PAR2 Participates in the Development of Cough Hypersensitivity in Guinea Pigs by Regulating TRPA1 Through PKC
Abstract
1. Introduction
2. Experiment and Methods
2.1. Animals
2.2. Establishment of a Citric Acid Induced Enhanced Cough Guinea Pig Model and Assessment of the Capsaicin-Induced Cough Response
2.3. Drug Intervention
2.4. Assessment of Vital Signs
2.5. Collection of Bronchoalveolar Lavage Fluid (BALF), Bronchial Tissues, and the Vagus Ganglion
2.6. Measurement of SP and CGRP Levels in BALF by ELISA
2.7. Western Blotting
2.8. Isolation and Culture of Nodose Ganglion Cells
2.9. Patch-Clamp Recording of Nodose Ganglion Neurons
2.10. Multiplex Immunohistochemistry and Laser-Scanning Confocal Microscopy
2.11. Statistical Analysis
3. Results
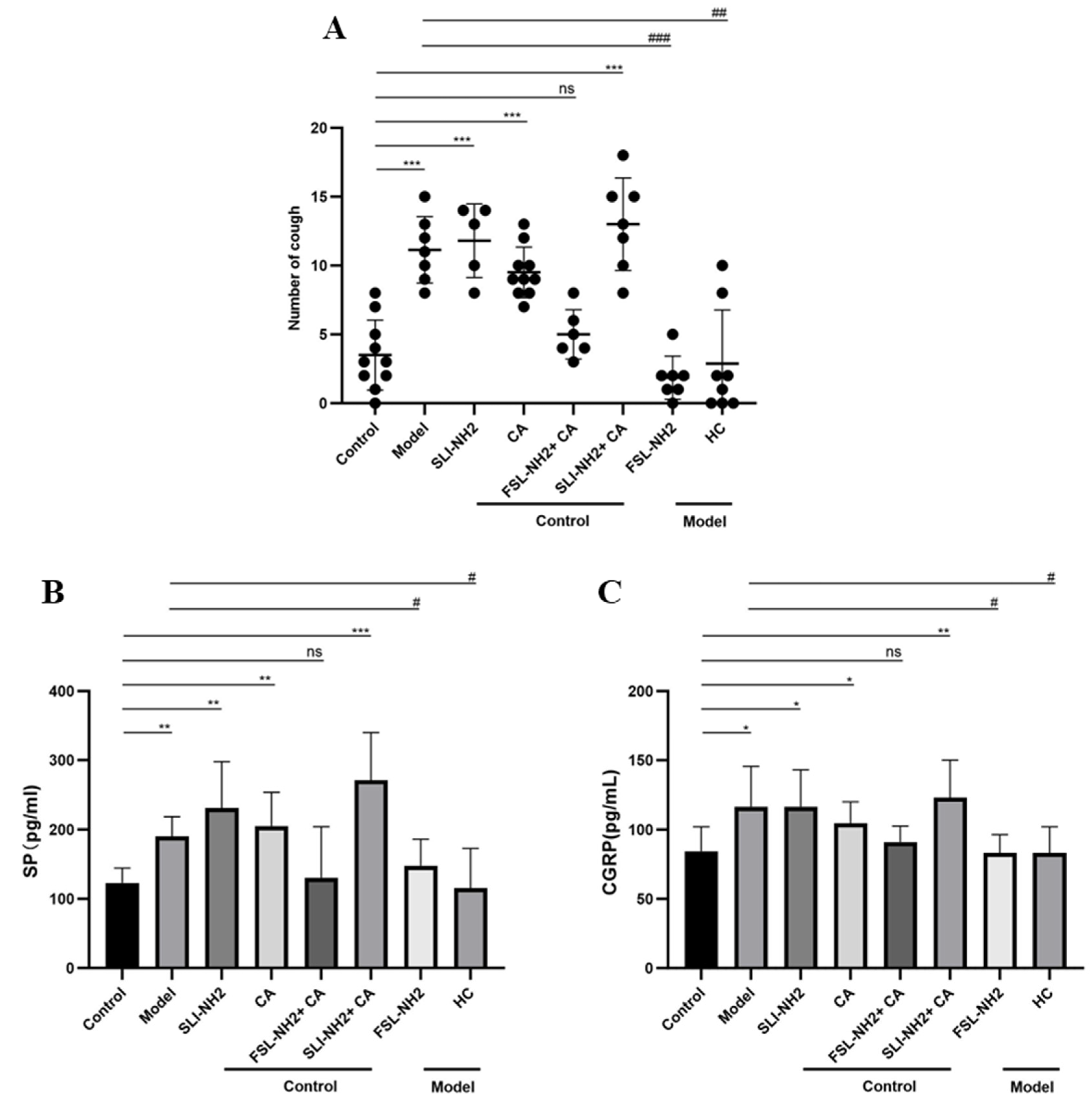
3.1. Cough Sensitivity to Different Drugs
3.2. SP and CGRP Levels in BALF
3.3. Assessment of Vital Signs After PAR2 Antagonist Application
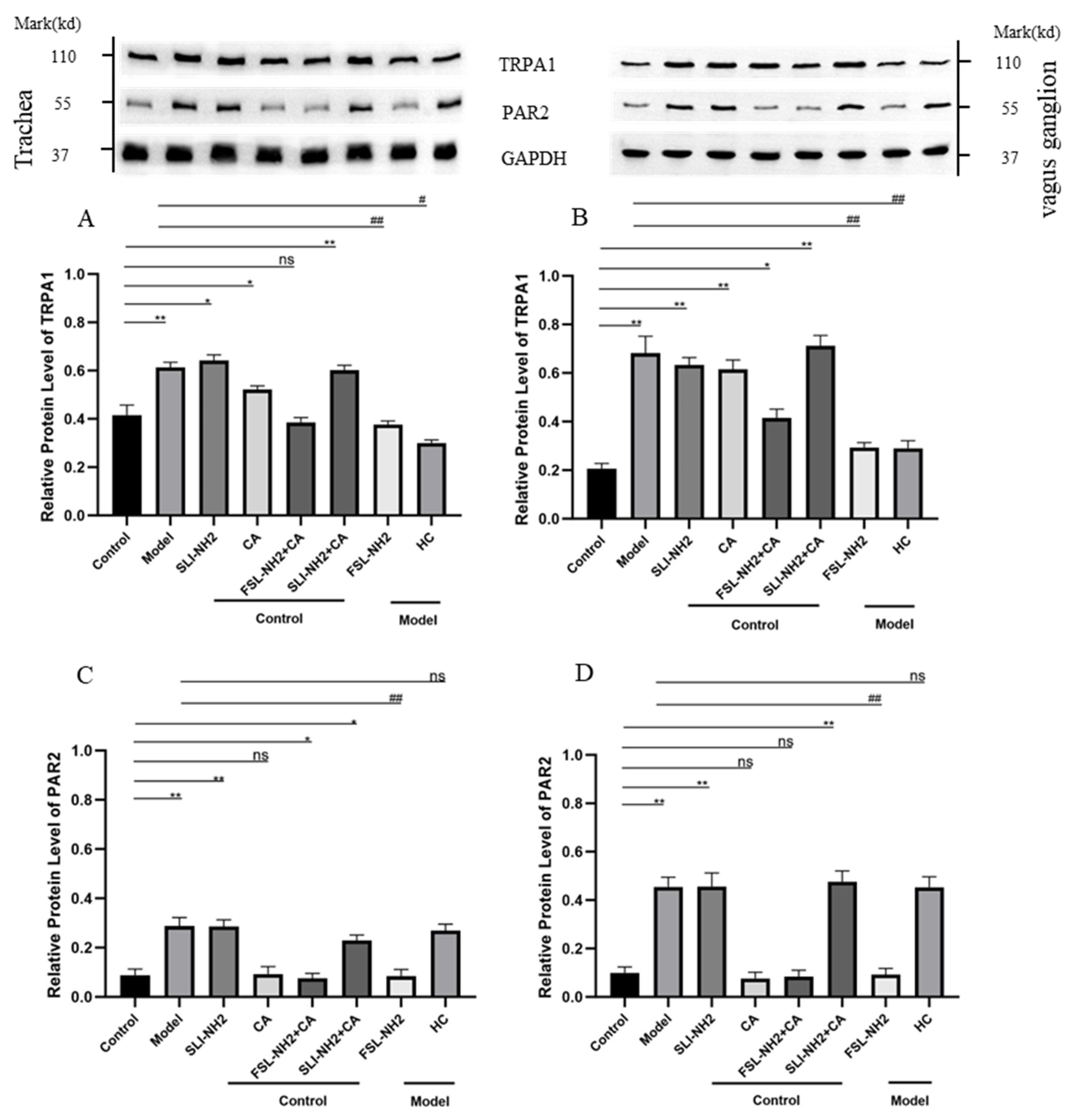
3.4. PAR2/PKC/TRPA1 Expression on Peripheral Nerve Fibers and Neurons in Guinea Pigs with a Cough
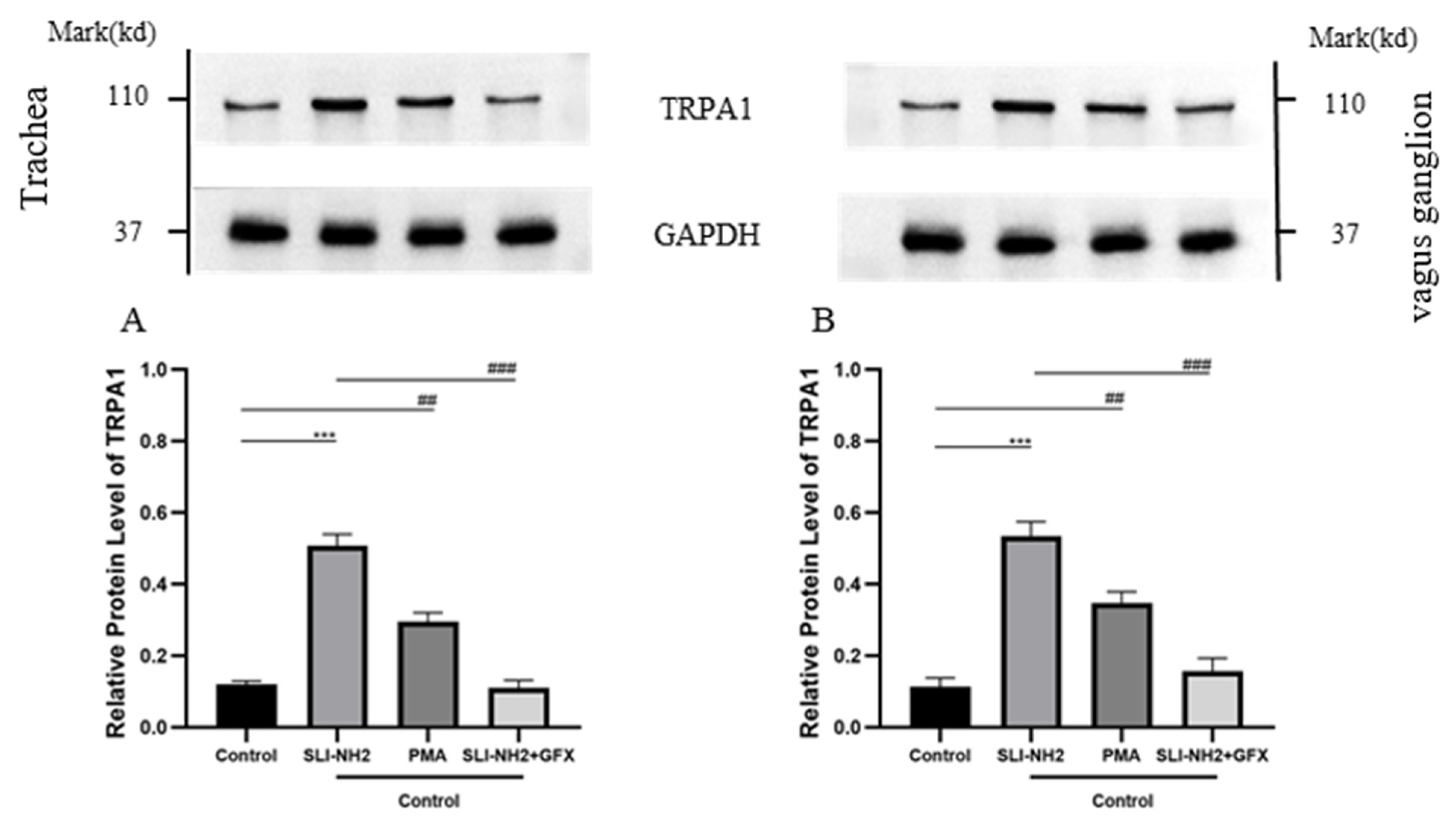
3.5. PAR2 Regulates TRPA1
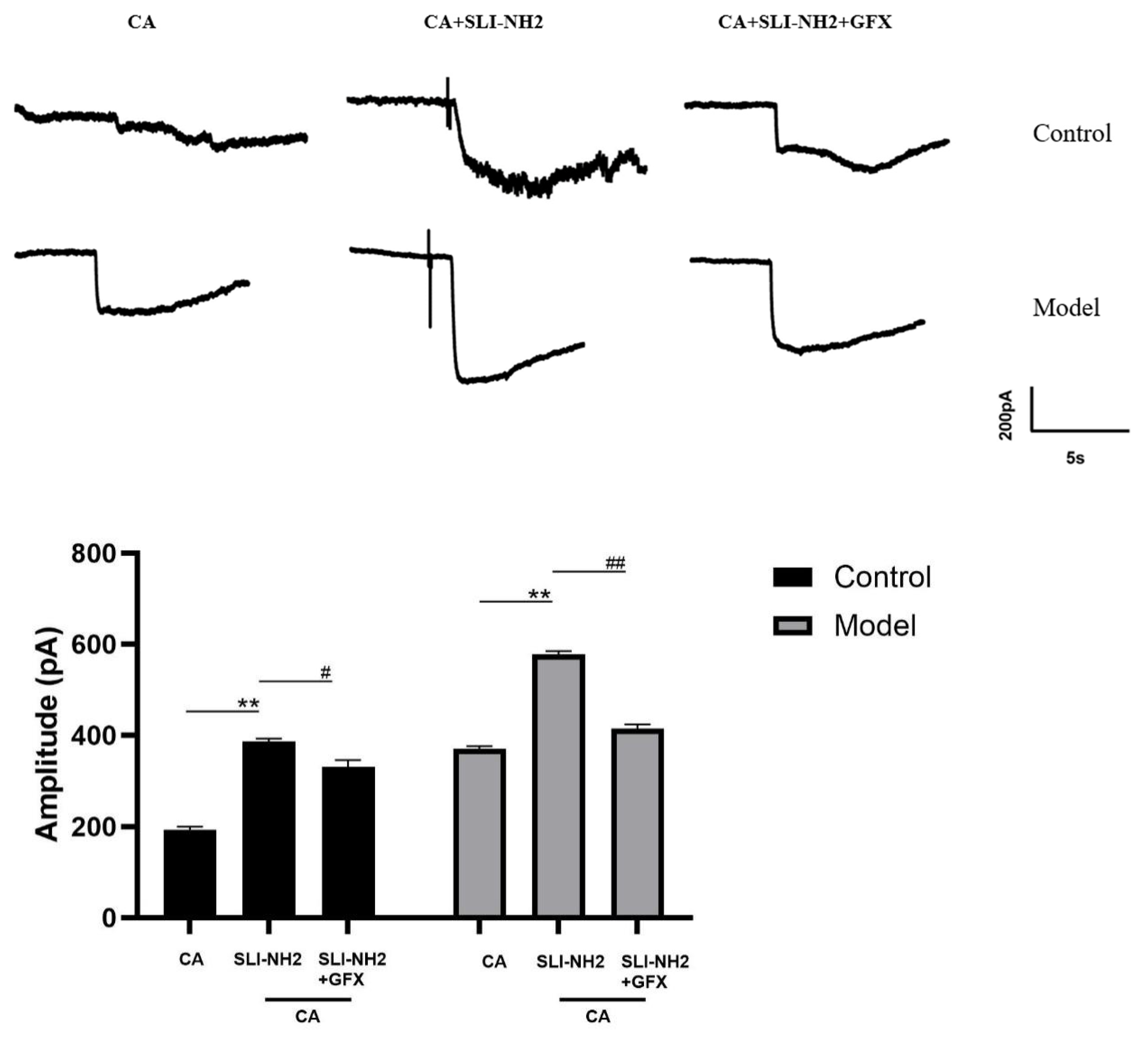
3.6. PAR2 Regulates Cough Hypersensitivity Through TRPA1 via a Mechanism Involving PKC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asthma Group; Respiratory Society; Chinese Medical Association. Guidelines for the iagnosis and treatment of cough (2021). Chin. J. Tuberc. Respir. J. 2022, 45, 34. [Google Scholar]
- McGarvey, L.; Gibson, P.; Wang, G. Treatment of Unexplained Chronic Cough: CHEST Guideline and Expert Panel Report. Chest J. Circ. Respir. Relat. Syst. 2016, 149, 27. [Google Scholar]
