Folic Acid Supplementation Inhibits Proliferative Retinopathy of Prematurity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Mouse Model of OIR
2.3. Folic Acid Deficiency in Maternal Diets
2.4. Folic Acid Supplementation
2.5. Measurement of Total Retinal Folate Levels
2.6. Single-Cell Analysis of Folate Cycle Metabolic Genes
2.7. Cell Culture
2.8. Real-Time PCR
2.9. Western Blot
2.10. Statistics
3. Results
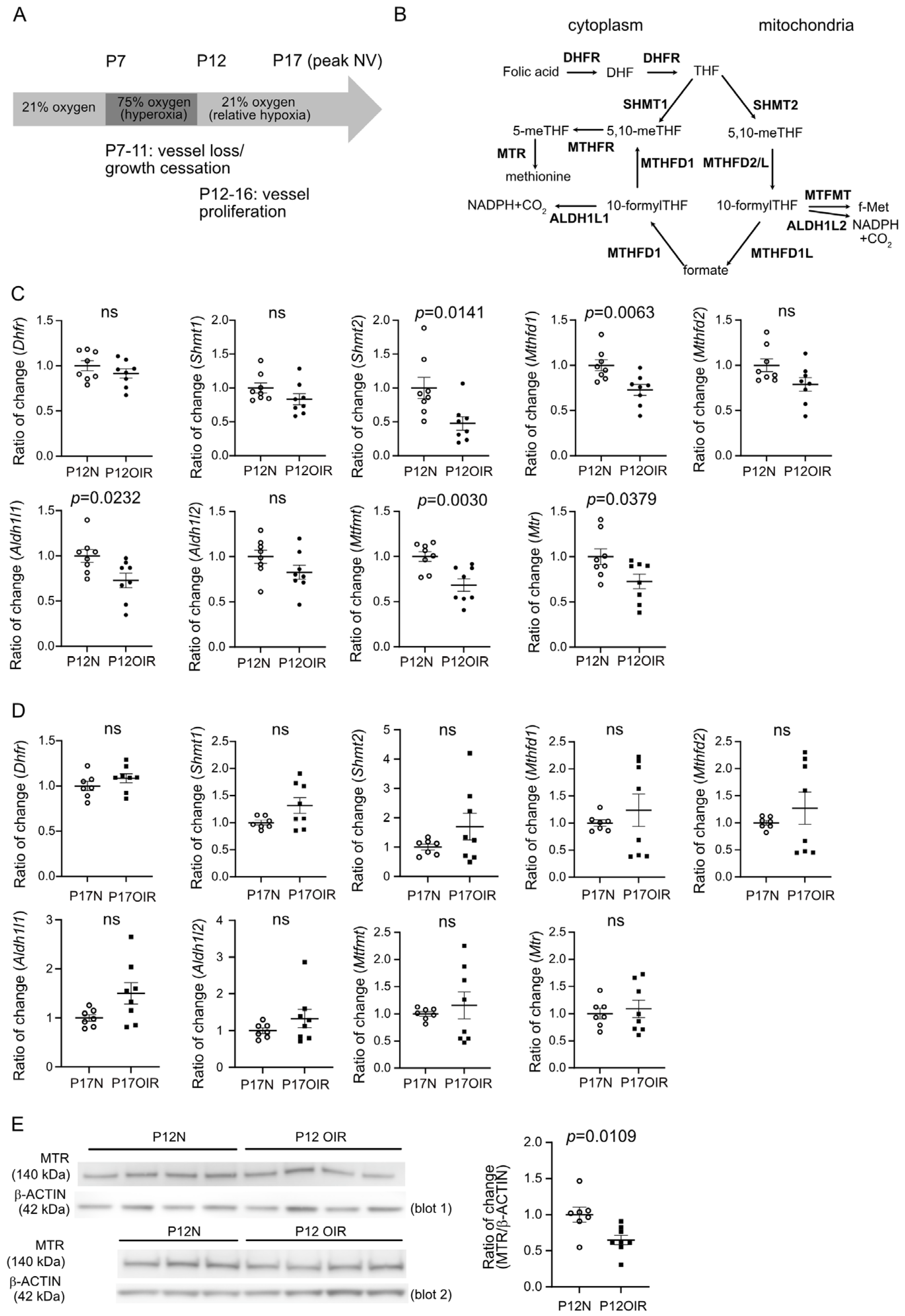
3.1. Compromised Expression of Genes Involved in Folate Cycle in OIR Retinas After Hyperoxic Exposure (Phase I)
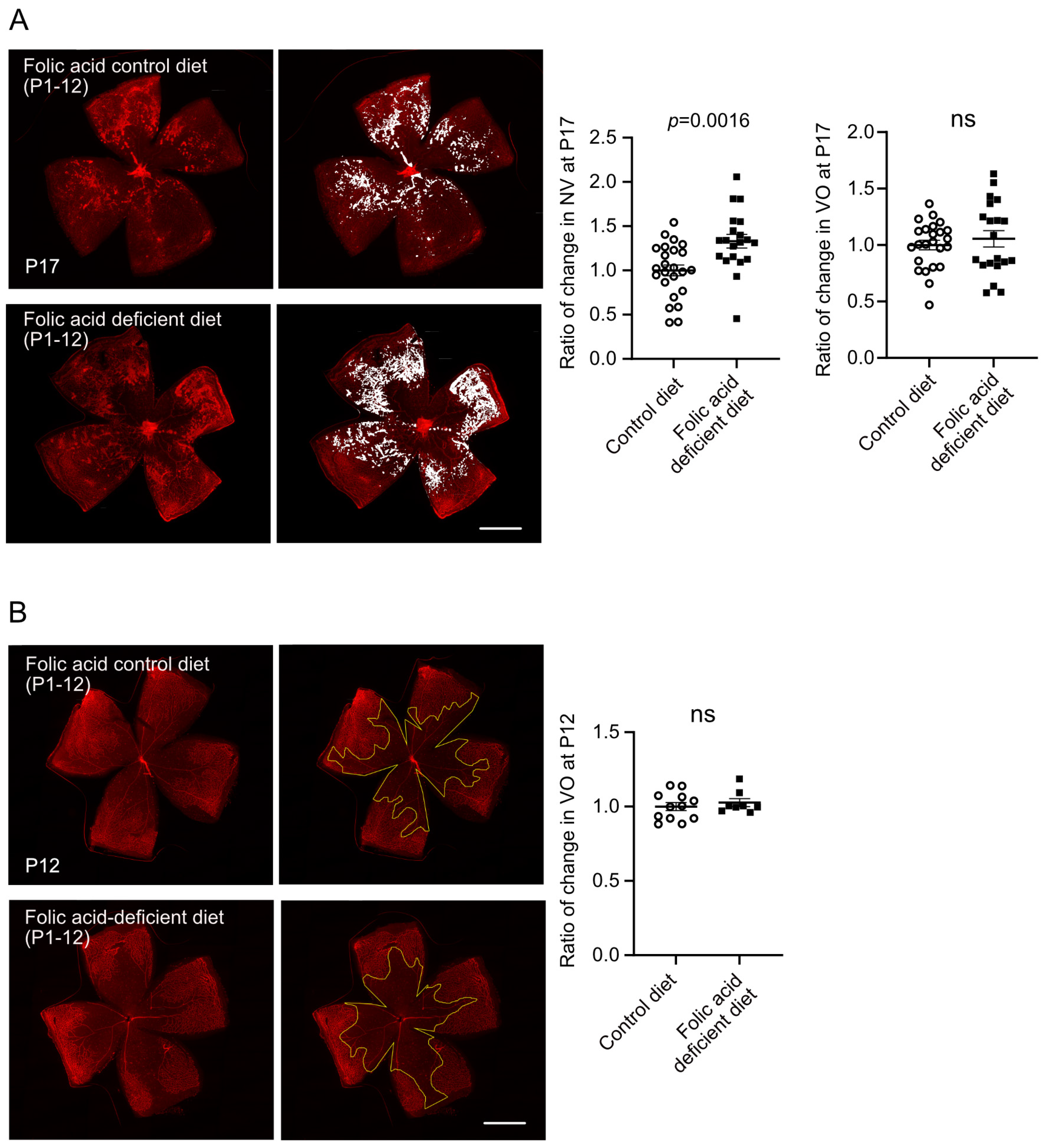
3.2. Early Folic Acid Deficiency in Maternal Diets Worsened Retinal Neovascularization
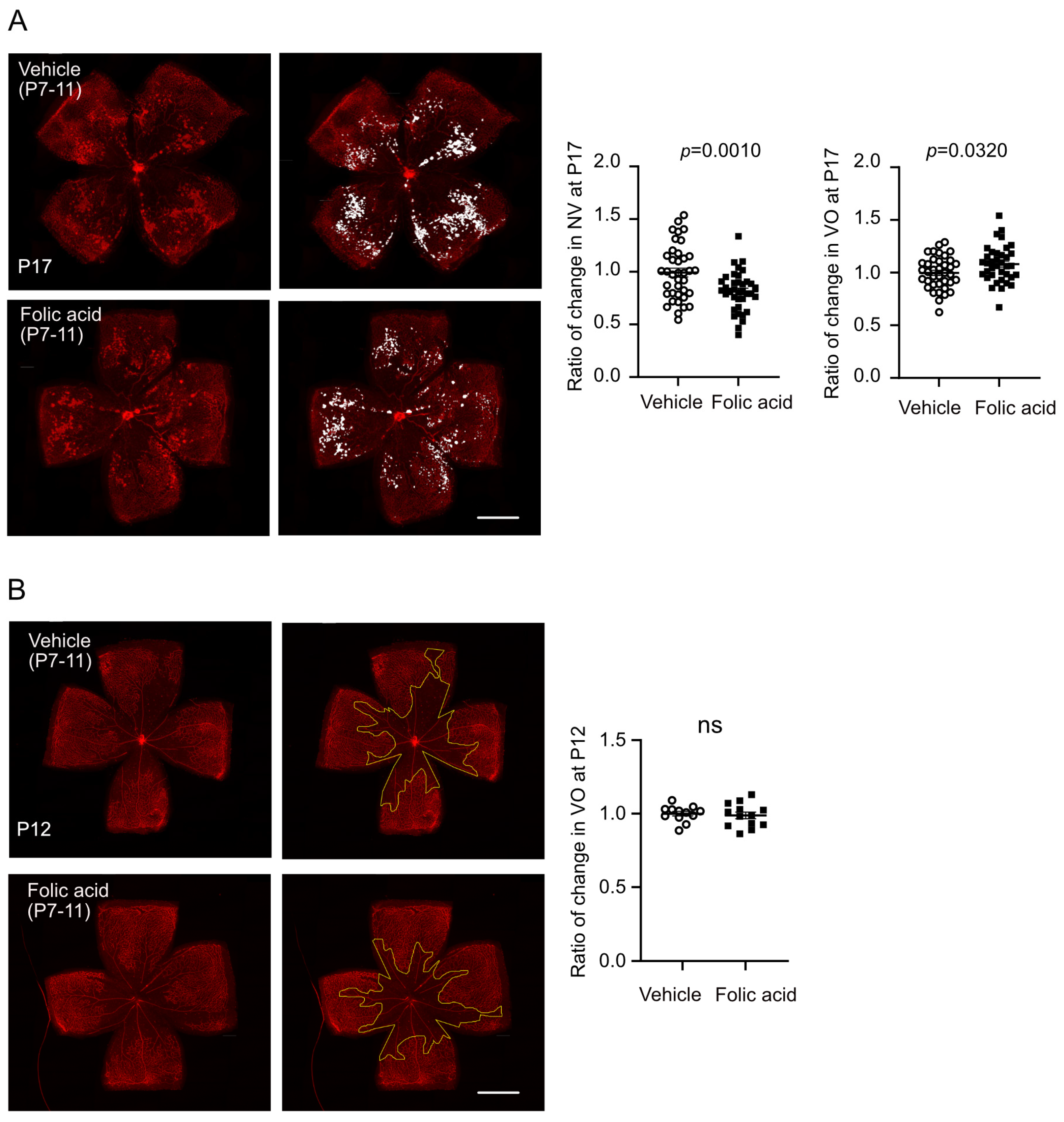
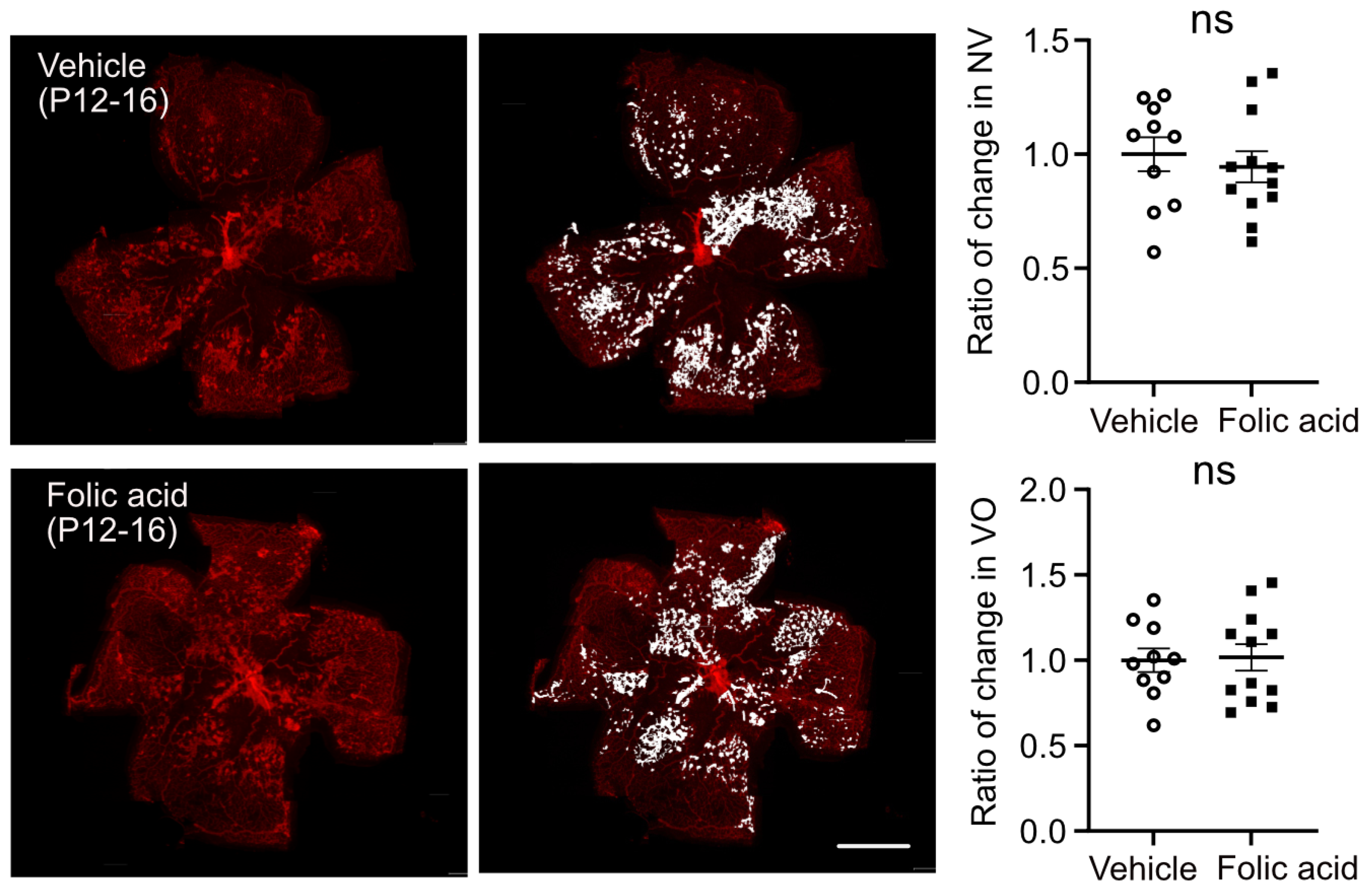
3.3. Folic Acid Supplementation During Hyperoxia (Phase I) Decreased Retinal Neovascularization
3.4. Folic Acid Decreased Pro-Angiogenic Response in Müller Glia/Astrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALDH1L1 | 10-formyltetrahydrofolate dehydrogenase family member L1 |
| ALDH1L2 | 10-formyltetrahydrofolate dehydrogenase family member L2 |
| BCA | bicinchoninic acid |
| Cat | catalase |
| CoCl2 | cobalt chloride |
| Cox2 | cyclooxygenase-2 |
| CycloA | cyclophilin a |
| DHFR | dihydrofolate reductase |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| ECL | enhanced chemiluminescence |
| FA | folic acid |
| Epo | erythropoietin |
| GFAP | glial fibrillary acidic protein |
| Gpx1 | glutathione Peroxidase 1 |
| Grx1 | glutaredoxin-1 |
| HIF1α | hypoxia-induced factor 1α |
| iNOS | nitric oxide synthase, inducible |
| MTFMT | mitochondrial methionyl-tRNA formyltransferase |
| MTHFD1 | methenyltetrahydrofolate cyclohydrolase 1 |
| MTHFD2 | methenyltetrahydrofolate cyclohydrolase 2 |
