Sirtuin Family in Acute Kidney Injury: Insights into Cellular Mechanisms and Potential Targets for Treatment
Abstract
1. Introduction
An Overview of the Sirtuin Family
2. Mechanisms of the Sirtuin Family in AKI
3. The Role of the Sirtuin Family in AKI Treatment
3.1. Traditional Chinese Medicine or Natural Compounds Targeting SIRTs in the Treatment of AKI
3.2. Nanoparticles Targeting SIRTs in the Treatment of AKI
3.3. Clinical Drugs Targeting SIRTs in the Treatment of AKI
3.4. Stem Cell Therapy Targeting SIRTs in the Treatment of AKI
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Booke, H.; Zarbock, A.; Meersch, M. Renal dysfunction in surgical patients. Curr. Opin. Crit. Care 2024, 30, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Cobussen, M.; Verhave, J.C.; Buijs, J.; Stassen, P.M. The incidence and outcome of AKI in patients with sepsis in the emergency department applying different definitions of AKI and sepsis. Int. Urol. Nephrol. 2023, 55, 183–190. [Google Scholar] [CrossRef]
- Monard, C.; Rimmelé, T.; Blanc, E.; Goguillot, M.; Bénard, S.; Textoris, J. Economic burden of in-hospital AKI: A one-year analysis of the nationwide French hospital discharge database. BMC Nephrol. 2023, 24, 343. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Han, S.; Liu, Y.; Gao, J.; Huang, Y.; Sun, W.; Wang, J.; Wang, C.; Zhao, J. Selenium nanoparticles alleviate ischemia reperfusion injury-induced acute kidney injury by modulating GPx-1/NLRP3/Caspase-1 pathway. Theranostics 2022, 12, 3882–3895. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Din, S.; Ye, Z.; Zhou, X.; Cheng, F.; Li, W. The SIRT6/BAP1/xCT signaling axis mediates ferroptosis in cisplatin-induced AKI. Cell. Signal. 2025, 125, 111479. [Google Scholar] [CrossRef]
- Yang, S.; Ye, Z.; Chen, W.; Wang, P.; Zhao, S.; Zhou, X.; Li, W.; Cheng, F. BMAL1 alleviates sepsis-induced AKI by inhibiting ferroptosis. Int. Immunopharmacol. 2024, 142, 113159. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhu, B.; Li, W.; Jiang, Q.; Zuo, Y.; Wen, J.; He, Y.; Xi, X.; Jiang, L. The effects of timing onset and progression of AKI on the clinical outcomes in AKI patients with sepsis: A prospective multicenter cohort study. Ren. Fail. 2023, 45, 2138433. [Google Scholar] [CrossRef]
- Magadi, W.; Peracha, J.; McKane, W.S.; Savino, M.; Braddon, F.; Steenkamp, R.; Nitsch, D. Do outcomes for patients with hospital-acquired Acute Kidney Injury (H-AKI) vary across specialties in England? BMC Nephrol. 2023, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Samoni, S.; De Rosa, S.; Ronco, C.; Castellano, G. Update on persistent acute kidney injury in critical illnesses. Clin. Kidney J. 2023, 16, 1813–1823. [Google Scholar] [CrossRef]
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin. Nephrol. 2020, 40, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhang, A.; Yao, M.; Xin, W.; Guan, X.; Qin, S.; Liu, Y.; Xiong, J.; Yang, K.; Xiong, L.; et al. REST contributes to AKI-to-CKD transition through inducing ferroptosis in renal tubular epithelial cells. JCI Insight 2023, 8. [Google Scholar] [CrossRef]
- Yang, S.; Ye, Z.; Chen, L.; Zhou, X.; Li, W.; Cheng, F. Circadian Clock Gene Bmal1: A Molecular Bridge from AKI to CKD. Biomolecules 2025, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Lumlertgul, N.; Jeong, R.; See, E.; Joannidis, M.; James, M. Acute kidney injury. Lancet 2025, 405, 241–256. [Google Scholar] [CrossRef]
