ONC201 (Dordaviprone) Induces Integrated Stress Response and Death in Cervical Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cytotoxicity Assay
2.3. Clonogenicity Assay
2.4. Cell Cycle Analysis
2.5. Annexin-V/PI Assay
2.6. Caspase-Glo® 3/7 Assay
2.7. Acridine Orange/Ethidium Bromide (AO/EB) Assay
2.8. Real-Time PCR
2.9. Western Blot Analysis
2.10. In Vitro Combination Assay
2.11. Statistical Analysis
3. Results
3.1. ONC201 Exerts Cytotoxicity in Cervical Cancer Cell Lines
3.2. ONC201 Induces S/G2-M Arrest in CC Cells
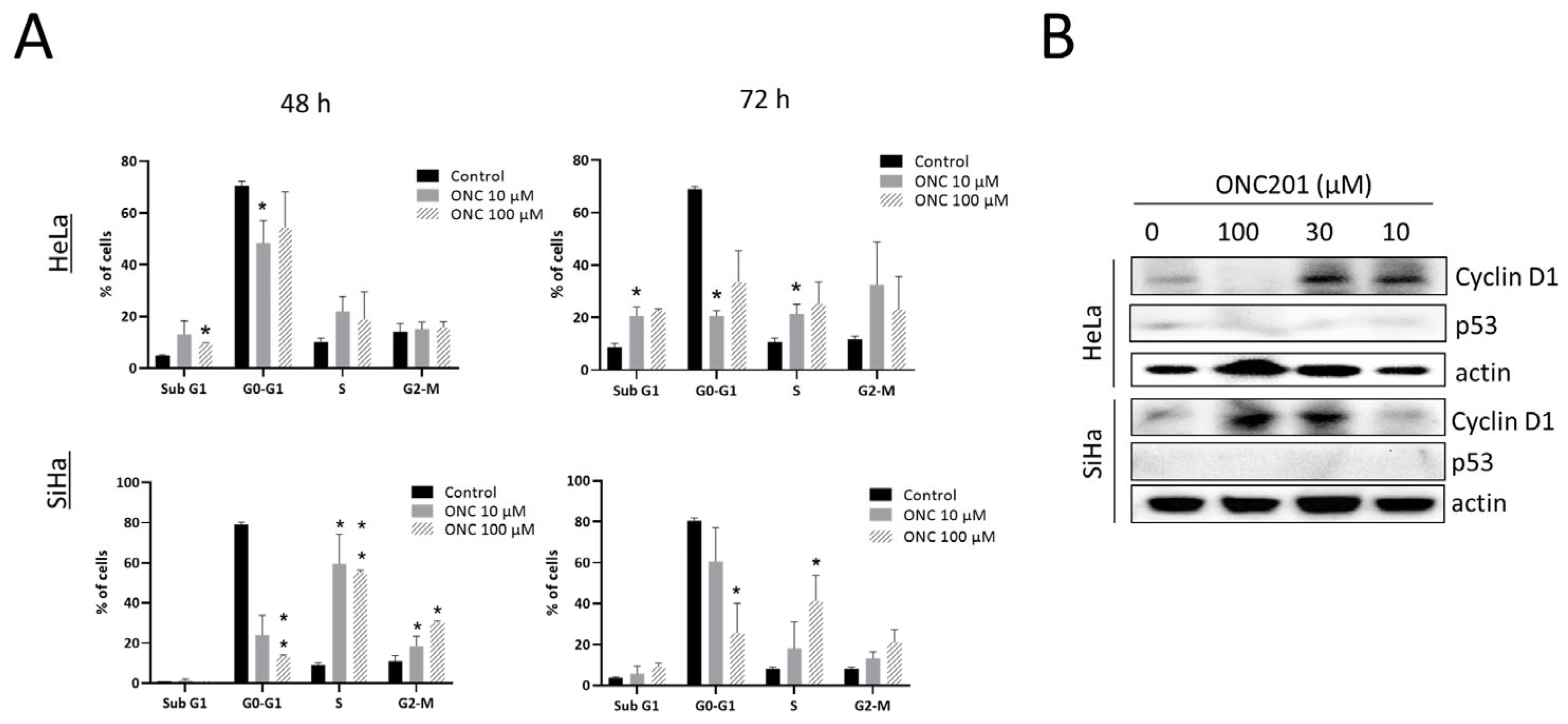
3.3. ONC201 Induces Integrated Stress Response While Downregulating Akt and Erk Phosphorylation

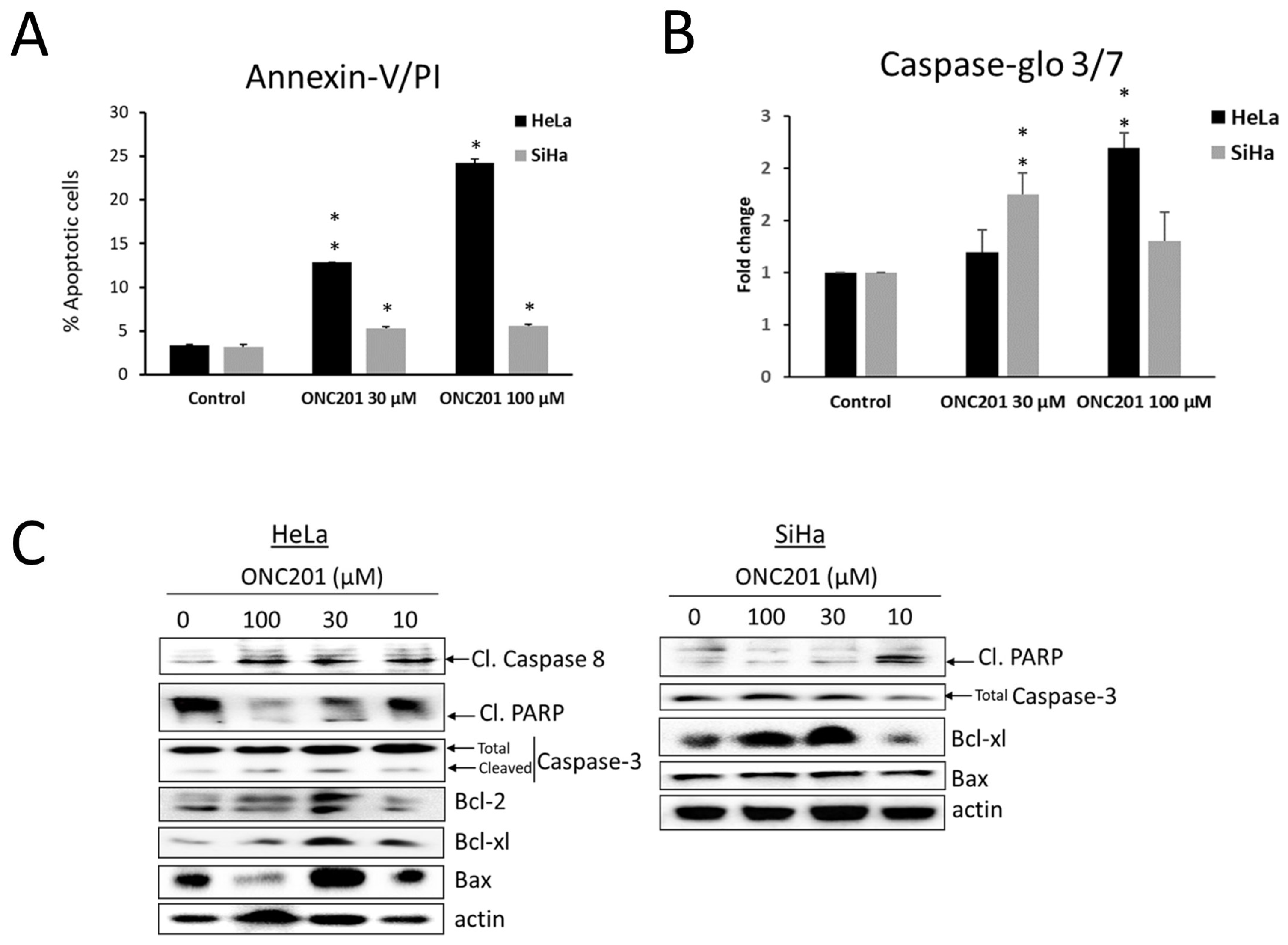
3.4. Apoptosis Is Induced by ONC201 in CC Cells
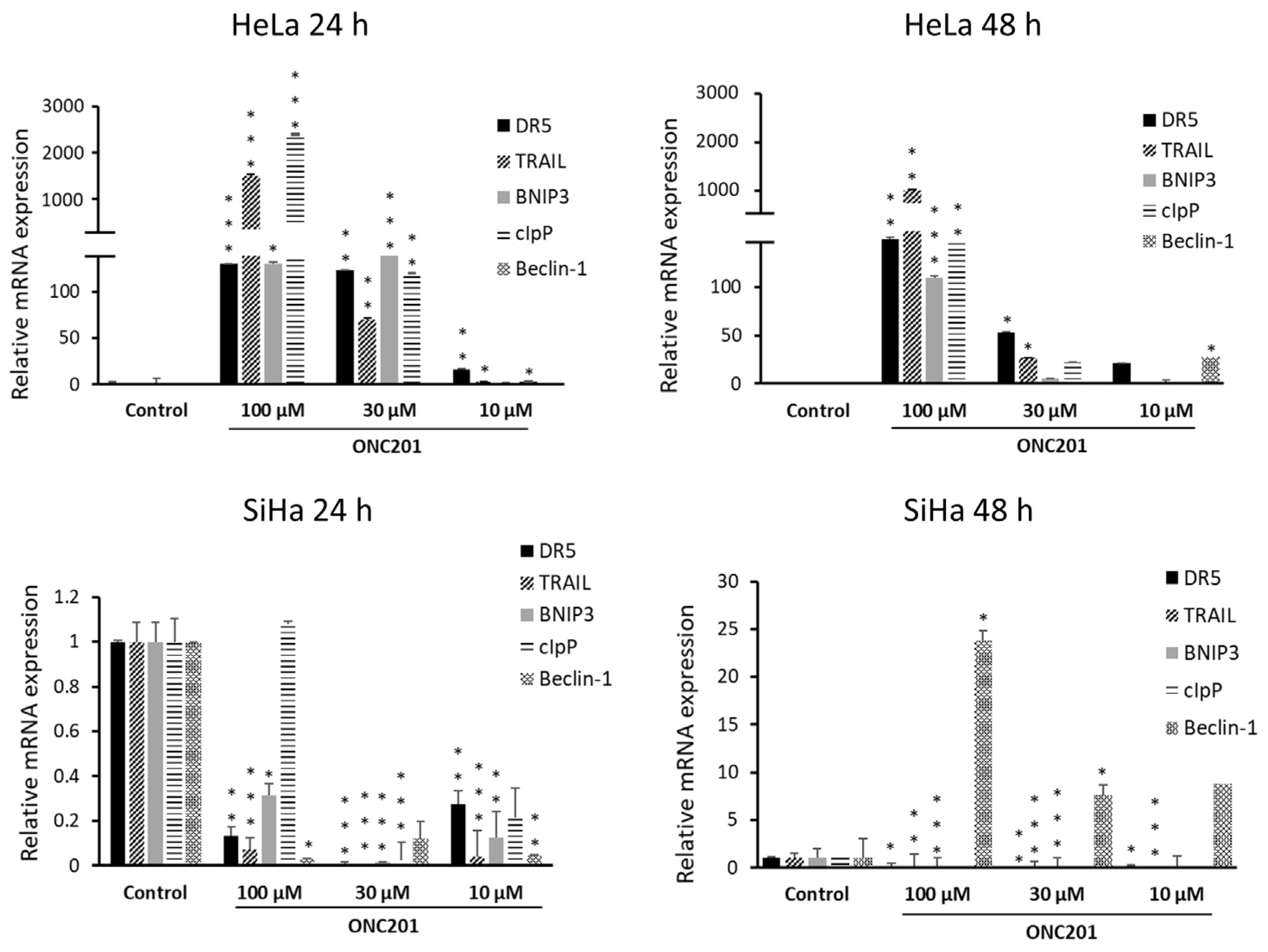
3.5. ONC201 Upregulates Pro-Apoptotic Gene Expression
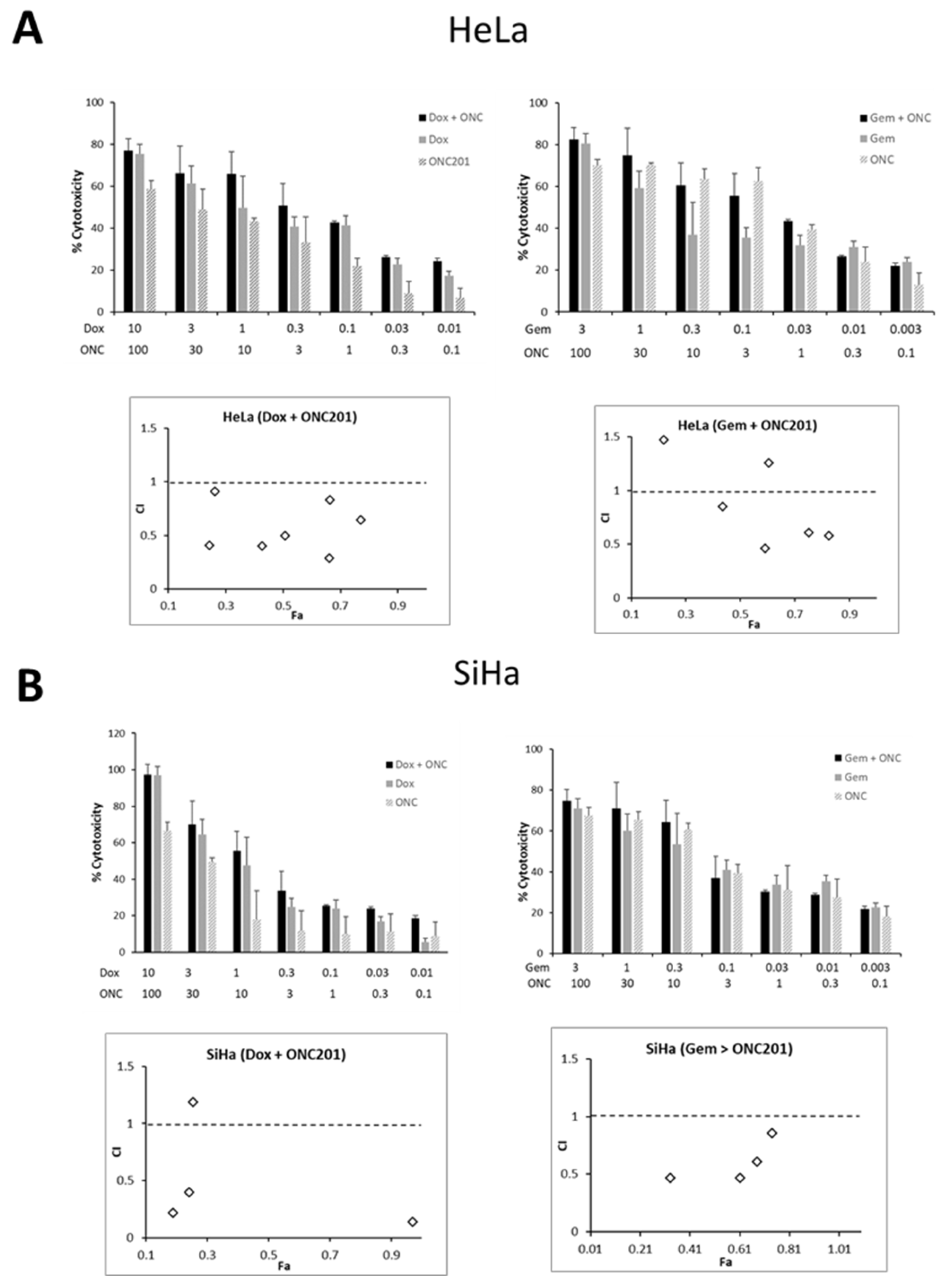
3.6. ONC201 Exhibits Synergism in Combination with Standard Chemotherapeutic Agents Against CC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef]
- Bouvard, V.; Wentzensen, N.; Mackie, A.; Berkhof, J.; Brotherton, J.; Giorgi-Rossi, P.; Kupets, R.; Smith, R.; Arrossi, S.; Bendahhou, K.; et al. The IARC perspective on cervical cancer screening. N. Engl. J. Med. 2021, 385, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.E.; Craig, D.J.; Vellani, S.D.; Hegazi, A.; Fredrickson, K.J.; Walter, A.; Stanbery, L.; Nemunaitis, J. Advances in targeted therapy for the treatment of cervical cancer. J. Clin. Med. 2023, 12, 5992. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Solazzo, M.C.; Bizzarri, N.; Ambrosio, D.; La Verde, M.; Torella, M.; Carotenuto, R.M.; Cobellis, L.; Colacurci, N.; De Franciscis, P. Fertility-sparing treatment for early-stage cervical cancer ≥ 2 cm: A problem with a thousand nuances-a systematic review of oncological outcomes. Ann. Surg. Oncol. 2022, 29, 8346–8358. [Google Scholar] [CrossRef]
