Catestatin and Advanced Glycation End-Products: Potential Indicators of Cardiovascular Risk in Hashimoto’s Thyroiditis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weetman, A.P. An update on the pathogenesis of Hashimoto’s thyroiditis. J. Endocrinol. Investig. 2021, 44, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef] [PubMed]
- Paglia, L. WHO: Healthy diet to prevent chronic diseases and caries. Eur. J. Paediatr. Dent. 2018, 19, 5. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Zhang, M.; Gharib, H.; Lerman, L.O.; Lerman, A. Hypothyroidism Is Associated With Coronary Endothelial Dysfunction in Women. J. Am. Heart Assoc. 2015, 4, e002225. [Google Scholar] [CrossRef]
- Niknam, N.; Khalili, N.; Khosravi, E.; Nourbakhsh, M. Endothelial dysfunction in patients with subclinical hypothyroidism and the effects of treatment with levothyroxine. Adv. Biomed. Res. 2016, 5, 38. [Google Scholar] [CrossRef]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Urlic, H.; Martinovic, D.; Vilovic, M.; Tomasovic Mrcela, N.; Borovac, J.A. Catestatin as a Biomarker of Cardiovascular Diseases: A Clinical Perspective. Biomedicines 2021, 9, 1757. [Google Scholar] [CrossRef]
- Kumric, M.; Dujic, G.; Vrdoljak, J.; Svagusa, K.; Kurir, T.T.; Supe-Domic, D.; Dujic, Z.; Bozic, J. CBD supplementation reduces arterial blood pressure via modulation of the sympatho-chromaffin system: A substudy from the HYPER-H21-4 trial. Biomed. Pharmacother. 2023, 160, 114387. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef]
- Grahovac, M.; Kumric, M.; Vilovic, M.; Martinovic, D.; Kreso, A.; Ticinovic Kurir, T.; Vrdoljak, J.; Prizmic, K.; Božić, J. Adherence to Mediterranean diet and advanced glycation endproducts in patients with diabetes. World J. Diabetes. 2021, 12, 1942–1956. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative Stress and Advanced Glycation End Products in Hashimoto’s Thyroiditis. Thyroid 2016, 26, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, I.M.; van de Zande, S.C.; Westra, J.; Zwerver, J.; Smit, A.J.; Mulder, D.J. The AGE Reader: A non-invasive method to assess long-term tissue damage. Methods 2021, 203, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Dosiou, C. Teprotumumab for Inactive Thyroid Eye Disease? The Jury Is Still Out. J. Clin. Endocrinol. Metab. 2024, 109, e1802–e1803. [Google Scholar] [CrossRef] [PubMed]
