RNA-Binding Protein Signature in Proliferative Cardiomyocytes: A Cross-Species Meta-Analysis from Mouse, Pig, and Human Transcriptomic Profiling Data
Abstract
1. Introduction
2. Materials and Methods
2.1. snRNA-Seq Datasets
2.2. RBP-Specific Analyses in snRNA-Seq Data
2.3. Generating and Analyzing Bulk RNA-Seq Data from hiPSC-CM
2.4. Combining RBPs Associated with Cardiomyocyte Proliferation in Mouse, Pig, and hiPSC-CM Data
2.5. Immunohistochemistry
3. Results
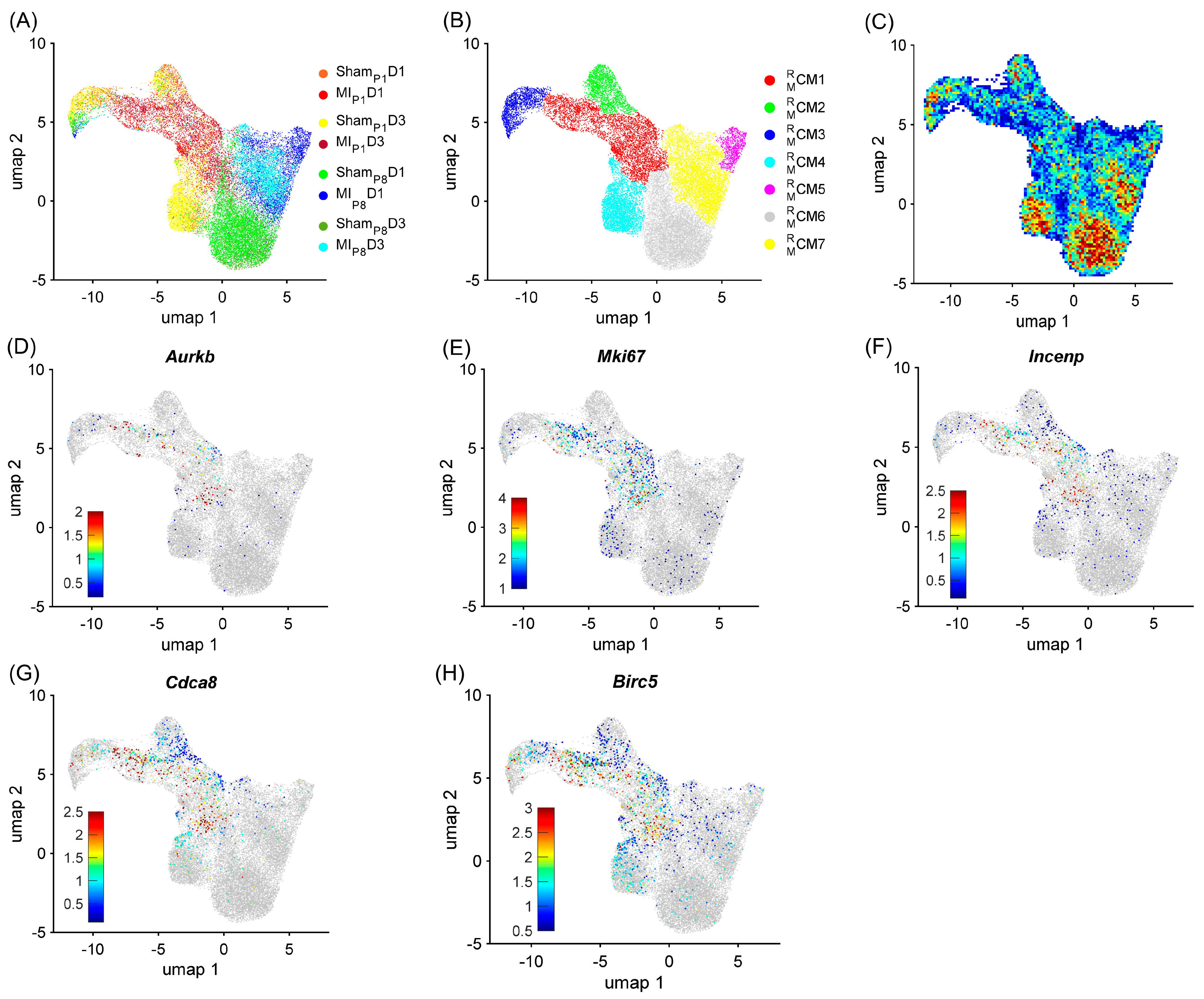
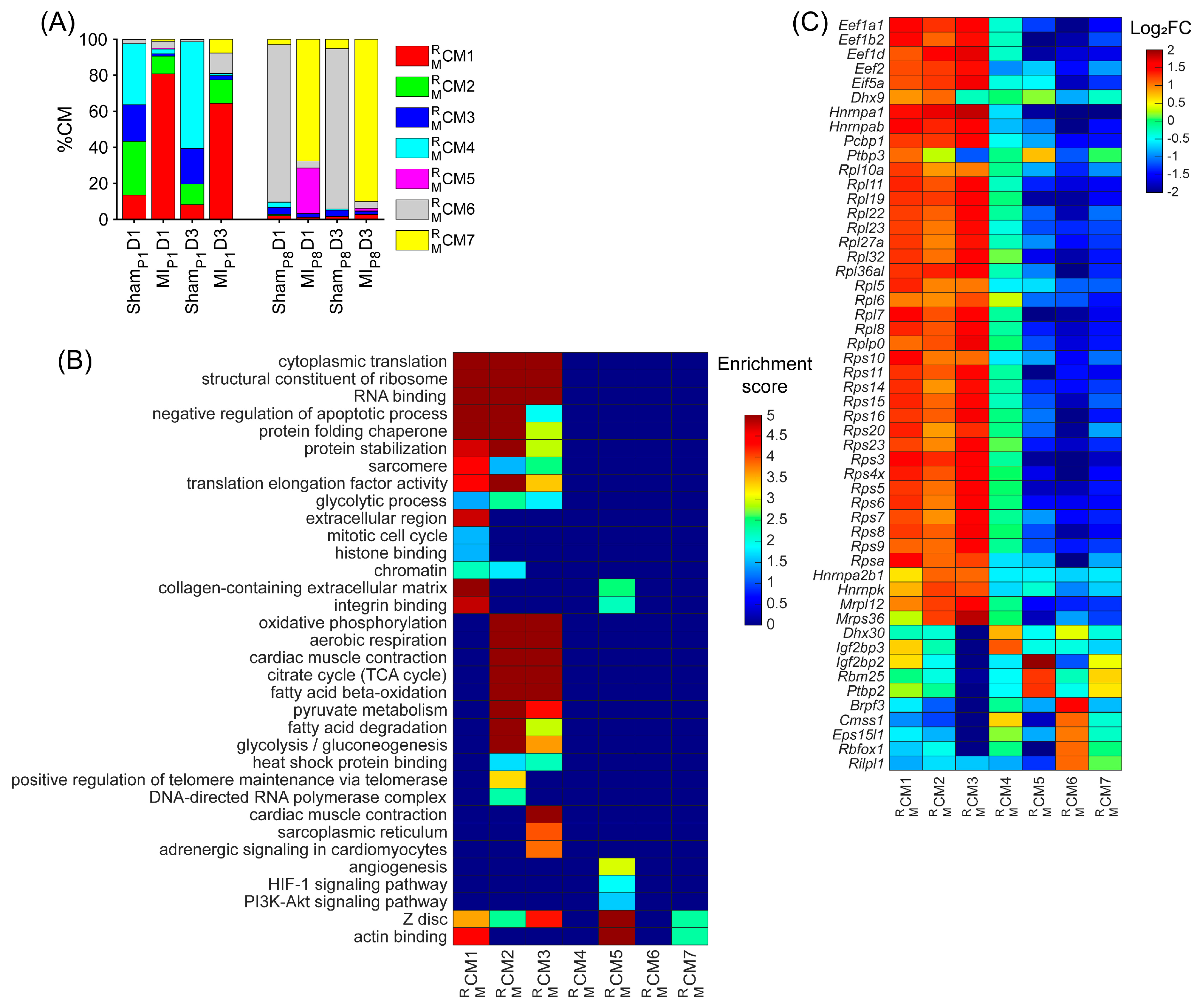
3.1. RBP-Specific Analysis Re-Identified One ‘Proliferating’ CM Cluster and Three ‘Proliferation-Associated’ CM Clusters in Regenerative Mouse Hearts
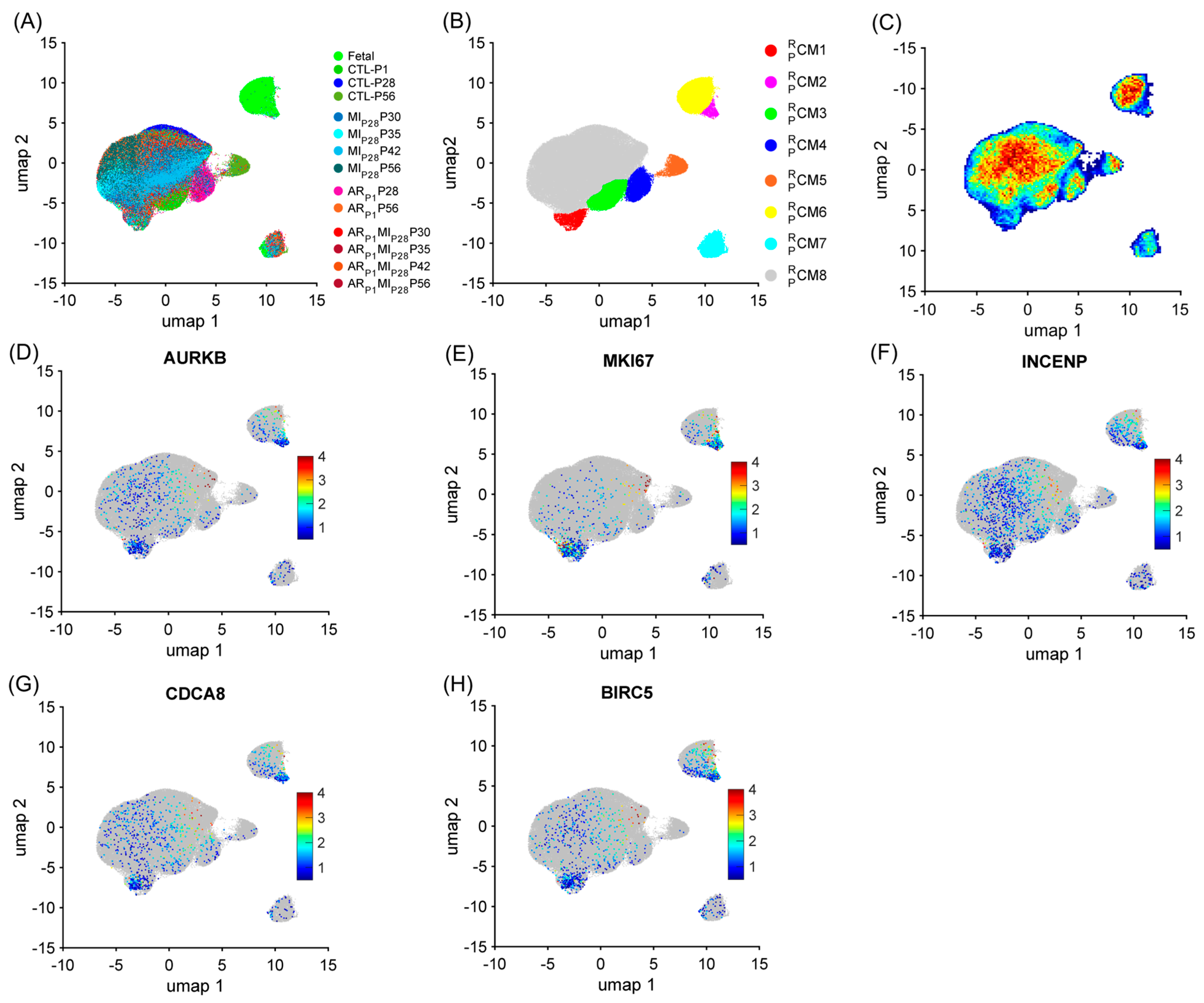
3.2. RBP-Specific Analysis Re-Identified Two ‘Proliferating’ CM Clusters and Two ‘Proliferation-Associated’ CM Clusters in Pig Hearts
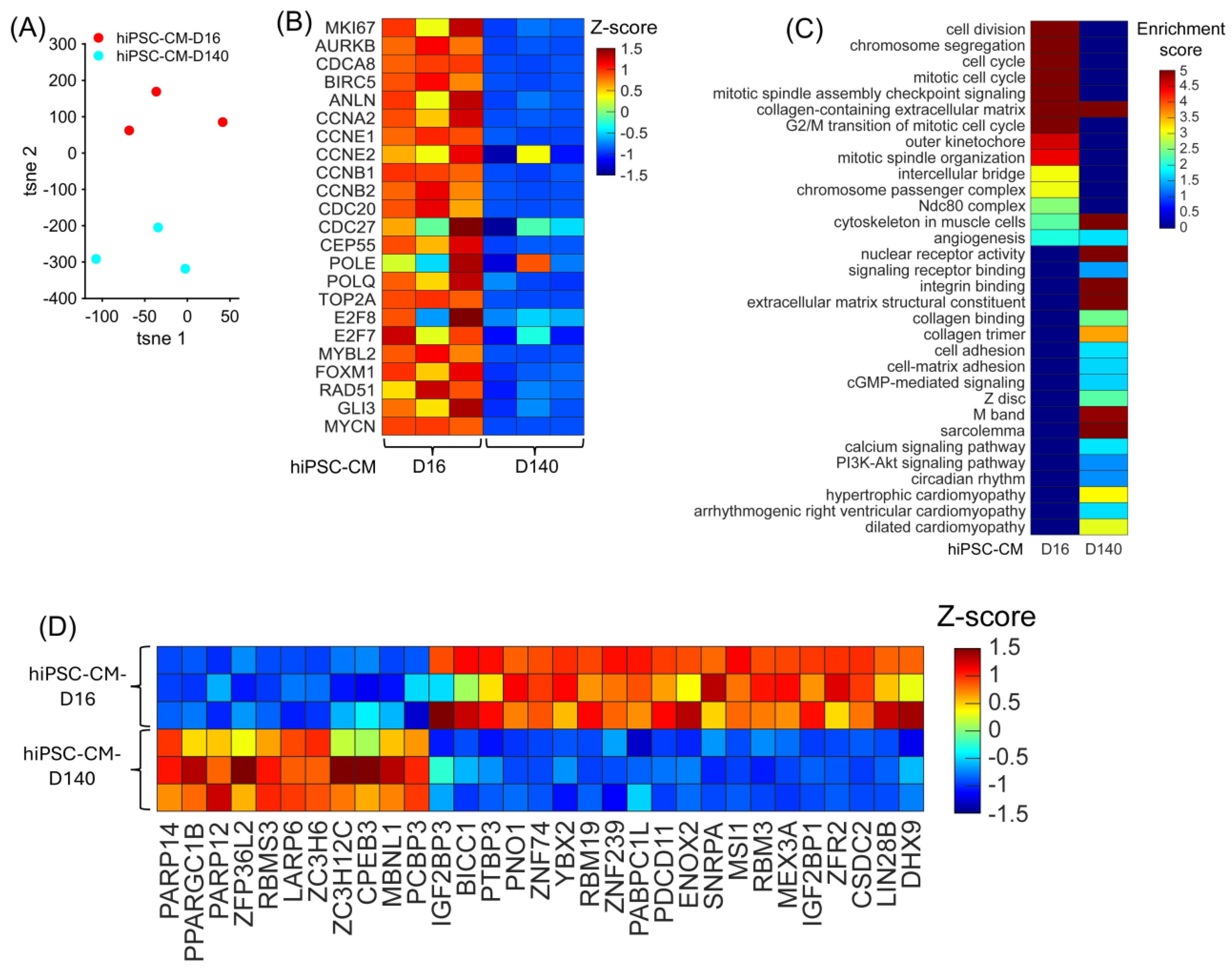
3.3. Differentially Expressed RBPs in hiPSC-CM Bulk RNA-Seq Analysis
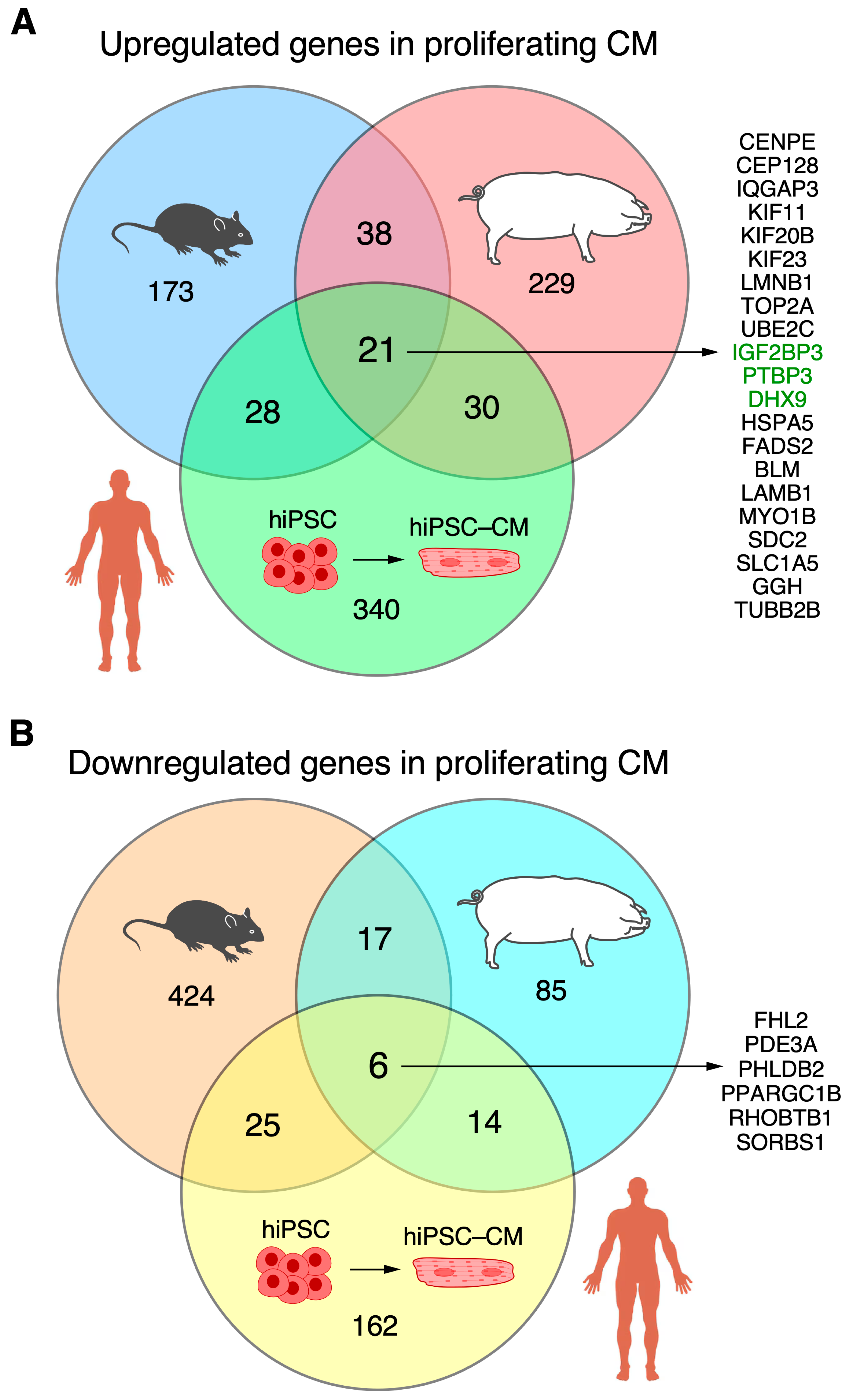
3.4. Overlapping CM-Proliferation-Associated RBP Among Mice, Pig, and hiPSC-CM Transcriptomic Profiling Data
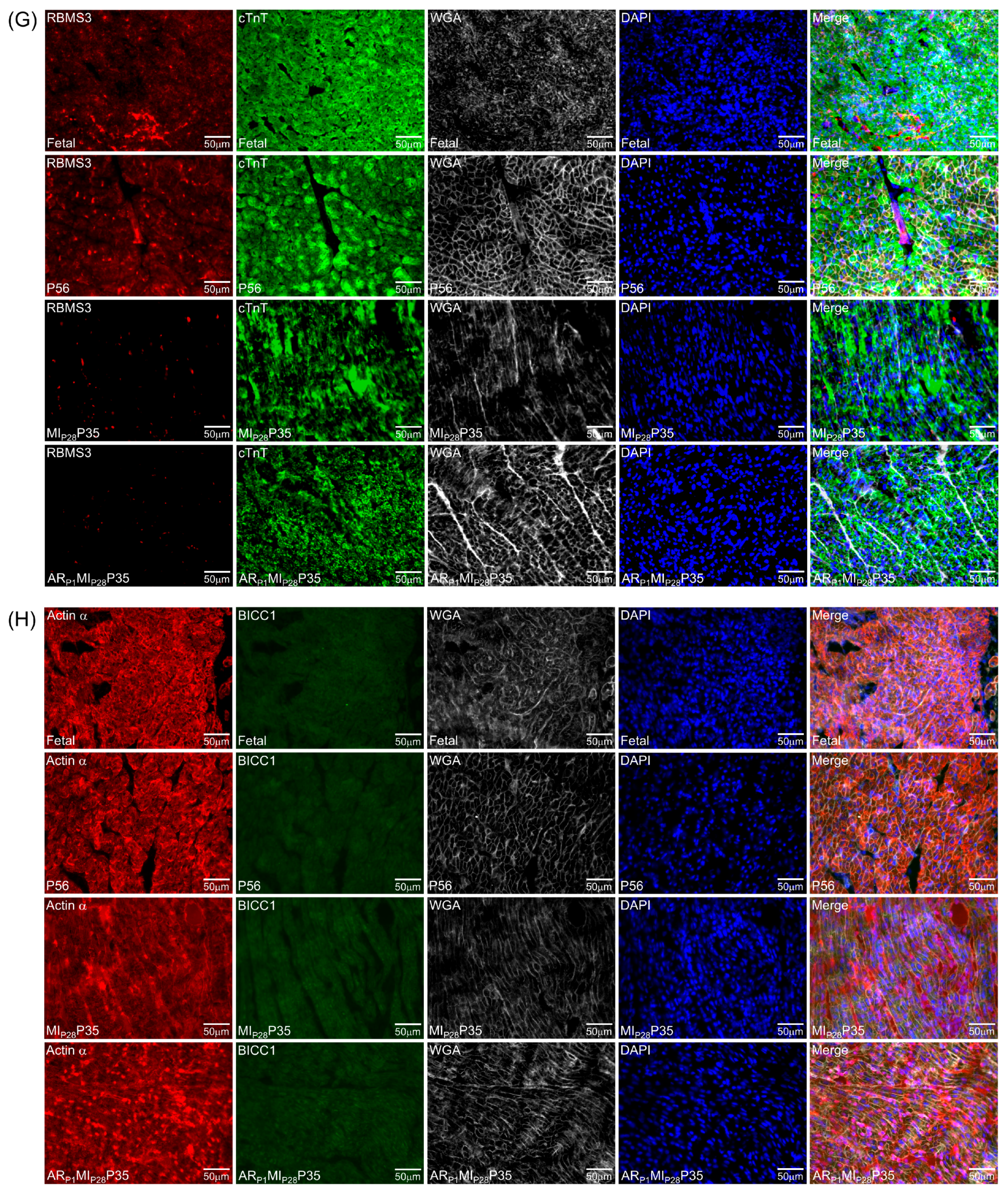