- Mahiou, K.; Vincent, L.; Peignaux-Casasnovas, K.; Costaz, H.; Padeano, M.M.; Amet, A.; Dabakuyo, S.; Mamguem Kamga, A.; Bengrine-Lefevre, L.; Coutant, C. Impact of therapeutic strategy on disease-free and overall survival of early-stage cervical cancer: Surgery alone versus preoperative radiation. Cancer Rep. 2023, 6, e1823. [Google Scholar] [CrossRef]
- Allen, J.E.; Krigsfeld, G.; Patel, L.; Mayes, P.A.; Dicker, D.T.; Wu, G.S.; El-Deiry, W.S. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol. Cancer. 2015, 14, 99, Erratum in Mol. Cancer. 2024, 23, 233. https://doi.org/10.1186/s12943-024-02158-w. [Google Scholar] [CrossRef]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013, 5, 171ra17. [Google Scholar] [CrossRef]
- Kline, C.L.; Van den Heuvel, A.P.; Allen, J.E.; Prabhu, V.V.; Dicker, D.T.; El-Deiry, W.S. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci. Signal. 2016, 9, ra18. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, G.; Fang, K.; Sun, H. Development of novel anticancer agents with a scaffold of Tetrahydropyrido [4, 3-d] pyrimidine-2, 4-dione. ACS Med. Chem. Lett. 2019, 10, 191–195. [Google Scholar] [CrossRef]
- Wang, S.; Dougan, D.A. The direct molecular target for Imipridone ONC201 is finally established. Cancer Cell 2019, 35, 707–708. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Morrow, S.; Rahman Kawakibi, A.; Zhou, L.; Ralff, M.; Ray, J.; Jhaveri, A.; Ferrarini, I.; Lee, Y.P.; Arker, C.; et al. ONC201 and imipridones: Anti-cancer compounds with clinical efficacy. Neoplasia 2020, 22, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Zarabi, S.F.; Davis, R.E.; Halgas, O.; Nii, T.; Jitkova, Y.; Zhao, R.; St-Germain, J.; Heese, L.E.; Egan, G.; et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell 2019, 35, 721–737.e9. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Kline, C.L.; Prabhu, V.V.; Wagner, J.; Ishizawa, J.; Madhukar, N.; Lev, A.; Baumeister, M.; Zhou, L.; Lulla, A.; et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget 2016, 7, 74380–74392. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Peterson, C.B.; Davis, A.; Cho, E.; Pearson, T.; Liu, H.; Hwang, M.; Ueno, N.T.; Lee, J. ONC201 and an MEK inhibitor Trametinib synergistically inhibit the growth of Triple-Negative Breast cancer cells. Biomedicines 2021, 9, 1410. [Google Scholar] [CrossRef]