- Birkelo, B.C.; Koyner, J.L.; Ostermann, M.; Bhatraju, P.K. The Road to Precision Medicine for Acute Kidney Injury. Crit. Care Med. 2024, 52, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Pais, T.; Jorge, S.; Lopes, J.A. Acute Kidney Injury in Sepsis. Int. J. Mol. Sci. 2024, 25, 5924. [Google Scholar] [CrossRef]
- Shen, H.; Qi, X.; Hu, Y.; Wang, Y.; Zhang, J.; Liu, Z.; Qin, Z. Targeting sirtuins for cancer therapy: Epigenetics modifications and beyond. Theranostics 2024, 14, 6726–6767. [Google Scholar] [CrossRef]
- Yaghoobi, A.; Rezaee, M.; Hedayati, N.; Keshavarzmotamed, A.; Khalilzad, M.A.; Russel, R.; Asemi, Z.; Rajabi Moghadam, H.; Mafi, A. Insight into the cardioprotective effects of melatonin: Shining a spotlight on intercellular Sirt signaling communication. Mol. Cell. Biochem. 2025, 480, 799–823. [Google Scholar] [CrossRef]
- Ding, Y.N.; Wang, H.Y.; Chen, X.F.; Tang, X.; Chen, H.Z. Roles of Sirtuins in Cardiovascular Diseases: Mechanisms and Therapeutics. Circ. Res. 2025, 136, 524–550. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, X.; Du, C.; Hao, H.; Liu, S.; Liu, G.; Zhang, G.; Fan, K.; Ma, J. Up-regulated succinylation modifications induce a senescence phenotype in microglia by altering mitochondrial energy metabolism. J. Neuroinflamm. 2024, 21, 296. [Google Scholar] [CrossRef]
- Lin, S.; Wu, B.; Hu, X.; Lu, H. Sirtuin 4 (Sirt4) downregulation contributes to chondrocyte senescence and osteoarthritis via mediating mitochondrial dysfunction. Int. J. Biol. Sci. 2024, 20, 1256–1278. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Guo, C.; Zeng, S.; Yu, K.; Liu, M.; Li, Y. Sirt6 overexpression relieves ferroptosis and delays the progression of diabetic nephropathy via Nrf2/GPX4 pathway. Ren. Fail. 2024, 46, 2377785. [Google Scholar] [CrossRef]
- Martino, E.; D’Onofrio, N.; Balestrieri, A.; Mele, L.; Sardu, C.; Marfella, R.; Campanile, G.; Balestrieri, M.L. MiR-15b-5p and PCSK9 inhibition reduces lipopolysaccharide-induced endothelial dysfunction by targeting SIRT4. Cell. Mol. Biol. Lett. 2023, 28, 66. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Shi, M.; Dong, S.; Li, Z.; Zhao, B.; Liu, D.; Li, G.; Cen, J.; Yu, L.; Liang, X.; et al. SIRT5-related lysine demalonylation of GSTP1 contributes to cardiomyocyte pyroptosis suppression in diabetic cardiomyopathy. Int. J. Biol. Sci. 2024, 20, 585–605. [Google Scholar] [CrossRef]
- Perrotta, F.; D’Agnano, V.; Mariniello, D.F.; Castaldo, G.; Vitale, M.; Cazzola, M.; Bianco, A.; Scialò, F. Potential role of SIRT-1 and SIRT-3 as biomarkers for the diagnosis and prognosis of idiopathic pulmonary fibrosis. Respir. Res. 2024, 25, 189. [Google Scholar] [CrossRef]
- Juszczak, F.; Arnould, T.; Declèves, A.E. The Role of Mitochondrial Sirtuins (SIRT3, SIRT4 and SIRT5) in Renal Cell Metabolism: Implication for Kidney Diseases. Int. J. Mol. Sci. 2024, 25, 6936. [Google Scholar] [CrossRef]
- Rine, J.; Herskowitz, I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics 1987, 116, 9–22. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin functions and modulation: From chemistry to the clinic. Clin. Epigenetics 2016, 8, 61. [Google Scholar] [CrossRef]
- Tao, Z.; Jin, Z.; Wu, J.; Cai, G.; Yu, X. Sirtuin family in autoimmune diseases. Front. Immunol. 2023, 14, 1186231. [Google Scholar] [CrossRef]
