Plant-Based Inhibitors of Protein Aggregation
Abstract
1. Introduction
2. Molecular Mechanism of Amyloid Fibril Inhibition
- The suppression of the early stages of fibril formation via the modulation of protein gathering and nuclei formation: In this pathway, the plant-derived inhibitor can alter fibril formation in at least two ways: (i) by increasing the protein’s stability in the monomeric form and (ii) by complexation with the monomer, thereby preventing monomer–monomer interactions and the conversion of α-helical protein intermediates to the β-sheet structures [56,57,58,59,60,61,62,63,64]. To exemplify, Wang et al. found that the major flavonoid of Scuttellaria baincalensis Georgi baicalein and baicalin inhibit IAPP fibrillation through concentration-dependent suppression of protein nucleation, thereby preventing the formation of β-sheet-rich aggregates [56]. Myricetin prevents the conformational change in the Aβ protein from a random coil to a β-sheet-rich structure by preferentially targeting Aβ monomers [57]. It was hypothesized that the ability of epigallocatechin gallate to inhibit the formation of the IAPP amyloid fibrils relates to the suppression of the early stages of protein self-aggregation, presumably IAPP dimer formation [59].
- The stabilization of protein oligomerization and the inhibition of pre-fibril formation: Plant bioactive compounds can suppress the formation of prefibrillar structures by stabilizing protein oligomers [65,66,67,68,69]. Moreover, recent studies indicate that natural amyloid inhibitors can remodel soluble protein oligomers into non-toxic protein aggregates [70,71,72,73,74,75]. Brazilin, a natural compound extracted from Caesalpinia sappan, serves as a typical example of amyloid inhibitors capable of suppressing the formation of the toxic-on pathway of Aβ oligomers, remodeling them to β-sheet aggregates with a higher molecular weight (above 70 kDa) [70]. Myricetin, tannic acid, and nordihydroguaiaretic acid also demonstrated an ability to remodel the amyloid fibrils, neutralizing the oligomer-specific conformational epitope at substoichiometric concentrations [71].
- The stabilization of protofibrils: Several natural compounds can stabilize the pre-fibrillar state, thus blocking further fibril growth [76,77,78,79]. Quercetin was shown to inhibit the fibrillization of the IAPP, slowing down the growth of the amyloid fibrils after 10.5 h of incubation and causing an increase in the fibril amount wherein the fibril size remained constant [76]. Moreover, nordihydroguaiaretic acid inhibits the direct protofibril–protofibril association of Aβ but does not alter protofibril elongation through monomer addition [78].
- The disassembly or remodeling of the mature amyloid fibrils: Many compounds were shown to inhibit the formation of amyloid fibrils, destabilizing the preformed protein assemblies by subsequently converting the mature fibrillar structures to non-toxic smaller intermediates [72,80,81,82,83,84,85,86]. For example, the ThT assay and electron microscopy studies demonstrated that baicalein disaggregates preformed mature amyloid fibrils of the Aβ peptide to an amorphous state [80]. Palhano and colleagues observed that epigallocatechin-3-gallate (EGCG) remodels mature amyloid fibrils of Aβ1–40 and IAPP8–24 through the formation of a Schiff base and hydrophobic interactions [81].
3. Polyphenols as Amyloid Inhibitors
3.1. Flavonoids
3.2. Non-Flavonoid Polyphenolic Compounds
4. Non-Phenolic Compounds as Amyloid Inhibitors
5. Plant Extracts as Inhibitors of Amyloid Aggregation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jucker, M.; Walker, L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Seghal, A.; Singh, S.; Sharma, N.; Zengin, G.; Bungau, S.; Andronie-Ciora, F.L.; Munteanu, M.A.; Brisc, M.C.; et al. Current trends in neurodegeneration: Cross-talks between oxidative stress, cell death, and inflammation. Int. J. Mol. Sci. 2021, 22, 7432. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Almeida, Z.L.; Brito, R.M.M. Structure and aggregation mechanisms in amyloids. Molecules 2020, 25, 1195. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein folding and misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Hardy, J. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci. 1997, 20, 154–159. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Jakes, R.; Rutherford, D.; Crowther, R.A. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 267, 22570–22574. [Google Scholar] [CrossRef]
- Trojanowski, J.Q.; Lee, V.M.Y. Aggregation of neurofilament and α-synuclein proteins in Lewy bodies: Implication for the pathogenesis of Parkinson Disease and Lewy body dimentia. Arch. Neurol. 1998, 55, 151–152. [Google Scholar] [CrossRef]
- Olanow, C.W.; Tatton, W.G. Ethiology and pathogenesis of Parkinson’s disease. Anu. Rev. Neurosci. 1999, 22, 123–144. [Google Scholar] [CrossRef]
- Westermark, M.Z.; Westermark, G.T. Amyloid in human islets of Langerhans: Immunologic evidence that islet amyloid polypeptide is modified in amyloidogenesis. Pancreas 2000, 21, 212–218. [Google Scholar]
- Christensen, M.; Skeby, K.K.; Schiott, B. Identification of key interactions in the initial self-assembly of amylin in a membrane environment. Biochemistry 2017, 56, 4884–4894. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Eisenberg, D. Recent atomic models of amyloid fibril structure. Curr. Opin. Struct. Biol. 2006, 16, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Lesné, S.; Kotilinek, L.; Ashe, K.H. Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neuroscience 2008, 151, 745–749. [Google Scholar] [CrossRef]
- Glabe, C.C. Amyloid accumulation and pathogensis of Alzheimer’s disease: Significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem. 2005, 38, 167–177. [Google Scholar]
- Walsh, D.M.; Selkoe, D.J. A beta oligomers—A decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef]
- Cascella, R.; Chen, S.W.; Bigi, A.; Camino, J.D.; Xu, C.K.; Dobson, C.M.; Chiti, F.; Cremades, N.; Cecchi, C. The release of toxic oligomers from α-synuclein fibrils induces dysfunction in neuronal cells. Nat. Commun. 2021, 12, 1814. [Google Scholar] [CrossRef]
- Sciacca, M.F.; Kotler, S.A.; Brender, J.R.; Chen, J.; Lee, D.K.; Ramamoorthy, A. Two-step mechanism of membrane disruption by Abeta through membrane fragmentation and pore formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef]
- Luth, E.S.; Stavrovskaya, I.G.; Bartels, T.; Kristal, B.S.; Selkoe, D.J. Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 2014, 289, 21490–21507. [Google Scholar] [CrossRef]
- Bartolini, M.; Andrisano, V. Strategies for the inhibition of protein aggregation in human diseases. ChemBioChem 2010, 11, 1018–1035. [Google Scholar] [PubMed]
- Aguzzi, A.; O’Connor, T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 2010, 9, 237–248. [Google Scholar] [PubMed]
- EEisele, Y.S.; Monteiro, C.; Fearns, C.; Encalada, S.E.; Wiseman, R.L.; Powers, E.T.; Kelly, J.W. Targeting protein aggregation for the treatment of degenerative diseases. Nat. Rev. Drug Discov. 2015, 14, 759–780. [Google Scholar]
- Debnath, K.; Sarkar, A.K.; Jana, N.R.; Jana, N.R. Inhibiting protein aggregation by small molecule-based colloidal nanoparticles. Acc. Mater. Res. 2022, 3, 54–66. [Google Scholar]
- Sonawane, S.K.; Ahmad, A.; Chinnathambi, S. Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer’s disease. ACS Omega 2019, 4, 12833–12840. [Google Scholar]
- Vus, K.; Tarabara, U.; Danylenko, I.; Pirko, Y.; Krupodorova, T.; Yemets, A.; Blume, Y.; Turchenko, V.; Klymchuk, D.; Smertenko, P.; et al. Silver nanoparticles as inhibitors of insulin amyloid formation: A fluorescence study. J. Mol. Liq. 2021, 342, 117508. [Google Scholar]
- Luo, S.; Ma, C.; Zhu, M.Q.; Ju, W.N.; Yang, Y.; Wang, X. Application of iron oxide nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on Alzheimer’s disease. Front. Cell Neurosci. 2020, 14, 21. [Google Scholar]
- Mitra, A.; Sarkar, N. Sequence and structure-based peptides as potent amyloid inhibitors: A review. Arch. Biochem. Biophys. 2020, 695, 108614. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Q.; Wang, C.; Zheng, J.; Luo, F.; Xie, J.; Li, Y.; Ma, X.; He, L.; Eisenberg, D.; et al. Structure-based peptide inhibitor design of amyloid-β aggregation. Front. Mol. Neurosci. 2019, 12, 54. [Google Scholar]
- Wang, X.; Wang, C.; Chan, H.N.; Ashok, I.; Krishnamoorthi, S.K.; Li, M.; Wong, M.S. Amyloid-β oligomer targeted theranostic probes for in vivo NIR imaging and inhibition of self-aggregation and amyloid-β induced ROS generation. Talanta 2021, 1, 121830. [Google Scholar]
- Necula, M.; Chirita, C.N.; Kyret, J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: Implications for the treatment of tauopathic neurodegenerative diseases. Bochemistry 2005, 44, 10227–10237. [Google Scholar]
- Rana, M.; Cho, H.J.; Roy, T.K.; Mirica, L.M.; Sharma, A.K. Azo-dyes based small bifunctional moleculaes for metal chelation and controlling amyloid formation. Inorganica Chim. Acta 2018, 471, 419–429. [Google Scholar] [PubMed]
- Wong, H.E.; Kwon, I. Xanthene food dye, as a modulator of Alzheimer’s disease amyloid-beta peptide aggregation and the associated impaired neuronal cell function. PLoS ONE 2011, 6, e25752. [Google Scholar]
- Solomon, B.; Koppel, R.; Hanan, E.; Katzav, T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc. Natl. Acad. Sci. USA 1996, 93, 452–455. [Google Scholar]
- Radbakhsh, S.; Barreto, G.E.; Bland, A.R.; Sahebkar, A. Curcumin: A small moleculae with big functionality against amyloid aggregation in neurodegenerative diseases and type 2 diabetes. BioFactors 2021, 47, 570–586. [Google Scholar]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin attenuates amyloid-β aggregate toxicity and modulates amyloid-β aggregation pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar]
- Yu, K.C.; Kwan, P.; Cheung, S.K.K.; Ho, A.; Baum, L. Effects of resveratrol and morin on insoluble tau in tau transgenic mice. Transl. Neurosci. 2018, 9, 54–60. [Google Scholar] [CrossRef]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of tau protein fibrillization by oleocanthal. J. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar]
- Siposova, K.; Kozar, T.; Huntosova, V.; Tomkova, S.; Musatov, A. Inhibition of amyloid formation and disassembly of pre-formed fibrils by natural polyphenol rottlerin. Biochim. Biophys. Acta. Proteins Proteom. 2019, 1867, 259–274. [Google Scholar] [CrossRef]
- Chua, S.W.; Cornejo, A.; van Eersel, J.; Stevens, C.H.; Vaca, I.; Cueto, M.; Kassiou, M.; Gladbach, A.; Macmillan, A.; Lewis, L.; et al. The polyphenol altenusin inhibits in vitro fibrillization of tau ad reduces induced tau pathology in primary neurons. ACS Chem. Neurosci. 2017, 8, 743–751. [Google Scholar]
- Alghamdi, A.; Birsh, D.J.S.; Vyshemirsky, V.; Rolinski, O.J. Impact of the flavonoid quercetin on β-amyloid aggregation revealed by intrinsic fluorescence. J. Phys. Chem. 2022, 126, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharifi-Rad, M.; Kumar, N.V.A.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Rochet, J.C.; Lansbury, P.T. Amyloid fibrillogenesis: Themes and variations. Curr. Opin. Struct. Biol. 2000, 10, 60–68. [Google Scholar] [CrossRef]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.S. Tau protein aggregation in Alzheimer’s disease: An attractive target for the development of novel therapeutic agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef]
- Gracia, P.; Camino, J.D.; Volpicelli-Daley, L.; Cremades, N. Multiplicity of α-synuclein aggregated species and their possible roles in disease. Int. J. Mol. Sci. 2020, 21, 8043. [Google Scholar] [CrossRef]
- Bhowmick, D.C.; Kudaibergenova, Z.; Burnatt, L.; Jeremic, A.M. Molecular mechanisms of amylin turnover, misfolding and toxicity in the pancreas. Molecules 2022, 27, 1021. [Google Scholar] [CrossRef]