- Kahrilas, P.J.; Altman, K.W.; Chang, A.B.; Field, S.K.; Harding, S.M.; Lane, A.P.; Lim, K.; McGarvey, L.; Smith, J.; Irwin, R.S.; et al. Chronic Cough Due to Gastroesophageal Reflux in Adults: CHEST Guideline and Expert Panel Report. Chest 2016, 150, 1341–1360. [Google Scholar] [CrossRef]
- Taylor-Clark, T.E. Role of Reactive Oxygen Species and TRP channels in the Cough Reflex. Cell Calcium. 2016, 60, 155–162. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Birrell, M.A.; Smith, J.A.; Belvisi, M.G. Targeting TRP channels for chronic cough: From bench to bedside. Naunyn-Schmiedebergs Arch. Pharmacol. 2015, 388, 401–420. [Google Scholar] [CrossRef]
- Bonvini, S.J.; Belvisi, M.G. Cough and airway disease: The role of ion channels. Pulm. Pharmacol. Ther. 2017, 47, 21–28. [Google Scholar] [CrossRef]
- Jo-Chiao, W.; Theo, C.; Sebastien, T. Analysis of Airway Vagal Neurons. In Asthma: Methods and Protocols; Springer: New York, NY, USA, 2022; p. 2506. [Google Scholar]
- Li, J.S.; Ma, J.L.; Shi, L.Q.; Wen, Z.H.; Wang, L.Y.; Ji, K.; Dong, S.J.; Li, N.N.; Wang, Y.J.; Li, X.L. The mechanism of transient receptor potential A1/V1 channel linkage in chronic cough. J. Cardio-Pulm. Vasc. 2021, 40, 4. [Google Scholar]
- Morice, A. TRPA1 receptors in chronic cough. Pulm. Pharmacol. Ther. 2017, 47, 42–44. [Google Scholar] [CrossRef]
- Patil, M.; Salas, M.; Bialuhin, S.; Boyd, J.; Jeske, N.; Akopian, A. Sensitization of small-diameter sensory neurons is controlled by TRPV1 and TRPA1 association. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 287–302. [Google Scholar] [CrossRef]
