Rapid Biochemical Analysis of Postmortem Serum and Myocardial Homogenates—An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Postmortem Interval Estimation (PMI)
2.3. Sample Collection and Preparation
2.4. Laboratory Assays
2.5. Mass Spectrometry Analysis (Proteomics)
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Pilot Study
3.1.1. HemogloBind Treatment
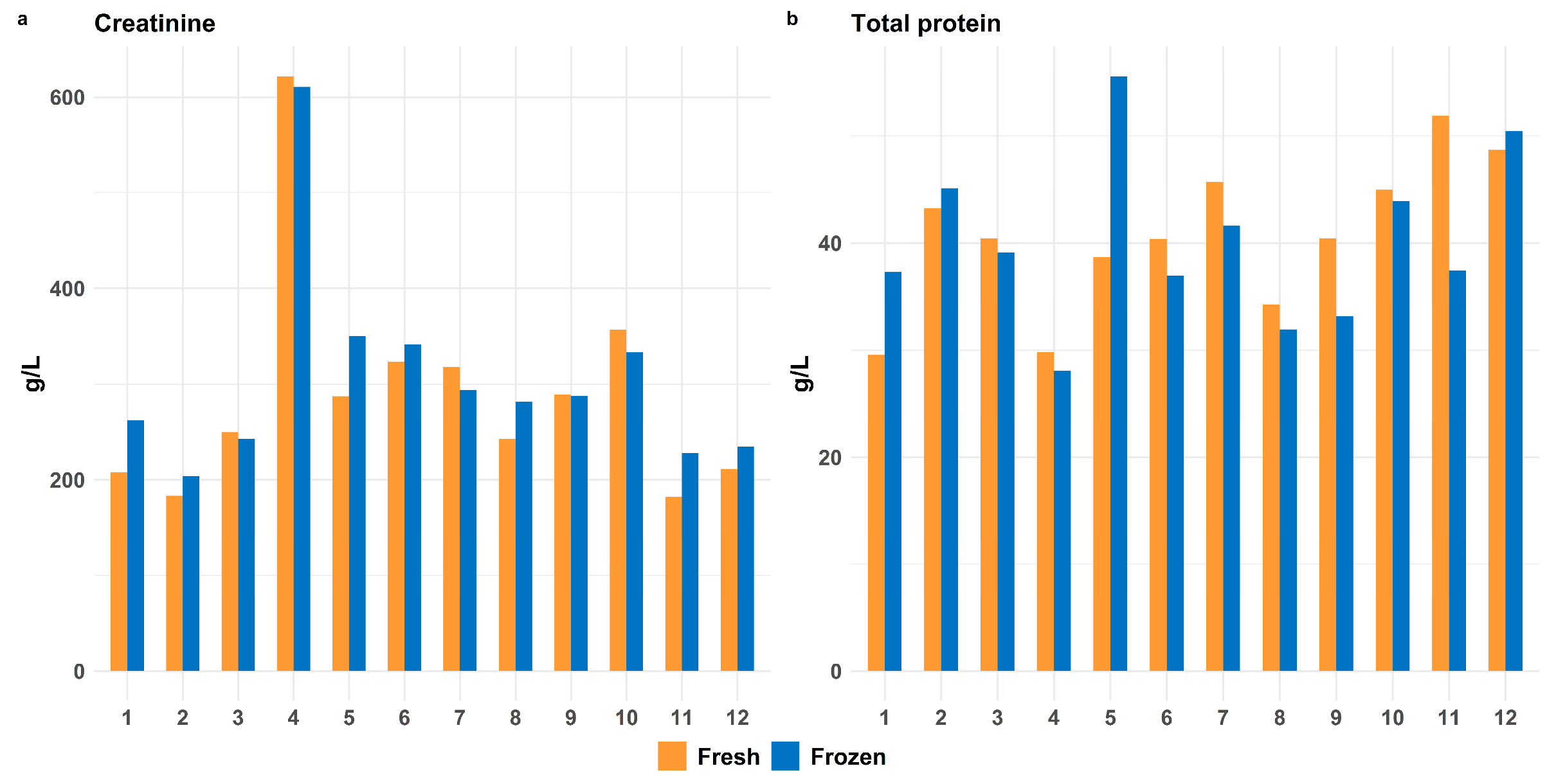
3.1.2. Impact of Freeze–Thaw Cycles on Postmortem Stability of Proteins
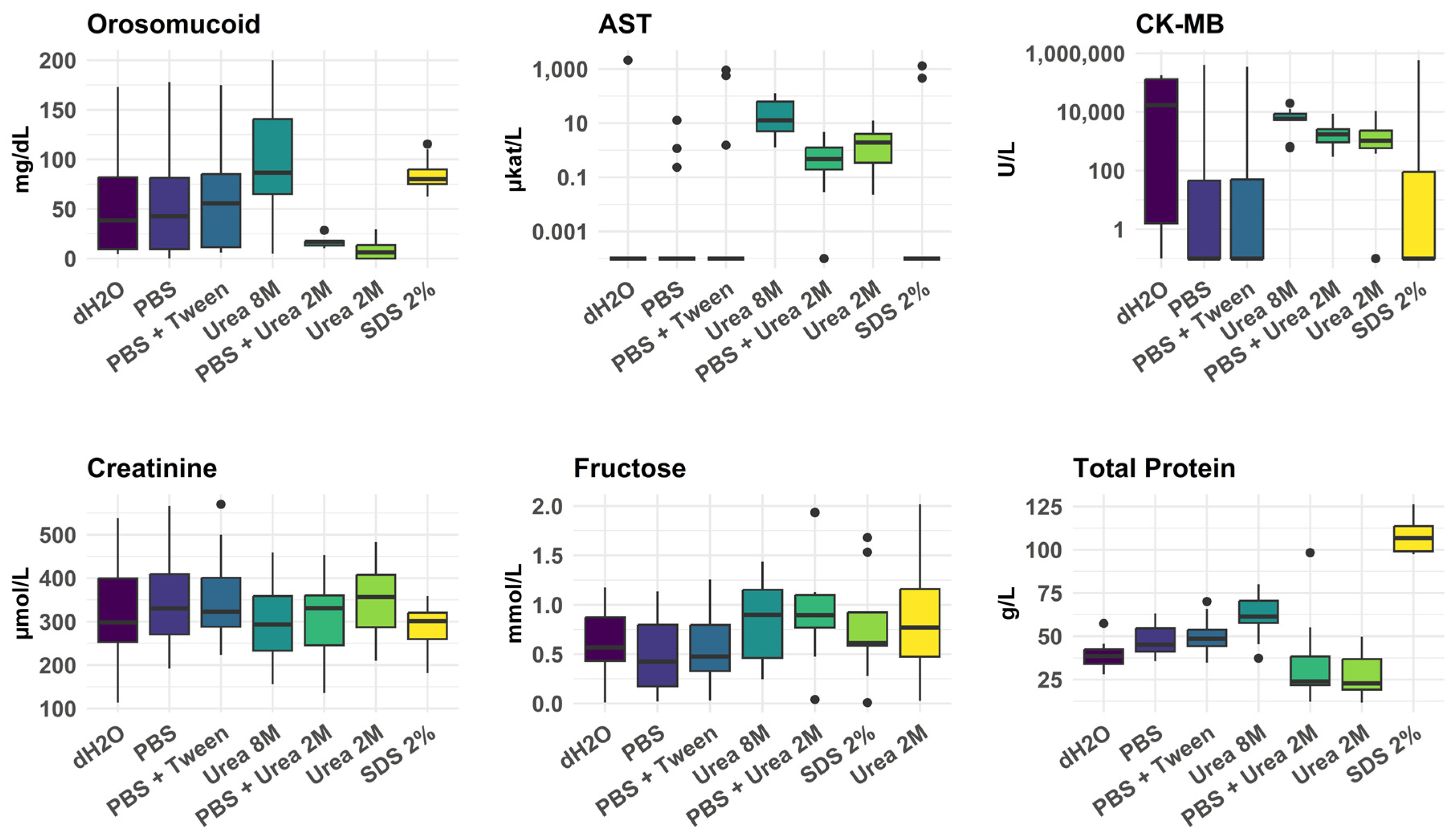
3.1.3. Efficacy of Different Myocardial Tissue Homogenization Conditions
3.2. Main Study
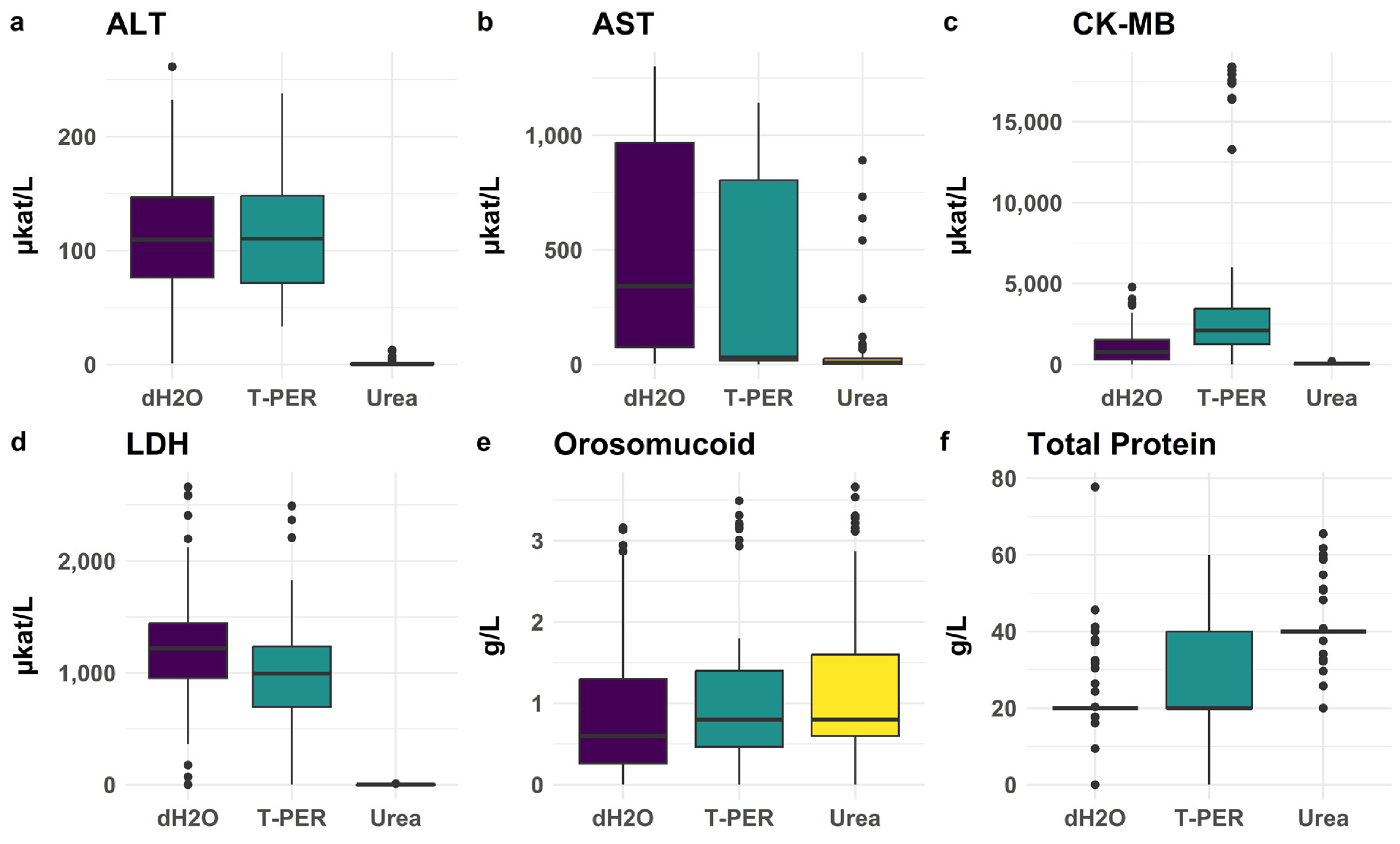
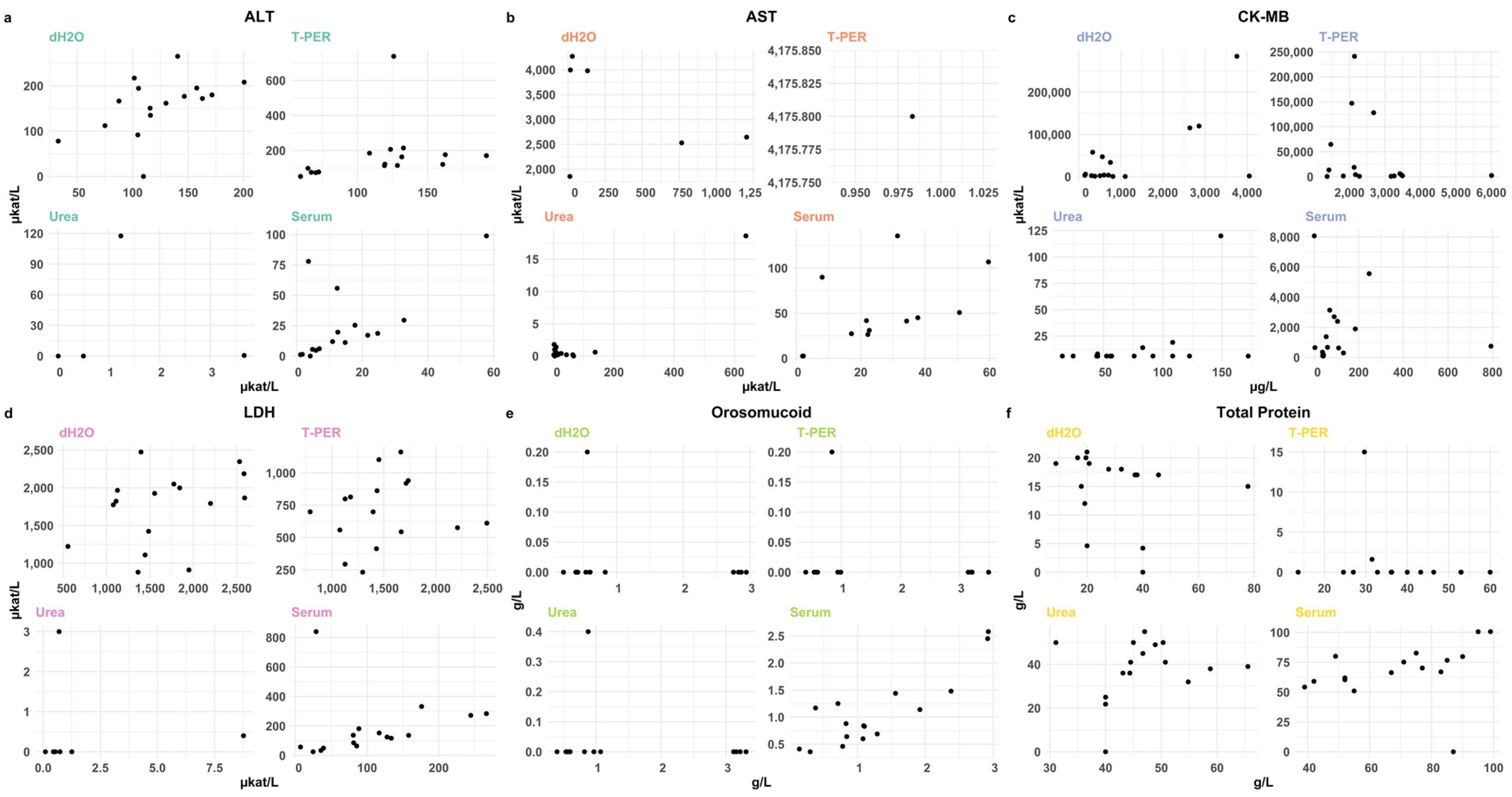
3.2.1. Evaluation of Extraction Methods for Effective Biomarker Yield from the Myocardium
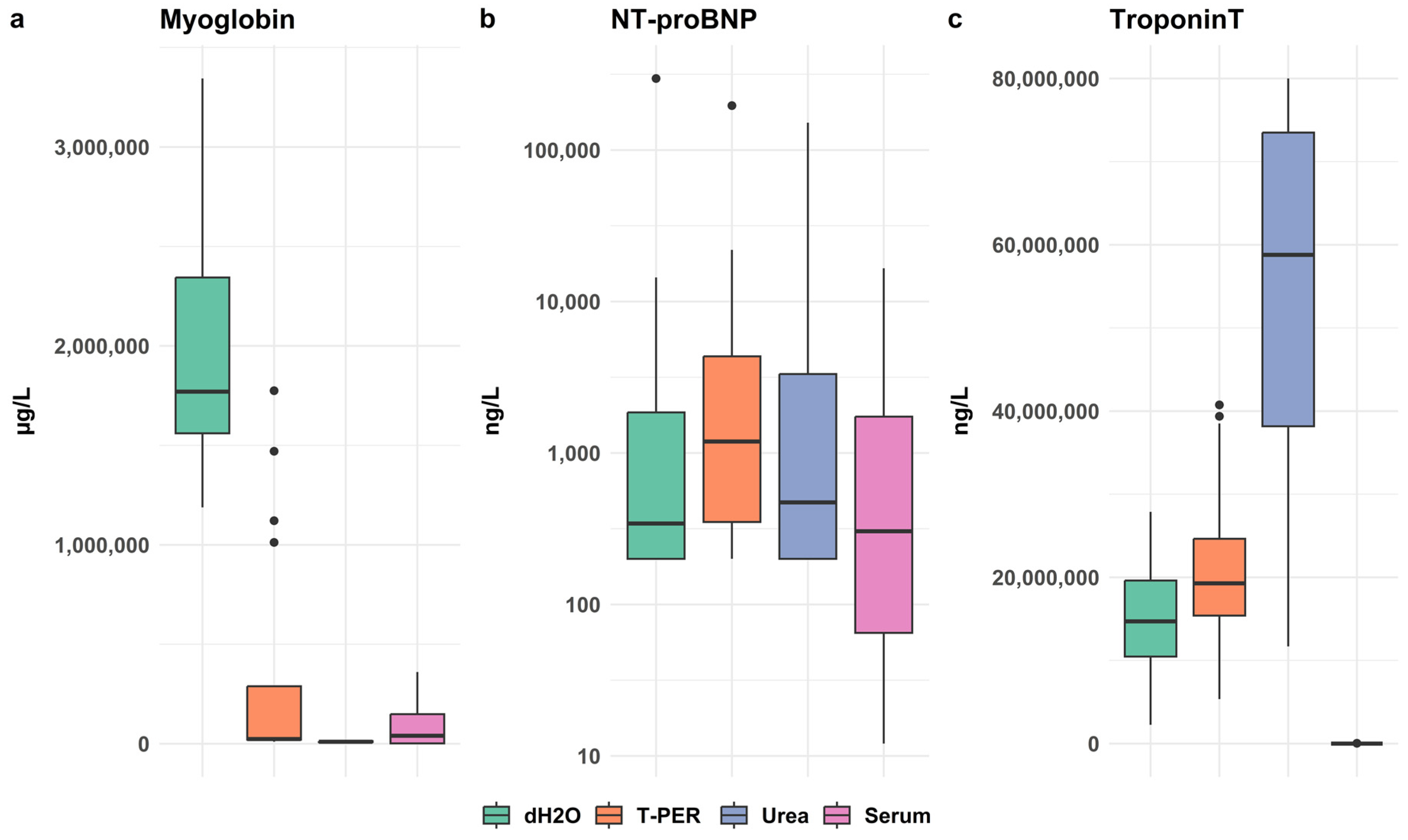
3.2.2. Evaluation of Extraction Methods for Myoglobin, NT-proBNP and cTnT
3.2.3. Comparative Biochemical Analysis of Posterior and Anterior Wall
3.2.4. Cross-Laboratory Validation of Extraction Methods
3.2.5. Comparison of Biomarkers Between SCD Cases and Controls
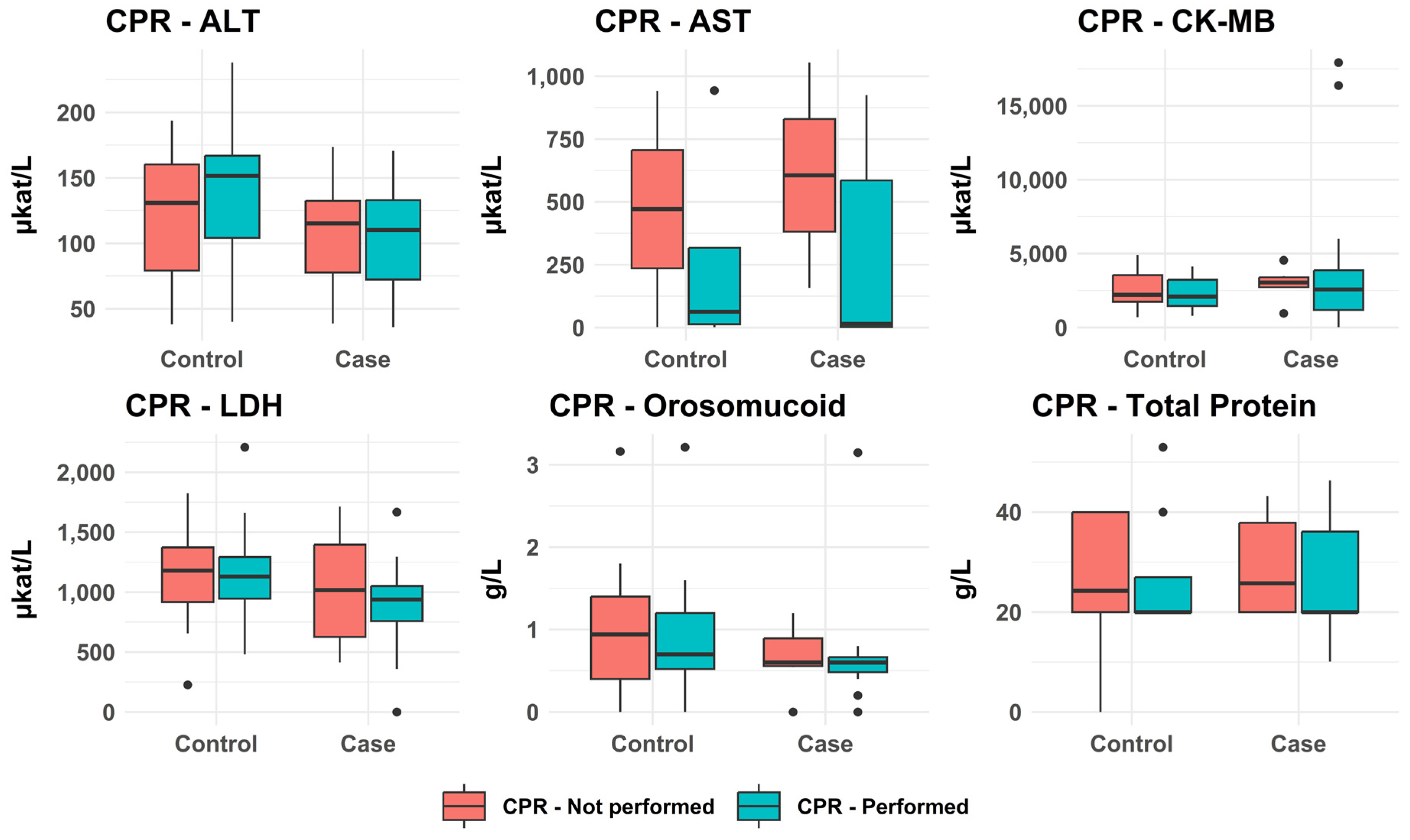
3.2.6. Impact of CPR on Analytical Results
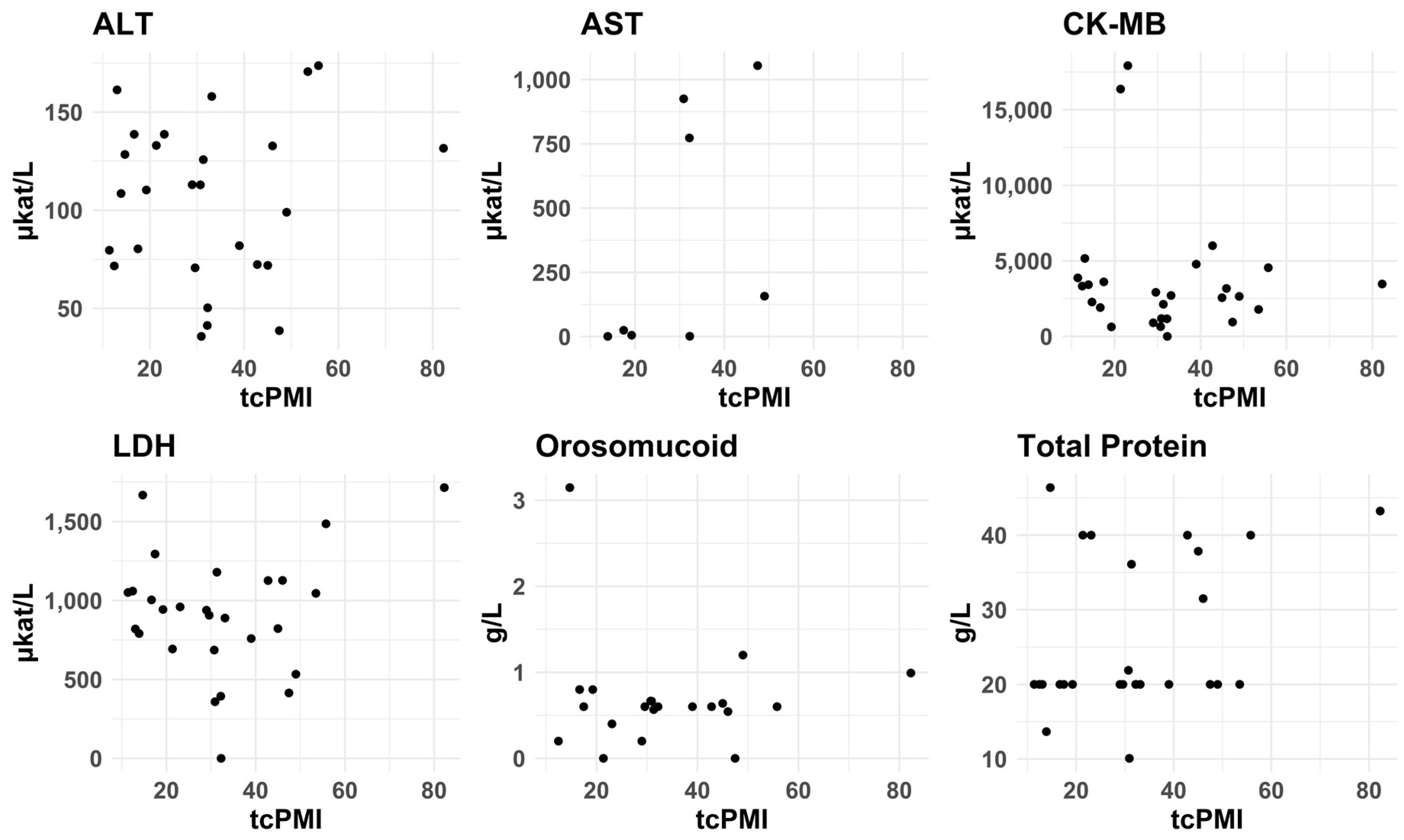
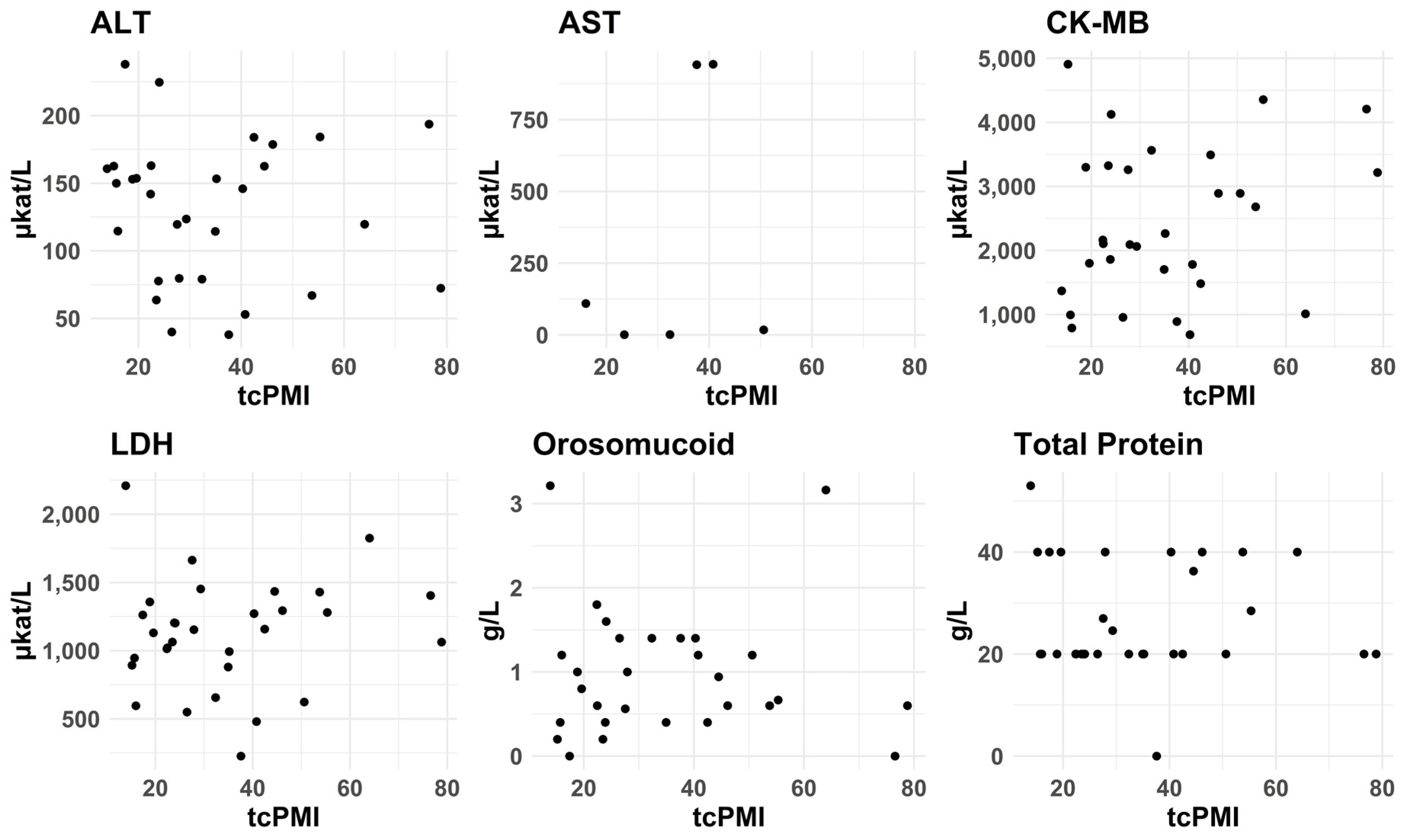