3.5. Immunohistochemistry Analysis of Upregulated Classical RBPs at the Proliferative Stages of Cardiomyocytes in Pig Hearts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient Regenerative Potential of the Neonatal Mouse Heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Nakada, Y.; Zhou, Y.; Gong, W.; Zhang, E.Y.; Skie, E.; Nguyen, T.; Wei, Y.; Zhao, M.; Chen, W.; Sun, J.; et al. Single Nucleus Transcriptomics: Apical Resection in Newborn Pigs Extends the Time Window of Cardio-myocyte Proliferation and Myocardial Regeneration. Circulation 2022, 145, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Wei, Y.; Nakada, Y.; Zhou, Y.; Zhang, J. Cardiomyocyte Cell-Cycle Regulation in Neonatal Large Mammals: Single Nucleus RNA-Sequencing Data Analysis via an Artificial-Intelligence–Based Pipeline. Front. Bioeng. Biotechnol. 2022, 10, 914450. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.P.; Martin, J.F. Cardiomyocyte Proliferation for Therapeutic Regeneration. Curr. Cardiol. Rep. 2018, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Van Amerongen, M.J.; Engel, F.B. Features of cardiomyocyte proliferation and its potential for cardiac regeneration. J. Cell Mol. Med. 2008, 12, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nakada, Y.; Wu, Y.; Zhao, J.; Garry, D.J.; Sadek, H.; Zhang, J. Cell-Cycle–Specific Autoencoding Improves Cluster Analysis of Cycling Cardiomyocytes. Stem Cells 2024, 42, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Grancharova, T.; Gerbin, K.A.; Rosenberg, A.B.; Roco, C.M.; Arakaki, J.E.; DeLizo, C.M.; Dinh, S.Q.; Donovan-Maiye, R.M.; Hirano, M.; Nelson, A.M.; et al. A comprehensive analysis of gene expression changes in a high replicate and open-source dataset of differentiating hiPSC-derived cardiomyocytes. Sci. Rep. 2021, 11, 15845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional Cardiomyocytes Derived From Human Induced Pluripotent Stem Cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Cui, M.; Wang, Z.; Chen, K.; Shah, A.M.; Tan, W.; Duan, L.; Sanchez-Ortiz, E.; Li, H.; Xu, L.; Liu, N.; et al. Dynamic Transcriptional Responses to Injury of Regenerative and Non-regenerative Cardiomyocytes Revealed by Single-Nucleus RNA Sequencing. Dev. Cell 2020, 55, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.T.; Sadek, H.A. Neonatal Heart Regeneration: Comprehensive Literature Review. Circulation 2018, 138, 412–423. [Google Scholar] [CrossRef] [PubMed]