- Ji, R. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm. Pharmacol. Ther. 2015, 35, 81–86. [Google Scholar] [CrossRef]
- Lavinka, P.C.; Dong, X. Molecular signaling and targets from itch: Lessons for cough. In Cough; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Balestrini, A.; Joseph, V.; Dourado, M.; Reese, R.M.; Shields, S.D.; Rougé, L.; Bravo, D.D.; Chernov-Rogan, T.; Austin, C.D.; Chen, H.; et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J. Exp. Med. 2021, 218, e20201637. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.P.; Nijboer-Brinksma, S.; Bos, I.S.T.; van den Berge, M.; Lamb, D.; van Faassen, M.; Kema, I.P.; Gosens, R.; Kistemaker, L.E. The novel TRPA1 antagonist BI01305834 inhibits ovalbumin-induced bronchoconstriction in guinea pigs. Respir. Res. 2021, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya Valeria, S.; Bunnett, N.W. Protease-Activated Receptors: Contribution to Physiology and Disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, K.; McGrath, S.; Huesa, C.; Dunning, L.; Litherland, G.; Crilly, A.; Hultin, L.; Ferrell, W.R.; Lockhart, J.C.; Goodyear, C.S. Rheumatic Disease: Protease-Activated Receptor-2 in Synovial Joint Pathobiology. Front. Endocrinol. 2018, 9, 257. [Google Scholar] [CrossRef]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Chambers, L.S.; Black, J.L.; Poronnik, P.; Johnson, P.R. Functional effects of protease–activated receptor–2 stimulation on human airway. Am. J. Physiol. 2001, 180, L1369–L1378. [Google Scholar] [CrossRef]