- Al-Shoumer, K.A.S.; Vasanthy, B.A. Serum Chromogranin A Concentration in Hyperthyroidism before and after Medical Treatment. J. Clin. Endocrinol. Metab. 2009, 94, 2321–2324. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M.; Corti, A.; Tota, B.; Curnis, F.; Angelone, T.; Colombo, B.; Cerra, M.C.; Bellocci, F.; Crea, F.; Maseri, A. Myocardial production of chromogranin A in human heart: A new regulatory peptide of cardiac function. Eur. Heart J. 2007, 28, 1117–1127. [Google Scholar] [CrossRef]
- Biswas, N.; Curello, E.; OConnor, D.T.; Mahata, S.K. Chromogranin/secretogranin proteins in murine heart: Myocardial production of chromogranin A fragment catestatin (Chga(364384)). Cell Tissue Res. 2010, 342, 353–361. [Google Scholar] [CrossRef]
- Choi, Y.; Miura, M.; Nakata, Y.; Sugasawa, T.; Nissato, S.; Otsuki, T.; Sugawara, J.; Iemitsu, M.; Kawakami, Y.; Shimano, H.; et al. A common genetic variant of the chromogranin A-derived peptide catestatin is associated with atherogenesis and hypertension in a Japanese population. Endocr. J. 2015, 62, 797–804. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, Y.K.; Lin, C.L.; Yeh, J.H.; Kao, C.H. Hashimoto’s thyroiditis, risk of coronary heart disease, and L-thyroxine treatment: A nationwide cohort study. J. Clin. Endocrinol. Metab. 2015, 100, 109–114. [Google Scholar] [CrossRef]
- Hu, Y.; Yao, Z.; Wang, G. The Relationship Between the Impairment of Endothelial Function and Thyroid Antibodies in Hashimoto’s Thyroiditis Patients with Euthyroidism. Horm. Metab. Res. 2020, 52, 642–646. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, S.H.; Park, K.S.; Park, S.W.; Cho, Y.W. Regression of the increased common carotid artery-intima media thickness in subclinical hypothyroidism after thyroid hormone replacement. Endocr. J. 2009, 56, 753–758. [Google Scholar] [CrossRef]