- Sazdova, I.; Hadzi-Petrushev, N.; Keremidarska-Markova, M.; Stojchevski, R.; Sopi, R.; Shileiko, S.; Mitrokhin, V.; Gagov, H.; Avtanski, D.; Lubomirov, L.T.; et al. SIRT-associated attenuation of cellular senescence in vascular wall. Mech. Ageing Dev. 2024, 220, 111943. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liang, W. SIRT1 and SIRT6: The role in aging-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166815. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yang, H.; Ni, W.; Gao, X.; Pei, X.; Jiang, H.; Su, J.; Weng, R.; Fei, Y.; Gao, Y.; et al. Attenuation of neuronal ferroptosis in intracerebral hemorrhage by inhibiting HDAC1/2: Microglial heterogenization via the Nrf2/HO1 pathway. CNS Neurosci. Ther. 2024, 30, e14646. [Google Scholar] [CrossRef]
- De Sá Fernandes, C.; Novoszel, P.; Gastaldi, T.; Krauß, D.; Lang, M.; Rica, R.; Kutschat, A.P.; Holcmann, M.; Ellmeier, W.; Seruggia, D.; et al. The histone deacetylase HDAC1 controls dendritic cell development and anti-tumor immunity. Cell Rep. 2024, 43, 114308. [Google Scholar] [CrossRef]
- Mielcarek, M.; Zielonka, D.; Carnemolla, A.; Marcinkowski, J.T.; Guidez, F. HDAC4 as a potential therapeutic target in neurodegenerative diseases: A summary of recent achievements. Front. Cell. Neurosci. 2015, 9, 42. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Ke, C.; Jin, Y.; Lao, W.; Wu, Y.; Liu, Y.; Kong, X.; Qiao, J.; Zhai, A.; et al. HDAC4: An emerging target in diabetes mellitus and diabetic complications. Eur. J. Med. Res. 2025, 30, 429. [Google Scholar] [CrossRef]
- Pande, S.; Raisuddin, S. Molecular and cellular regulatory roles of sirtuin protein. Crit. Rev. Food Sci. Nutr. 2023, 63, 9895–9913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Wen, R.; Liu, C.F.; Zhang, T.N.; Yang, N. Cellular and molecular biology of sirtuins in cardiovascular disease. Biomed. Pharmacother. 2023, 164, 114931. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zheng, C.C.; Yang, J.; Li, S.J.; Xu, T.Y.; Wei, X.; Chen, W.Y.; Jiang, Z.L.; Xu, J.J.; Zhang, G.G.; et al. Lysine butyrylation of HSP90 regulated by KAT8 and HDAC11 confers chemoresistance. Cell Discov. 2023, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging targets in neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef]
- Li, N.; Bai, N.; Zhao, X.; Cheng, R.; Wu, X.; Jiang, B.; Li, X.; Xue, M.; Xu, H.; Guo, Q.; et al. Cooperative effects of SIRT1 and SIRT2 on APP acetylation. Aging Cell 2023, 22, e13967. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Jin, W.; Liu, W.; Li, Z.; Fan, Z.; Zou, L.; Chen, S.; Zhu, L.; Zhao, Q.; et al. 2-APQC, a small-molecule activator of Sirtuin-3 (SIRT3), alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis. Signal Transduct. Target. Ther. 2024, 9, 133. [Google Scholar] [CrossRef]