- Barrios, V.E.; Jarosinski, M.A.; Wright, C.D. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem. Pharmacol. 2003, 66, 519–525. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Steinhoff, M.; Amadesi, S.; Guerrini, R.; Tognetto, M.; Trevisani, M.; Creminon, C.; Bertrand, C.; Bunnett, N.W.; Fabbri, L.M.; et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am. J. Respir. Crit. Care Med. 2000, 161, 1672–1680. [Google Scholar] [CrossRef]
- Schmidlin, F.; Amadesi, S.; Dabbagh, K.; Lewis, D.E.; Knott, P.; Bunnett, N.W.; Gater, P.R.; Geppetti, P.; Bertrand, C.; Stevens, M.E. Protease-Activated Receptor 2 Mediates Eosinophil Infiltration and Hyperreactivity in Allergic Inflammation of the Airway. J. Immunol. 2002, 169, 5315–5321. [Google Scholar] [CrossRef]
- Cory, E.; Paul, F.; Jason, N.; John, R.G.; Morley, H.; Harissios, V. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J. Allergy Clin. Immunol. 2005, 115. [Google Scholar]
- Xing, J.; Li, J. Proteinase-Activated Receptor-2 Sensitivity of Amplified TRPA1 Activity in Skeletal Muscle Afferent Nerves and Exercise Pressor Reflex in Rats with Femoral Artery Occlusion. Cell. Physiol. Biochem. 2017, 44, 163–171. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.; Tian, F.; Niu, T.; Yi, G. Blocking proteinase-activated receptor 2 alleviated neuropathic pain evoked by spinal cord injury. Physiol. Res. 2016, 65, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Gao, D.; Li, J. Inhibition of PAR2 and TRPA1 signals alleviates neuropathic pain evoked by chemotherapeutic bortezomib. J. Biol. Regul. Homeost. Agents. 2017, 31, 977–983. [Google Scholar] [PubMed]