- Knapp, M.; Lisowska, A.; Sobkowicz, B.; Tycinska, A.; Sawicki, R.; Musial, W.J. Myocardial perfusion and intima-media thickness in patients with subclinical hypothyroidism. Adv. Med. Sci. 2013, 58, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Fried, L.P.; Arnold, A.M.; Danese, M.D.; Kuller, L.H.; Burke, G.L.; Tracy, R.P.; Ladenson, P.W. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 2006, 295, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Rodondi, N.; Newman, A.B.; Vittinghoff, E.; de Rekeneire, N.; Satterfield, S.; Harris, T.B.; Bauer, D.C. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern. Med. 2005, 165, 2460–2466. [Google Scholar] [CrossRef]
- Taddei, S.; Caraccio, N.; Virdis, A.; Dardano, A.; Versari, D.; Ghiadoni, L.; Ferrannini, E.; Salvetti, A.; Monzani, F. Low-Grade Systemic Inflammation Causes Endothelial Dysfunction in Patients with Hashimoto’s Thyroiditis. J. Clin. Endocrinol. Metab. 2006, 91, 5076–5082. [Google Scholar] [CrossRef]
- Matsuura, E.; Atzeni, F.; Sarzi-Puttini, P.; Turiel, M.; Lopez, L.R.; Nurmohamed, M.T. Is atherosclerosis an autoimmune disease? BMC Med. 2014, 12, 47. [Google Scholar] [CrossRef]
- Tièche, M.; Lupi, G.A.; Gutzwiller, F.; Grob, P.J.; Studer, H.; Bürgi, H. Borderline low thyroid function and thyroid autoimmunity. Risk factors for coronary heart disease? Br. Heart J. 1981, 46, 202–206. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Mattace-Raso, F.U.; van der Lugt, A.; Ikram, M.A.; Franco, O.H.; Peeters, R.P.; Kavousi, M. Thyroid Function and the Risk of Atherosclerotic Cardiovascular Morbidity and Mortality: The Rotterdam Study. Circ. Res. 2017, 121, 1392–1400. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Labis, B.; Metz-Boutigue, M.H.; Bernstein, C.N.; Ghia, J.E. Catestatin decreases macrophage function in two mouse models of experimental colitis. Biochem. Pharmacol. 2014, 89, 386–398. [Google Scholar] [CrossRef]
- Kennedy, B.P.; Mahata, S.K.; OConnor, D.T.; Ziegler, M.G. Mechanism of cardiovascular actions of the chromo- granin A fragment catestatin in vivo. Peptides 1998, 19, 1241–1248. [Google Scholar] [CrossRef]