- Wagner, J.; Kline, C.L.; Zhou, L.; Khazak, V.; El-Deiry, W.S. Anti-tumor effects of ONC201 in combination with VEGF-inhibitors significantly impacts colorectal cancer growth and survival in vivo through complementary non-overlapping mechanisms. J. Exp. Clin. Cancer Res. 2018, 37, 11, Erratum in Exp. Clin. Cancer Res. 2024, 43, 257. https://doi.org/10.1186/s13046-024-03185-7. [Google Scholar] [CrossRef]
- Ray, J.E.; Ralff, M.D.; Jhaveri, A.; Zhou, L.; Dicker, D.T.; Ross, E.A.; El-Deiry, W.S. Antitumorigenic effect of combination treatment with ONC201 and TRAIL in endometrial cancer in vitro and in vivo. Cancer Biol. Ther. 2021, 22, 554–563. [Google Scholar] [CrossRef]
- Ralff, M.D.; Jhaveri, A.; Ray, J.E.; Zhou, L.; Lev, A.; Campbell, K.S.; Dicker, D.T.; Ross, E.A.; El-Deiry, W.S. TRAIL receptor agonists convert the response of breast cancer cells to ONC201 from anti-proliferative to apoptotic. Oncotarget 2020, 11, 3753–3769. [Google Scholar] [CrossRef]
- Amoroso, F.; Glass, K.; Singh, R.; Liberal, F.; Steele, R.E.; Maguire, S.; Tarapore, R.; Allen, J.E.; Van Schaeybroeck, S.; Butterworth, K.T.; et al. Modulating the unfolded protein response with ONC201 to impact on radiation response in prostate cancer cells. Sci. Rep. 2021, 11, 4252. [Google Scholar] [CrossRef]
- Stein, M.N.; Bertino, J.R.; Kaufman, H.L.; Mayer, T.; Moss, R.; Silk, A.; Chan, N.; Malhotra, J.; Rodriguez, L.; Aisner, J.; et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin. Cancer Res. 2017, 23, 4163–4169. [Google Scholar] [CrossRef]
- Stein, M.N.; Malhotra, J.; Tarapore, R.S.; Malhotra, U.; Silk, A.W.; Chan, N.; Rodriguez, L.; Aisner, J.; Aiken, R.D.; Mayer, T.; et al. Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J. Immunother. Cancer 2019, 7, 136. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Manohar, S.M. A flow cytometric journey into cell cycle analysis. Bioanalysis 2021, 13, 1627–1644. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.M.; Rathos, M.J.; Sonawane, V.; Rao, S.V.; Joshi, K.S. Cyclin-dependent kinase inhibitor, P276-00 induces apoptosis in multiple myeloma cells by inhibition of Cdk9-T1 and RNA polymerase II-dependent transcription. Leuk. Res. 2011, 35, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Shah, P.; Biswas, S.; Mukadam, A.; Joshi, M.; Viswanathan, G. Combining fluorescent cell barcoding and flow cytometry-based phospho-ERK1/2 detection at short time scales in adherent cells. Cytometry A 2019, 95, 192–200. [Google Scholar] [CrossRef]
- Manohar, S.M.; Joshi, K.S. Multitarget CDK inhibitors roscovitine and UCN-01 induce apoptosis in colorectal cancer cells by inhibiting cell cycle progression and transcription. Braz. J. Pharm. Sci. 2025, 61, e24402. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef]
- Chou, T.C. Preclinical versus clinical drug combination studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef]
- Buch, K.; Peters, T.; Nawroth, T.; Sänger, M.; Schmidberger, H.; Langguth, P. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT Assay—A comparative study. Radiat. Oncol. 2012, 7, 1. [Google Scholar] [CrossRef]
- Manohar, S.M.; Shah, P.; Nair, A. Flow cytometry: Principles, applications and recent advances. Bioanalysis 2021, 13, 181–198. [Google Scholar] [CrossRef]
- Manohar, S.M.; Joshi, K.S. Promising anticancer activity of multitarget cyclin dependent kinase inhibitors against human colorectal carcinoma cells. Curr. Mol. Pharmacol. 2022, 15, 1024–1033. [Google Scholar] [CrossRef]
- Lines, C.L.; McGrath, M.J.; Dorwart, T.; Conn, C.S. The integrated stress response in cancer progression: A force for plasticity and resistance. Front. Oncol. 2023, 13, 1206561. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cui, M.; Wang, D.; Guo, B.; Zhang, L. Tumor necrosis factor-related apoptosis inducing ligand overexpression and Taxol treatment suppresses the growth of cervical cancer cells in vitro and in vivo. Oncol. Lett. 2018, 15, 5744–5750. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liao, Q.; Su, M.; Huang, K.; Jin, J.; Cao, D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019, 459, 30–40. [Google Scholar] [CrossRef]