3.2.7. PMI—tcPMI—Postmortem Interval Correlation with Biomarkers Levels
3.2.8. Comparison of Coronary Arteriosclerosis and Biomarker Levels
3.2.9. Proteomics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCD | Sudden Cardiac Death |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CK-MB | Creatine Kinase-MB |
| LDH | Lactate Dehydrogenase |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| PMI | Post-Mortem Interval |
| CRP | C-Reactive Protein |
| AMI | Acute Myocardial Infarction |
| MR | Magnetic Resonance |
| tcPMI | Temperature Corrected Post-Mortem Interval |
| PBS | Phosphate-Buffered Saline |
| SDS | Sodium Dodecyl Sulfate |
| NADH | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| ECLIA | Electrochemiluminescence Immunoassay |
| cTnT | Cardiac Troponin T |
| ECG | Electrocardiogram |
| CT | Computed Tomography |
References
- Kuijpers, C.C.; Fronczek, J.; van de Goot, F.R.; Niessen, H.W.; van Diest, P.J.; Jiwa, M. The value of autopsies in the era of high-tech medicine: Discrepant findings persist. J. Clin. Pathol. 2014, 67, 512–519. [Google Scholar] [CrossRef]
- Dettmeyer, R.B. The role of histopathology in forensic practice: An overview. Forensic Sci. Med. Pathol. 2014, 10, 401–412. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Palmiere, C.; Mangin, P. Postmortem chemistry update part I. Int. J. Legal Med. 2012, 126, 187–198. [Google Scholar] [CrossRef]