- Cohen, S.I.A.; Vendruscolo, M.; Welland, M.E.; Dobson, C.M.; Terentjev, E.M.; Knowles, T.P.J. Nucleated polymerization with secondary pathways. I. Time evolution of the principal moments. J. Chem. Phys. 2011, 135, 065105. [Google Scholar] [CrossRef]
- Linse, S. Monomer-dependent secondary nucleation in amyloid formation. Biophys. Rev. 2017, 9, 329–338. [Google Scholar] [CrossRef]
- Ramachandran, G.; Udgaonkar, J.B. Evidence for the existence of a secondary pathway for fibril growth during the aggregation of tau. J. Mol. Biol. 2012, 421, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Meisl, G.; Buell, A.K.; Young, L.; Kaminski, C.F.; Knowles, T.P.J.; Sparr, E.; Linse, S. Secondary nucleation of monomers on fibril surface dominates α -synuclein aggregation and provides autocatalytic amyloid amplification. Q. Rev. Biophys. 2017, 50, e6. [Google Scholar]
- Padrick, S.B.; Miranker, A.D. Islet Amyloid: Phase Partitioning and Secondary Nucleation Are Central to the Mechanism of Fibrillogenesis. Biochemistry 2002, 41, 4694–4703. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Melki, R.; Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2010, 11, 301–307. [Google Scholar] [CrossRef]
- Kfoury, N.; Holmes, B.B.; Jiang, H.; Holtzman, D.M.; Diamond, M.I. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 2012, 287, 19440–19451. [Google Scholar]
- Wang, Y.; Hu, T.; Wei, J.; Yin, X.; Gao, Z.; Li, H. Inhibitory activities of flavonoids from Scutellaria baicalensis Georgi on amyloid aggregation related to type 2 diabetes and the possible structural requirements for polyphenol in inhibiting the nucleation phase of hIAPP aggregation. Int. J. Biol. Macromol. 2022, 215, 531–540. [Google Scholar]
- Bartolini, M.; Naldi, M.; Fiori, J.; Valle, F.; Biscarini, F.; Nicolau, D.V.; Andrisano, V. Kinetic characterization of amyloid-beta 1-42 aggregation with a multimethodological approach. Anal. Biochem. 2011, 414, 215–225. [Google Scholar]
- Cao, P.; Raleigh, D.P. Analysis of the inhibition and remodeling of islet amyloid polypeptide amyloid fibers by flavanols. Biochemistry 2012, 51, 2670–2683. [Google Scholar]
- Abioye, R.O.; Udenigwe, C.C. Potential of peptides and phytochemicals in attenuating different phases of islet amyloid polypeptide fibrillation for type 2 diabetes management. Food Sci. Hum. Welness 2021, 10, 259–269. [Google Scholar] [CrossRef]
- Aitken, J.F.; Loomes, K.M.; Riba-Garcia, I.; Unwin, R.D.; Prijic, G.; Phillips, A.S.; Phillips, A.R.; Wu, D.; Poppitt, S.D.; Ding, K.; et al. Rutin suppresses human-amylin\hIAPP misfolding and oligomer formation in-vitro, and ameliorates diabetes and it impacts in human-amylin/hIAPP transgenic mice. Biochem. Biophys. Res. Commun. 2017, 482, 625–631. [Google Scholar]
- Alvarez-Barbel, I.; Espargaro, A.; Viayna, A.; Caballero, A.; Busquets, M.A.; Gamez, P.; Luque, F.J.; Sabate, R. Three to tango: Inhibitory effect of quercetin and apigenin on acetylcholinesterase, amyloid-β aggregation and acetylcholinesterase-amyloid interaction. Pharmaceutics 2022, 14, 2342. [Google Scholar] [CrossRef] [PubMed]
- Lemkul, J.A.; Bevan, D.R. Morin inhibits the early stages of amyloid β-peptide aggregation by altering tertiary and quaternary interactions to produce “off-pathway” structures. Biochemistry 2012, 51, 5990–6009. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Suzuki, N.; Taniguchi, S.; Oikawa, T.; Nonaka, T.; Iwatsubo, T.; Hisanaga, S.-I.; Goedert, M.; Hasegawa, M. Small molecular inhibitors of α-synuclein filament assembly. Biochemistry 2006, 45, 6085–6094. [Google Scholar] [CrossRef] [PubMed]
- Tapa, A.; Woo, E.R.; Chi, E.Y.; Sharoar, M.G.; Jin, H.G.; Shin, S.Y.; Park, I.S. Biflavonoids are superior to monoflavonoids in inhibiting amyloid-β toxicity and fibrillogenesis via accumulation of nontoxic oligomer-like structures. Biochemistry 2011, 50, 2445–2455. [Google Scholar] [CrossRef]
- Nedumpully-Govindan, P.; Kakinen, A.; Pilkington, E.H.; Davis, T.P.; Ke, P.C.; Ding, F. Stabilizing off-pathway oligomers by polyphenol nanoassemblies for IAPP aggregation inhibition. Sci. Rep. 2016, 6, 19463. [Google Scholar] [CrossRef]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L.; Han, F.; Mao, X.; Ikeda, T.; Takasaki, J.-I.; Nishijo, H.; et al. Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef]
- Palazzi, L.; Bruzzone, E.; Bisello, G.; Leri, M.; Stefani, M.; Bucciantini, M.; de Laureto, P.P. Oleuropein aglycone stabilizes the monomeric α-synuclein and favours the growth of non-toxic aggregates. Sci. Rep. 2018, 8, 8337. [Google Scholar] [CrossRef]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Menon, S.A.; Khair, S.B.A.; Lu, J.-H.; Safieh-Garabedian, B.; Al-Hayani, A.A.; Eliezer, D.; Li, M.; et al. Ginsenoside Rb1 inhibits fibrillation and toxicity of alpha-synuclein and disaggregates preformed fibrils. Neurobiol. Dis. 2015, 74, 89–101. [Google Scholar] [CrossRef]
- Kai, T.; Zhang, L.; Wang, X.; Jing, A.; Zhao, B.; Yu, X.; Zheng, J.; Zhou, F. Tabersonine inhibits amyloid fibril formation and cytotoxicity of Aβ(1-42). ACS Chem. Neurosci. 2015, 6, 879–888. [Google Scholar] [CrossRef]
- Du, W.J.; Guo, J.J.; Gao, M.T.; Hu, S.Q.; Dong, X.Y.; Han, Y.F.; Liu, F.F.; Jiang, S.; Sun, Y. Brazilin inhibits amyloid β-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity. Sci. Rep. 2014, 5, 7992. [Google Scholar] [CrossRef]
- Ladiwala, A.R.A.; Dordick, J.S.; Tessier, P.M. Aromatic small molecules remodel toxic soluble oligomers of amyloid β through three independent pathways. J. Biol. Chem. 2011, 286, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.A.; Lin, J.C.; Bale, S.S.; Marcelino-Cruz, A.M.; Bhattacharya, M.; Dordick, J.S.; Tessier, P.M. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Aβ into off-pathway conformers. J. Biol. Chem. 2010, 285, 24228–24237. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Vendruscolo, M.; Dobson, C.M.; Knowles, T. Chemical kinetics for drug discovery to combat protein aggregation diseases. Trends Pharmacol. Sci. 2014, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Velander, P.; Brown, A.M.; Wang, Y.; Liu, D.; Bevan, D.R.; Zhang, S.; Xu, B. Rosmarinic acid potently detoxifies amylin amyloid and ameliorates diabetic pathology in a transgenic rat model of type 2 diabetes. ACS Pharmacol. Trans. Sci. 2021, 4, 1322–1337. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, C.; Yin, C.; Hu, X.; Guang, P.; Cheng, Y. Structural remodeling of the toxic amyloid fibrillary mediated by epigallocatechin-3-gallate. ACS Omega 2022, 7, 48047–48058. [Google Scholar] [CrossRef]
- Abioye, R.O.; Okagu, O.D.; Udenigwe, C. Inhibition of islet amyloid polypeptide fibrillation by structurally diverse phenolic compounds and fibril disaggregation potential of rutin and quercetin. J. Agric. Food Chem. 2022, 70, 392–402. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of β-strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef]
- Moss, M.A.; Varvel, N.H.; Nichols, M.R.; Reed, D.K.; Rosenberry, T.L. Nordihydroguaiaretic acid does not disaggregate β-amyloid (1–40) protofibrils but does inhibit growth arising from direct protofibril association. Mol. Pharmacol. 2004, 66, 592–600. [Google Scholar] [CrossRef]
- Lu, J.H.; Ardah, M.T.; Durairajan, S.S.K.; Liu, L.F.; Xie, L.X.; Fong, W.F.D.; Hasan, M.Y.; Huang, J.D.; El-Agnaf, O.M.A.; Li, M. Baicalein inhibits formation of α-synuclein oligomers within living cells and prevents Aβ-peptide fibrillation and oligomerization. ChemBioChem 2011, 12, 615–624. [Google Scholar] [CrossRef]
- Palhano, F.L.; Lee, J.; Grimster, N.P.; Kelly, J.W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013, 135, 7503–7510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Y.; Jin, L.; Liang, G. Revealing the mechanism of EGCG, genistein, rutin, quercetin, and silybinin against hIAPP aggregation via computation simulation. Interdiscip. Sci. Comput. Life Sci. 2020, 12, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin inhibits tau aggregation and disintegrates preformed tau filaments in vitro. J. Alzheimers Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Fazili, N.A.; Naeem, A. Anti-fibrillation potency of caffeic acid against an antidepressant-induced fibrillogenesis of human a-synuclein: Implications for Parkinson’s disease. Biochimie 2015, 108, 178–185. [Google Scholar] [CrossRef]
- Dubey, R.; Patil, K.; Dantu, S.C.; Sardesai, D.M.; Bhatia, P.; Malik, N.; Acharya, J.D.; Sarkar, S.; Ghosh, S.; Chakrabarti, R.; et al. Azadirachtin inhibits amyloid formation, disaggregates pre-formed fibrils and protects pancreatic β-cells from human islet amyloid polypeptide/amylin induced cytotoxicity. Biochem. J. 2019, 476, 889–907. [Google Scholar] [CrossRef]
- Brunhofer, G.; Fallarero, A.; Karlsoon, D.; Batista-Gonzalez, A.; Shinde, P.; Gopi Mohan, C.; Vuorele, P. Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta-amyloid aggregation: The case of chelerythrine. Bioorg. Med. Chem. 2012, 20, 6669–6679. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Manning-Bog, A.B.; Di Monte, D.A.; Fink, A.L. Dopamine and L-dopa disaggregate amyloid fibrils: Implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004, 18, 962–964. [Google Scholar] [CrossRef]
- Ma, L.; Yang, C.; Zheng, J.; Chen, Y.; Xiao, Y.; Huang, K. Non-polyphenolic natural inhibitors of amyloid aggregation. Eur. J. Med. Chem. 2020, 192, 112197. [Google Scholar] [CrossRef]
- Zhu, M.; Rajamani, S.; Kaylor, J.; Han, S.; Zhou, F.; Fink, A.L. The flavonoid baicalein inhibits fibrillation of α-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004, 26, 26846–26857. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Balmik, A.A.; Boral, D.; Ramasamy, S.; Chinnathambi, S. Baicalein suppress repeat tau fibrillization by sequestering oligomers. Arch. Biochem. Biophys. 2019, 675, 108119. [Google Scholar] [CrossRef]
- Bras, N.F.; Ashibaev, S.S.; Zipse, H. Combined in Silico and in vitro approaches to uncover the oxidation and Schiff base reaction of baicalein as an inhibitor of amyloid protein aggregation. Chem. Eur. J. 2022, 28, e202104240. [Google Scholar]
- Matos, A.M.; Cristovao, J.S.; Yashunsky, D.V.; Nifantiev, N.E.; Viana, A.S.; Gomes, C.M.; Rauter, A.P. Synthesis and effects of flavonoid structure variation on the amyloid-β aggregation. Pure Appl. Chem. 2017, 89, 1305–1320. [Google Scholar]
- Ruan, C.; Kong, J.; He, X.; Hu, B.; Zeng, X. Interaction between polyphenols and amyloids: From the view of prevention of protein misfolding disorders related diseases. Food Mater. Res. 2022, 2, 2. [Google Scholar]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.-I.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sperry, J.B.; Crowe, A.; Trojanowski, J.Q.; Smith, A.B.; Lee, W.M. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar]
- George, R.C.; Lew, G.; Graves, D.G. Interactions of cinnamaldehyde and epicatechin with tau: Implications of beneficial effect in modulating Alzheimer’s disease pathogenesis. J. Alzheimers Dis. 2013, 36, 21–40. [Google Scholar] [CrossRef]
- Velander, P.; Wu, L.; Henderson, F.; Zhang, S.; Bevan, D.R.; Xu, B. Natural product-based amyloid inhibitors. Biochem. Pharmacol. 2017, 139, 40–55. [Google Scholar]
- Luo, Z.; Gao, G.; Ma, Z.; Liu, Z.; Gao, X.; Tang, X.; Gao, Z.; Li, C.; Sun, T. Cichoric acid from witloof inhibits misfolding aggregation and fibrillation of hIAPP. Int. J. Biol. Macromol. 2020, 148, 1272–1279. [Google Scholar]
- Fan, Q.; Liu, Y.; Wang, X.; Zhang, Z.; Fu, Y.; Liu, L.; Wang, P.; Ma, H.; Ma, H.; Seeram, N.P.; et al. Ginnalin A inhibits aggregation, reverses fibrillogenesis and alleviates cytotoxicity of amyloid β(1-42). ACS Chem. Neurosci. 2020, 11, 638–647. [Google Scholar] [CrossRef]
- Pomier, K.M.; Ahmed, R.; Melacini, G. Catechins as tools to understand the molecular basis of neurodegeneration. Molecules 2020, 25, 3571. [Google Scholar] [CrossRef]