- Burton, E.; Ozer, B.H.; Boris, L.; Brown, D.; Theeler, B. Imipridones and dopamine receptor antagonism in the therapeutic management of gliomas. Adv. Oncol. 2024, 4, 101–110. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Clark, L.H.; Sun, W.; Yin, Y.; Kong, W.; Pierce, S.R.; West, L.; Sullivan, S.A.; Tran, A.Q.; et al. ONC201 demonstrates anti-tumorigenic and anti-metastatic activity in uterine serous carcinoma in vitro. Am. J. Cancer Res. 2018, 8, 1551–1563. [Google Scholar]
- Hayes-Jordan, A.A.; Ma, X.; Menegaz, B.A.; Lamhamedi-Cherradi, S.E.; Kingsley, C.V.; Benson, J.A.; Camacho, P.E.; Ludwig, J.A.; Lockworth, C.R.; Garcia, G.E.; et al. Efficacy of ONC201 in desmoplastic small round cell tumor. Neoplasia 2018, 20, 524–532. [Google Scholar] [CrossRef]
- Ralff, M.D.; Kline, C.L.B.; Küçükkase, O.C.; Wagner, J.; Lim, B.; Dicker, D.T.; Prabhu, V.V.; Oster, W.; El-Deiry, W.S. ONC201 demonstrates antitumor effects in both triple-negative and non-triple-negative breast cancers through TRAIL-dependent and TRAIL-Independent mechanisms. Mol. Cancer Ther. 2017, 16, 1290–1298. [Google Scholar] [CrossRef]
- Greer, Y.E.; Porat-Shliom, N.; Nagashima, K.; Stuelten, C.; Crooks, D.; Koparde, V.N.; Gilbert, S.F.; Islam, C.; Ubaldini, A.; Ji, Y.; et al. ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget 2018, 9, 18454–18479. [Google Scholar] [CrossRef]
- Ishizawa, J.; Kojima, K.; Chachad, D.; Ruvolo, P.; Ruvolo, V.; Jacamo, R.O.; Borthakur, G.; Mu, H.; Zeng, Z.; Tabe, Y.; et al. ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci. Signal. 2016, 9, ra17. [Google Scholar] [CrossRef]
- Endo Greer, Y.; Lipkowitz, S. ONC201: Stressing tumors to death. Sci. Signal. 2016, 9, fs1. [Google Scholar] [CrossRef]