- I Mahmoud, A.; Porrello, E.R.; Kimura, W.; Olson, E.N.; A Sadek, H. Surgical models for cardiac regeneration in neonatal mice. Nat. Protoc. 2014, 9, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Belmonte, J.C.I. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef]
- Lien, C.-L.; Schebesta, M.; Makino, S.; Weber, G.J.; Keating, M.T. Gene Expression Analysis of Zebrafish Heart Regeneration. PLOS Biol. 2006, 4, e260. [Google Scholar] [CrossRef]
- Zhang, E.; Nguyen, T.; Zhao, M.; Dang, S.D.H.; Chen, J.Y.; Bian, W.; Walcott, G.P. Identifying the key regulators that promote cell-cycle activity in the hearts of early neonatal pigs after myo-cardial injury. PLoS ONE 2020, 15, e0232963. [Google Scholar]
- Hu, B.; Lelek, S.; Spanjaard, B.; El-Sammak, H.; Simões, M.G.; Mintcheva, J.; Aliee, H.; Schäfer, R.; Meyer, A.M.; Theis, F.; et al. Origin and function of activated fibroblast states during zebrafish heart regeneration. Nat. Genet. 2022, 54, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Gemberling, M.; Karra, R.; Rosenfeld, G.E.; Evans, T.; Poss, K.D. An Injury-Responsive Gata4 Program Shapes the Zebrafish Cardiac Ventricle. Curr. Biol. 2013, 23, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, Y.