- Chen, Y.; Yang, C.; Wang, Z.J. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.; Andre, E.; Amadesi, S.; Dinh, T.Q.; Fischer, A.; Bunnett, N.W.; Harrison, S.; Geppetti, P.; Trevisani, M. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J. Appl. Physiol. 2006, 101, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Schiffers, C.; Hristova, M.; Habibovic, A.; Dustin, C.M.; Vliet, A.V.D. The Transient Receptor Potential Channel Vanilloid 1 (TRPV1) is Critical in Innate Airway Epithelial Responses to Protease Allergens. Am. J. Respir. Cell Mol. Biol. 2020, 63, 198–208. [Google Scholar] [CrossRef]
- Wang, S.; Dai, Y.; Kobayashi, K.; Zhu, W.; Kogure, Y.; Yamanaka, H.; Wan, Y.; Zhang, W.; Noguchi, K. Potentiation of the P2X3 ATP receptor by PAR-2 in rat dorsal root ganglia neurons, through protein kinase-dependent mechanisms, contributes to inflammatory pain. Eur. J. Neurosci. 2012, 36, 2293–2301. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Q.; Qiu, Z.; Shi, C.; Ding, H.; Wang, L.; Lv, H.; Yu, L. Association of cough hypersensitivity with tracheal TRPV1 activation and neurogenic inflammation in a novel guinea pig model of citric acid-induced chronic cough. J. Int. Med. Res. 2018, 46, 2913–2924. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Preti, D.; Szallasi, A.; Patacchini, R. TRP channels as therapeutic targets in airway disorders: A patent review. Expert Opin. Ther. Patents 2012, 22, 663–695. [Google Scholar] [CrossRef]