- Rumman, M.; Buck, S.; Polin, L.; Dzinic, S.; Boerner, J.; Winer, I.S. ONC201 induces the unfolded protein response (UPR) in high- and low-grade ovarian carcinoma cell lines and leads to cell death regardless of platinum sensitivity. Cancer Med. 2021, 10, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Hougardy, B.M.; van der Zee, A.G.; van den Heuvel, F.A.; Timmer, T.; de Vries, E.G.; de Jong, S. Sensitivity to Fas-mediated apoptosis in high-risk HPV-positive human cervical cancer cells: Relationship with Fas, caspase-8, and Bid. Gynecol. Oncol. 2005, 97, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Gadet, R.; Jabbour, L.; Nguyen, T.T.M.; Lohez, O.; Mikaelian, I.; Gonzalo, P.; Luyten, T.; Chalabi-Dchar, M.; Wierinckx, A.; Marcillat, O.; et al. The endoplasmic reticulum pool of Bcl-xL prevents cell death through IP3R-dependent calcium release. Cell Death Discov. 2024, 10, 346. [Google Scholar] [CrossRef]
- Pathak, S.O.; Manohar, S.M. Molecular milieu of autophagy in cervical cancer and its therapeutic implications. Curr. Cancer Drug Targets 2023, 23, 843–857. [Google Scholar] [CrossRef]
- Mei, Y.; Glover, K.; Su, M.; Sinha, S.C. Conformational flexibility of BECN1: Essential to its key role in autophagy and beyond. Protein Sci. 2016, 25, 1767–1785. [Google Scholar] [CrossRef]
- Huang, R.; Gao, S.; Han, Y.; Ning, H.; Zhou, Y.; Guan, H.; Liu, X.; Yan, S.; Zhou, P.K. BECN1 promotes radiation-induced G2/M arrest through regulation CDK1 activity: A potential role for autophagy in G2/M checkpoint. Cell Death Discov. 2020, 6, 70. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, B.; Yan, J.; Zhao, W.; Dou, P.; Sun, N.; Wang, Y.; Huang, X. BNIP3 Upregulation characterizes cancer cell subpopulation with increased fitness and proliferation. Front. Oncol. 2022, 12, 923890. [Google Scholar] [CrossRef]
- Cao, J.; Cao, F.; Wang, C.; Jiao, Z.; You, Y.; Wang, X.; Zhao, W. ONC206 targeting ClpP induces mitochondrial dysfunction and protective autophagy in hepatocellular carcinoma cells. Neoplasia 2024, 55, 101015. [Google Scholar] [CrossRef]