- Astrup, B.S.; Thomsen, J.L. The routine use of C-reactive protein in forensic investigations. Forensic Sci. Int. 2007, 172, 49–55. [Google Scholar] [CrossRef]
- Zilg, B.; Alkass, K.; Kronstrand, R.; Berg, S.; Druid, H. A Rapid Method for Postmortem Vitreous Chemistry-Deadside Analysis. Biomolecules 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Johns, S.H.; Wist, A.A.; Najam, A.R. Spot Tests: A Color Chart Reference for Forensic Chemists. J. Forensic Sci. 1979, 24, 631–649. [Google Scholar] [CrossRef]
- Yagoda, H. Applications of Confined Spot Tests in Analytical Chemistry: Preliminary Paper. Ind. Eng. Chem. Anal. Ed. 1937, 9, 79–82. [Google Scholar] [CrossRef]
- Eckart, R.E.; Shry, E.A.; Burke, A.P.; McNear, J.A.; Appel, D.A.; Castillo-Rojas, L.M.; Avedissian, L.; Pearse, L.A.; Potter, R.N.; Tremaine, L.; et al. Sudden death in young adults: An autopsy-based series of a population undergoing active surveillance. J. Am. Coll. Cardiol. 2011, 58, 1254–1261. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018, 15, e190–e252. [Google Scholar] [CrossRef]
