Role of NMDA Receptors. Biochemistry 2023, 88, 1404–1415. [Google Scholar] [CrossRef]
- Alilova, G.A.; Tikhonova, L.A.; Kosenko, E.A. NMDA Receptors and Indices of Energy Metabolism in Erythrocytes: Missing Link to the Assessment of Efficiency of Oxygen Transport in Hepatic Encephalopathy. Biochemistry 2024, 89, 1490–1508. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamane, K.; Shinohara, K.; Doi, K.; Inokuchi, R.; Hiruma, T.; Nakajima, S.; Noiri, E.; Yahagi, N. Hyperammonemia in Idiopathic Epileptic Seizure. Am. J. Emerg. Med. 2013, 31, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, E.; Luxenberg, J. Valproate Preparations for Agitation in Dementia. Cochrane Database Syst. Rev. 2009, CD003945. [Google Scholar] [CrossRef] [PubMed]
- König, S.A.; Knolle, J.; Friedewald, S.; Koelfen, W.; Longin, E.; Lenz, T.; Hannak, D. Effects of Valproic Acid, Carbamazepine, and Phenobarbitone on the Fatty Acid Composition of Erythrocyte Membranes in Children. Epilepsia 2003, 44, 708–711. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red Blood Cell Oxidative Stress Impairs Oxygen Delivery and Induces Red Blood Cell Aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Stromme, J.H.; Eldjarn, L. The Role of the Pentose Phosphate Pathway in the Reduction of Methaemoglobin in Human Erythrocytes. Biochem. J. 1962, 84, 406–410. [Google Scholar] [CrossRef]
- Hassan, K.S.; Al-Riyami, A.Z.; Al-Huneini, M.; Al-Farsi, K.; Al-Khabori, M. Methemoglobinemia in an Elderly Patient with Glucose-6-Phosphate Dehydrogenase Deficiency: A Case Report. Oman Med. J. 2014, 29, 135–137. [Google Scholar] [CrossRef]
- Titheradge, H.; Nolan, K.; Sivakumar, S.; Bandi, S. Methaemoglobinaemia with G6PD Deficiency: Rare Cause of Persistently Low Saturations in Neonates. Acta Paediatr. 2011, 100, e47–e48. [Google Scholar] [CrossRef]
- Lichtman, M.A.; Miller, D.R.; Cohen, J.; Waterhouse, C. Reduced Red Cell Glycolysis, 2, 3-Diphosphoglycerate and Adenosine Triphosphate Concentration, and Increased Hemoglobin-Oxygen Affinity Caused by Hypophosphatemia. Ann. Intern. Med. 1971, 74, 562–568. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonova, L.; Maevsky, E.; Montoliu, C.; Kosenko, E. Valproate Damaging Effect on Erythrocyte Metabolism as a Decisive Factor in the Development of Encephalopathy. Biomolecules 2025, 15, 588. https://doi.org/10.3390/biom15040588
Tikhonova L, Maevsky E, Montoliu C, Kosenko E. Valproate Damaging Effect on Erythrocyte Metabolism as a Decisive Factor in the Development of Encephalopathy. Biomolecules. 2025; 15(4):588. https://doi.org/10.3390/biom15040588
Chicago/Turabian StyleTikhonova, Lyudmila, Eugene Maevsky, Carmina Montoliu, and Elena Kosenko. 2025. "Valproate Damaging Effect on Erythrocyte Metabolism as a Decisive Factor in the Development of Encephalopathy" Biomolecules 15, no. 4: 588. https://doi.org/10.3390/biom15040588
APA StyleTikhonova, L., Maevsky, E., Montoliu, C., & Kosenko, E. (2025). Valproate Damaging Effect on Erythrocyte Metabolism as a Decisive Factor in the Development of Encephalopathy. Biomolecules, 15(4), 588. https://doi.org/10.3390/biom15040588