- Wang, T.; Wu, X.; Al Rudaisat, M.; Song, Y.; Cheng, H. Curcumin induces G2/M arrest and triggers autophagy, ROS generation and cell senescence in cervical cancer cells. J. Cancer 2020, 11, 6704–6715. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Fang, Z.; Pierce, S.R.; West, L.; Staley, A.; Tucker, K.; Yin, Y.; Sun, W.; Kong, W.; et al. Anti-tumor and anti-invasive effects of ONC201 on ovarian cancer cells and a transgenic mouse model of serous ovarian cancer. Front. Oncol. 2022, 12, 789450. [Google Scholar] [CrossRef]
- Song, Y.; Lu, M.; Qiu, H.; Yin, J.; Luo, K.; Zhang, Z.; Jia, X.; Zheng, G.; Liu, H.; He, Z. Activation of FOXO3a reverses 5-Fluorouracil resistance in human breast cancer cells. Exp. Mol. Pathol. 2018, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, H.; Ran, L.; Zhang, Z.; Jiang, R. The preclinical evaluation of TIC10/ONC201 as an anti-pancreatic cancer agent. Biochem. Biophys. Res. Commun. 2016, 476, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Lev, A.; Lulla, A.R.; Wagner, J.; Ralff, M.D.; Kiehl, J.B.; Zhou, Y.; Benes, C.H.; Prabhu, V.V.; Oster, W.; Astsaturov, I.; et al. Anti-pancreatic cancer activity of ONC212 involves the unfolded protein response (UPR) and is reduced by IGF1-R and GRP78/BIP. Oncotarget 2017, 8, 81776–81793. [Google Scholar] [CrossRef] [PubMed]
- Talekar, M.K.; Allen, J.E.; Dicker, D.T.; El-Deiry, W.S. ONC201 induces cell death in pediatric non-Hodgkin’s lymphoma cells. Cell Cycle 2015, 14, 2422–2428. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Allen, J.E.; Dicker, D.T.; El-Deiry, W.S. Small-Molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res. 2015, 75, 1423–1432. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Koivusalo, R.; Hietanen, S. The cytotoxicity of chemotherapy drugs varies in cervical cancer cells depending on the p53 status. Cancer Biol. Ther. 2004, 3, 1177–1183. [Google Scholar] [CrossRef]
- Bustany, S.; Cahu, J.; Guardiola, P.; Sola, B. Cyclin D1 sensitizes myeloma cells to endoplasmic reticulum stress-mediated apoptosis by activating the unfolded protein response pathway. BMC Cancer 2015, 15, 262. [Google Scholar] [CrossRef]