- Ahmed, R.; Huang, J.; Lifshitz, R.; Pomier, K.M.; Melacini, G. Structural determinants of the interactions of catechins with Aβ oligomers and lipid membranes. J. Biol. Chem. 2022, 298, 101502. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, P.B.; Renno Sodero, A.C.; Cordeiro, Y. Green tea epigallocatechin-3-gallate (EGCG) targeting protein misfolding in drug discovery for neurodegenerative diseases. Biomolecules 2021, 11, 767. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduce cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (−)-Epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef]
- Seidler, P.M.; Murray, K.A.; Boyer, D.R.; Ge, P.; Sawaya, M.R.; Hu, C.J.; Cheng, X.; Abskharon, R.; Pan, H.; De Ture, M.A.; et al. Structure-based discovery of small molecules that disaggregate Alzheimer’s disease tissue derived tau fibrils in vitro. Nat. Commun. 2022, 13, 5451. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Chidambaran, H.; Boral, D.; Gorantla, N.V.; Balmik, A.A.; Dangi, A.; Ramasamy, S.; Marelli, U.K.; Chinnathambi, S. EGCG impedes human tau aggregation and interacts with tau. Sci. Rep. 2020, 10, 12579. [Google Scholar] [CrossRef]
- Liu, M.; Chen, F.; Sha, L.; Wang, S.; Tao, L.; Yao, L.; He, M.; Yao, Z.; Liu, H.; Zhu, Z.; et al. (−)-Epigallocatechin-3-gallate ameliorates learning and memory deficits by adjusting the balance of TrkA/p75NTR signaling in APP/PS1 transgenic mice. Mol. Neurobiol. 2014, 49, 1350–1356. [Google Scholar] [CrossRef]
- Yao, Y.; Tang, Y.; Wei, G. Epigallocatechin Gallate Destabilizes α-Synuclein Fibril by Disrupting the E46−K80 Salt-Bridge and Inter-protofibril Interface. ASC Chem. Neurosci. 2020, 11, 4351–4361. [Google Scholar] [CrossRef]
- Franko, A.; Camargo, D.C.R.; Böddrich, A.; Garg, D.; Camargo, A.R.; Rathkolb, B.; Janik, D.; Aichler, M.; Feuchtinger, A.; Neff, F.; et al. Epigallocatechin gallate (EGCG) reduces the intensity of pancreatic amyloid fibrils in human islet amyloid polypeptide (hIAPP) transgenic mice. Sci. Rep. 2018, 8, 1116. [Google Scholar] [CrossRef]
- Liu, F.F.; Dong, X.Y.; He, L.; Middelber, A.P.; Sun, Y. Molecular insight into conformational transition of amyloid β-peptide 42 inhibited by (−)-Epigallocatechin-3-gallate probed by molecular simulations. J. Phys. Chem. B 2011, 115, 11879–11887. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Derreumaux, P.; Mu, Y. Molecular mechanism of the inhibition of EGCG on the Alzheimer Aβ(1-42) dimer. J. Phys. Chem. B 2013, 117, 3993–4002. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Stockmann, J.; Beyer, L.; Rudack, T.; Nabers, A.; Gumbart, J.C.; Gerwert, K.; Batista, V.S. The effect of (−)-Epigallocatechin-3-gallate on the amyloid-β secondary structure. Biophys. J. 2020, 119, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Chen, Y.; Tang, Y.; Wei, G. Green tea extracts EGCG and EGC display distinct mechanisms in disrupting Aβ42 protofibril. ACS Chem. Neurosci. 2020, 11, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, F.; Pedone, A.; Menziani, M.C. Insights into the effect of curcumin and (−)-epigallocatechin-3-gallate on the aggregation of Aβ(1-40) monomers by means of molecular dynamics. Int. J. Mol. Sci. 2020, 21, 5462. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-Epigallocatechin-3gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.E.; Kim, S. Baicalein as a potent neuroprotective agent: A review. Biomed. Pharmacother. 2017, 95, 1021–1032. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic potential of baicalein in Alzheimer’s and Parkinson’s disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef]
- Hu, Q.; Uversky, V.N.; Huang, M.; Kang, H.; Xu, F.; Liu, X.; Lian, L.; Liang, Q.; Jiang, H.; Liu, A.; et al. Baicalein inhibits α-synuclein oligomer formation and prevents progression of α-synuclein accumulation in a rotenoma mouse model of Parkinson’s disease. Biochim. Biophys. Acta 2016, 1862, 1883–1890. [Google Scholar] [CrossRef]
- Yao, Y.; Tang, Y.; Zhou, Y.; Yang, Z.; Wei, G. Baicalein exhibits differential effects and mechanisms towards disruption of α-synuclein fibrils with different polymorphs. Int. J. Biol. Mol. 2022, 220, 316–325. [Google Scholar] [CrossRef]
- Song, M.S.; Wang, Y.X.; Xiong, M.L.; Qu, B.L.; Xu, M.T. AFM and fluorescence spectroscopy investigation for disaggregation of existing Aβ fibrils by baicalein. Chin. Chem. Lett. 2012, 23, 595–598. [Google Scholar] [CrossRef]
- Hong, D.P.; Fink, A.L.; Uversky, V. Structural characteristics of α-synuclein oligomers stabilized by the flavonoid baicalein. J. Mol. Biol. 2008, 283, 214–223. [Google Scholar]
- Velander, P.; Wu, L.; Ray, W.K.; Helm, R.F.; Xu, B. Amylin amyloid inhibition by flavonoid baicalein: Key roles of its vicinal groups of the catechol moiety. Biochemistry 2016, 55, 4255–4258. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.; Ravikumar, S.; Venkataramanan, S.; Purohit, R.; Rajasekaran, R. Molecular mechanics and quantum chemical calculations unveil the combating effect of baicalein on human islet amyloid polypeptide aggregates. Mol. Simul. 2016, 45, 1538–1548. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnakumar, V.G.; Morya, V.; Gupta, S.; Datta, B. Nanobiocatalyst facilitated aglycosidic quercetin as a potent inhibitor of tau protein aggregation. Int. J. Biol. Mol. 2019, 138, 168–180. [Google Scholar] [CrossRef]
- Jimenez-Aliaga, K.; Bermejo-Bescos, P.; Benedi, J.; Martin-Aragon, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef]
- Zhu, M.; Han, S.; Fink, A.L. Oxidized quercetin inhibits α-synuclein fibrillization. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 2872–2881. [Google Scholar]
- HHirohata, M.; Hasegawa, K.; Tsutsumi-Yasuhara, S.; Ohhashi, Y.; Ookoshi, T.; Ono, K.; Yamada, M.; Naiki, H. The anti-amyloidogenic effect is exerted against Alzheimer’s β-amyloid fibrils in vitro by preferential and reversible binding of flavonoids to the amyloid fibril structure. Biochemistry 2007, 46, 1888–1899. [Google Scholar] [CrossRef]
- Mancini, R.S.; Wang, Y.; Weaver, D.F. Phenylindanes in brewed coffee inhibit amyloid-beta and tau aggregation. Front. Neurosci. 2018, 12, 735. [Google Scholar] [CrossRef]
- Marsh, D.T.; Das, S.; Ridell, J.; Smid, S.D. Structure-activity relationships for flavone interactions with amyloid β reveal a novel anti-aggregatory and neuroprotective effect of 2′,3′,4′-trihydroxyflavone (2-D08). Bioorg. Med. Chem. 2017, 25, 3827–3834. [Google Scholar] [CrossRef]
- King, K.M.; Bevan, D.; Brown, A.M. Molecular dynamics simulations indicate aromaticity as a key factor in the inhibition of IAPP(20-29) aggregation. ACS Chem. Neurosci. 2022, 13, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, D. Mulberry fruit extract alleviates cognitive impairment by promoting the clearance of amyloid-β and inhibiting neuroinflammation in Alzheimer’s disease mice. Neurochem. Res. 2020, 45, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, J. Myricetin as a promising molecule for the treatment of post-ischemic brain neurodegeneration. Nutrients 2021, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Dai, B.; Zhong, T.; Chen, Z.-X.; Chen, W.; Zhang, N.; Liu, X.-L.; Wang, L.-Q.; Chen, J.; Liang, Y. Myricetin slows liquid-liquid phase separation of tau and activates ATG5-dependent autophagy to suppress tau toxicity. J. Biol. Chem. 2021, 297, 101222. [Google Scholar] [CrossRef]
- Dubey, R.; Kulkarni, S.H.; Dantu, S.C.; Panigrahi, R.; Sardesai, D.M.; Malik, N.; Acharya, J.D.; Chugh, J.; Sharma, S.; Kumar, A. Myricetin protects pancreatic β-cells from human islet amyloid polypeptide (hIAPP) induced cytotoxicity and restores islet function. Biol. Chem. 2021, 402, 179–194. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, C.; Huang, X.; Du, W. Regulation of artemisinin and its derivatives on the assembly behavior and cytotoxicity of amyloid polypeptides hIAPP and Aβ. ACS Chem. Neurosci. 2019, 10, 4522–4534. [Google Scholar] [CrossRef]
- Taniguchi, S.; Suzuki, N.; Masuda, M.; Hisanaga, S.I.; Iwatsubo, T.; Goedert, M.; Hasegawa, M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005, 280, 7614–7623. [Google Scholar] [CrossRef]
- Berhanu, W.; Masunov, A.E. Atomistic mechanism of polyphenol amyloid aggregation inhibitors: Molecular dynamics study of curcumin, exifone, and myricetin interaction with the segment of tau peptide oligomer. J. Biomol. Struct. Dyn. 2015, 33, 1399–1411. [Google Scholar] [PubMed]
- Caruana, M.; Högen, T.; Levin, J.; Hillmer, A.; Giese, A.; Vassallo, N. Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011, 585, 1113–1120. [Google Scholar] [PubMed]
- Takahashi, R.; Ono, K.; Takamura, Y.; Mizuguchi, M.; Ikeda, T.; Nishijo, H.; Yamada, M. Phenolic compounds prevent the oligomerization of α-synuclein and reduce synaptic toxicity. J. Neurochem. 2015, 134, 943–955. [Google Scholar] [PubMed]
- Zelus, C.; Fox, A.; Calsiano, A.; Faridian, B.S.; Nogaj, L.A.; Moffet, D.A. Myricetin inhibits islet amyloid polypeptide (IAPP) aggregation and rescue living mammalian cells from IAPP toxicity. Open. Biochem. J. 2012, 6, 66–70. [Google Scholar]
- Jia, L.; Zhao, W.; Sang, J.; Wang, W.; Wei, W.; Wang, Y.; Zhao, F.; Lu, F.; Liu, F. Inhibitory effect of a flavonoid dihydromyricetin against Aβ40 amyloidogenesis and its associated cytotoxicity. ACS Chem. Neurosci. 2019, 10, 4696–4703. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Sang, J.; Cui, W.; Zhao, W.; Wei, W.; Chen, B.; Lu, F.; Liu, F. Dihydromyricetin inhibits α-synuclein aggregation, disrupts preformed fibrils, and protects neuronal cells in culture against amyloid-induced cytotoxicity. J. Agric. Food. Chem. 2019, 67, 3946–3955. [Google Scholar]
- Wu, J.-Z.; Ardah, M.; Haikal, C.; Svanbergsson, A.; Diepenbroek, M.; Vaikath, N.N.; Li, W.; Wang, Z.-Y.; Outeiro, T.F.; El-Agnaf, O.M.; et al. Dihydromyricetin and Salvianolic acid B inhibit alpha-synuclein aggregation and enhance chaperone-mediated autophagy. Transl. Neurodegener. 2019, 8, 18. [Google Scholar] [CrossRef]
- Wang, S.-W.; Wang, Y.-J.; Su, Y.-J.; Zhou, W.-W.; Yang, S.-G.; Zhang, R.; Zhao, M.; Li, Y.-N.; Zhang, Z.-P.; Zhan, D.-W.; et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. NeuroToxicology 2012, 33, 482–490. [Google Scholar]
- Xu, P.-X.; Wang, S.-W.; Yu, X.-L.; Su, Y.-J.; Wang, T.; Zhou, W.-W.; Zhang, H.; Wang, Y.-J.; Liu, R.-T. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014, 264, 173–180. [Google Scholar] [CrossRef]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to Bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar]
- Sun, X.Y.; Li, L.; Dong, Q.X.; Zhu, J.; Huangm, Y.R.; Hou, S.J.; Liu, R.T. Rutin prevents tau pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Staats, R.; Michaels, T.C.; Flagmeier, P.; Chia, S.; Horne, R.I.; Habchi, J.; Linse, S.; Knowles, T.P.; Dobson, C.M.; Vendruscolo, M. Screening of small molecules using the inhibition of oligomer formation in α-synuclein aggregation as a selection parameter. Commun. Chem. 2020, 3, 191. [Google Scholar] [CrossRef] [PubMed]
- Noor, H.; Cao, P.; Raleigh, P. Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregate amyloid fibers. Prot. Sci. 2012, 21, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, S.; Wei, W.; Yao, X.; Liu, H.; Hu, Z. Computational insights into the inhibition and destabilization of morin on the oligomer of full-length human islet amyloid popypeptide. Phys. Chem. Chem. Phys. 2015, 17, 29103–29112. [Google Scholar] [CrossRef]
- Tanaka, M.; Saito, S.; Inoue, T.; Satoh-asahara, N.; Ihara, M. Novel therapeutic potentials of taxifolin for amyloid-β-associated neurodegenerative diseases and other diseases: Recent advances and future perspectives. Int. J. Mol. Sci. 2019, 20, 2139. [Google Scholar] [CrossRef]
- Sato, M.; Murakami, K.; Uno, M.; Ikubo, H.; Nakagawa, Y.; Katayama, S.; Akagi, K.-I.; Irie, K. Structure-activity relationship for (+)-taxifolin isolated from silymarin as an inhibitor of amyloid β aggregation. Biosci. Biotechnol. Biochem. 2013, 77, 1100–1103. [Google Scholar] [CrossRef]