| MTHFR | methylenetetrahydrofolate reductase |
| MTR | methionine synthase |
| NV | neovascularization |
| OIR | oxygen-induced retinopathy |
| PBS | phosphate-buffered saline |
| Prdx1 | peroxiredoxin 1 |
| RIPA | radioimmuoprecipitation assay |
| rMC-1 | rat Müller cell-1 |
| ROP | retinopathy of prematurity |
| SHMT1 | serine hydroxymethyltransferase 1 |
| SHMT2 | serine hydroxymethyltransferase 2 |
| Sod2 | superoxide dismutase 2 |
| Trx2 | thioredoxin 2 |
| VEGF | vascular endothelial growth factor |
| VO | vaso-obliteration |
References
- Shah, P.K.; Prabhu, V.; Karandikar, S.S.; Ranjan, R.; Narendran, V.; Kalpana, N. Retinopathy of prematurity: Past, present and future. World J. Clin. Pediatr. 2016, 5, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stahl, A.; Hellstrom, A.; Smith, L.E. Current update on retinopathy of prematurity: Screening and treatment. Curr. Opin. Pediatr. 2011, 23, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.K.; Dray, P.B. Retinopathy of prematurity. Dis. A Mon. DM 2014, 60, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Azad, R.; Dave, V.; Jalali, S. Use of intravitreal anti-VEGF: Retinopathy of prematurity surgeons’ in Hamlet’s dilemma? Indian J. Ophthalmol. 2011, 59, 421–422. [Google Scholar] [CrossRef]
- Mandala, V.K.; Urakurva, A.K.; Gangadhari, S.; Kotha, R., Sr. The Effects of Early Enteral and Parental Nutrition on Retinopathy of Prematurity: A Systematic Review. Cureus 2023, 15, e49029. [Google Scholar] [CrossRef]
- Scholl, T.O.; Hediger, M.L.; Schall, J.I.; Khoo, C.S.; Fischer, R.L. Dietary and serum folate: Their influence on the outcome of pregnancy. Am. J. Clin. Nutr. 1996, 63, 520–525. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic Acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Roberts, P.M.; Arrowsmith, D.E.; Lloyd, A.V.; Monk-Jones, M.E. Effect of folic acid treatment on premature infants. Arch. Dis. Child. 1972, 47, 631–634. [Google Scholar] [CrossRef][Green Version]