- Goldstein, S. The necessity of a uniform definition of sudden coronary death: Witnessed death within 1 hour of the onset of acute symptoms. Am. Heart J. 1982, 103, 156–159. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Aljakna, A.; Fracasso, T.; Sabatasso, S. Molecular tissue changes in early myocardial ischemia: From pathophysiology to the identification of new diagnostic markers. Int. J. Legal Med. 2018, 132, 425–438. [Google Scholar] [CrossRef]
- Isbister, J.; Semsarian, C. Sudden cardiac death: An update. Intern. Med. J. 2019, 49, 826–833. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Hayward-Jones, P.M.; Nolasco-Hipolito, C.; Barradas-Dermitz, D.M.; Calderón-Garcidueñas, A.L.; López-Amador, N. Use of Cardiac Injury Markers in the Postmortem Diagnosis of Sudden Cardiac Death. J. Forensic Sci. 2017, 62, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; Leone, O.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Hawkes, J.A.; Dittmar, T.; Patriarca, C.; Tranvik, L.; Bergquist, J. Evaluation of the Orbitrap Mass Spectrometer for the Molecular Fingerprinting Analysis of Natural Dissolved Organic Matter. Anal. Chem. 2016, 88, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- UniProt Tools. Proteomes · Homo sapiens (Human). Available online: https://www.uniprot.org/proteomes/UP000005640 (accessed on 12 September 2025).