- Ginex, T.; Trius, M.; Luque, F.J. Computational study of the Aza-Michael addition of the flavonoid (+)-taxifolin in the inhibition of β-amyloid fibril aggregation. Chem. Eur. J. 2018, 24, 5813–5824. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Bao, X.; Ding, Y.; Shentu, J.; Cui, W.; Chen, X.; Wei, X.; Xu, S. Taxifolin prevents ß-amyloid-induced impairments of synaptic formation and deficits of memory via the inhibition of cytosolic phospholipase A 2/prostaglandin E2 content. Metab. Brain Dis. 2018, 33, 1069–1079. [Google Scholar] [CrossRef]
- ChChurches, Q.I.; Caine, J.; Cavanagh, K.; Epa, V.C.; Waddington, L.; Tranberg, C.E.; Meyer, A.G.; Varghese, J.N.; Streltsov, V.; Duggan, P.J. Naturally occurring polyphenolic inhibitors of amyloid beta aggregation. Bioorg. Med. Chem. Lett. 2014, 24, 3108–3112. [Google Scholar] [CrossRef]
- Das, S.; Pukala, T.L.; Smid, S.D. Exploring the structural diversity in inhibitors of α-synuclein amyloidogenic folding, aggregation and neurotoxicity. Front. Chem. 2018, 6, 181. [Google Scholar] [CrossRef]
- Inden, M.; Takagi, A.; Kitai, H.; Ito, T.; Kurita, H.; Honda, R.; Kamatari, Y.O.; Nozaki, S.; Wen, X.; Hijioka, M.; et al. Kaempferol has potent protective and antifibrillogenic effects for α-synuclein neurotoxicity in vitro. Int. J. Mol. Sci. 2021, 22, 11484. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, M.; Murakami, K.; Akagi, K.; Irie, K. Structural insights into mechanisms for inhibiting amyloid beta42 aggregation by non-catechol-type flavonoids. Bioorg. Med. Chem. 2016, 24, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Bhat, W.F.; Bhat, S.A.; Bano, B. Evaluation of polyphenols as possible therapeutics for amyloidoses: Comparative analysis of Kaempferol and Catechin. Int. J. Biol. Macromol. 2016, 81, 60–68. [Google Scholar]
- Kim, J.K.; Shin, E.-C.; Kim, C.R.; Park, G.G.; Choi, S.J.; Park, C.-S.; Shin, D.-H. Effects of brussels sprouts and their phytochemical components on oxidative stress-induced neuronal damages in PC12 cells and ICR mice. J. Med. Food. 2013, 16, 1057–1061. [Google Scholar]
- Akaishi, T.; Morimoto, T.; Shibao, M.; Watanabe, S.; Sakai-kato, K.; Utsunomiya-Tate, N.; Abe, K. Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid β protein. Neurosci. Lett. 2008, 444, 280–285. [Google Scholar]
- Rane, A.R.; Paithankar, H.; Hosur, R.V.; Choudharym, S. Modulation of α-synuclein fibrillation by plant metabolites, daidzein, fisetin, scopoletin under physiological conditions. Int. J. Biol. Macromol. 2021, 182, 1278–1291. [Google Scholar]
- Rosado-Ramos, R.; Godinho-Pereira, J.; Marques, D.; Figueira, I.; Fleming Outeiro, T.; Menezes, R.; Nunes dos Santos, C. Small Molecule Fisetin Modulates Alpha–Synuclein Aggregation. Molecules 2021, 26, 3353. [Google Scholar] [CrossRef]
- Espargaró, A.; Ginex, T.; Vadell, M.D.; Busquets, M.A.; Estelrich, J.; Muñoz-Torrero, D.; Luque, F.J.; Sabate, R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-Alzheimer drugs. J. Nat. Prod. 2017, 80, 278–289. [Google Scholar]
- Fang, M.; Zhang, Q.; Guan, P.; Su, K.; Wang, X.; Hu, X. Insights into molecular mechanism of EGCG and apigenin on disrupting amyloid-beta protofibrils based on molecular dynamics simulations. J. Phys. Chem. 2022, 122, 8155–8165. [Google Scholar]
- Caruana, M.; Neuner, J.; Högen, T.; Schmidt, F.; Kamp, F.; Scerri, C.; Giese, A.; Vassallo, N. Polyphenolic compounds are novel protective agents against lipid membrane damage by α-synuclein aggregates in vitro. Biophys. Biochem. Acta 2012, 1818, 2502–2510. [Google Scholar]
- Chiang, N.N.; Lin, T.H.; Teng, Y.S.; Sun, Y.C.; Chang, K.H.; Lin, C.Y.; Hsieh-Li, H.M.; Su, M.T.; Chen, C.M.; Lee-Chen, G.J. Flavones 7, 8-DHF, Quercetin, and Apigenin Against Tau Toxicity via Activation of TRKB Signaling in ΔK280 TauRD-DsRed SH-SY5Y Cells. Front. Aging Neurosci. 2021, 13, 758895. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liu, Y.; Zhang, Y.; Cai, Y.; Gong, X.; Chang, Y.; Xu, L.; Zheng, J. Genistein: A dual inhibitor of both amyloidβ and human islet amyloid polypeptides. ACS Chem. Neurosci. 2018, 9, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Roghani, M.; Joghataei, M.T.; Mohseni, S. Genistein inhibits aggregation of exogenous amyloid-beta1-40 and alleviates astrogliosis in the hippocampus of rats. Brain Res. 2012, 1429, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Zhang, Y.; Yin, F. A novel antagonistic role of natural compound icariin on neurotoxicity of amyloid β peptide. Indian J. Med. Res. 2015, 142, 190–195. [Google Scholar]
- Zhang, L.; Shen, C.; Chu, J.; Zhang, R.; Li, Y.; Li, L. Icariin decreases the expression of APP and BACE-1 and reduces the beta-amyloid burden in an APP transgenic mouse model of Alzheimer’s disease. Int. J. Biol. Sci. 2014, 10, 181–191. [Google Scholar] [CrossRef]
- Duan, S.; Guan, X.; Lin, R.; Liu, X.; Yan, Y.; Lin, R.; Zhang, T.; Chen, X.; Huang, J.; Sun, X.; et al. Silibinin inhibits acetylcholinesterase activity and amyloid ß peptide aggregation: A dual-target drug for the treatment of Alzheimer’s disease. Neurobiol. Aging 2015, 36, 1792–1807. [Google Scholar] [CrossRef]
- Murata, N.; Murakami, K.; Ozawa, Y.; Kinoshita, N.; Irie, K.; Shirasawa, T.; Shimizu, T. Silymarin attenuated the amyloid ß plaque burden and improved behavioral abnormalities in an Alzheimer’s disease mouse model. Biosci. Biotechnol. Biochem. 2010, 74, 2299–2306. [Google Scholar] [CrossRef]
- Khodabandeh, A.; Yakhchian, R.; Hasan, A.; Paray, B.A.; Shahi, F.; Rasti, B.; Mirpour, M.; Sharifi, M.; Derakhshankhah, H.; Akhtari, K.; et al. Silybin as a potent inhibitor of a-synuclein aggregation and associated cytotoxicity against neuroblastoma cells induced by zinc oxide nanoparticles. J. Mol. Liq. 2020, 310, 113198. [Google Scholar] [CrossRef]
- García-Viñuales, S.; Ilie, I.M.; Santoro, A.M.; Romanucci, V.; Zarrelli, A.; Di Fabio, G.; Caflisch, A.; Milardi, D. Silybins inhibit human IAPP amyloid growth and toxicity through stereospecific interactions. Biophys. Biochem. J. 2022, 1870, 140772. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.; Ji, X.; Wang, Y.; Zidichouski, J.; Zhang, J. Silibinin: A novel inhibitor of Aβ aggregation. Neurochem. Int. 2011, 58, 399–403. [Google Scholar] [CrossRef]
- Meng, X.; Munishkina, L.A.; Fink, A.L.; Uversky, V. Effects of various flavonoids on the α-synuclein fibrillation process. Parkinson’s Dis. 2010, 2010, 650794. [Google Scholar] [CrossRef] [PubMed]
- Yanagisava, D.; Taguchi, H.; Morikawa, S.; Kato, T.; Hirao, K.; Shirai, N.; Tooyama, I. Novel curcumin derivatives as potent inhibitors of amyloid β aggregation. Biochem. Biophys. Rep. 2015, 4, 357–368. [Google Scholar]
- Reinke, A.A.; Gestwicki, E. Structure–activity relationships of amyloid beta-aggregation inhibitors based on curcumin: Influence of linker length and flexibility. Chem. Bio. Drug Des. 2007, 70, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Liang, F.; Wan, Y.; Schaak, D.; Ward, J.; Shen, X.; Tanzi, R.E.; Zhang, C.; Quan, Q. Nanoplasmonic fiber tip probe detects significant reduction of intracellular Alzheimer’s disease-related oligomers by curcumin. Sci. Rep. 2017, 7, 5722. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borelli, L.A.; Rozkalne, A.; Hyman, T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, distrupts existing plaques and partially restores distorted neurites in an Alzheimer mouse model. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar]
- Zhao, L.N.; Chiu, S.-W.; Benoit, J.; Chew, L.Y.; Mu, Y. The effect of curcumin on the stability of Aβ dimers. J. Phys. Chem. B 2012, 116, 7428–7435. [Google Scholar] [CrossRef]
- Kumaraswamy, P.; Sethuraman, S.; Krishnan, U.M. Mechanistic insights of curcumin interactions with the core-recognition motif of β-amyloid peptide. J. Agric. Food Chem. 2013, 61, 3278–3285. [Google Scholar] [CrossRef]
- Masuda, Y.; Fukuchi, M.; Yatagawa, T.; Tada, M.; Takeda, K.; Irie, K.; Akagi, K.-I.; Monobe, Y.; Imazawa, T.; Takegoshi, K. Solid-state NMR analysis of interaction sites of curcumin and 42-residue amyloid β-protein fibrils. Bioorg. Med. Chem. 2011, 19, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.P.N.; Mohamed, T.; Teckwani, K.; Tin, G. Curcumin binding to beta amyloid: A computation study. Chem. Biol. Drug Des. 2015, 86, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.-P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Vohra, S.; Yamamoto, S.; Suzuki, K.; Pavel, K.; Shulga, S.; Blume, Y.; Kurita, N. Specific interactions between tau protein and curcumin derivatives: Molecular docking and ab initio molecular orbital simulation. J. Mol. Graph. Mod. 2020, 98, 107611. [Google Scholar] [CrossRef]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin modulates α-synuclein aggregation and toxicity. ACS Chem. Nerosci. 2013, 4, 393–407. [Google Scholar] [CrossRef]
- Ahmad, B.; Lapidus, L.J. Curcumin prevents aggregation in α-synuclein by increasing reconfiguration rate. J. Biol. Chem. 2012, 287, 193–199. [Google Scholar] [CrossRef]
- Gautam, S.; Karmakar, S.; Bose, A.; Chowdhury, P.K. β-cyclodextrin and curcumin, a potent cocktail for disaggregation and/or inhibiting amyloids: A case study with α-synuclein. Biochemistry 2014, 53, 4081–4083. [Google Scholar] [CrossRef]
- Kamelabad, M.R.; Sardroodi, J.J.; Ebrahimzadeh, A.R.; Ajamgard, M. Influence of curcumin and rosmarinic acid on disrupting the general properties of alpha-synuclein oligomer: Molecular dynamics simulation. J. Mol. Graph. Model. 2021, 107, 107963. [Google Scholar] [CrossRef]
- Sparks, S.; Liu, G.; Robbins, K.J.; Lazo, N.D. Curcumin modulates the self-assembly of the islet amyloid polypeptide by disassembling α-helix. Biochem. Biophys. Res. Commun. 2012, 422, 551–555. [Google Scholar] [CrossRef]
- Mirhashemi, S.M.; Aarabi, M.-H. Effect of two herbal polyphenol compounds on human amylin amyloid formation and destabilization. J. Med. Plants Res. 2012, 6, 3207–3212. [Google Scholar]
- Daval, M.; Bedrood, S.; Gurlo, T.; Huang, C.-J.; Costes, S.; Butler, P.C.; Langen, R. The effect of curcumin on human islet amyloid polypeptide misfolding and toxicity. Amyloid 2010, 17, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Singh, A.; Mandal, D.; Chandra, A.; Gupta, A.N. Quantification of protein aggregation rates and quenching effects of amylin–inhibitor complexes. Phys. Chem. Chem. Phys. 2019, 21, 20083–20094. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.; Pawlus, A.D.; Iglesias, M.L.; Pedrot, E.; Waffo-Teguo, P.; Merrilon, J.M.; Monti, J.P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 103–108. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.-P.; Yang, S.-G.; Wang, Y.-J.; Zhang, X.; Du, X.-T.; Sun, X.-X.; Zhao, M.; Huang, L.; Liu, R.-T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Dong, X.; Wei, G. Molecular mechanisms of resveratrol and EGCG in the inhibition of Ab42 aggregation and disruption of Ab42 protofibril: Similarities and differences. Phys. Chem. Chem. Phys. 2021, 23, 18843. [Google Scholar] [CrossRef]
- Sun, X.Y.; Dong, Q.X.; Zhu, X.; Sun, X.; Zhang, L.F.; Qiu, M.; Yu, X.L.; Liu, R.T. Resveratrol rescues tau-induced cognitive deficits abd neurophatology in a mouse model of tauopathy. Curr. Alzheimer Res. 2019, 16, 710–722. [Google Scholar] [CrossRef]
- Zhang, L.F.; Yu, X.L.; Ji, M.; Liu, S.Y.; Wu, X.L.; Wang, Y.J.; Liu, R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef]
- Gautam, S.; Karmakar, S.; Batra, R.; Sharma, P.; Pradhan, P.; Singh, J.; Kundu, B.; Chowdhury, P.K. Polyphenols in combination with β-cyclodextrin can inhibit and disaggregate α-synuclein amyloids under cell mimicking conditions: A promising therapeutic alternative. Biochim. Biophys. Acta 2017, 1865, 589–603. [Google Scholar] [CrossRef]
- Mishra, R.; Sellin, D.; Radovan, D.; Gohle, A.; Winter, R. Inhibiting islet amyloid polypeptide fibril formation by the red wine compound resveratrol. ChemBioChem 2009, 10, 445–449. [Google Scholar] [CrossRef]