- Julius, D. TRP Channels and Pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, T.; Wei, W.; Zhang, M.; Qiu, Z. A Cold Environment Aggravates Cough Hyperreactivity in Guinea Pigs With Cough by Activating the TRPA1 Signaling Pathway in Skin. Front. Physiol. 2020, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Canning, B. Central regulation of the cough reflex: Therapeutic implications. Pulm. Pharmacol. Ther. 2009, 22, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cao, P. Neural Mechanisms Underlying the Coughing Reflex. Neurosci. Bull. 2023, 39, 1823–1839. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gao, G.; Peterson, B.Z.; Ouyang, A.; Brozmanova, M.; Mazurova, L.; Liu, Z.; Hu, Y.; Yu, X.; Xi, J.; et al. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G34. [Google Scholar] [CrossRef] [PubMed]
- Hung-Te, H.; Yu-Ting, T.; Wen-Jhe, W.; Chi-Ming, L.; Yi-Ching, L. Resveratrol prevents nanoparticles-induced inflammation and oxidative stress via downregulation of PKC-α and NADPH oxidase in lung epithelial A549 cells. BMC Complement. Altern. Med. 2018, 18, 211. [Google Scholar]
- Amadesi, S.; Cottrell, G.S.; Divino, L.; Chapman, K.; Bunnett, N.W. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J. Physiol. 2010, 575 Pt 2, 555–571. [Google Scholar] [CrossRef]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef]
- Baffi, T.R.; Newton, A.C. mTOR Regulation of AGC Kinases: New Twist to an Old Tail. Mol. Pharmacol. 2022, 101, 213–218. [Google Scholar] [CrossRef]
- Zou, W.; Zhan, X.; Li, M.; Song, Z.; Liu, C.; Peng, F.; Guo, Q. Identification of differentially expressed proteins in the spinal cord of neuropathic pain models with PKCgamma silence by proteomic analysis. Brain Res. 2012, 1440, 34–46. [Google Scholar] [CrossRef]