- Abraham, R.A.; Rana, G.; Agrawal, P.K.; Johnston, R.; Sarna, A.; Ramesh, S.; Acharya, R.; Khan, N.; Porwal, A.; Kurundkar, S.B.; et al. The Effects of a Single Freeze-Thaw Cycle on Concentrations of Nutritional, Noncommunicable Disease, and Inflammatory Biomarkers in Serum Samples. J. Lab. Physicians 2021, 13, 6–13. [Google Scholar] [CrossRef]
- Reimers, T.J.; McCann, J.P.; Cowan, R.G.; Concannon, P.W. Effects of Storage, Hemolysis, and Freezing and Thawing on Concentrations of Thyroxine, Cortisol, and Insulin in Blood Samples. Proc. Soc. Exp. Biol. Med. 1982, 170, 509–516. [Google Scholar] [CrossRef]
- Mattana, J.; Singhal, P.C. Determinants of elevated creatine kinase activity and creatine kinase MB-fraction following cardiopulmonary resuscitation. Chest 1992, 101, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Palmiere, C.; Tettamanti, C.; Bonsignore, A.; De Stefano, F.; Vanhaebost, J.; Rousseau, G.; Scarpelli, M.P.; Bardy, D. Cardiac troponins and NT-proBNP in the forensic setting: Overview of sampling site, postmortem interval, cardiopulmonary resuscitation, and review of the literature. Forensic Sci. Int. 2018, 282, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Polena, S.; Shen, K.H.; Mamakos, E.; Chuang, P.J.; Sharma, M.; Griciene, P.; Ponomarev, A.A.; Gintautas, J.; Maniar, R. Correlation between cardiac enzyme elevation and the duration of cardiopulmonary resuscitation. Proc. West. Pharmacol. Soc. 2005, 48, 136–138. [Google Scholar] [PubMed]
- Sacco, M.A.; Aquila, V.R.; Gualtieri, S.; Raffaele, R.; Verrina, M.C.; Tarda, L.; Gratteri, S.; Aquila, I. Quantification of Myocardial Biomarkers in Sudden Cardiac Deaths Using a Rapid Immunofluorescence Method for Simultaneous Biomarker Analysis. Biomedicines 2025, 13, 193. [Google Scholar] [CrossRef]
- Voss, E.M.; Sharkey, S.W.; Gernert, A.E.; Murakami, M.M.; Johnston, R.B.; Hsieh, C.C.; Apple, F.S. Human and canine cardiac troponin T and creatine kinase-MB distribution in normal and diseased myocardium. Infarct sizing using serum profiles. Arch. Pathol. Lab. Med. 1995, 119, 799–806. [Google Scholar]
- Ricchiuti, V.; Sharkey, S.W.; Murakami, M.M.; Voss, E.M.; Apple, F.S. Cardiac troponin I and T alterations in dog hearts with myocardial infarction: Correlation with infarct size. Am. J. Clin. Pathol. 1998, 110, 241–247. [Google Scholar] [CrossRef]
- Rossky, P.J. Protein denaturation by urea: Slash and bond. Proc. Natl. Acad. Sci. USA 2008, 105, 16825–16826. [Google Scholar] [CrossRef]
- Wright, M.C.; Mi, R.; Connor, E.; Reed, N.; Vyas, A.; Alspalter, M.; Coppola, G.; Geschwind, D.H.; Brushart, T.M.; Hoke, A. Novel roles for osteopontin and clusterin in peripheral motor and sensory axon regeneration. J. Neurosci. 2014, 34, 1689–1700. [Google Scholar] [CrossRef]
- Frostadottir, D.; Welinder, C.; Perez, R.; Dahlin, L.B. Refinement of Protein Extraction Protocols for Human Peripheral Nerve Tissue. ACS Omega 2025, 10, 5111–5118. [Google Scholar] [CrossRef]
- Kutlu, E.; Cil, N.; Avci, E.; Bir, F.; Kilic, I.D.; Dereli, A.K.; Acar, K. Significance of postmortem biomarkers and multimarker strategy in sudden cardiac death. Leg. Med. 2023, 61, 102212. [Google Scholar] [CrossRef]
- Sharkey, S.W.; Murakami, M.M.; Smith, S.A.; Apple, F.S. Canine myocardial creatine kinase isoenzymes after chronic coronary artery occlusion. Circulation 1991, 84, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Zhu, B.L.; Ishikawa, T.; Quan, L.; Michiue, T. Significance of postmortem biochemistry in determining the cause of death. Leg. Med. 2009, 11 (Suppl. 1), S46–S49. [Google Scholar] [CrossRef]
- Pélissier-Alicot, A.L.; Gaulier, J.M.; Champsaur, P.; Marquet, P. Mechanisms underlying postmortem redistribution of drugs: A review. J. Anal. Toxicol. 2003, 27, 533–544. [Google Scholar] [CrossRef]
- Zilg, B.; Thelander, G.; Giebe, B.; Druid, H. Postmortem blood sampling-Comparison of drug concentrations at different sample sites. Forensic Sci. Int. 2017, 278, 296–303. [Google Scholar] [CrossRef]
- Matsui, Y.; Hashimoto, H.; Tsukamoto, H.; Okumura, K.; Ito, T.; Ogawa, K.; Satake, T. Disappearance and appearance of isoenzymes of creatine kinase, lactate dehydrogenase and aspartate aminotransferase in the myocardium undergoing infarction. Cardiovasc. Res. 1989, 23, 249–253. [Google Scholar] [CrossRef]
- Van der Laarse, A.; Dijkshoorn, N.J.; Hollaar, L.; Caspers, T. The (iso)enzyme activities of lactate dehydrogenase, alpha-hydroxybutyrate dehydrogenase, creatine kinase and aspartate aminotransferase in human myocardial biopsies and autopsies. Clin. Chim. Acta 1980, 104, 381–391. [Google Scholar] [CrossRef]
- Ali, M.; Laraia, S.; Angeli, R.; Fayemi, A.O.; Braun, E.V.; Davis, E.; Palladino, P.H. Immunochemical CK-MB assay for myocardial infarction. Am. J. Clin. Pathol. 1982, 77, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, W.; Ljungdahl, L.; Herbert, A.K. Troponin-T and CK MB (mass) in early diagnosis of ischemic myocardial injury. The Helsingborg Study, 1992. Clin. Biochem. 1993, 26, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Haridas, N. Evaluation of clinical utility of serum enzymes and troponin-T in the early stages of acute myocardial infarction. Indian. J. Clin. Biochem. 2003, 18, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]


| Case | Age | Sex | Cause of Death | Freeze Thaw | HemogloBind | Heart | Serum |
|---|---|---|---|---|---|---|---|
| P01 | 77 | M | Bronchopneumonia | 1 | 1 | ||
| P02 | 47 | M | Lobar pneumonia | 1 | 1 | ||
| P03 | 20 | F | Hanging | 1 | 1 | ||
| P04 | 26 | M | Intoxication drugs | 1 | 1 | ||
| P05 | 54 | M | Hanging | 1 | 1 | ||
| P06 | 18 | M | Undetermined | 1 | 1 | ||
| P07 | 55 | M | Lung embolism | 1 | |||
| P08 | 64 | F | Fracture complication | 1 | |||
| P09 | 68 | F | AMI with rupture | 1 | 1 | 1 | |
| P10 | 66 | M | Bronchopneumonia | 1 | 1 | ||
| P11 | 50 | M | Hanging | 1 | 1 | ||
| P12 | 50 | M | Hanging | 1 | 1 | ||