- Zhang, D.; Shooshtarizadeh, P.; Laventie, B.J.; Colin, D.A.; Chich, J.F.; Vidic, J.; de Barry, J.; Chasserot-Golaz, S.; De- lalande, F.; Van Dorsselaer, A.; et al. Two chromo- granin a-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated calcium independent phospholipase A2. PLoS ONE 2009, 4, e4501. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Munyaka, P.M.; Eissa, N.; Metz-Boutigue, M.H.; Khafipour, E.; Ghia, J.E. Human Catestatin Alters Gut Microbiota Composition in Mice. Front. Microbiol. 2017, 7, 2151. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Ozawa, N.; Mori, Y. Catestatin Prevents Macrophage-Driven Atherosclerosis but Not Arterial Injury–Induced Neointimal Hyperplasia. Thromb Haemost. 2018, 118, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Manish, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 2017, 26, 001–011. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Wautier, J.L.; Stern, D. Activation of receptor for advanced glycation end products: A mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ. Res. 1999, 84, 489–897. [Google Scholar] [CrossRef]
- Sara, J.D.S.; Prasad, M.; Zhang, M.; Lennon, R.J.; Herrmann, J.; Lerman, L.O.; Lerman, A. High-sensitivity C-reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non-obstructive coronary artery disease. Am. Heart J. 2017, 190, 1–11. [Google Scholar] [CrossRef]
- Ranganadane, R.; Raghavan, S.A.; Shafiulla, A.; Subramanian, G.; Nachimuthu, M.K.S. Cardiovascular Risk in Hashimoto’s Thyroiditis: Role of Thyroid Autoimmunity. J. Basic Clin. Appl. Health Sci. 2021, 4, 20–22. [Google Scholar] [CrossRef]
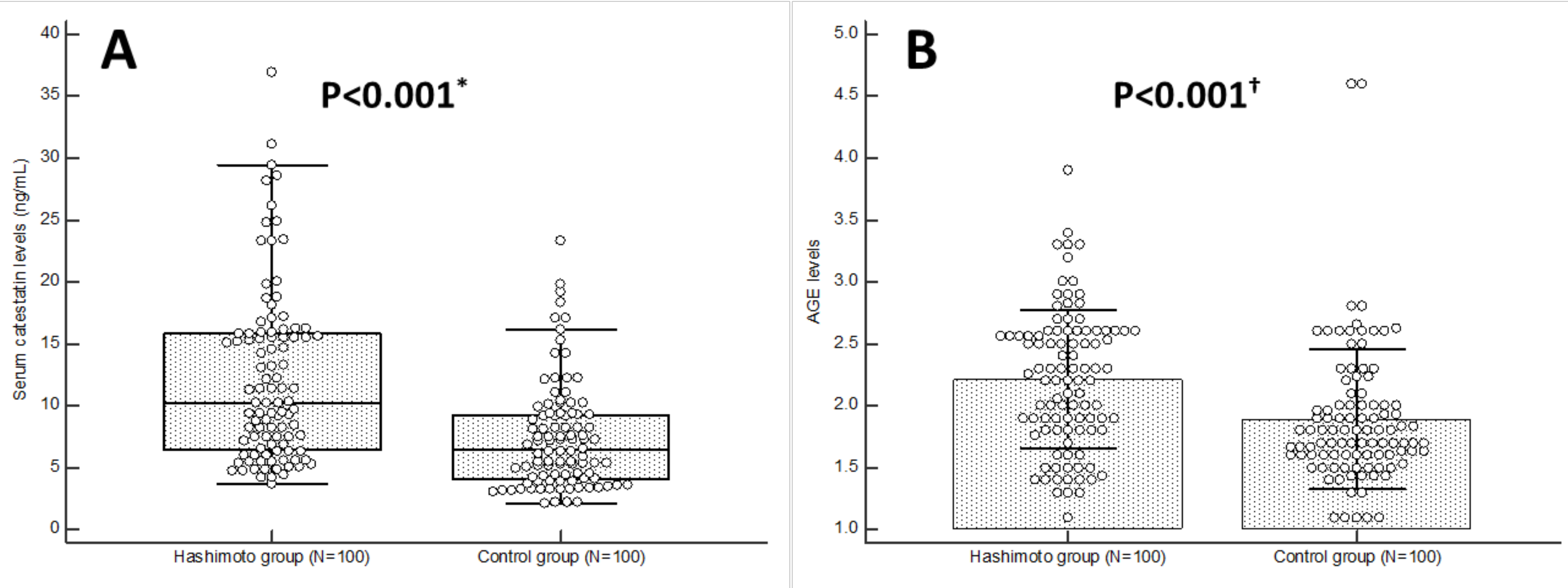
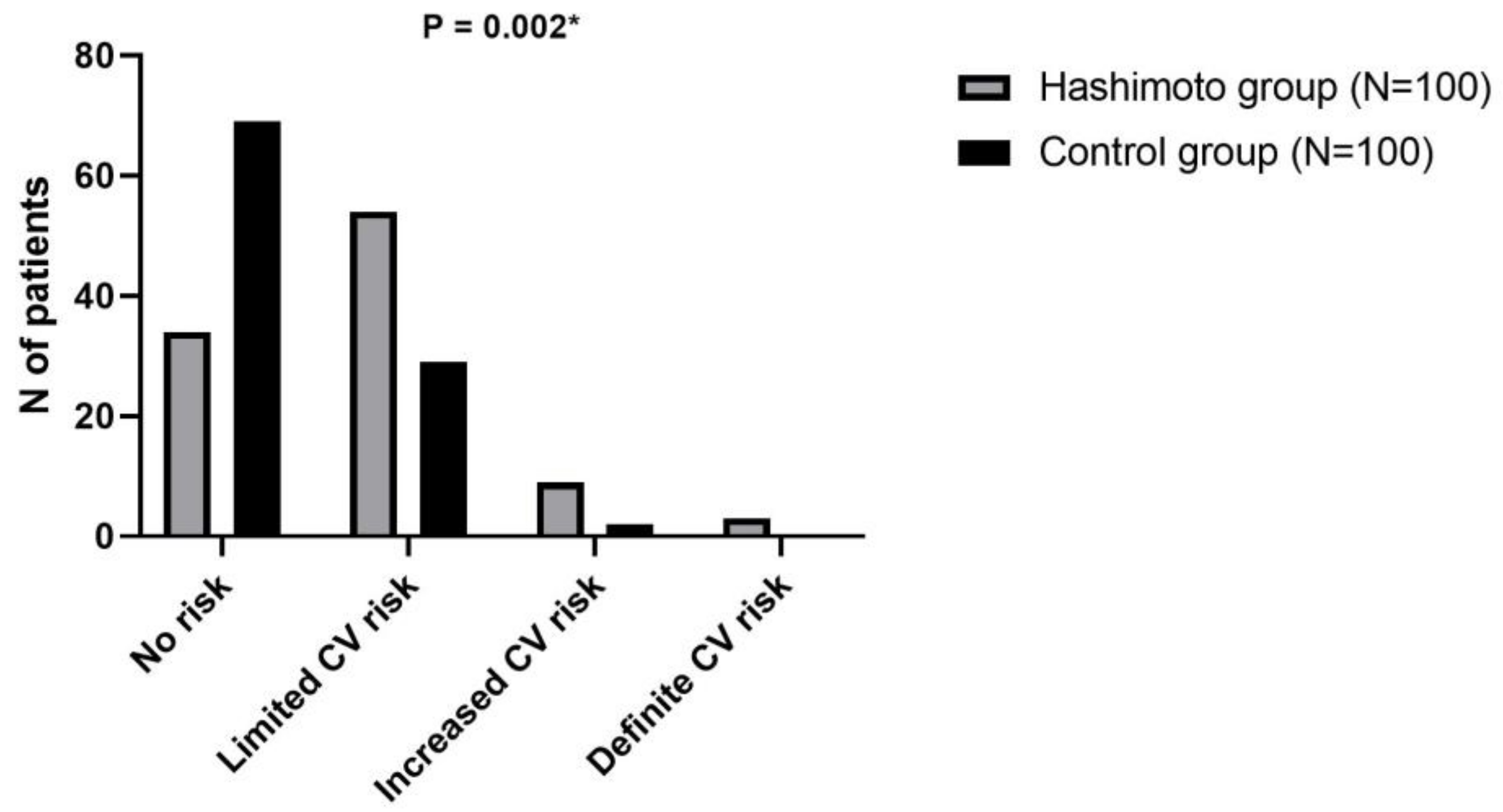
| Parameter | Hashimoto Group (N = 100) | Control Group (N = 100) | p * |
|---|---|---|---|
| Age (years) | 36.8 ± 12.6 | 35.2 ± 10.5 | 0.345 |
| Body height (cm) | 170.3 ± 6.6 | 171.5 ± 5.9 | 0.372 |
| Body weight (kg) | 68.2 ± 12.2 | 66.6 ± 10.6 | 0.326 |
| Body mass index (kg/m2) | 23.3 ± 4.9 | 22.7 ± 3.5 | 0.398 |
| Waist circumference (cm) | 79.2 ± 12.2 | 79.1 ± 10.5 | 0.978 |
| Hip circumference (cm) | 101.8 ± 9.1 | 100.5 ± 9.4 | 0.327 |
| WHR | 1.30 ± 0.09 | 1.28 ± 0.09 | 0.298 |
| Systolic blood pressure (mmHg) | 110.2 ± 15.5 | 112.4 ± 11.5 | 0.269 |
| Diastolic blood pressure (mmHg) | 68.1 ± 9.5 | 68.2 ± 7.7 | 0.935 |