- Dianat, M.; Radan, M.; Badavi, M.; Mard, S.A.; Bayati, V.; Ahmadizadeh, M. Crocin attenuates cigarette smoke-induced lung injury and cardiac dysfunction by anti-oxidative effects: The role of Nrf2 antioxidant system in preventing oxidative stress. Respir. Res. 2018, 19, 58. [Google Scholar] [CrossRef]
- Qiao, Y.; Tam, J.K.C.; Tan, S.S.; Tai, Y.K.; Chin, C.Y.; Stewart, A.G.; Ashman, L.; Sekiguchi, K.; Langenbach, S.Y.; Stelmack, G.; et al. CD151, a laminin receptor showing increased 4 expression in asthma, contributes to airway 5 hyperresponsiveness through calcium signaling. J. Allergy Clin. Immunol. 2017, 139, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell Signaling through Protein Kinase C Oxidation and Activation. Int. J. Mol. Sci. 2012, 13, 10697–10721. [Google Scholar] [CrossRef] [PubMed]
- Bahia, P.K.; Hadley, S.H.; Barannikov, I.; Sowells, I.; Kim, S.H.; Taylor-Clark, T.E. Antimycin A increases bronchopulmonary C-fiber excitability via protein kinase C alpha. Respir. Physiol. Neurobiol. 2020, 278, 103446. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, T.; Okamura, K.; Sasaki, Y.; Ishii, H.; Ohmori, K. KW-4679-induced inhibition of tachykininergic contraction in the guinea-pig bronchi by prejunctional inhibition of peripheral sensory nerves. Br. J. Pharmacol. 1996, 118, 967–973. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Control | FSL-NH2 (µg/kg) | p Value | |||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 400 | |||