-F.; Pang, M.; Chang, N.; Yu, C.; Li, Q.; Xiong, J.-W.; Peng, Y.; Zhang, R. BMP and Notch Signaling Pathways differentially regulate Cardiomyocyte Proliferation during Ventricle Regeneration. Int. J. Biol. Sci. 2021, 17, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Budzko, L.; Hoffa-Sobiech, K.; Jackowiak, P.; Figlerowicz, M. Engineered deaminases as a key component of DNA and RNA editing tools. Mol. Ther.-Nucleic Acids 2023, 34, 102062. [Google Scholar] [CrossRef] [PubMed]
- Baade, I.; Hutten, S.; Sternburg, E.L.; Pörschke, M.; Hofweber, M.; Dormann, D.; Kehlenbach, R.H. The RNA-binding protein FUS is chaperoned and imported into the nucleus by a network of import receptors. J. Biol. Chem. 2021, 296, 100659. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-Y.; Yang, B.; Shi, C.-P.; Deng, W.-X.; Deng, J.-S.; Cen, M.-F.; Zheng, B.-Q.; Zhan, Z.-L.; Liang, Q.-L.; Wang, J.-E.; et al. RBPWorld for exploring functions and disease associations of RNA-binding proteins across species. Nucleic Acids Res. 2024, 53, D220–D232. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, B.M.; Castello, A.; Medenbach, J. The expanding universe of ribonucleoproteins: Of novel RNA-binding proteins and unconventional interactions. Pflüg. Arch.-Eur. J. Physiol. 2016, 468, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Shi, D.-L. RNA-Binding Proteins as Critical Post-Transcriptional Regulators of Cardiac Regeneration. Int. J. Mol. Sci. 2023, 24, 12004. [Google Scholar] [CrossRef]
- Nguyen, T.; Wei, Y.; Nakada, Y.; Chen, J.Y.; Zhou, Y.; Walcott, G.; Zhang, J. Analysis of Cardiac Single-cell RNA-sequencing Data Can be Improved by the Use of Artifi-cial-Intelligence-based Tools. Sci. Rep. 2023, 13, 6821. [Google Scholar]
- Caudron-Herger, M.; E Jansen, R.; Wassmer, E.; Diederichs, S. RBP2GO: A comprehensive pan-species database on RNA-binding proteins, their interactions and functions. Nucleic Acids Res. 2020, 49, D425–D436. [Google Scholar] [CrossRef]
- McInnes, L.; Healy, J.; Melville, J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Meehan, S. Uniform Manifold Approximation and Projection (UMAP). 2021. Available online: https://www.mathworks.com/matlabcentral/fileexchange/71902-uniform-manifold-approximation-and-projection-umap (accessed on 25 January 2025).