- Celik, F.C.; Aygun, C.; Gulten, S.; Bedir, A.; Cetinoglu, E.; Kucukoduk, S.; Bek, Y. Assessment of different folic acid supplementation doses for low-birth-weight infants. Turk. Arch. Pediatr. 2016, 51, 210–216. [Google Scholar] [CrossRef]
- Dallman, P.R. Iron, vitamin E, and folate in the preterm infant. J. Pediatr. 1974, 85, 742–752. [Google Scholar] [CrossRef]
- Hoffbrand, A.V. Folate deficiency in premature infants. Arch. Dis. Child. 1970, 45, 441–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oncel, M.Y.; Calisici, E.; Ozdemir, R.; Yurttutan, S.; Erdeve, O.; Karahan, S.; Dilmen, U. Is folic acid supplementation really necessary in preterm infants ≤32 weeks of gestation? J. Pediatr. Gastroenterol. Nutr. 2014, 58, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H. Benefits and risks of folic acid to the nervous system. J. Neurol. Neurosurg. Psychiatry 2002, 72, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Englert, J.A.; Saunders, R.A.; Purohit, D.; Hulsey, T.C.; Ebeling, M. The effect of anemia on retinopathy of prematurity in extremely low birth weight infants. J. Perinatol. 2001, 21, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sijilmassi, O. Folic acid deficiency and vision: A review. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1573–1580. [Google Scholar] [CrossRef]
- George, A.K.; Majumder, A.; Ice, H.; Homme, R.P.; Eyob, W.; Tyagi, S.C.; Singh, M. Genes and genetics in hyperhomocysteinemia and the “1-carbon metabolism”: Implications for retinal structure and eye functions. Can. J. Physiol. Pharmacol. 2020, 98, 51–60. [Google Scholar] [CrossRef]