| Active smoking (yes) | 37 (37.0) | 32 (32.0) | 0.458 |
| Parameter | Hashimoto Group (N = 100) | Control Group (N = 100) | p * |
|---|---|---|---|
| TSH (mIU/L) | 1.65 (1.4–2.9) | 1.58 (1.22–2.12) | 0.017 |
| fT3 (pmol/L) | 4.4 (4.3–4.85) | 4.45 (4.2–4.8) | 0.562 |
| fT4 (pmol/L) | 13.1 ± 1.81 | 13.7 ± 1.99 | 0.027 |
| anti-TG (IU/mL) | 119.1 (16.4–274.0) | 9.2 (6.6–13.4) | <0.001 |
| anti-TPO (IU/mL) | 71.9 (21.7–243.0) | 1.9 (1.3–4.1) | <0.001 |
| hsCRP (mg/L) | 2.1 (1.3–3.2) | 1.2 (0.4–1.6) | <0.001 |
| Total cholesterol (mmol/L) | 5.28 ± 1.01 | 4.69 ± 1.42 | 0.088 |
| HDL-cholesterol (mmol/L) | 1.55 ± 0.31 | 1.53 ± 0.48 | 0.822 |
| LDL-cholesterol (mmol/L) | 3.24 ± 0.90 | 2.91 ± 1.12 | 0.025 |
| Triglycerides (mmol/L) | 1.44 ± 0.67 | 1.37 ± 1.08 | 0.573 |
| Fasting glucose (mmol/L) | 5.11 ± 0.49 | 4.93 ± 0.81 | 0.109 |
| Creatinine (µmol/L) | 69.3 ± 15.5 | 67.6 ± 10.7 | 0.358 |
| Urea (mmol/L) | 5.5 ± 1.77 | 5.31 ± 1.51 | 0.416 |
| Parameter | Catestatin r (p †) | AGE r (p *) |
|---|---|---|
| Age (years) | 0.052 (0.466) | 0.226 (0.001) |
| Body mass index (kg/m2) | 0.101 (0.155) | 0.151 (0.033) |
| Waist circumference (cm) | 0.053 (0.454) | 0.166 (0.019) |
| Systolic blood pressure (mmHg) | 0.003 (0.965) | −0.005 (0.945) |
| Diastolic blood pressure (mmHg) | 0.051 (0.475) | 0.057 (0.419) |
| TSH (mIU/L) | 0.182 (0.010) | 0.086 (0.225) † |
| fT3 (pmol/L) | 0.016 (0.822) | 0.038 (0.591) † |
| fT4 (pmol/L) | −0.119 (0.0937) | −0.067 (0.344) |
| anti-TG (IU/mL) | 0.405 (<0.001) | 0.278 (<0.001) † |
| anti-TPO (IU/mL) | 0.416 (<0.001) | 0.397 (<0.001) † |
| hsCRP (mg/L) | 0.204 (0.004) | 0.138 (0.051) † |
| Total cholesterol (mmol/L) | 0.075 (0.289) | −0.003 (0.967) |
| HDL-cholesterol (mmol/L) | −0.043 (0.548) | −0.019 (0.792) |
| LDL-cholesterol (mmol/L) | 0.056 (0.429) | 0.068 (0.338) |
| Triglycerides (mmol/L) | 0.011 (0.881) | −0.006 (0.933) |
| Fasting glucose (mmol/L) | 0.064 (0.365) | 0.043 (0.543) |
| Creatinine (µmol/L) | 0.050 (0.478) | 0.005 (0.940) |
| Parameter | Catestatin r (p †) | AGE r (p *) |
|---|---|---|
| Age (years) | 0.151 (0.134) | 0.247 (0.013) |
| Body mass index (kg/m2) | 0.142 (0.159) | 0.200 (0.046) |