| Breathing Rate (brpm) | 85.50 ± 13.65 | 85.70 ± 15.19 | 85.02 ± 13.02 | 87.10 ± 14.67 | 85.20 ± 16.14 | 0.998 |
| Pulse Distention (µm) | 112.10 ± 23.77 | 105.40 ± 19.72 | 97.71 ± 18.96 | 107.50 ± 18.96 | 104.50 ± 21.71 | 0.652 |
| Body Temperature (°C) | 38.53 ± 0.89 | 38.06 ± 0.85 | 38.63 ± 0.76 | 38.35 ± 0.74 | 38.32 ± 0.81 | 0.578 |
| Heart Rate (bpm) | 210.00 ± 19.89 | 220.60 ± 30.80 | 208.20 ± 22.71 | 223.80 ± 30.44 | 218.90 ± 28.27 | 0.630 |
| Oxygen Saturation (%) | 94.79 ± 2.99 | 95.00 ± 2.89 | 94.68 ± 2.98 | 94.49 ± 2.25 | 94.59 ± 3.02 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhang, T.; Bai, H.; Li, W.; Wang, S.; Xu, X.; Yu, L. PAR2 Participates in the Development of Cough Hypersensitivity in Guinea Pigs by Regulating TRPA1 Through PKC. Biomolecules 2025, 15, 208. https://doi.org/10.3390/biom15020208
Zhu Y, Zhang T, Bai H, Li W, Wang S, Xu X, Yu L. PAR2 Participates in the Development of Cough Hypersensitivity in Guinea Pigs by Regulating TRPA1 Through PKC. Biomolecules. 2025; 15(2):208. https://doi.org/10.3390/biom15020208
Chicago/Turabian StyleZhu, Yiqing, Tongyangzi Zhang, Haodong Bai, Wanzhen Li, Shengyuan Wang, Xianghuai Xu, and Li Yu. 2025. "PAR2 Participates in the Development of Cough Hypersensitivity in Guinea Pigs by Regulating TRPA1 Through PKC" Biomolecules 15, no. 2: 208. https://doi.org/10.3390/biom15020208
APA StyleZhu, Y., Zhang, T., Bai, H., Li, W., Wang, S., Xu, X., & Yu, L. (2025). PAR2 Participates in the Development of Cough Hypersensitivity in Guinea Pigs by Regulating TRPA1 Through PKC. Biomolecules, 15(2), 208. https://doi.org/10.3390/biom15020208