- Sofi, F.; Marcucci, R.; Bolli, P.; Giambene, B.; Sodi, A.; Fedi, S.; Menchini, U.; Gensini, G.F.; Abbate, R.; Prisco, D. Low vitamin B6 and folic acid levels are associated with retinal vein occlusion independently of homocysteine levels. Atherosclerosis 2008, 198, 223–227. [Google Scholar] [CrossRef]
- Shute, C. A case report of branch retinal artery occlusion in a teenager due to hyperhomocysteinaemia; the interplay of genetic and nutritional defects. BMC Ophthalmol. 2018, 18 (Suppl. S1), 220. [Google Scholar] [CrossRef]
- Taubert, M.; Dowd, T.C.; Wood, A. Malnutrition and bilateral central retinal vein occlusion in a young woman: A case report. J. Med. Case Rep. 2008, 2, 77. [Google Scholar] [CrossRef]
- Maestro-de-las-Casas, C.; Perez-Miguelsanz, J.; Lopez-Gordillo, Y.; Maldonado, E.; Partearroyo, T.; Varela-Moreiras, G.; Martinez-Alvarez, C. Maternal folic acid-deficient diet causes congenital malformations in the mouse eye. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 587–596. [Google Scholar] [CrossRef]
- Sijilmassi, O.; Del Rio Sevilla, A.; Maldonado Bautista, E.; Barrio Asensio, M.D.C. Gestational folic acid deficiency alters embryonic eye development: Possible role of basement membrane proteins in eye malformations. Nutrition 2021, 90, 111250. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, Y.; Liegl, R.; Wang, Z.; Liu, C.H.; Meng, S.S.; Burnim, S.B.; Saba, N.J.; Fredrick, T.W.; Morss, P.C.; et al. FGF21 Administration Suppresses Retinal and Choroidal Neovascularization in Mice. Cell Rep. 2017, 18, 1606–1613. [Google Scholar] [CrossRef]
- Fu, Z.; Lofqvist, C.A.; Shao, Z.; Sun, Y.; Joyal, J.S.; Hurst, C.G.; Cui, R.Z.; Evans, L.P.; Tian, K.; SanGiovanni, J.P.; et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am. J. Clin. Nutr. 2015, 101, 879–888. [Google Scholar] [CrossRef]