| P13 | 59 | M | Anoxic brain injury due to AMI | 1 | 1 | ||
| P14 | 45 | M | Undetermined | 1 | |||
| P15 | 55 | M | Rupture of esophageal varices | ||||
| P16 | 60 | M | Blood loss due to gastric ulcer | 1 | |||
| P17 | 99 | M | Coronary arteriosclerosis | ||||
| P18 | 57 | M | Hanging | 1 | 1 | ||
| P19 | 61 | M | Hanging | 1 | 1 | ||
| P20 | 52 | M | Aspiration pneumonia | 1 | |||
| P21 | 41 | M | Hanging | 1 | 1 | ||
| P22 | 58 | M | AMI | 1 | 1 | 1 | |
| P23 | 78 | M | Drowning | 1 | 1 | ||
| P24 | 67 | M | Acute pyelonephritis | 1 | 1 | 1 | |
| P25 | 84 | F | Bronchopneumonia | 1 | 1 | 1 | |
| P26 | 53 | M | Traumatic subarachnoid bleeding | ||||
| P27 | 64 | M | Hanging | 1 | 1 | ||
| P28 | 60 | M | Intoxication drugs and ethanol | 1 | |||
| P29 | 56 | F | Multiple trauma | ||||
| P30 | 68 | M | Intoxication ethanol | 1 | |||
| P31 | 76 | M | Coronary arteriosclerosis | 1 | |||
| P32 | 52 | M | Intoxication drugs | 1 | 1 | ||
| P33 | 59 | F | Intoxication drugs | 1 | 1 | ||
| P34 | 92 | M | Multiple trauma | 1 | 1 | ||
| P35 | 40 | M | Dissecting aortic aneurysm | 1 | 1 | ||
| P36 | 81 | M | Cervical spine injury | 1 | 1 | ||
| P37 | 27 | M | Intoxication drugs | 1 | |||
| P38 | 40 | F | Traumatic brain injury | 1 | 1 | ||
| P39 | 19 | M | Drowning | 1 | 1 | ||
| P40 | 83 | M | Traumatic brain injury | 1 | 1 | ||
| P41 | 83 | M | Lobar pneumonia | 1 | 1 | ||
| P42 | 49 | F | Intoxication carbon monoxide | 1 | 1 | ||
| P43 | 40 | M | Traumatic brain injury | 1 | 1 |
| Case | Age | Sex | Cause of Death | Group | Height (cm) | BW (kg) | Heart (g) | Asc LAD | Asc Post | Fibrosis | CKD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M01 | 88 | M | Drowning | 165 | 82 | 492 | 1 | 1 | 1 | 0 | |
| M02 | 51 | M | Hemopericardium | 181 | 92 | 560 | 0 | 0 | 0 | 0 | |
| M03 | 20 | F | Anoxic brain injury | 162 | 42 | 205 | 0 | 0 | 0 | 0 | |
| M04 | 55 | M | Bronchopneumonia | 171 | 66 | 400 | 1 | 2 | 0 | 0 | |
| M05 | 54 | M | Alcoholic ketoacidosis | 186 | 100 | 394 | 0 | 0 | 0 | 0 | |
| M06 | 73 | M | Lung embolism | Control | 179 | 114 | 504 | 0 | 0 | 0 | 1 |
| M07 | 71 | M | Coronary arteriosclerosis | Case | 177 | 57 | 330 | 2 | 2 | 0 | 0 |
| M08 | 75 | M | AMI | Case | 190 | 80 | 505 | 2 | 0 | 0 | 0 |
| M09 | 66 | F | Hanging | Control | 158 | 48 | 320 | 0 | 0 | 0 | 0 |
| M10 | 38 | M | Ashyxia plastic bag | Control | 175 | 69 | 375 | 0 | 0 | 0 | 0 |
| M11 | 64 | M | Cardiomegaly | Case | 182 | 138 | 790 | 1 | 0 | 0 | 0 |
| M12 | 73 | M | AMI | Case | 186 | 85 | 540 | 2 | 2 | 2 | 1 |
| M13 | 29 | F | Hanging | Control | 165 | 49 | 218 | 0 | 0 | 0 | 0 |
| M14 | 53 | M | Multiple trauma | 186 | 119 | 586 | 0 | 0 | 0 | 2 | |
| M15 | 49 | M | TBI | 188 | 128 | 544 | 0 | 0 | 0 | 0 | |
| M16 | 55 | M | Intoxication opioids | 197 | 136 | 642 | 1 | 1 | 0 | 0 | |
| M17 | 38 | M | Hanging | Control | 181 | 75 | 335 | 0 | 0 | 0 | 0 |
| M18 | 58 | M | Lung cancer | 183 | 95 | 372 | 0 | 0 | 0 | 0 | |
| M19 | 52 | M | AMI | Case | 188 | 104 | 490 | 1 | 1 | 0 | 0 |
| M20 | 70 | M | Blood loss | Control | 166 | 82 | 450 | 0 | 0 | 0 | 0 |
| M21 | 83 | M | Fractures with fat embolism | 183 | 62 | 550 | 0 | 1 | 0 | 0 | |
| M22 | 49 | M | TBI | Control | 179 | 90 | 470 | 0 | 0 | 0 | 0 |
| M23 | 44 | M | AMI | Case | 185 | 105 | 500 | 1 | 0 | 2 | 0 |
| M24 | 77 | M | Drowning | 186 | 72 | 556 | 2 | 1 | 1 | 0 | |
| M25 | 68 | M | Intox. drugs and ethanol | 185 | 127 | 605 | 0 | 1 | 0 | 0 | |
| M26 | 58 | M | Coronary arteriosclerosis | Case | 178 | 85 | 428 | 1 | 1 | 0 | 0 |
| M27 | 64 | M | Intoxication drugs | 174 | 73 | 355 | 2 | 1 | 0 | 0 | |
| M28 | 52 | M | Hanging | Control | 180 | 81 | 474 | 0 | 0 | 0 | 0 |
| M29 | 19 | F | Hanging | Control | 159 | 42 | 185 | 0 | 0 | 0 | 0 |
| M30 | 76 | F | Heart failure | Case | 164 | 71 | 530 | 1 | 1 | 1 | 0 |
| M31 | 64 | M | Possibly SCD | 183 | 74 | 395 | 0 | 0 | 0 | 0 | |
| M32 | 65 | M | Coronary arteriosclerosis | 193 | 105 | 582 | 1 | 2 | 1 | 0 | |
| M33 | 69 | M | AMI | Case | 183 | 88 | 550 | 2 | 1 | 1 | 1 |
| M34 | 32 | M | Brain hemorrhage | Control | 176 | 73 | 345 | 0 | 0 | 0 | 0 |
| M35 | 36 | M | Causa ignota | 177 | 73 | 350 | 0 | 0 | 0 | 0 | |
| M36 | 53 | M | Coronary arteriosclerosis | Case | 184 | 118 | 465 | 1 | 1 | 1 | 0 |
| M37 | 23 | M | Intoxication drugs | 183 | 107 | 470 | 0 | 0 | 0 | 0 | |
| M38 | 63 | M | Gunshot in the head | Control | 174 | 118 | 522 | 0 | 0 | 0 | 0 |
| M39 | 55 | F | Coronary arteriosclerosis | 178 | 98 | 450 | 1 | 0 | 0 | 0 | |
| M40 | 29 | F | Lung embolism | Control | 165 | 131 | 414 | 0 | 0 | 0 | 0 |
| M41 | 43 | M | Cardiomegaly | Case | 171 | 112 | 596 | 0 | 0 | 2 | 0 |
| M42 | 67 | M | AMI | 167 | 56 | 340 | 2 | 2 | 2 | 1 | |
| M43 | 56 | M | Acute pleuritis | 170 | 88 | 450 | 0 | 0 | 0 | 0 | |
| M44 | 58 | F | Anoxic brain injury | 162 | 84 | 440 | 1 | 2 | 0 | 0 | |
| M45 | 57 | F | Intox. drugs and ethanol | Control | 171 | 79 | 405 | 0 | 0 | 0 | 0 |
| M46 | 21 | M | Intoxication drugs | Control | 180 | 59 | 310 | 0 | 0 | 0 | 0 |
| M47 | 77 | M | Cardiomegaly | 171 | 76 | 520 | 0 | 0 | 0 | 0 | |
| M48 | 86 | F | Bolus death | Control | 171 | 84 | 482 | 0 | 0 | 0 | 0 |
| M49 | 66 | F | Alcoholic ketoacidosis | 165 | 47 | 352 | 1 | 1 | 0 | 0 | |
| M50 | 72 | F | AMI | Case | 168 | 49 | 500 | 2 | 2 | 0 | 0 |
| M51 | 33 | M | Hanging | Control | 167 | 77 | 290 | 0 | 0 | 0 | 0 |
| M52 | 39 | M | Hanging | Control | 170 | 75 | 380 | 0 | 0 | 0 | 0 |
| M53 | 67 | M | Coronary arteriosclerosis | Case | 175 | 93 | 400 | 0 | 0 | 0 | 1 |
| M54 | 18 | M | Hanging | Control | 185 | 88 | 324 | 0 | 0 | 0 | 0 |
| M55 | 82 | M | TBI | 175 | 68 | 440 | 2 | 1 | 0 | 0 | |
| M56 | 49 | M | Acute tonsillitis | 180 | 88 | 530 | 1 | 1 | 0 | 0 | |
| M57 | 79 | M | AMI | 183 | 81 | 520 | 2 | 2 | 1 | 0 | |
| M58 | 67 | M | Brain hemorrhage | Control | 165 | 49 | 310 | 0 | 0 | 0 | 1 |
| M59 | 53 | M | Hanging | Control | 181 | 76 | 420 | 0 | 0 | 0 | 0 |