- Hilton, J.F. The Appropriateness of the Wilcoxon Test in Ordinal Data. Stat. Med. 1996, 15, 631–645. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huntley, R.P.; Sawford, T.; Mutowo-Meullenet, P.; Shypitsyna, A.; Bonilla, C.; Martin, M.J.; O’Donovan, C. The GOA database: Gene Ontology annotation updates for 2015. Nucleic Acids Res. 2014, 43, D1057–D1063. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Barber, R.F. Multiple Testing with the Structure-Adaptive Benjamini–Hochberg Algorithm. J. R. Stat. Soc. Ser. B Stat. Methodol. 2018, 81, 45–74. [Google Scholar] [CrossRef]
- Wang, L.; Nguyen, T.; Rosa-Garrido, M.; Zhou, Y.; Cleveland, D.C.; Zhang, J. Comparative analysis of the cardiomyocyte differentiation potential of induced pluripotent stem cells repro-grammed from human atrial or ventricular fibroblasts. Front. Bioeng. Biotechnol. 2023, 11, 1108340. [Google Scholar]
- Krueger, F. Trim Galore!: A Wrapper Around Cutadapt and FastQC to Consistently Apply Adapter and Quality Trimming to FastQ files, with Extra Functionality for RRBS Data; Babraham Institute: Cambridge, UK, 2015. [Google Scholar]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ensembl Genome Browser. Available online: http://useast.ensembl.org/Homo_sapiens/Info/Index (accessed on 5 September 2011).
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Geng, X.; Wei, Y.; Ye, L.; Garry, D.J.; Zhang, J. Single-cell RNA sequencing analysis identifies one subpopulation of endothelial cells that proliferates and another that undergoes the endothelial-mesenchymal transition in regenerating pig hearts. Front. Bioeng. Biotechnol. 2024, 11, 1257669. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Bonetti, D.; Lockhart, A.; Serhal, K.; Kellner, V.; Maicher, A.; Jolivet, P.; Teixeira, M.T.; Luke, B. Telomere Length Determines TERRA and R-Loop Regulation through the Cell Cycle. Cell 2017, 170, 72–85.e14. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhou, P.; von Gise, A.; Gu, F.; Ma, Q.; Chen, J.; Guo, H.; van Gorp, P.R.; Wang, D.-Z.; Pu, W.T. Pi3kcb Links Hippo-YAP and PI3K-AKT Signaling Pathways to Promote Cardiomyocyte Proliferation and Survival. Circ. Res. 2015, 116, 35–45. [Google Scholar] [CrossRef]
- Sorensen, D.W.; van Berlo, J.H. The Role of TGF-beta Signaling in Cardiomyocyte Proliferation. Curr. Heart Fail. Rep. 2020, 17, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Li, S.; Shen, S.; Xu, H.; Cai, S.; Yuan, X.; Wang, C.; Zhang, X.; Chen, S.; Chen, J.; Shi, D.-L.; et al. IGF2BP3 promotes adult myocardial regeneration by stabilizing MMP3 mRNA through interaction with m6A modification. Cell Death Discov. 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Yen, T.J.; Compton, D.A.; Wise, D.; Zinkowski, R.P.; Brinkley, B.R.; Earnshaw, W.C.; Cleveland, D.W. CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 1991, 10, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Mönnich, M.; Borgeskov, L.; Breslin, L.; Jakobsen, L.; Rogowski, M.; Doganli, C.; Schrøder, J.M.; Mogensen, J.B.; Blinkenkjær, L.; Harder, L.M.; et al. CEP128 Localizes to the Subdistal Appendages of the Mother Centriole and Regulates TGF-beta/BMP Signaling at the Primary Cilium. Cell Rep. 2018, 22, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Mjelle, R.; Hegre, S.A.; Aas, P.A.; Slupphaug, G.; Drabløs, F.; Sætrom, P.; Krokan, H.E. Cell cycle regulation of human DNA repair and chromatin remodeling genes. DNA Repair 2015, 30, 53–67. [Google Scholar] [CrossRef]
- Venere, M.; Horbinski, C.; Crish, J.F.; Jin, X.; Vasanji, A.; Major, J.; Burrows, A.C.; Chang, C.; Prokop, J.; Wu, Q.; et al. The mitotic kinesin KIF11 is a driver of invasion, proliferation, and self-renewal in glioblastoma. Sci. Transl. Med. 2015, 7, 304ra143. [Google Scholar] [CrossRef] [PubMed]
- Lecland, N.; Lüders, J. The dynamics of microtubule minus ends in the human mitotic spindle. Nat. Cell Biol. 2014, 16, 770–778. [Google Scholar] [CrossRef]