- Heyden, K.E.; Fiddler, J.L.; Xiu, Y.; Malysheva, O.V.; Handzlik, M.K.; Phinney, W.N.; Stiles, L.; Stabler, S.P.; Metallo, C.M.; Caudill, M.A.; et al. Reduced methionine synthase expression results in uracil accumulation in mitochondrial DNA and impaired oxidative capacity. PNAS Nexus 2023, 2, pgad105. [Google Scholar] [CrossRef]
- Suh, J.R.; Oppenheim, E.W.; Girgis, S.; Stover, P.J. Purification and properties of a folate-catabolizing enzyme. J. Biol. Chem. 2000, 275, 35646–35655. [Google Scholar] [CrossRef]
- Bensadoun, A.; Weinstein, D. Assay of proteins in the presence of interfering materials. Anal. Biochem. 1976, 70, 241–250. [Google Scholar] [CrossRef]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.M.; Buscarlet, M.; Mawambo, G.T.; Howard, J.P.; et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020, 369, eaay5356. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., 3rd; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587 e3529. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Ichiki, T.; Sankoda, C.; Takahara, Y.; Ikeda, J.; Inoue, E.; Tokunou, T.; Kitamoto, S.; Sunagawa, K. Suppression of abdominal aortic aneurysm formation by inhibition of prolyl hydroxylase domain protein through attenuation of inflammation and extracellular matrix disruption. Clin. Sci. 2014, 126, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Fraisl, P.; Aragones, J.; Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat. Rev. Drug Discov. 2009, 8, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Miwa, Y.; Wu, J.; Shoda, C.; Jeong, H.; Kawagishi, H.; Tsubota, K.; Kurihara, T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules 2020, 10, 1405. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, Z.; Liu, C.H.; Gong, Y.; Cakir, B.; Liegl, R.; Sun, Y.; Meng, S.S.; Burnim, S.B.; Arellano, I.; et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes 2018, 67, 974–985. [Google Scholar] [CrossRef]