- Radowan, D.; Opitz, N.; Winter, R. Fluorescence microscopy studies on islet amyloid polypeptide fibrillation at heterogeneous and cellular membrane interfaces and its inhibition by resveratrol. FEBS Lett. 2009, 583, 1439–1445. [Google Scholar] [CrossRef]
- Evers, F.; Jeworrek, C.; Tiemeyer, S.; Weise, K.; Sellin, D.; Paulus, M.; Struth, B.; Tolan, M.; Winter, R. Elucidating the mechanism of lipid membrane-induced IAPP fibrillogenesis and its inhibition by the red wine compound resveratrol: A synchrotron x-ray reflectivity study. J. Am. Chem. Soc. 2009, 131, 9516–9521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ning, L.; Niu, Y.; Liu, H.; Yao, X. Molecular mechanism of the inhibition and remodeling of human islet amyloid polypeptide (hIAPP1−37) oligomer by resveratrol from molecular dynamics simulation. J. Phys. Chem. B 2015, 119, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.H.; Young, L.M.; Wong, A.G.; Ashcroft, A.E.; Radford, S.E.; Raleigh, D.P. Mutational analysis of the ability of resveratrol to inhibit amyloid formation by islet amyloid polypeptide: Critical evaluation of the importance of aromatic−inhibitor and histidine−inhibitor interactions. Biochemistry 2015, 54, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Li, W.; Shea, J.E.; Mu, Y. Resveratrol inhibits the formation of multiple-layered β-sheet oligomers of the Human Islet Amyloid Polypeptide Segment 22–27. Biophys. J. 2011, 100, 1550–1558. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Sang, J.; Wei, W.; Zhao, W.; Beibei, C.; Zhao, F.; Jia, L.; Lu, F. Brazilin Inhibits α-Synuclein Fibrillogenesis, Disrupts Mature Fibrils, and Protects against Amyloid-Induced Cytotoxicity. J. Agric. Food. Chem. 2019, 67, 11769–11777. [Google Scholar] [CrossRef]
- Nahass, G.R.; Sun, Y.; Xu, Y.; Batchelor, M.; Reilly, M.; Benilova, I.; Kedia, N.; Spehar, K.; Sobott, F.; Sessions, R.B.; et al. Brazilin Removes Toxic Alpha-Synuclein and Seeding Competent Assemblies from Parkinson Brain by Altering Conformational Equilibrium. J. Mol. Biol. 2021, 433, 166878. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Li, L.; Wenyu, L. Brazilin inhibits fibrillogenesis of human islet amyloid polypeptide, disassembles mature fibrils, and alleviates cytotoxicity. RCS Adv. 2017, 7, 43491. [Google Scholar] [CrossRef]
- Pitt, J.; Roth, W.; Lacor, P.; Smith, A.B.; Blankeship, P.V.; Felice, F.; Breslin, P.; Klein, W.L. Alzheimer’s-associated aβ oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol. Appl. Pharmacol. 2009, 240, 189–197. [Google Scholar] [CrossRef]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Nichino, D.; Relini, A.; Berti, A.; Stefani, M. Aβ(1–42) aggregates into non-toxic amyloid assemblies in the presence of the natural polyphenol oleuropein aglycon. Curr. Alzheimer Res. 2011, 8, 841–852. [Google Scholar] [CrossRef]
- Luccarini, I.; Grossi, C.; Rigacci, S.; Coppi, E.; Pugliese, A.M.; Pantano, D.; la Marca, G.; Dami, T.E.; Berti, A.; Stefani, M.; et al. Oleuropein aglycone protects against pyroglutamylated-3 amyloid-Δ toxicity: Biochemical, epigenetic and functional correlates. Neurobiol. Aging 2015, 36, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L.; Rigacci, S.; Romeo, M.; Stefani, M.; Salmona, M. Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ42 by reducing plaque load and motor deficit. PLoS ONE 2013, 8, e58893. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 726–735. [Google Scholar] [CrossRef]
- Andradem, S.; Loureiro, J.A.; Pereire, M.C. Caffeic acid for the prevention and treatments of Alzheimer’s disease: The effect of lipid membranes on the inhibition of aggregation and disruption of Aβ fibrils. Int. J. Biol. Macromol. 2021, 190, 853–861. [Google Scholar] [CrossRef]
- Cheng, B.; Liu, X.; Gong, H.; Huang, L.; Chen, H.; Zhang, X.; Li, C.; Yang, M.; Ma, B.; Jiao, L.; et al. Coffee Components Inhibit Amyloid Formation of Human Islet Amyloid Polypeptide In Vitro: Possible Link Between Coffee Consumption and Diabetes Mellitus. J. Agric. Food Chem. 2011, 59, 13147–13155. [Google Scholar] [CrossRef]
- Pena-Diaz, S.; Ventura, S. One ring is sufficient to inhibit α-synuclein aggregation. Neural. Regen. Res. 2022, 17, 508–511. [Google Scholar]
- Liu, Y.; Pukala, T.L.; Musgrave, I.F.; Williams, D.M.; Dehle, F.C.; Carver, J.A. Gallic acid is the major component of grape seed extract that inhibits amyloid fbril formation. Bioorg. Med. Chem. Lett. 2013, 23, 6336–6340. [Google Scholar] [CrossRef]
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta 2014, 1844, 1481–1485. [Google Scholar] [CrossRef]
- Yao, J.; Gao, X.; Sun, W.; Yao, T.; Shi, S.; Ji, L. Molecular Hairpin: A Possible model for inhibition of tau aggregation by tannic acid. Biochemistry 2013, 52, 1893–1902. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, X.; Lu, Y.; Shi, S.; Yang, D.; Yao, T. New strategy for reducing tau aggregation cytologically by a hairpin-like molecular inhibitor, tannic acid encapsulated in liposome. ACS Chem. Neurosci. 2020, 11, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, M.; Kimura, Y.; Aguirre, C.; Kakuda, K.; Takeuchi, T.; Choong, C.-J.; Doi, J.; Nabekura, K.; Yamaguchi, K.; Nakajima, K.; et al. Two-step screening method to identify α-synuclein aggregation inhibitors for Parkinson’s disease. Sci. Rep. 2022, 12, 351. [Google Scholar]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Antiamyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim. Biophys. Acta 2004, 1690, 193–202. [Google Scholar] [CrossRef]
- Bruno, E.; Pereira, C.; Roman, K.P.; Takiguchi, M.; Kao, P.Y.; Nogaj, L.A.; Moffet, D.A. IAPP aggregation and cellular toxicity are inhibited by1,2,3,4,6-penta-O-galloyl-b-D-glucose. Amyloid 2013, 20, 34–38. [Google Scholar] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, Y.; Cao, H.; Wang, Y.; Teng, T.; Mao, G.; Li, Y.; Li, K.; Zhang, Y. Ferulic acid inhibits the transition of amyloid-β42 monomers to oligomers but accelerates the transition from oligomers to fibrils. J. Alzheimer’s Dis. 2013, 37, 19–28. [Google Scholar]
- Bramanti, E.; Fulgentini, L.; Bizzarri, R.; Lenci, F.; Sgarbossa, A. β-amyloid amorphous aggregates induced by the small natural molecule ferulic acid. J. Phys. Chem. B 2013, 117, 13816–13821. [Google Scholar]
- Wu, Y.; Shi, Y.-G.; Zheng, X.-L.; Dang, Y.-L.; Zhu, C.-M.; Zhang, R.-R.; Fu, Y.-Y.; Zhou, T.-Y.; Li, J.-H. Lipophilic ferulic acid derivatives protect PC12 cells against oxidative damage via modulating β-amyloid aggregation and activating Nrf2 enzymes. Food Funct. 2020, 11, 4707–4718. [Google Scholar]
- Ono, K.; Yamada, M. Antioxidants compounds have potent anti-fibrillogenic and fibril-destabilizing effects for α-synuclein fibrils in vitro. J. Neurochem. 2006, 97, 105–115. [Google Scholar]
- Medvedeva, M.; Barinova, K.; Melnikova, A.; Semenyuk, P.; Kolmogorov, V.; Gorelkin, P.; Erofeev, A.; Muronetz, V. Naturally occurring cinnamic acid derivatives prevent amyloid transformation of α-synuclein. Biochimie 2020, 170, 128–139. [Google Scholar] [CrossRef]
- Chaari, A.; Abdellatif, B.; Nabi, F.; Khan, R. Date palm (Phoenix dactylifera L.) fruit’s polyphenols as potential inhibitors for human amylin fibril formation and toxicity in type 2 diabetes. Int. J. Biol. Macromol. 2020, 164, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, G.; Shigemori, H. Inhibitory activity on amyloid aggregation of rosmarinic acid and its substructures from Isodon japonicus. Nat. Prod. Commun. 2019, 164, 14. [Google Scholar] [CrossRef]
- Airoldi, C.; Sironi, E.; Dias, C.; Marcelo, F.; Martins, A.; Rauter, A.P.; Nicotra, F.; Jimenez-Barbero, J. Natural compounds against alzheimer’s disease: Molecular recognition of aβ1–42 peptide by Salvia sclareoides extract and its major component, rosmarinic acid, as investigated by NMR. Chem. Asian J. 2013, 8, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, A.; Sandoval, F.A.; Caballero, L.; Machuca, L.; Muñoz, P.; Caballero, J.; Perry, G.; Ardiles, A.; Areche, C.; Melo, F. Rosmarinic acid prevents fibrillization and diminishes vibrational modes associated to β sheet in tau protein linked to Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2017, 32, 945–953. [Google Scholar] [CrossRef]
- Terry, C. Insights from nature: A review of natural compounds that target protein misfolding in vivo. Curr. Res. Biotechnol. 2020, 2, 131–134. [Google Scholar]
- Malishev, R.; Shaham-Niv, S.; Nandi, S.; Kolusheva, S.; Gazit, E.; Jelinek, R. Bacoside-A, an Indian traditional-medicine substance, inhibits β-amyloid cytotoxicity; fibrillation, and membrane interaction. ACS Chem. Neurosci. 2017, 8, 884–891. [Google Scholar] [CrossRef]
- Tangrodchanapong, T.; Sobhon, P.; Meemon, K. Frondoside A attenuates amyloid-β proteotoxicity in transgenic Caenorhabditis elegans by suppressing its formation. Front. Farmacol. 2020, 11, 553579. [Google Scholar]
- Chalorak, P.; Sanguanphun, T.; Limboonreung, T.; Meemon, K. Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease. Molecules 2021, 26, 4843. [Google Scholar] [CrossRef]
- Zhi, L.; Hang, L.; Chunbui, Z.; Cui, L.; Changjia, Z.; Wenfeng, X.; Wensheng, Z. Protective effect of notoginsenoside R1 on an APP/PS1 mouse model of alzheimer&aposs disease by up-regulating insulin-degrading enzyme and inhibiting Aβ accumulation. CNS Neurol. Disord.-Drug Targets. 2015, 14, 360–369. [Google Scholar]
- Wu, A.-G.; Wong, V.K.-W.; Xu, S.-W.; Chan, W.-K.; Ng, C.-I.; Liu, L.; Law, B.Y.-K. Onjisaponin B Derived from Radix Polygalae Enhances Autophagy and Accelerates the Degradation of Mutant α-Synuclein and Huntingtin in PC-12 Cells. Int. J. Mol. Sci. 2013, 14, 22618–22641. [Google Scholar] [CrossRef]
- Fujihara, K.; Koike, S.; Ogasawara, Y.; Takahashi, K.; Koyama, K.; Kinoshita, K. Inhibition of amyloid β aggregation and protective effect on SH-SY5Y cells by triterpenoid saponins from the cactus Polaskia chichipe. Bioorg. Med. Chem. 2017, 25, 3377–3383. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.H.; Han, H.; Hu, X.D.; Shi, L.L. Protective effect of ginsenoside Rb1 on β—Amyloid protein (1-42)-induced neurotoxicity in cortical neurons. Neurol. Res. 2009, 31, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.L.; Park, B.H.; Hou, J.; Oh, J.P.; Han, J.H.; Kim, S.C. Ginsenoside F1 protects the brain against amyloid Beta-induced toxicity by regulating IDE and NEP. Life 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. RCS Adv. 2022, 12, 18746–18758. [Google Scholar] [CrossRef]
- Othman, A.; Sayed, A.M.; Amen, Y.; Shimizu, K. Possible neuroprotective effects of amide alkaloids from Bassia indica and Agathophora alopecuroides: In vitro and in silico investigation. Molecules 2021, 26, 728. [Google Scholar] [CrossRef]
- Plazas, E.; Hagenow, S.; Murillo, M.A.; Stark, H.; Cuca, L.E.; Plazas, E.; Suarez, L.C. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ1-42 aggregation. Bioorg. Chem. 2020, 98, 103722. [Google Scholar] [CrossRef]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; De Pandis, M.F.; Roviello, G.N. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine based compounds on β-amyloid aggregation. Chem.-Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef]
- Shi, A.; Huang, L.; Lu, C.; He, F.; Li, X. Synthesis, biological evaluation and molecular modeling of novel triazole-containing berberine derivatives as acetylcholinesterase and β-amyloid aggregation inhibitors. Bioorg. Med. Chem. 2011, 19, 2298–2305. [Google Scholar] [CrossRef]
- Shan, W.J.; Huang, L.; Zhou, Q.; Meng, F.C.; Li, X.S. Synthesis, biological evaluation of 9-N-substituted berberine derivatives as multi-functional agents of antioxidant, inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-β aggregation. Eur. J. Med. Chem. 2011, 46, 5885–5893. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef]