- Waterhouse, D.N.; Gelmon, K.A.; Klasa, R.; Chi, K.; Huntsman, D.; Ramsay, E.; Wasan, E.; Edwards, L.; Tucker, C.; Zastre, J.; et al. Development and assessment of conventional and targeted drug combinations for use in the treatment of aggressive breast cancers. Curr. Cancer Drug Targets 2006, 6, 455–489. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, Y.Y.; Lu, P.H.; Peng, Y.; Yuan, Q.; Gu, X.S.; Jin, Y.; Chen, M.B.; Bai, X.M. Identification of DNA-PKcs as a primary resistance factor of TIC10 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 28385–28394. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Talekar, M.K.; Lulla, A.R.; Kline, L.B.; Zhou, L.; Hall, J.; Van den Heuvel, A.P.J.; Dicker, D.T.; Babar, J.; Grupp, S.A.; et al. Single agent and synergistic combinatorial efficacy of first-in-class small molecule imipridone ONC201 in hematological malignancies. Cell Cycle 2018, 17, 468–478. [Google Scholar] [CrossRef] [PubMed]
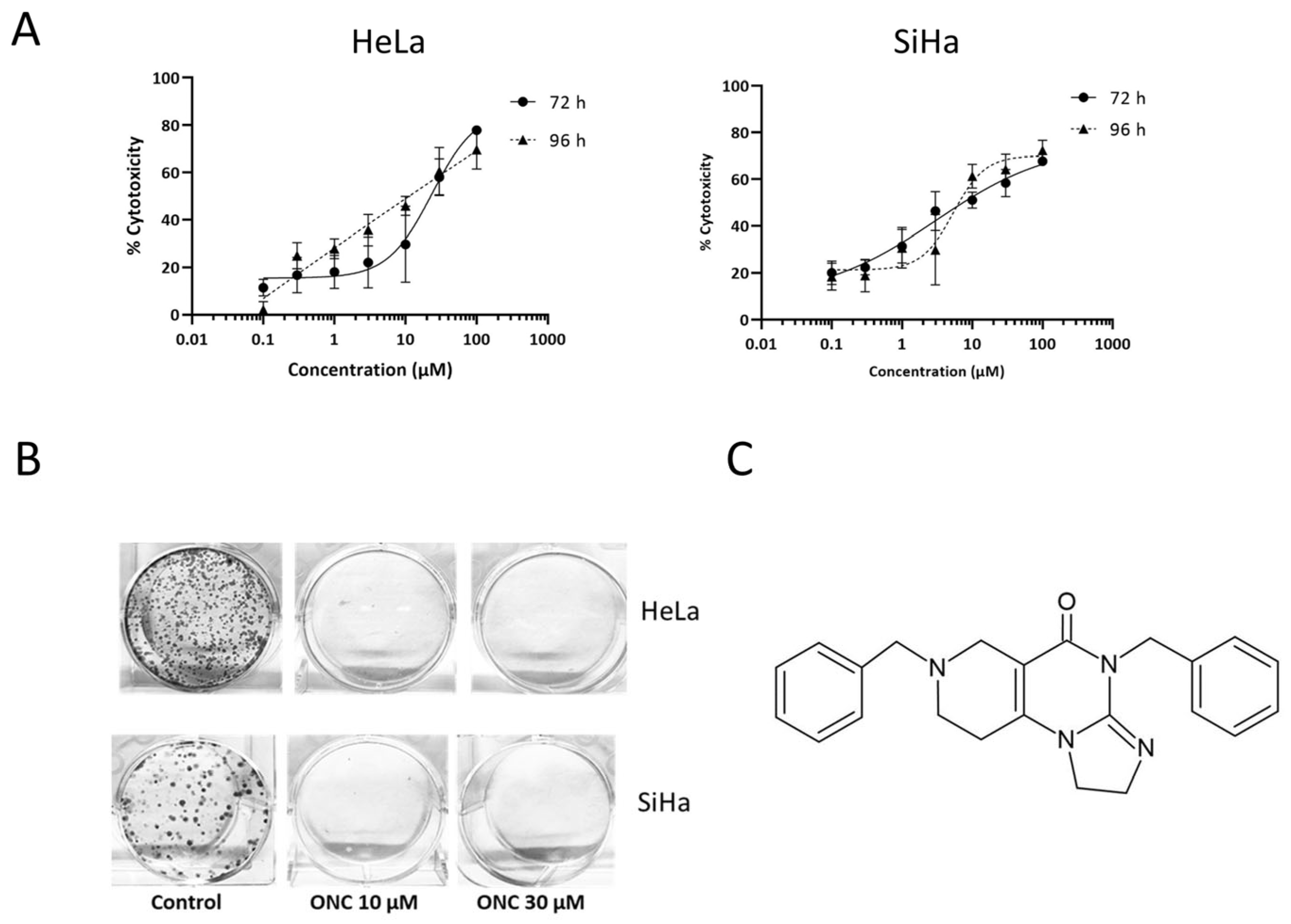
| Timepoint | IC50 (µM) | |
|---|---|---|
| HeLa | SiHa | |
| 72 h | 23.6 ± 2 | 16.3 ± 0.3 |
| 96 h | 17.8 ± 4 | 12.9 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, S.O.; Manohar, S.M. ONC201 (Dordaviprone) Induces Integrated Stress Response and Death in Cervical Cancer Cells. Biomolecules 2025, 15, 463. https://doi.org/10.3390/biom15040463
Pathak SO, Manohar SM. ONC201 (Dordaviprone) Induces Integrated Stress Response and Death in Cervical Cancer Cells. Biomolecules. 2025; 15(4):463. https://doi.org/10.3390/biom15040463
Chicago/Turabian StylePathak, Sneha O., and Sonal M. Manohar. 2025. "ONC201 (Dordaviprone) Induces Integrated Stress Response and Death in Cervical Cancer Cells" Biomolecules 15, no. 4: 463. https://doi.org/10.3390/biom15040463
APA StylePathak, S. O., & Manohar, S. M. (2025). ONC201 (Dordaviprone) Induces Integrated Stress Response and Death in Cervical Cancer Cells. Biomolecules, 15(4), 463. https://doi.org/10.3390/biom15040463