- Fu, Z.J.; Li, S.Y.; Kociok, N.; Wong, D.; Chung, S.K.; Lo, A.C. Aldose reductase deficiency reduced vascular changes in neonatal mouse retina in oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5698–5712. [Google Scholar] [CrossRef][Green Version]
- Sears, J.E.; Hoppe, G.; Ebrahem, Q.; Anand-Apte, B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proc. Natl. Acad. Sci. USA 2008, 105, 19898–19903. [Google Scholar] [CrossRef]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef]
- Mowat, F.M.; Luhmann, U.F.; Smith, A.J.; Lange, C.; Duran, Y.; Harten, S.; Shukla, D.; Maxwell, P.H.; Ali, R.R.; Bainbridge, J.W. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS ONE 2010, 5, e11103. [Google Scholar] [CrossRef]
- Chen, J.; Connor, K.M.; Aderman, C.M.; Willett, K.L.; Aspegren, O.P.; Smith, L.E. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1329–1335. [Google Scholar] [CrossRef]
- Pan, S.; Fan, M.; Liu, Z.; Li, X.; Wang, H. Serine, glycine and onecarbon metabolism in cancer (Review). Int. J. Oncol. 2021, 58, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov. 2014, 4, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Maharaj, A.S.; Walshe, T.E.; Tucker, B.A.; Sekiyama, E.; Kurihara, T.; Darland, D.C.; Young, M.J.; D’Amore, P.A. Endogenous VEGF is required for visual function: Evidence for a survival role on muller cells and photoreceptors. PLoS ONE 2008, 3, e3554. [Google Scholar] [CrossRef]