- Cao, C.; Cirrito, J.R.; Lin, X.; Wang, L.; Verges, D.K.; Dickson, A.; Mamcarz, M.; Zhang, C.; Mori, T.; Arendash, G.W.; et al. Caffeine suppresses amyloid-β levels in plasma and brain of Alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 2009, 71, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Kalita, S.; Paul, A.; Mandal, B.; Paul, S. The role of caffeine as an inhibitor in the aggregation of amyloid forming peptides: A unified molecular dynamics simulation and experimental study. J. Agric. Food. Chem. 2011, 59, 13147–13155. [Google Scholar]
- Zeng, H.; Zhang, Y.; Peng, L.-J.; Shao, H.; Menon, N.K.; Yang, J.; Salomon, A.R.; Freidland, R.P.; Zagorski, M.G. Nicotine and amyloid formation. Biol. Psychiatry 2001, 49, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Kardani, J.; Sethi, R.; Roy, I. Nicotine slows down oligomerization of α-synuclein and ameliorates cytotoxicity in a yeast model of Parkinson’s disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1454–1463. [Google Scholar] [CrossRef]
- Ono, K.; Hirohata, M.; Yamada, M. Anti-fibrillogenic and fibril-destabilizing activity of nicotine in vitro: Implications for the prevention and therapeutics of Lewy body diseases. Exp. Neurol. 2007, 205, 414–424. [Google Scholar]
- Matharu, B.; Gibson, G.; Parsons, R.; Huckerby, T.N.; Moore, S.A.; Cooper, L.J.; Millichamp, R.; Allsop, D.; Austen, B. Galantamine inhibits β-amyloid aggregation and cytotoxicity. J. Neurol. Sci. 2009, 280, 49–58. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Patal, K.; Hu, R.; Chuang, S.; Zhang, G.; Zheng, J. Tanshinones inhibit amyloid aggregation by amyloid-β peptide, disaggregate amyloid fibrils and protect cultured cells. ACS Chem. Neurosci. 2013, 4, 1004–1015. [Google Scholar]
- Ren, B.; Liu, Y.; Zhang, Y.; Sun, Y.; Liang, G.; Xu, J.; Zheng, J. Tanshinones inhibit hIAPP aggregation, disaggregate preformed hIAPP fibrils, and protect cultured cells. J. Mater. Chem. B 2018, 6, 56–67. [Google Scholar]
- Cai, N.; Chen, J.; Bi, D.; Gu, L.; Yao, L.; Li, X.; Xu, H.; Hu, Z.; Liu, Q.; Xu, X. Specific degradation of endogenous tau protein and inhibition of tau fibrillation by tanshinone IIA through the ubiquitin-proteasome pathway. J. Agric. Food Chem. 2020, 68, 2054–2062. [Google Scholar]
- Ji, K.; Zhao, Y.; Yu, T.; Wang, Z.; Gong, H.; Yang, X.; Liu, Y.; Huang, K. Inhibition effects of tanshinone on the aggregation of α-synuclein. Food Funct. 2016, 7, 409–416. [Google Scholar]
- Ding, Y.; Qiao, A.; Wang, Z.; Goodwin, J.S.; Lee, E.-S.; Block, M.L.; Allsbrook, M.; McDonald, M.P.; Fan, G.-H. Retinoic acid attenuates beta-amyloid deposition and reduces memory deficits in an Alzheimer’s disease transgenic mouse model. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 11622–11634. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gattoni-Celli, M.; Zhu, H.; Bhat, N.R.; Sambamurti, K.; Gattoni-Celli, S.; Kindy, M.S. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J. Alzheimer’s Dis. 2011, 25, 295–307. [Google Scholar]
- Ibrahim, N.F.; Yanagisawa, D.; Durani, L.W.; Hamezah, H.S.; Damanhuri, H.A.; Wan Ngah, W.Z.; Tsuji, M.; Kiuchi, Y.; Ono, K.; Tooyama, I. Tocotrienolrich fraction modulates amyloid pathology and improves cognitive function in AβPP/PS1 mice. J. Alzheimer’s Dis. 2016, 55, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, J.; Ono, K.; Yoshiike, Y.; Hirohata, M.; Ikeda, T.; Morinaga, A.; Takashima, A.; Yamada, M. Vitamin A has anti-oligomerization effects on amyloid-β in vitro. J. Alzheimer’s Dis. 2014, 27, 271–280. [Google Scholar]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Vitamin A exhibits potent anti-amyloidogenic and fibril-destabilizing effects in vitro. Exp. Neurol. 2004, 189, 380–392. [Google Scholar] [CrossRef]
- Alam, P.; Siddiqi, M.K.; Malik, S.; Chaturvedi, S.K.; Uddin, M.; Khan, R.H. Elucidating the inhibitory potential of vitamin A against fibrillation and amyloid associated cytotoxicity. Int. J. Biol. Macromol. 2019, 129, 333–338. [Google Scholar]
- Alam, P.; Siddiqi, M.K.; Chaturvedi, S.K.; Zaman, M.; Khan, R.H. Vitamin b12 offers neuronal cell protection by inhibiting abeta-42 amyloid fibrillation. Int. J. Biol. Macromol. 2017, 99, 477–482. [Google Scholar]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Vitamin b12 inhibits abeta fibrillation and disaggregates preformed fibrils in the presence of synthetic neuronal membranes. ACS Chem. Neurosci. 2021, 12, 2491–2502. [Google Scholar]
- Lauer, A.A.; Grimm, H.S.; Apel, B.; Golobrodska, N.; Kruse, L.; Ratanski, E.; Schulten, N.; Schwarze, L.; Slawik, T.; Sperlich, S.; et al. Mechanistic Link Between Vitamin B12 and Alzheimer’s Disease. Biomolecules 2022, 12, 129. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Wei, W.; Zhao, W.; Lu, F.; Liu, F. Vitamin B12 inhibits α-synuclein fibrillogenesis and protects against amyloid-induced cytotoxicity. Food Funct. 2019, 10, 2861–2870. [Google Scholar]
- Rafiee, S.; Assadollahi, K.; Riazi, G.; Ahmadian, S.; Saboury, A.A. Vitamin B12 inhibits tau fibrillization via binding to cysteine residues of tau. ACS Chem. Neurosci. 2017, 8, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L.; Cerqueira, E.C.; Freitas, M.S.; Goncalves, D.L.; Costa, L.T.; Follmer, C. Vitamin K interact with N-terminus α-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and α-synuclein. Neurochem. Int. 2013, 62, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Chaturvedi, S.K.; Siddiqi, M.K.; Rajpoot, R.K.; Ajmal, M.R.; Zaman, M.; Khan, R.H. Vitamin K3 inhibits protein aggregation: Implication in the treatment of amyloid diseases. Sci. Rep. 2016, 6, 26759. [Google Scholar] [CrossRef]
- Chasemzadeh, S.; Riazi, G.H. Inhibition of tau amyloid fibril formation by folic acid: In-vitro and theoretical studies. Int. J. Biol. Maromol. 2020, 1546, 1505–1516. [Google Scholar] [CrossRef]
- Loópez, L.C.; Varea, O.; Navarro, S.; Carrodeguas, J.A.; Sanchez de Groot, N.; Ventura, S.; Sancho, J. Benzbromarone, Quercetin, and Folic Acid Inhibit Amylin Aggregation. Int. J. Mol. Sci. 2016, 17, 964. [Google Scholar] [CrossRef]
- Ghahghaei, A.; Bathaie, S.Z.; Kheirkhan, H.; Bahraminejad, E. The protective effect of crocin on the amyloid fibril formation of aβ peptide in vitro. Cell. Mol. Biol. Lett. 2013, 18, 328–339. [Google Scholar]
- Tigan, M.G.; Ghahghaei, A.; Lagzian, M. In-vitro and in-silico investigation of protective mechanisms of crocin against E46K α-synuclein amyloid formation. Mol. Biol. Rep. 2019, 46, 4279–4292. [Google Scholar] [CrossRef]
- Saffari, B.; Amininasab, M. Crocin inhibits the fibrillation of Human α-synuclein and disassembly mature fibrils: Experimental findings and mechanistic insights from molecular dynamics simulation. ACS Chem. Neurosci. 2021, 12, 4037–4057. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Doens, D.; Kumar, J.; Murillo, E.; Fernández, P.L.; Rao, K.; Durant, A. Anti-amyloid aggregation activity of novel carotenoids: Implications for Alzheimer’s drug discovery. Clin. Interv. Aging 2017, 12, 815–822. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin inhibits β-amyloid assembly and attenuates β-amyloid oligomer-induced cognitive impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ(1–42)) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2022, 25, 2167–2180. [Google Scholar] [CrossRef]
- Mohammad-Beigi, H.; Kjaer, L.; Eskandari, H.; Aliakbari, F.; Christiansen, G.; Ruvo, G.; Ward, J.L.; Otzen, D.E. A possible connection between plant longevity and the absence of protein fibrillation: Basis for identifying aggregation inhibitors in plants. Front. Plant Sci. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Ogara, T.; Takahashi, T.; Yasui, H.; Uwai, K.; Tokuraku, K. Evaluation of the effects of amyloid β aggregation from seaweed extracts by a microliter-scale high-throughput screening system with a quantum dot nanoprobe. J. Biosci. Bioeng. 2015, 120, 45–50. [Google Scholar] [CrossRef]
- Boubakri, A.; Leri, M.; Bucciantini, M.; Najjaa, H.; Ben Arfa, A.; Stefani, M.; Neffati, M. Allium roseum L. extract inhibits amyloid beta aggregation and toxicity involved in Alzheimer’s disease. PLoS ONE 2020, 15, e0223815. [Google Scholar] [CrossRef]
- Li, Q.; Yanbei, T.; Zhu, C.; Luo, W.; Huang, W.; Liu, W.; Li, Y. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crop Prod. 2017, 108, 512–519. [Google Scholar] [CrossRef]
- Steffi, W.; Raiker, W.; Raivo, V.; Ago, S. Medical plants and nutraceuticals for amyloid-β fibrillation inhibition. J. Alzheimer’s Dis. Rep. 2018, 2, 239–252. [Google Scholar]
- Holcomb, L.A.; Dhanasekaran, M.; Hitt, A.R.; Young, K.A.; Riggs, M.; Manyam, B.V. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J. Alzheimers Dis. 2006, 9, 243–251. [Google Scholar] [CrossRef]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Dubey, T.; Kushwaha, P.; Thulsairam, H.V.; Chandrashekar, M.; Chinnathambi, S. Bacopa monnieri reduces tau aggregation and tau-mediated toxicity in cells. Int. J. Biol. Macromol. 2023, 234, 123171. [Google Scholar] [CrossRef] [PubMed]
- Jadiya, P.; Khan, A.; Sammi, S.R.; Kaur, S.; Mir, S.S.; Nazir, A. Anti-Parkinsonian effects of Bacopa monnieri: Insights from transgenic and pharmacological Caenorhabditis elegans models of Parkinson’s disease. Biochem. Biopys. Res. Commun. 2022, 25, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Kleinrichert, K.; Alappat, B. Comparative Analysis of Antioxidant and Anti-Amyloidogenic Properties of Various Polyphenol Rich Phytoceutical Extracts. Antioxidants 2019, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.L.; Hennessy, K.; Pascual, J.; Pepe, N.; Wang, I.; Santiago, A.; Chaggan, C.; Martinez, J.; Rivera, E.; Cota, P.; et al. Identification of plant extracts that inhibit the formation of diabetes-linked IAPP amyloid. J. Herb. Med. 2016, 6, 37–41. [Google Scholar] [CrossRef]
- Ravi, S.K.; Narasingappa, R.B.; Prasad, M.; Javagal, M.R.; Vincent, B. Cassia tora prevents Aβ1-42 aggregation, inhibits acetylcholinesterase activity and protects against Aβ1-42-induced cell death and oxidative stress in human neuroblastoma cells. Pharmacol. Rep. 2019, 71, 1151–1159. [Google Scholar] [CrossRef]
- Berrocal, R.; Vasudevaraju, P.; Indi, S.S.; Sambasiva Rao, K.R.S.; Rao, K.S. In vitro evidence that an aqueous extracts of Centella asiatica modulates α-synuclein aggregation dynamics. J. Alzheimers Dis. 2014, 39, 457–465. [Google Scholar] [CrossRef]
- Chen, C.L.; Tsai, W.H.; Chen, C.J.; Pan, T.Z. Centella asiatica extract protects against amyloid β1-40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J. Tradt. Complement. Med. 2015, 6, 362–369. [Google Scholar] [CrossRef]
- Peterson, D.W.; George, R.C.; Scaramozzino, F.; Lapointe, N.F.; Anderson, R.A.; Graves, D.J.; Lew, J. Cinnamon extract inhibits tau aggregation associated with Alzheimer’s disease in vitro. J. Alzheimer’s Dis. 2009, 17, 585–597. [Google Scholar] [CrossRef]
- Manalo, R.V.; Silvestre, M.A.; Barbosa, A.L.A.; Medina, P.M. Coconut (Cocos nucifera) Ethanolic leaf extract reduces Amyloid-β (1–42) aggregation and paralysis prevalence in transgenic Caenorhabditis elegans independently of free radical scavenging and acetylcholinesterase inhibition. Biomedicines 2017, 5, 17. [Google Scholar] [CrossRef]
- Tu, Y.; Huang, J.; Li, Y. Anticholinesterase, antioxidant, and beta-amyloid aggregation inhibitory constituents from Cremastra appendiculata. Med. Chem. Res. 2017, 27, 857–863. [Google Scholar] [CrossRef]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory activity on amyloid-β aggregation and antioxidant properties of Crocus sativus Stigmas extract and its crocin constituents. J. Agric. Food. Chem. 2006, 54, 8762–8768. [Google Scholar] [CrossRef] [PubMed]
- Inoue, E.; Shimizu, Y.; Masui, R.; Hayakawa, T.; Tsubonoya, T.; Hori, S.; Sudoh, K. Effects of saffron and its constituents, crocin-1, crocin-2 and crocetin on α-synuclein fibrils. J. Nat. Med. 2018, 72, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Bharate, S.S.; Kumar, V.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B.; Kaddoumi, A. Crocus sativus extract tightens the blood-brain barrier, reduces amyloid β load and related toxicity in 5XFAD mice. ACS Chem. Neurosci. 2017, 8, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, D.; Aliakbari, F.; Tayaranian- Marvian, A.; Fassini, A.; Pan-Montojo, F.; Perez-Sanchez, H. Cuminaldehyde as the major component of Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J. Food. Sci. 2015, 80, H2336–H2345. [Google Scholar] [CrossRef]