| M60 | 34 | F | Hanging | Control | 185 | 66 | 440 | 0 | 0 | 0 | 0 |
| M61 | 22 | M | TBI | Control | 165 | 62 | 314 | 0 | 0 | 0 | 0 |
| M62 | 77 | M | Hanging | Control | 174 | 77 | 430 | 2 | 2 | 0 | 0 |
| M63 | 43 | M | Burns | Control | 174 | 80 | 510 | 0 | 0 | 0 | 0 |
| M64 | 30 | M | Intoxication drugs | 193 | 98 | 430 | 0 | 0 | 0 | 0 | |
| M65 | 20 | F | Intoxication drugs | 181 | 90 | 362 | 0 | 0 | 0 | 0 | |
| M66 | 42 | M | Intoxication drugs | 200 | 182 | 858 | 0 | 0 | 0 | 0 | |
| M67 | 70 | F | Alcoholic ketoacidosis | 159 | 75 | 514 | 0 | 0 | 0 | 0 | |
| M68 | 71 | F | Diabetic coma | 163 | 45 | 270 | 1 | 1 | 0 | 0 | |
| M69 | 34 | M | Intoxication drugs | Control | 178 | 80 | 335 | 0 | 0 | 0 | 0 |
| M70 | 73 | M | Intoxication drugs | 191 | 158 | 476 | 0 | 0 | 0 | 0 | |
| M71 | 68 | F | Alcoholic ketoacidosis | 160 | 66 | 365 | 0 | 0 | 0 | 0 | |
| M72 | 61 | M | Intoxication CO | Control | 176 | 89 | 395 | 0 | 0 | 0 | 0 |
| M73 | 76 | M | Coronary arteriosclerosis | 173 | 77 | 560 | 2 | 2 | 1 | 1 | |
| M74 | 71 | M | Coronary arteriosclerosis | 174 | 70 | 388 | 2 | 2 | 0 | 0 | |
| M75 | 76 | F | Cardiac arrhythmia | 168 | 70 | 302 | 0 | 0 | 0 | 0 | |
| M76 | 63 | M | AMI | Case | 174 | 87 | 465 | 1 | 2 | 0 | 0 |
| M77 | 70 | F | Intoxication drugs | Control | 151 | 83 | 425 | 1 | 1 | 0 | 0 |
| M78 | 84 | F | Intoxication drugs | 170 | 74 | 516 | 2 | 2 | 1 | 0 | |
| M79 | 54 | M | Intoxication drugs | Control | 168 | 68 | 320 | 0 | 0 | 0 | 0 |
| M80 | 32 | M | Ketoacidosis NOS | 191 | 71 | 342 | 0 | 0 | 0 | 0 | |
| M81 | 67 | F | AMI | Case | 159 | 68 | 515 | 2 | 2 | 0 | 0 |
| M82 | 57 | F | Fat embolism | 164 | 43 | 315 | 0 | 0 | 0 | 0 | |
| M83 | 65 | M | Esophageal bleeding | 178 | 58 | 295 | 0 | 0 | 0 | 0 | |
| M84 | 32 | M | Intoxication drugs | 185 | 99 | 460 | 0 | 0 | 0 | 0 | |
| M85 | 48 | M | Hypothermia | 167 | 62 | 320 | 0 | 0 | 0 | 0 | |
| M86 | 81 | F | Hanging | Control | 157 | 41 | 342 | 0 | 0 | 0 | 0 |
| M87 | 64 | M | AMI | Case | 177 | 84 | 625 | 2 | 2 | 2 | 2 |
| M88 | 67 | F | Lobar pneumonia—sepsis | 170 | 290 | 0 | 0 | 0 | 0 | ||
| M89 | 65 | M | AMI | Case | 175 | 45 | 574 | 2 | 2 | 1 | 0 |
| M90 | 75 | F | Spleen injury | 157 | 45 | 275 | 0 | 0 | 0 | 0 | |
| M91 | 59 | M | Cardiomegaly | 189 | 103 | 578 | 0 | 0 | 0 | 0 | |
| M92 | 64 | M | Drowning | Control | 177 | 103 | 380 | 0 | 0 | 0 | 0 |
| M93 | 19 | F | Intox. drugs + hypothermia | 171 | 49 | 170 | 1 | 1 | 1 | 1 | |
| M94 | 82 | F | Coronary arteriosclerosis | Case | 152 | 61 | 695 | 2 | 2 | 1 | 1 |
| M95 | 76 | F | Cardiomegaly | 173 | 100 | 635 | 1 | 1 | 0 | 0 | |
| M96 | 41 | M | Cardiomegaly | 183 | 107 | 550 | 0 | 0 | 0 | 0 | |
| M97 | 59 | M | AMI | Case | 185 | 99 | 465 | 1 | 1 | 1 | 0 |
| M98 | 53 | M | Cardiomegaly | 179 | 116 | 600 | 0 | 0 | 0 | 0 | |
| M99 | 56 | M | Coronary arteriosclerosis | 182 | 122 | 470 | 0 | 0 | 0 | 0 | |
| M100 | 56 | M | AMI | Case | 180 | 71 | 482 | 2 | 1 | 0 | 0 |
| M101 | 43 | M | AMI | 179 | 66 | 480 | 0 | 0 | 0 | 0 | |
| M102 | 59 | M | Intoxication CO | 189 | 77 | 476 | 2 | 1 | 1 | 0 | |
| M103 | 46 | F | Multi-organ failure | 170 | 121 | 498 | 0 | 0 | 0 | 1 | |
| M104 | 58 | M | AMI | Case | 186 | 120 | 594 | 1 | 1 | 0 | 0 |
| M105 | 62 | M | Myocardial fibrosis | Case | 189 | 91 | 516 | 0 | 0 | 2 | 0 |
| M106 | 76 | M | Coronary arteriosclerosis | Case | 175 | 90 | 496 | 2 | 2 | 1 | 0 |
| M107 | 89 | M | Stab wound in heart | 175 | 76 | 380 | 0 | 0 | 0 | 0 | |
| M108 | 54 | M | AMI | Case | 174 | 75 | 360 | 1 | 1 | 0 | 0 |
| M109 | 22 | M | Intoxication drug | 182 | 79 | 412 | 0 | 0 | 0 | 0 | |
| M110 | 55 | F | AMI | Case | 175 | 68 | 520 | 0 | 0 | 0 | 0 |
| M111 | 76 | M | AMI | Case | 192 | 96 | 740 | 2 | 2 | 1 | 0 |
| M112 | 41 | M | Lung embolism | Control | 183 | 84 | 420 | 0 | 0 | 0 | 0 |
| M113 | 77 | M | AMI | Case | 177 | 71 | 420 | 2 | 2 | 2 | 0 |
| Posterior Myocardial Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analayte | Extraction | SCD Cases | Controls | ||||||
| Range | Q1:Q3 | Median | N | Range | Q1:Q3 | Median | N | ||
| ALT | Serum | 0.0–984.1 | 1.9; 19.8 | 6.5 | 27 | 0.1–343.5 | 5.8; 25.4 | 16.8 | 31 |
| AST | Serum | 0.0–102.1 | 2.2; 31.4 | 8.5 | 25 | 0.0–196.8 | 8.5; 42.4 | 21.8 | 26 |
| CK-MB | Serum | 0.0–7901 | 14.4; 85.0 | 41.7 | 27 | 0.3–981.6 | 36.1; 128.4 | 66.9 | 30 |
| LDH | Serum | 0.0–3745 | 17.9; 133.1 | 72.4 | 27 | 0.1–1575 | 86.6; 209.3 | 133.8 | 31 |
| Orosomucoid | Serum | 0.0–7.4 | 0.4; 1.1 | 0.7 | 27 | 0.1–8.5 | 0.1; 1.3 | 0.9 | 31 |
| Total Protein | Serum | 0.0–686.0 | 44.0; 79.0 | 71.0 | 27 | 6.0–1176 | 57.0; 92.0 | 75.0 | 31 |
| ALT | dH2O | 1.0–171.0 | 82.2; 138.9 | 109.7 | 27 | 42.7–261.0 | 97; 171.0 | 132.0 | 31 |
| AST | dH2O | 8.0–1299 | 42.0; 1000 | 429.3 | 17 | 4.3–4632 | 296; 2697 | 755.3 | 13 |
| CK-MB * | dH2O | 6.7–3664 | 179.3; 650.6 | 449.7 | 27 | 65.0–120,000 | 499.3; 2051 | 1088 | 31 |
| LDH | dH2O | 69.7–2198 | 958.2; 1339.7 | 1157 | 27 | 0.3–2585 | 1116; 1520 | 1329 | 31 |
| Orosomucoid | dH2O | 0.0–2.8 | 0.2; 0.5 | 0.4 | 21 | 0.0–3.2 | 0.2; 1.3 | 0.6 | 27 |
| Total Protein | dH2O | 0.0–40.0 | 20.0; 20.0 | 20.0 | 27 | 0.0–45.6 | 20; 20.2 | 20.0 | 27 |
| ALT | T-PER | 35.8–73.7 | 72.1; 132.9 | 110.3 | 8 | 38.0–238.0 | 79.2; 163.0 | 144.0 | 30 |
| AST | T-PER | 1.0–1054 | 4.3; 811.0 | 91.0 | 27 | 0.7–943.0 | 5.1; 733.0 | 63.2 | 6 |
| CK-MB | T-PER | 4.0–17,925 | 1487.1; 3740 | 2715 | 27 | 685.0–4906 | 1537; 3289 | 2134 | 30 |
| LDH | T-PER | 0.4–1715 | 726; 1093 | 938.7 | 27 | 227.0–2209 | 920; 1325 | 1154 | 31 |
| Orosomucoid | T-PER | 0.0–3.1 | 0.5; 0.7 | 0.6 | 21 | 0.0–3.2 | 0.4; 1.4 | 0.8 | 29 |
| Total Protein | T-PER | 10.0–46.4 | 20.0; 37.0 | 20.0 | 27 | 0.0–53.0 | 20.0; 40.0 | 20.0 | 31 |
| Anterior myocardial samples | |||||||||
| ALT | Serum | 0.0–984.0 | 1.9; 19.8 | 6.5 | 27 | 0.1–343.5 | 5.8; 25.4 | 16.8 | 31 |
| AST | Serum | 0.0–102.1 | 2.2; 31.4 | 8.5 | 27 | 0.0–196.8 | 8.5; 42.4 | 21.8 | 31 |