- Becker, S.; Wang, H.; Simmons, A.B.; Suwanmanee, T.; Stoddard, G.J.; Kafri, T.; Hartnett, M.E. Targeted Knockdown of Overexpressed VEGFA or VEGF164 in Muller cells maintains retinal function by triggering different signaling mechanisms. Sci. Rep. 2018, 8, 2003. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, J.X.; Guo, J.; Wang, J.; Zhu, M.; Chen, Y.; Le, Y.Z. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 2009, 219, 446–454. [Google Scholar] [CrossRef]
- Davis, J.M.; Auten, R.L. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal Med. 2010, 15, 191–195. [Google Scholar] [CrossRef]
- Lee, J.W.; Davis, J.M. Future applications of antioxidants in premature infants. Curr. Opin. Pediatr. 2011, 23, 161–166. [Google Scholar] [CrossRef]
- Lynch, A.M.; Wagner, B.D.; Mandava, N.; Palestine, A.G.; Mourani, P.M.; McCourt, E.A.; Oliver, S.C.; Abman, S.H. The Relationship of Novel Plasma Proteins in the Early Neonatal Period With Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5076–5082. [Google Scholar] [CrossRef]
- Boskabadi, H.; Marefat, M.; Maamouri, G.; Abrishami, M.; Abrishami, M.; Shoeibi, N.; Sanjari, M.S.; Mobarhan, M.G.; Shojaei, S.R.H.; Tavallaei, S.; et al. Evaluation of pro-oxidant antioxidant balance in retinopathy of prematurity. Eye 2021, 36, 148–152. [Google Scholar] [CrossRef]
- Ozieblo-Kupczyk, M.; Bakunowicz-Lazarczyk, A.; Dzienis, K.; Skrzydlewska, E.; Szczepanski, M.; Waszkiewiczz, E. The estimation of selected parameters in antioxidant system in red blood cells in ROP screening of premature infants. Klin. Ocz. 2006, 108, 413–415. [Google Scholar]
- Gansemer, E.R.; McCommis, K.S.; Martino, M.; King-McAlpin, A.Q.; Potthoff, M.J.; Finck, B.N.; Taylor, E.B.; Rutkowski, D.T. NADPH and Glutathione Redox Link TCA Cycle Activity to Endoplasmic Reticulum Homeostasis. iScience 2020, 23, 101116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Siow, Y.L.; Au-Yeung, K.K.; House, J.; Karmin, O. Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney. Am. J. Physiol. Ren. Physiol. 2011, 300, F189–F198. [Google Scholar] [CrossRef] [PubMed]