- Janisch, K.M.; McNeely, K.C.; Dardick, J.M.; Lim, S.H.; Dwyer, N.D. Kinesin-6 KIF20B is required for efficient cytokinetic furrowing and timely abscission in human cells. Mol. Biol. Cell 2018, 29, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Simsek, D.; Tiu, G.C.; Flynn, R.A.; Byeon, G.W.; Leppek, K.; Xu, A.F.; Chang, H.Y.; Barna, M. The Mammalian Ribo-interactome Reveals Ribosome Functional Diversity and Heterogeneity. Cell 2017, 169, 1051–1065.e18. [Google Scholar] [CrossRef]
- Liao, Y.; Castello, A.; Fischer, B.; Leicht, S.; Föehr, S.; Frese, C.K.; Ragan, C.; Kurscheid, S.; Pagler, E.; Yang, H.; et al. The Cardiomyocyte RNA-Binding Proteome: Links to Intermediary Metabolism and Heart Disease. Cell Rep. 2016, 16, 1456–1469. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gregorich, Z.R.; Cai, W.; Zhang, P.; Young, B.; Gu, Y.; Zhang, J.; Ge, Y. Quantitative Proteomics and Immunohistochemistry Reveal Insights into Cellular and Molecular Processes in the Infarct Border Zone One Month after Myocardial Infarction. J. Proteome Res. 2017, 16, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-W.; Andrade, M.; Bae, S.; Kim, S.; Kim, J.E.; Jang, E.Y.; Lee, S.; Husain, A.; Sutliff, R.L.; Calvert, J.W.; et al. Polycomb Group Protein CBX7 Represses Cardiomyocyte Proliferation Through Modulation of the TARDBP/RBM38 Axis. Circulation 2023, 147, 1823–1842. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.J.; Qin, Y.; Zhou, S.J.; Dong, B.H.; Yang, J.S.; Zou, P.; Wang, L.P.; Zhao, Y.T. Hapln1 promotes dedifferentiation and proliferation of iPSC-derived cardiomyocytes by promoting versi-can-based GDF11 trapping. J. Pharm. Anal. 2024, 14, 335–347. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, Y.; Kang, J.X. Down-regulation of the M6P/IGF-II receptor increases cell proliferation and reduces apoptosis in neonatal rat cardiac myocytes. BMC Cell Biol. 2004, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, V.O.C.; Hoy, R.C.; Kurian, J.; Zarka, C.; Behanan, M.; Brosious, I.; Pennise, J.; Patel, T.; Wang, T.; Johnson, J.; et al. RNA-Binding Protein LIN28a Regulates New Myocyte Formation in the Heart Through Long Noncoding RNA-H19. Circulation 2023, 147, 324–337. [Google Scholar] [CrossRef]
- Bailey, L.R.; Bugg, D.; Reichardt, I.M.; Ortaç, C.D.; Nagle, A.; Gunaje, J.; Martinson, A.; Johnson, R.; MacCoss, M.J.; Sakamoto, T.; et al. MBNL1 Regulates Programmed Postnatal Switching Between Regenerative and Differentiated Cardiac States. Circulation 2024, 149, 1812–1829. [Google Scholar] [CrossRef]
- Zhao, K.; Yang, C.; Zhang, J.; Sun, W.; Zhou, B.; Kong, X.; Shi, J. METTL3 improves cardiomyocyte proliferation upon myocardial infarction via upregulating miR-17-3p in a DGCR8-dependent manner. Cell Death Discov. 2021, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-R.; Wei, T.-W.; Tang, C.-P.; Sun, J.-T.; Shan, T.-K.; Fan, Y.; Yang, T.-T.; Li, Y.-F.; Ma, Y.; Wang, S.-B.; et al. MNK2-eIF4E axis promotes cardiac repair in the infarcted mouse heart by activating cyclin D1. J. Mol. Cell. Cardiol. 2022, 166, 91–106. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Hao, K.; Nakada, Y.; Guragain, B.; Yao, P.; Zhang, J. RNA-Binding Protein Signature in Proliferative Cardiomyocytes: A Cross-Species Meta-Analysis from Mouse, Pig, and Human Transcriptomic Profiling Data. Biomolecules 2025, 15, 310. https://doi.org/10.3390/biom15020310
Nguyen T, Hao K, Nakada Y, Guragain B, Yao P, Zhang J. RNA-Binding Protein Signature in Proliferative Cardiomyocytes: A Cross-Species Meta-Analysis from Mouse, Pig, and Human Transcriptomic Profiling Data. Biomolecules. 2025; 15(2):310. https://doi.org/10.3390/biom15020310
Chicago/Turabian StyleNguyen, Thanh, Kaili Hao, Yuji Nakada, Bijay Guragain, Peng Yao, and Jianyi Zhang. 2025. "RNA-Binding Protein Signature in Proliferative Cardiomyocytes: A Cross-Species Meta-Analysis from Mouse, Pig, and Human Transcriptomic Profiling Data" Biomolecules 15, no. 2: 310. https://doi.org/10.3390/biom15020310
APA StyleNguyen, T., Hao, K., Nakada, Y., Guragain, B., Yao, P., & Zhang, J. (2025). RNA-Binding Protein Signature in Proliferative Cardiomyocytes: A Cross-Species Meta-Analysis from Mouse, Pig, and Human Transcriptomic Profiling Data. Biomolecules, 15(2), 310. https://doi.org/10.3390/biom15020310