| CK-MB | Serum | 0.0–7901 | 14.4; 85.0 | 41.7 | 27 | 0.3–981.6 | 36.1; 128.4 | 66.9 | 31 |
| LDH | Serum | 0.0–3745 | 17.9; 133.1 | 72.4 | 27 | 0.1–1575 | 86.6; 209.3 | 133.8 | 31 |
| Orosomucoid | Serum | 0.0–7.4 | 0.4; 1.1 | 0.7 | 27 | 0.1–8.5 | 0.1; 1.3 | 0.9 | 31 |
| Total Protein | Serum | 0.0–686.0 | 44.0; 79.0 | 71.0 | 27 | 6.0–1176 | 57.0; 92.0 | 75.0 | 31 |
| ALT | dH2O | 1.3–223.7 | 25.7; 81.3 | 55.7 | 21 | 0.3–211.0 | 43.3; 139.0 | 93.7.0 | 22 |
| AST | dH2O | 3.3–1390 | 41.0; 703.3 | 199.0 | 19 | 0.7–1215 | 65.3; 998.7 | 215.3 | 18 |
| CK-MB * | dH2O | 4.7–1822 | 42.2; 388.2 | 68.2 | 22 | 22.7–2439 | 193.3; 1082 | 559.3 | 22 |
| LDH | dH2O | 2.0–2515 | 982.7; 1977.3 | 1407 | 21 | 0.3–2098 | 899.3; 1143 | 1521 | 22 |
| Orosomucoid | dH2O | 0.0–0.0 | 0.0; 0.0 | 0.0 | 4 | 0.0–0.6 | 0.0; 0.2 | 0.2 | 19 |
| Total Protein | dH2O | 0.0–40.0 | 20.0; 40.0 | 20.0 | 22 | 0.0–40.0 | 20.0; 20.0 | 20.0 | 25 |
| ALT ** | T-PER | 39.7–236.0 | 88.6; 179.3 | 152.5 | 22 | 10.7–189.0 | 43.3; 112.0 | 62.0 | 25 |
| AST | T-PER | 0.0–1171 | 0.3; 855.7 | 92,3 | 5 | 0.3–1217 | 14.3; 470.0 | 65.7 | 17 |
| CK-MB ** | T-PER | 5.7–17,650 | 1627; 12,631 | 3768 | 22 | 327.7–4405 | 851.7; 2455 | 1262 | 25 |
| LDH ** | T-PER | 254.7–1735 | 919; 1534 | 1371 | 22 | 187.0–1413 | 436; 784.7 | 624.7 | 25 |
| Orosomucoid | T-PER | 0.0–0.4 | 0.2; 0.2 | 0.2 | 10 | 0.0–1.0 | 0.0; 0.4 | 0.2 | 20 |
| Total Protein | T-PER | 20.0–60.0 | 40.0; 40.0 | 40.0 | 22 | 20.0–40.0 | 20.0; 40.0 | 40.0 | 25 |
| Analyte | Position | ASC Grade 0 + 1 | ASC Grade 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Q1:Q3 | Median | N | Range | Q1:Q3 | Median | N | ||
| ALT | LAD vs. anterior wall | 4.7–224 | 59.3; 146 | 102 | 28 | 0.3–265 | 34.3; 135 | 87.7 | 25 |
| AST | LAD vs. anterior wall | 8.0–3980 | 123; 1020 | 469 | 20 | 3.3–4299 | 36.8; 1242 | 316 | 22 |
| CK-MB | LAD vs. anterior wall | 14.7–3664 | 96.3; 3664 | 425 | 28 | 4.7–47,340 | 60.2; 65 | 1210 | 27 |
| LDH | LAD vs. anterior wall | 363.0–2499 | 838; 2499 | 1278 | 28 | 2.0–2515 | 1111; 1498 | 1251 | 26 |
| ALT | A cor. dexter vs. posterior wall | 4.7–224 | 56.6; 141 | 104 | 30 | 0.3–265 | 37.0; 130 | 87.6 | 23 |
| AST | A cor. dexter vs. posterior wall | 8.0–3980 | 74.8; 1000 | 317 | 22 | 3.3–4299 | 40.3; 1266 | 513 | 20 |
| CK-MB | A cor. dexter vs. posterior wall | 14.7–3664 | 99.2; 805 | 425 | 30 | 4.7–47,340 | 59.7; 702 | 154 | 25 |
| LDH | A cor. dexter vs. posterior wall | 362.7–2499 | 1080; 1762 | 1278 | 30 | 2.0–2515 | 957.0; 1526 | 1215 | 24 |
| Case | Tissue | AST | Myoglobin | TcnT | CK-M-Type | LDH | Orosomucoid | ALT | CK-B-Type |
|---|---|---|---|---|---|---|---|---|---|
| N001 | Homogenates | 2.8 × 109 | 2.1 × 1010 | 4.8 × 108 | 5.1 × 109 | 6.3 × 109 | 1.1 × 109 | 1.6 × 108 | 8.4 × 108 |
| N003 | Homogenates | 6.2 × 109 | 1.3 × 1010 | 2.8 × 108 | 9.3 × 109 | 8.5 × 109 | 4.0 × 109 | 5.0 × 108 | 6.0 × 107 |
| N008 | Homogenates | 1.9 × 109 | 1.9 × 1010 | 1.3 × 109 | 4.1 × 109 | 4.5 × 109 | 2.2 × 109 | 1.3 × 108 | 6.1 × 107 |
| N009 | Homogenates | 2.8 × 109 | 2.3 × 1010 | 9.1 × 108 | 5.7 × 109 | 5.1 × 109 | 5.3 × 108 | 2.6 × 108 | 3.2 × 107 |
| N011 | Homogenates | 1.1 × 108 | 4.2 × 1010 | 1.4 × 109 | 3.1 × 108 | 1.0 × 108 | 1.2 × 109 | 4.6 × 107 | ND |
| N013 | Homogenates | 2.7 × 109 | 4.1 × 1010 | 5.7 × 108 | 4.1 × 109 | 1.1 × 109 | 8.9 × 108 | 3.7 × 108 | 6.3 × 108 |
| N040 | Homogenates | 2.7 × 108 | 4.9 × 1010 | 1.5 × 109 | 6.6 × 108 | 8.0 × 108 | 2.3 × 109 | ND | ND |
| N044 | Homogenates | 3.9 × 108 | 4.9 × 1010 | 1.3 × 109 | 7.7 × 108 | 8.5 × 108 | 4.1 × 109 | 4.1 × 107 | ND |
| N085 | Homogenates | 1.4 × 107 | 4.1 × 1010 | 3.6 × 108 | 1.7 × 108 | 5.1 × 107 | 3.0 × 109 | ND | 4.2 × 107 |
| N086 | Homogenates | 1.7 × 109 | 2.6 × 1010 | 1.2 × 108 | 5.3 × 109 | 3.1 × 109 | 2.0 × 109 | 1.0 × 108 | 2.1 × 109 |
| Mean | 1.89 × 109 | 3.24 × 1010 | 8.24 × 108 | 3.55 × 109 | 3.05 × 109 | 2.13 × 109 | 2.01 × 108 | 5.33 × 108 | |
| N001 | Serum | ND | 1.4 × 109 | ND | ND | ND | 5.0 × 1010 | ND | ND |
| N003 | Serum | ND | 1.7 × 109 | ND | ND | ND | 3.6 × 1010 | ND | ND |
| N008 | Serum | ND | 9.4 × 107 | ND | ND | ND | 5.4 × 1010 | ND | ND |
| N009 | Serum | ND | 2.8 × 108 | ND | ND | 4.0 × 107 | 3.1 × 1010 | ND | ND |
| N011 | Serum | ND | 2.9 × 108 | ND | ND | ND | 3.4 × 1010 | ND | ND |
| N013 | Serum | 1.3 × 107 | 9.4 × 108 | ND | 1.2 × 108 | 2.4 × 107 | 2.8 × 1010 | ND | ND |
| N040 | Serum | ND | 7.6 × 108 | ND | 4.8 × 107 | 1.5 × 107 | 2.6 × 1010 | ND | ND |
| N044 | Serum | ND | 3.4 × 109 | ND | 8.9 × 107 | ND | 2.5 × 1010 | ND | ND |
| N085 | Serum | ND | 4.7 × 109 | ND | 9.0 × 107 | ND | 2.5 × 1010 | ND | ND |
| N086 | Serum | 8.6 × 107 | 1.4 × 109 | ND | 5.3 × 107 | 4.1 × 107 | 2.0 × 1010 | ND | ND |
| Mean | Serum | 4.93 × 107 | 1.49 × 109 | ND | 7.98 × 107 | 2.939 × 107 | 3.31 × 1010 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarri, N.; Druid, H.; Rezaie, A.-R.; Osinga, K.; Sultana, N.; Alkass, K. Rapid Biochemical Analysis of Postmortem Serum and Myocardial Homogenates—An Exploratory Study. Biomolecules 2025, 15, 1483. https://doi.org/10.3390/biom15101483
Sarri N, Druid H, Rezaie A-R, Osinga K, Sultana N, Alkass K. Rapid Biochemical Analysis of Postmortem Serum and Myocardial Homogenates—An Exploratory Study. Biomolecules. 2025; 15(10):1483. https://doi.org/10.3390/biom15101483
Chicago/Turabian StyleSarri, Niki, Henrik Druid, Ali-Reza Rezaie, Klaske Osinga, Nargis Sultana, and Kanar Alkass. 2025. "Rapid Biochemical Analysis of Postmortem Serum and Myocardial Homogenates—An Exploratory Study" Biomolecules 15, no. 10: 1483. https://doi.org/10.3390/biom15101483
APA StyleSarri, N., Druid, H., Rezaie, A.-R., Osinga, K., Sultana, N., & Alkass, K. (2025). Rapid Biochemical Analysis of Postmortem Serum and Myocardial Homogenates—An Exploratory Study. Biomolecules, 15(10), 1483. https://doi.org/10.3390/biom15101483