- Loewen, N.; Chen, J.; Dudley, V.J.; Sarthy, V.P.; Mathura, J.R., Jr. Genomic response of hypoxic Muller cells involves the very low density lipoprotein receptor as part of an angiogenic network. Exp. Eye Res. 2009, 88, 928–937. [Google Scholar] [CrossRef]
- Liang, Y.; Zhen, X.; Wang, K.; Ma, J. Folic acid attenuates cobalt chloride-induced PGE(2) production in HUVECs via the NO/HIF-1alpha/COX-2 pathway. Biochem. Biophys. Res. Commun. 2017, 490, 567–573. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nian, S.; Zeng, Y.; Heyden, K.E.; Cagnone, G.; Yagi, H.; Boeck, M.; Lee, D.; Hirst, V.; Hua, Z.; Lee, J.; et al. Folic Acid Supplementation Inhibits Proliferative Retinopathy of Prematurity. Biomolecules 2025, 15, 309. https://doi.org/10.3390/biom15020309
Nian S, Zeng Y, Heyden KE, Cagnone G, Yagi H, Boeck M, Lee D, Hirst V, Hua Z, Lee J, et al. Folic Acid Supplementation Inhibits Proliferative Retinopathy of Prematurity. Biomolecules. 2025; 15(2):309. https://doi.org/10.3390/biom15020309
Chicago/Turabian StyleNian, Shen, Yan Zeng, Katarina E. Heyden, Gaël Cagnone, Hitomi Yagi, Myriam Boeck, Deokho Lee, Victoria Hirst, Zhanqing Hua, Jeff Lee, and et al. 2025. "Folic Acid Supplementation Inhibits Proliferative Retinopathy of Prematurity" Biomolecules 15, no. 2: 309. https://doi.org/10.3390/biom15020309
APA StyleNian, S., Zeng, Y., Heyden, K. E., Cagnone, G., Yagi, H., Boeck, M., Lee, D., Hirst, V., Hua, Z., Lee, J., Wang, C., Neilsen, K., Joyal, J.-S., Field, M. S., & Fu, Z. (2025). Folic Acid Supplementation Inhibits Proliferative Retinopathy of Prematurity. Biomolecules, 15(2), 309. https://doi.org/10.3390/biom15020309