| Waist circumference (cm) | 0.124 (0.219) | 0.203 (0.042) |
| Systolic blood pressure (mmHg) | −0.045 (0.661) | 0.036 (0.721) |
| Diastolic blood pressure (mmHg) | 0.071 (0.482) | 0.093 (0.357) |
| TSH (mIU/L) | 0.123 (0.223) | 0.045 (0.660) † |
| fT3 (pmol/L) | 0.163 (0.105) | 0.073 (0.469) † |
| fT4 (pmol/L) | −0.090 (0.374) | −0.124 (0.218) |
| anti-TG (IU/mL) | 0.353 (<0.001) | 0.215 (0.032) † |
| anti-TPO (IU/mL) | 0.229 (0.024) | 0.276 (0.005) † |
| hsCRP (mg/L) | 0.277 (0.005) | 0.141 (0.163) † |
| Total cholesterol (mmol/L) | 0.040 (0.689) | −0.082 (0.416) |
| HDL-cholesterol (mmol/L) | 0.092 (0.361) | 0.092 (0.361) |
| LDL-cholesterol (mmol/L) | −0.029 (0.776) | 0.010 (0.922) |
| Triglycerides (mmol/L) | −0.113 (0.263) | −0.003 (0.978) |
| Fasting glucose (mmol/L) | −0.007 (0.946) | −0.057 (0.576) |
| Creatinine (µmol/L) | 0.089 (0.378) | −0.006 (0.952) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punda, P.; Kumric, M.; Baric Zizic, A.; Sladic, S.; Vuletic, M.; Supe Domic, D.; Vilovic, M.; Rusic, D.; Bozic, J. Catestatin and Advanced Glycation End-Products: Potential Indicators of Cardiovascular Risk in Hashimoto’s Thyroiditis. Biomolecules 2025, 15, 169. https://doi.org/10.3390/biom15020169
Punda P, Kumric M, Baric Zizic A, Sladic S, Vuletic M, Supe Domic D, Vilovic M, Rusic D, Bozic J. Catestatin and Advanced Glycation End-Products: Potential Indicators of Cardiovascular Risk in Hashimoto’s Thyroiditis. Biomolecules. 2025; 15(2):169. https://doi.org/10.3390/biom15020169
Chicago/Turabian StylePunda, Petra, Marko Kumric, Ana Baric Zizic, Sanda Sladic, Marko Vuletic, Daniela Supe Domic, Marino Vilovic, Doris Rusic, and Josko Bozic. 2025. "Catestatin and Advanced Glycation End-Products: Potential Indicators of Cardiovascular Risk in Hashimoto’s Thyroiditis" Biomolecules 15, no. 2: 169. https://doi.org/10.3390/biom15020169
APA StylePunda, P., Kumric, M., Baric Zizic, A., Sladic, S., Vuletic, M., Supe Domic, D., Vilovic, M., Rusic, D., & Bozic, J. (2025). Catestatin and Advanced Glycation End-Products: Potential Indicators of Cardiovascular Risk in Hashimoto’s Thyroiditis. Biomolecules, 15(2), 169. https://doi.org/10.3390/biom15020169