- Thakur, A.; Chun, Y.S.; October, N.; Yang, H.O.; Maharaj, V. Potential of South African medicinal plants targeting the reduction of Aβ42 protein as a treatment of Alzheimer’s disease. J. Ethnopharmacol. 2019, 231, 363–373. [Google Scholar] [CrossRef]
- Paul, K.; Ganguly, U.; Chakrabarti, S.; Bhattacharjee, P. Is 1,8-cineole-rich extract of small cardamon seeds more effective in preventing Alzheimer’s disease than 1,8-cineole alone. Neuromol. Med. 2020, 22, 150–158. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar] [CrossRef]
- Luo, Y. Alzheimer’s disease, the nematode Caenorhabditis elegans, and Ginkgo biloba leaf extract. Life Sci. 2006, 78, 2066–2072. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Lobbens, E.S.; Breydo, L.; Skamris, T.; Vestergaard, B.; Jager, A.K.; Jorgensen, L.; Uversky, V.; de Weert, M. Mechanistic study of the inhibitory activity of Geum urbanum extract against α-synuclein fibrillation. Biochem. Biophys. Acta 2016, 1864, 1160–1169. [Google Scholar] [CrossRef]
- Liao, M.; Zhao, Y.; Huang, L.; Cheng, B.; Huang, K. Isoliquiritigenin and liquiritin from Glycyrrhiza uralensis inhibit α-synuclein amyloid formation. RSC Adv. 2016, 6, 86640–86649. [Google Scholar]
- Tan, M.A.; Lagamayo, M.W.D.; Alejandro, G.J.D.; An, S.S.A. Anti-Amyloidogenic and Cyclooxygenase Inhibitory Activity of Guettarda speciosa. Molecules 2019, 24, 4112. [Google Scholar] [CrossRef] [PubMed]
- Kleawyothatis, W.; Jattujan, P.; Chumphoochai, K.; Chalorak, P.; Sobhon, P. Holothuria scarba extracts confer neuprotactive effect in C. elegans model of Alzheimer’s disease by attenuating amyloid-β aggregation and toxicity. J. Tradit. Complement. Med. 2023, 13, 93–104. [Google Scholar] [PubMed]
- Chauchan, N.; Wang, K.C.; Wegiel, J.; Malik, M.N. Walnut extract inhibits the fibrilization of amyloid-beta protein and also destabilizes its preformed fibrils. Curr. Alzheimer Res. 2004, 1, 183–188. [Google Scholar]
- Hanaki, M.; Murakami, K.; Gunji, H.; Irie, K. Activity-differential search for amyloid-β aggregation inhibitors using LC-MS combined with principal component analysis. Bioorg. Med. Chem. Lett. 2022, 61, 128613. [Google Scholar]
- Dhouafli, Z.; Rigacci, S.; Leri, M.; Bucciantini, M.; Mahjoub, B.; Tounsi, M.S.; Wannes, W.A.; Stefani, M.; Hayounti, E.A. Screening for amyloid-β aggregation inhibitor and neuronal toxicity of eight Tunisian medicinal plants. Ind. Crops Prod. 2018, 111, 823–833. [Google Scholar] [CrossRef]
- Meena, V.K.; Kumar, V.; Karalia, S. Inhibitory effect of naturally occurring Ocimum sanctum extract on α-synuclein aggregation in aqueous solution. J. Mol. Liq. 2021, 336, 116176. [Google Scholar]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef]
- Basli, A.; Delaunay, J.C.; Pedrot, E.; Bernillon, S.; Madani, K.; Monti, J.P.; Mérillon, J.M.; Chibane, M.; Richard, T. New cyclolignans from Origanum glandulosum active against β-amyloid aggregation. Rec. Nat. Prod. 2014, 8, 208–216. [Google Scholar]
- Fujiwara, H.; Tabuchi, M.; Yamaguchi, T.; Iwasaki, K.; Furukawa, K.; Sekiguchi, K.; Ikarashi, Y.; Kudo, Y.; Higuchi, M.; Saido, T.C.; et al. A traditional medicinal herb Paeonia suffruticosa and its active constituents 1,2,3,4,6,-penta-O-galloyl-β-d-glucopyranose have potent anti-aggregation effects on Alzheimer’s amyloid βproteins in vitro and in vivo. J. Neurochem. 2009, 109, 1648–1657. [Google Scholar]
- Kawamoto, H.; Takeshita, F.; Murata, K. Inhibitory effects of essential oil extracts from Panax plants against β-secretase, cholinesterase, and amyloid aggregation. Nat. Prod. Commun. 2019, 14, 1934578X19881549. [Google Scholar]
- Shimamori, K.; Nambu, T.; Kawamata, D.; Kuragano, M.; Nishishita, N.; Iimori, T.; Yamanaka, S.; Uwai, K.; Tokuraku, K. Cultivation Factors That Affect Amyloid-β Aggregation Inhibitory Activity in Perilla frutescens var. crispa. Foods 2023, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, H.; Ghanadian, M.; Noleto-Dias, C.; Lomax, C.; Tawfike, A.; Christiansen, G.; Sutherland, D.S.; Ward, J.L.; Mohammad-Beigi, H.; Otzen, D.E. Inhibitors of α-synuclein fibrillation and oligomer toxicity in Rosa damascene: The all-pervading powers of flavonoids and phenolic glycosides. ACS Chem. Neurosci. 2020, 11, 3161–3173. [Google Scholar] [CrossRef] [PubMed]
- Hovnanyan, K.; Sharoyan, S.; Antonyan, A.; Hovnanyan, N.; Mardanyan, S. Effects of extract and phenol glycoside from Rose Petals on the amylin fibrils. OALib 2017, 4, 1–9. [Google Scholar]
- Sashourpour, M.; Zahri, S.; Radjabin, T.; Ruf, V.; Pan-montojo, F.; Morshedi, D. A study on the modulation of alpha-synuclein fibrillation by Scutellaria pinnatifida extracts and its neuroprotective properties. PLoS ONE 2017, 12, e0184483. [Google Scholar]
- Fujiwara, H.; Iwasaki, K.; Furukawa, K.; Seki, T.; He, M.; Maruyama, M.; Tomita, N.; Kudo, Y.; Higuchi, M.; Saido, T.C.; et al. Uncaria rhynchophylla, a Chinese medicinal Herb, has potent antiaggregation effects on Alzheimer’s β-amyloid proteins. J. Neurosci. Res. 2006, 84, 427–433. [Google Scholar]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon rain forest pant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci. Rep. 2019, 9, 561. [Google Scholar]
- Miyazaki, H.; Okamoto, Y.; Motoi, A.; Watanabe, T.; Katayama, S.; Kawahara, S.-I.; Makabe, H.; Fujii, H.; Yonekura, S. Adzuki bean (Vigna angularis) extract reduces amyloid-β aggregation and delays cognitive impairment in Drosophila models of Alzheimer’s disease. Nutr. Res. Pract. 2019, 13, 64–69. [Google Scholar]
- Floris, S.; Fais, A.; Medda, R.; Pintus, F.; Piras, A.; Kumar, A.; Kus, P.M.; Westermark, G.T.; Era, B. Washingtonia filifera seed extracts inhibits the islet amyloid polypeptide fibrils formations and α-amylase and α-glucosidase activity. J. Enzyme Inhib. Med. Chem. 2021, 1, 517–524. [Google Scholar] [CrossRef]
- Kumar, S.; Harris, R.J.; Seal, H.C.; Okello, E.J. An aqueous extract of Withania somnifera root inhibits amyloid βfibril formation in vitro. Phytother. Res. 2012, 26, 113–117. [Google Scholar]
- Sehgal, N.; Gupta, A.; Valli, R.K.; Joshi, S.D.; Mills, J.T.; Hamel, E.; Khanna, P.; Jain, S.C.; Thakur, S.S.; Ravindranath, V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA 2012, 109, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, J.; Vidyashankar, R.; Godbole, A. Differential effect of nootropics on C. elegans models of Parkinson’s disease. J. Ayurveda Integr. Med. 2020, 11, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Khan, R.H. Protein misfolding and related human diseases: A comprehensive review of toxicity, proteins involved, and current therapeutic strategies. Int. J. Biol. Macromol. 2022, 223 Pt A, 143–160. [Google Scholar] [CrossRef]
- Valastyan, J.S.; Lindquist, S. Mechanisms of protein-folding diseases at a glance. Dis. Models Mech. 2014, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Amendola, G.; Cosconati, S. PyRMD: A new fully automated ai-powered ligand-based virtual screening tool. J. Chem. Inf. Model. 2021, 61, 3835–3845. [Google Scholar] [CrossRef]
- Kang, S.; Kim, M.; Sun, J.; Lee, M.; Min, K. Prediction of protein aggregation propensity via Data-driven approaches. ACS Biomater. Sci. Eng. 2023, 9, 6451–6463. [Google Scholar] [CrossRef]
- Segura, L.Y.; Santos, N.; Cheng, K.H.K.; Sikazwe, D.; Flores, R.; Rogers, V.B.; Mcgibbon, M. Targeting anti-aggregation and anti-oxidation of amyloid deposits for therapeutic intervention of Alzheimer’s using machine learning-enhanced molecular docking. Biophys. J. 2024, 123, 416a. [Google Scholar] [CrossRef]
- Das, B.; Mathew, A.T.; Baidya, A.T.K.; Devi, B.; Salmon, R.R.; Kumar, R. Artificial intelligence assisted identification of potential tau aggregation inhibitors: Ligand- and structure-based virtual screening, in silico ADME, and molecular dynamics study. Mol. Divers. 2024, 28, 2013–2031. [Google Scholar] [CrossRef]
- Levin, J.; Maaß, S.; Schuberth, M.; Giese, A.; Oertel, W.H.; Poewe, W.; Trenkwalder, C.; Wenning, G.K.; Mansmann, U.; Südmeyer, M.; et al. Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019, 18, 724–735. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Salehi, B.; Calina, D.; Docea, A.O.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Castillo, C.M.S.; Martorell, M.; et al. Curcumin’s nanomedicine formulations for therapeutic application in neurological diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
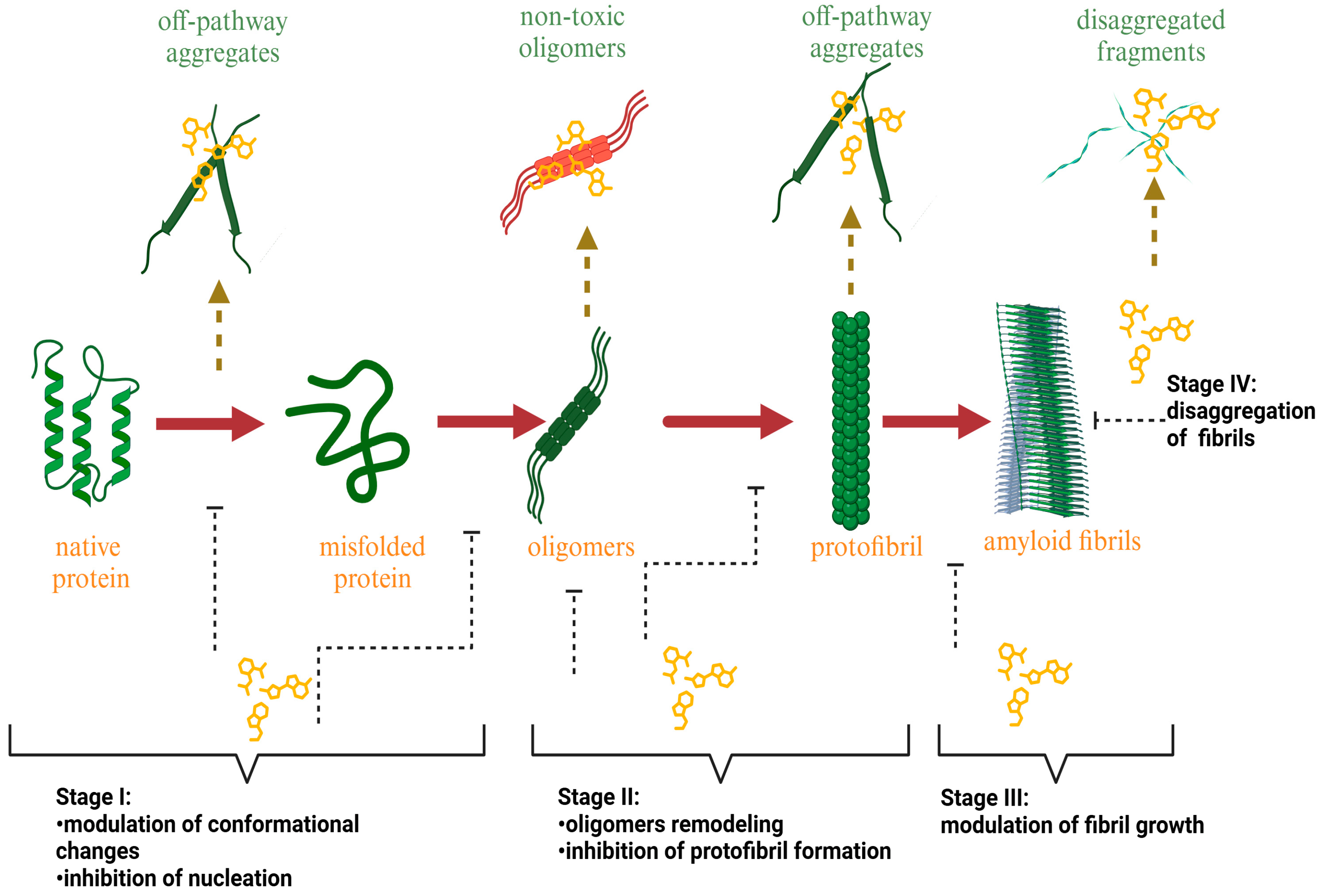
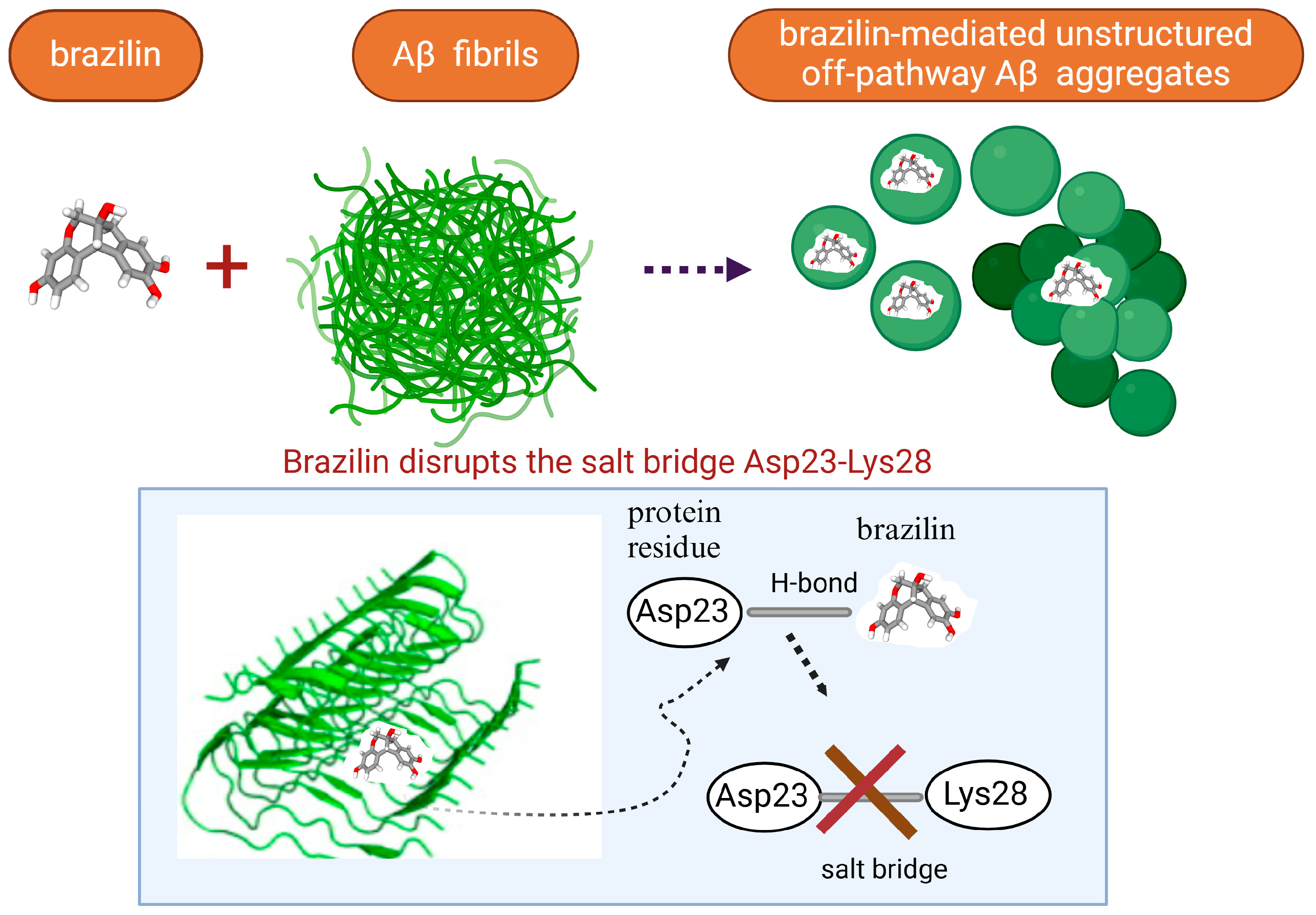
| Plant-Based Compound | Main Source | Targeted Protein | IC50 | Effects | References |
|---|---|---|---|---|---|
(−)Epigallocatechin-3-gallate (EGCG) | Green tea | Aβ | 15 µM (Aβ42) 3 µM (Aβ40) |
| [58,81,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116] |
| Tau | Nd ˟ | ||||
| synuclein | Nd | ||||
| IAPP | Nd | ||||
Baicalein | Scutellaria baicalensis | Aβ42 | 1.35 μM |
| [80,89,90,91,117,118,119,120,121,122,123,124] |
| Tau | 35.8 μM | ||||
| synuclein | Nd | ||||
| IAPP | 92.1 μM | ||||
Quercetin | fruits, vegetables, red wine | Aβ42 | 15.3 μM |
| [8,41,61,76,94,125,126,127,128,129,130,131] |
| synuclein | Nd | ||||
| IAPP | Nd | ||||
| tau | >200 μM | ||||
Myricetin | fruits, vegetables, red wine | Aβ42 | 15.1 μM |
| [66,71,76,94,132,133,134,135,136,137,138,139,140,141,142,143,144] |
| Tau | Nd | ||||
| synuclein | 3.57 μM | ||||
| IAPP | Nd | ||||
Dihydromyricetin | herb Ampelopsis grossedentata | Aβ40 Ab42 | 18.96 μM 25.3 μM |
| [94,145,146,147] |
| synuclein | Nd | ||||
Rutin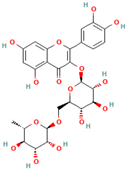 | Buckwheat, citrus fruits, apicots, blackberries, apple, cherries, red wine. | Aβ, Tau, IAPP | Nd |
| [41,76,82,126,148,149,150,151] |
Morin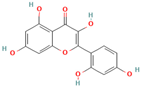 | almond hulls, guava leaves, old fustic, onion, apple, tea, red wine | Aβ1-42 | 30.3 μM |
| [37,62,94,152,153,154] |
| Tau | 13 µM | ||||
| synuclein | Nd | ||||
| IAPP | Nd | ||||
(+)-Taxifolin | onions, milk thistle, Douglas fir bark | Aβ | 33 μM |
| [63,94,131,155,156,157,158] |
| Tau | >200 μM | ||||
| synuclein | >40 μM | ||||
| IAPP | Nd | ||||
Rottlerin | Kamala powder of the Mallotus philippinensis fruits | Aβ | 19.55 μM |
| [39] |
Transilitin | Luculia pinceana, Acacia cyperophylla | Aβ | 5.4 μM |
| [130,159,160] |
| synuclein | Nd | ||||
Kaempferol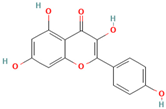 | fruits, vegetables, and medicinal plants | Aβ | 75.1 μM |
| [94,135,161,162,163,164] |
| synuclein | Nd | ||||
Fisetin | fruits and vegetables | Aβ | 43.7 μM |
| [56,77,165,166,167] |
| Tau | 41.45 μM | ||||
| synuclein | Nd | ||||
| hIAPP | Nd | ||||
Apigenin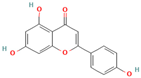 | fruits and vegetables | Aβ | Nd |
| [61,64,160,168,169,170,171] |
| tau | Nd | ||||
| synuclein | Nd | ||||
Genistein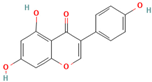 | soybeans and soy products | Aβ | Nd |
| [82,172,173] |
| hIAPP | Nd | ||||
Icariin | Chinese herbal medicine Herba Epimedii | Aβ | 0.48 μM |
| [174,175] |
Silybin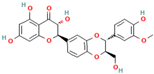 | Silybum Marianum | Aβ | Nd |
| [82,176,177,178,179,180] |
| synuclein | Nd | ||||
| hIAPP | Nd | ||||
Luteolin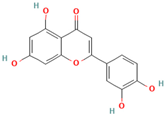 | celery, parsley, peppermint, thyme, oregano | Aβ | 6.4 μM |
| [73,92,159,181] |
| Plant-Based Compound | Main Source | Targeted Protein | IC50 | Effects | References |
|---|---|---|---|---|---|
Curcumin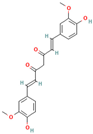 | Curcuma longa | Aβ1-40 | 0.8 µM |
| [35,36,65,83,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203] |
| Tau | 3.0 µM | ||||
| synuclein | Nd | ||||
| IAPP | Nd | ||||
Resveratrol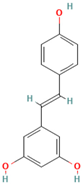 | red grapes | Aβ1-42 | 11 µM |
| [37,72,183,190,204,205,206,207,208,209,210,211,212,213,214] |
| Tau | 10 µM | ||||
| synuclein | Nd | ||||
| IAPP | 3.3 µM | ||||
Brazilin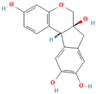 | wood of Caesalpinia echinata or Caesalpinia sappan | Aβ1-42 | 1.5 µM |
| [70,215,216,217,218,219,220,221] |
| synuclein | Nd | ||||
| IAPP | Nd | ||||
Altenusin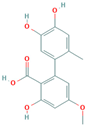 | Fungal endophyte Alternaria | Tau | Nd |
| [40] |
Oleocanthal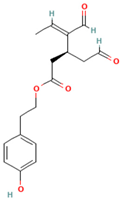 | Olive oil | Aβ | 2.9 µM |
| [38,95,219] |
| Tau | |||||
| Nd | |||||
Oleuropein aglycone | olive oil | Aβ | Nd |
| [67,78,220,221,222,223,224] |
| Tau | 1.4 μM | ||||
| synuclein | Nd | ||||
| IAPP | Nd | ||||
Caffeic acid | coffee beans, fruits, potatoes, carrots and propolis | Aβ | 17.9 µM |
| [76,84,225,226,227] |
| Tau | Nd | ||||
| synuclein | Nd | ||||
| IAPP | 57.6 µM | ||||
Tannic acid | Tara pods, gallnuts or Sicilian sumac leaves | Aβ | 0.1 μM |
| [230,231,232,233,234] |
| Tau | 3.5 μM | ||||
| synuclein | Nd | ||||
| IAPP | Nd | ||||
Ferulic acid | cereal grains, fruits, vegetables | Aβ | Nd |
| [3,76,235,236,237,238,239,240,241] |
| Tau | Nd | ||||
| synuclein | 13 μM | ||||
| IAPP | 40 μM | ||||
Gallic acid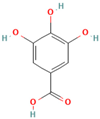 | fruits, teas, cloves, and vinegars. | Aβ | Nd |
| [76,227,228,229,230] |
| Tau | 92 μM | ||||
| synuclein | Nd | ||||
| IAPP | Nd | ||||
Rosmarinic acid | species in the Labiatae and Boraginaceae family of plants. | Aβ | 4.8 μM |
| [74,199,239,242,243,244] |
| Tau | 7.7 μM | ||||
| synuclein | Nd | ||||
| IAPP | 3.1 μM |
| Plant Extract | Extract Type | Model System | Effects | References |
|---|---|---|---|---|
| Acer saccharum | acetone, water, and ethanol | in vitro, α-synuclein, and Aβ1-40 | inhibits the formation of amyloid fibrils | [294] |
| Alaria crassifolia | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Allium Roseum | ethanolic | in vitro, Aβ1-42, and SH-SY5Y cells | inhibits amyloid-β aggregation and amyloid-induced cytotoxicity | [296] |
| Alisma orientale | ethanolic | in vitro, Aβ1-42 | 25% of protein aggregation inhibition | [297] |
| Bacopa monnieri | aqueous | in vitro and MAβ1-40 PSAPP mice | inhibits the formation of amyloid fibrils and reduces amyloid levels in PSAPP mice | [298,299,300] |
| ethanolic | in vitro, tau, and tau-stressed Neuro2A neuronal cells | inhibits tau aggregation and reduces tau-mediated toxicity in cells | [301] | |
| NL5901 strain of C. elegans | reduces synuclein aggregation in NL5901 worms | [302] | ||
| Camellia sinensis | methanolic | in vitro and Aβ1-42 | inhibits amyloid-β aggregation | [303] |
| Capsicum annuum | ethyl acetate | In vitro, IAPP, and HeLa cells | attenuates IAPP aggregation and protects cells from amyloid-induced toxicity | [304] |
| Cassi Tore | ethyl acetate | in vitro, Aβ1-42, SK-N-SH, and SH-SY5Y cells | inhibits amyloid fibrillation in a dose-dependent manner and alleviates Aβ-induced oxidative stress in human neuroblastoma cell lines. | [305] |
| Centella asiatica | ethanolic | in vitro and MAβ1-40 PC12 cells | reduces the formation of amyloid fibrils and protects PC12 cells from amyloid-induced toxicity | [298] |
| aqueous | in vitro and α-synuclein | inhibits protein aggregation and dissociates fibrils | [306,307] | |
| Chondrous yendoi | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Cinnamomumverum | aqueous | in vitro and tau187 | inhibits tau filament formation, disaggregates preformed tau aggregates, and causes alterations in the morphology of AD brain-isolated fibrils | [308] |
| Cocos nucifera | ethanolic | Transgenic C. elegans | reduces Aβ deposits by 30.3% in CL2006 | [309] |
| Cystoseira hakodatensis | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Cremastra appendiculata | ethanolic | in vitro and Aβ1-42 | inhibits amyloid-β aggregation | [310] |
| Crocus sativus L. (Saffron) | water/methanol 50:50 | in vitro and Aβ1-40 | inhibits the formation of amyloid fibrils in a concentration-dependent manner | [311] |
| DMSO/water 10:90 | in vitro and α-synuclein | dose-dependently inhibits protein aggregation and dissociates fibrils | [312] | |
| water/ethanol (1:1) | AD mouse model | significantly reduced (53%) the total Aβ levels in 5XFAD brains hippocampi and decreased Aβ brain deposits | [313] | |
| Cuminum cyminum | Essential oil | in vitro and α-synuclein | dose-dependently inhibits protein aggregation | [314] |
| Cussonia paniculata | dichloromethane/methanol | in vitro and HeLa cells | reduces Aβ production in HeLa cells | [315] |
| Curcuma longa | methanolic | in vitro and Aβ1-42 | inhibits amyloid-β aggregation | [303] |
| Elettaria cardamomum | 1,8-cineole-rich extract | in vitro, Aβ1-42, and SH-SY5Y cells | prevents Aβ oligomerization and protects SH-SY5Y cells against iron-induced death | [316] |
| Gardenia jasminoides | ethanolic | in vitro and Aβ1-42 | 32% of protein aggregation inhibition | [297] |
| Geum urbanum | ethanolic | in vitro and α-synuclein | inhibits protein aggregation and disaggregates fibrils | [317] |
| Gloiopeltis furcata | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Ginkgo giloba | standardized EGb761 | in vitro, Aβ1-40, neuroblastoma cell lines, and C. elegans | inhibits amyloid fibrillation and prevents Aβ aggregation in the medium of Aβ-producing cells | [318,319,320] |
| Glycyrrhiza uralensis | ethanolic | in vitro, α-synuclein, and C. elegans NL5901 | inhibits protein aggregation and alleviates amyloid-induced toxicity | [321] |
| Guettarda speciosa | methanol, chloroform | in vitro, Aβ1-42, and SH-SY5Y cells | reduces the formation of amyloid fibrils | [322] |
| Holothuria scarba | ethanol, buthanol and ethyl acetate | Caenorhabditis elegans | attenuates amyloid-β aggregation and toxicity | [323] |
| Juglas regia | methanolic | in vitro and Aβ | inhibits amyloid fibrillation and defibrillizes performed fibrils | [324] |
| Juncus effusus | ethanolic | in vitro and Aβ1-42 | 68% of protein aggregation inhibition | [297] |
| Juncus setchuensis | ethanolic | in vitro and Aβ1-42 | 68% of protein aggregation inhibition | [297] |
| Lawsonia inermis L. | methanolic | in vitro, Aβ1-42, and SH-SY5Y cells | inhibits Aβ1-42 aggregation in the early stage of β-sheet-rich structure formation and reduces amyloid-induced cytotoxicity | [325] |
| Mazzaella japonica | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation, alters fibril morphology | [295] |
| Mentha sachalinensis | ethyl acetate | In vitro, IAPP, and HeLa cells | attenuates IAPP aggregation and protects cells from amyloid-induced toxicity | [304] |
| Mentha piperite | ethyl acetate | In vitro, IAPP, and HeLa cells | attenuates IAPP aggregation, protects cells from amyloid-induced toxicity | [304] |
| Nardostachys chinensi | ethanolic | in vitro and Aβ1-42 | 62.8% of protein aggregation inhibition | [297] |
| Nelumbinis folium | ethanolic | in vitro and Aβ1-42 | inhibits protein aggregation | [326] |
| Ocimum sanctum | DMSO | in vitro, α-synuclein, and mouse Neuro2a cells | inhibits protein aggregation, disaggregates preformed fibrils, and protects mouse neuroblastoma cells against α-synuclein amyloid-induced cytotoxicity. | [327] |
| Olea europaea | acetone/water | transgenic worms | prevents β-amyloid aggregation in Caenorhabditis elegans | [328] |
| Origanum glandulosum | ethyl acetate | in vitro and Aβ25-35 | reduces amyloid fibril formation in a dose-dependent manner | [329] |
| Panionia suffruticosa | water | in vitro, Aβ1-42, Aβ1-40, in vivo, and Tg2576 mice | inhibits fibril formation, destabilizes preformed amyloid fibrils, and inhibits the accumulation of Aβ in the brain of Tg2576 transgenic mice | [330] |
| Panax notoginseng | Essential oil | in vitro and Aβ1-42 | 57.3% inhibition at 500 μg/mL against Aβ aggregation | [331] |
| Perila frutescens | ethanolic | in vitro and Aβ1-42 | inhibits the formation of amyloid fibrils in a concentration-dependent manner | [332] |
| Pistacia lentiscus L. | methanolic | in vitro, Aβ1-42, and SH-SY5Y cells | hinders Aβ1-42 aggregation during the early secondary structure transition to amyloid | [325] |
| Punica granatum L. | methanolic | in vitro, Aβ1-42, and SH-SY5Y cells | hinders Aβ1-42 aggregation during the early secondary structure transition to amyloid | [325] |
| Rheum officinale | ethanolic | in vitro and Aβ1-42 | 92% of protein aggregation inhibition | [297] |
| Rosa damascena | methanolic | in vitro, α-synuclein, and SH_SY5Y cells | inhibits α-syn fibrillation in a concentration-dependent manner and reduces the toxicity of oligomers to SH-SY5Y cells. | [333] |
| ethanolic | in vitro and IAPP | modulates IAPP aggregation | [334] | |
| Saccharina japonica | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Saccharina sculpera | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Sargassum fusitorme | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Sargassum horneri | ethanolic and boiling water | in vitro and Aβ | inhibits amyloid fibrillation and alters fibril morphology | [295] |
| Schotia brachypetala | dichloromethane/methanol | in vitro and HeLa cells | reduces the Aβ production in HeLa cells | [306] |
| Spatholobus suberectus Dunn | ethanolic | in vitro and Aβ1-42 | 90.5% of protein aggregation inhibition | [297] |
| Scutellaria pinnatidifa | dichloromethane, n-butanol | in vitro, α-synuclein, PC12 cells, and dopaminergic neurons | dose-dependently inhibits protein fibrillization and protects against amyloid-induced cytotoxicity | [335] |
| Thymus vulgaris | ethyl acetate | In vitro, IAPP, and HeLa cells | attenuates IAPP aggregation and protects cells from amyloid-induced toxicity | [304] |
| Uncaria rhynchophyla | ethanolic, methanolic, aqueous | in vitro and Aβ | inhibits Aβ fibril formation and disassembles preformed Aβ fibrils | [336] |
| Uncaria tomentosa | Water | in vitro, Aβ, and tau | inhibits Aβ40 and tau protein amyloid fibril formation and disrupts preformed Aβ42 and tau protein fibrils to amorphous non-toxic aggregates | [337] |
| Vigna angularis | ethanolic | in vitro, Aβ1-42, and drosophila models | dose-dependently inhibits protein fibrillization and reduces the Aβ level in the brain of Aβ-overexpressing Drosophila | [338] |
| Washingtonia filifera seed | aqueous, methanolic, ethanolic | in vitro and IAPP | inhibits α-amylase, α-glucosidase enzyme activity, and IAPP fibril formation | [339] |
| Withania somnifera | aqueous | in vitro and Aβ1-42 | reduces protein fibrillation in a concentration-dependent manner and inhibits cholesterol-induced Aβ1-42 aggregation | [340] |
| residual material of chloroform-methanol extract | middle-aged and old APP/PS1 Alzheimer’s disease transgenic mice | reduces amyloid plaques, β-amyloid peptides levels, and oligomers in the brains of middle-aged and old APP/PS1 Alzheimer’s disease transgenic mice | [341] | |
| methanolic | Caenorhabditis elegans and BZ555 and NL5901 strains | exhibits neuroprotective and α-synuclein aggregation-mitigating effects | [342] | |
| Xysmalobium undulatum | dichloromethane /methanol | in vitro and HeLa cells | reduces Aβ production in HeLa cells | [317] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhytniakivska, O.; Chaturvedi, T.; Thomsen, M.H. Plant-Based Inhibitors of Protein Aggregation. Biomolecules 2025, 15, 481. https://doi.org/10.3390/biom15040481
Zhytniakivska O, Chaturvedi T, Thomsen MH. Plant-Based Inhibitors of Protein Aggregation. Biomolecules. 2025; 15(4):481. https://doi.org/10.3390/biom15040481
Chicago/Turabian StyleZhytniakivska, Olha, Tanmay Chaturvedi, and Mette Hedegaard Thomsen. 2025. "Plant-Based Inhibitors of Protein Aggregation" Biomolecules 15, no. 4: 481. https://doi.org/10.3390/biom15040481
APA StyleZhytniakivska, O., Chaturvedi, T., & Thomsen, M. H. (2025). Plant-Based Inhibitors of Protein Aggregation. Biomolecules, 15(4), 481. https://doi.org/10.3390/biom15040481