- Hu, S.H.; Feng, Y.Y.; Yang, Y.X.; Ma, H.D.; Zhou, S.X.; Qiao, Y.N.; Zhang, K.H.; Zhang, L.; Huang, L.; Yuan, Y.Y.; et al. Amino acids downregulate SIRT4 to detoxify ammonia through the urea cycle. Nat. Metab. 2023, 5, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jing, Y.; Jiang, X.; Zhang, X.; Liu, F.; Huang, H.; Zhang, Z.; Wang, H.; Sun, S.; Ma, S.; et al. SIRT5 safeguards against primate skeletal muscle ageing via desuccinylation of TBK1. Nat. Metab. 2025, 7, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Kim, C.J.; Coleman, A.; Little, A.; Perez, M.M.; Clarke, E.J.; Diekman, B.; Peffers, M.J.; Chubinskaya, S.; Tomlinson, R.E.; et al. Cartilage-specific Sirt6 deficiency represses IGF-1 and enhances osteoarthritis severity in mice. Ann. Rheum. Dis. 2023, 82, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, H.; Yang, Y.; Wang, H.; Zhang, H.; Guo, S.; Chen, J.; Du, J.; Tian, Y.; Ma, J.; et al. SIRT7 orchestrates melanoma progression by simultaneously promoting cell survival and immune evasion via UPR activation. Signal Transduct. Target. Ther. 2023, 8, 107. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. Yi Chuan Xue Bao 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Zeng, C.; Chen, M. Progress in Nonalcoholic Fatty Liver Disease: SIRT Family Regulates Mitochondrial Biogenesis. Biomolecules 2022, 12, 1079. [Google Scholar] [CrossRef]
- Bi, S.; Jiang, X.; Ji, Q.; Wang, Z.; Ren, J.; Wang, S.; Yu, Y.; Wang, R.; Liu, Z.; Liu, J.; et al. The sirtuin-associated human senescence program converges on the activation of placenta-specific gene PAPPA. Dev. Cell 2024, 59, 991–1009.e1012. [Google Scholar] [CrossRef]
- Mao, J.; Wang, D.; Wang, D.; Wu, Q.; Shang, Q.; Gao, C.; Wang, H.; Wang, H.; Du, M.; Peng, P.; et al. SIRT5-related desuccinylation modification of AIFM1 protects against compression-induced intervertebral disc degeneration by regulating mitochondrial homeostasis. Exp. Mol. Med. 2023, 55, 253–268. [Google Scholar] [CrossRef]
- Kumar, V.; Kundu, S.; Singh, A.; Singh, S. Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr. Neuropharmacol. 2022, 20, 158–178. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. CMLS 2022, 79, 297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Liu, C.; Zhang, H. Sirtuins in osteoarthritis: Current understanding. Front. Immunol. 2023, 14, 1140653. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, G. Roles of sirtuins in asthma. Respir. Res. 2022, 23, 251. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, K.; Gao, T.; Gao, Y.; Yang, Y.; Li, Z.; Zuo, D. SIRT1 silencing promotes EMT and Crizotinib resistance by regulating autophagy through AMPK/mTOR/S6K signaling pathway in EML4-ALK L1196M and EML4-ALK G1202R mutant non-small cell lung cancer cells. Mol. Carcinog. 2024, 63, 2133–2144. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Jiang, G.; Fang, J.; You, Z.; Shao, G.; Zhang, Z.; Jiao, A.; Peng, X. FGF21 promotes non-small cell lung cancer progression by SIRT1/PI3K/AKT signaling. Life Sci. 2021, 269, 118875. [Google Scholar] [CrossRef]
- Park, J.; Chen, Y.; Tishkoff, D.X.; Peng, C.; Tan, M.; Dai, L.; Xie, Z.; Zhang, Y.; Zwaans, B.M.; Skinner, M.E.; et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 2013, 50, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Cui, K.; Yao, S.; Fei, B.; Ling, F.; Li, C.; Huang, Z. SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration. Cell Death Differ. 2024, 31, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Fan, T.; Gao, P.; Peng, Y.; Li, M.; Li, J.; Qin, M.; Hao, R.; Wang, L.; Li, M.; et al. SIRT5-Mediated Desuccinylation of RAB7A Protects Against Cadmium-Induced Alzheimer’s Disease-Like Pathology by Restoring Autophagic Flux. Adv. Sci. 2024, 11, e2402030. [Google Scholar] [CrossRef]
- Yin, J.Y.; Lu, X.T.; Hou, M.L.; Cao, T.; Tian, Z. Sirtuin1-p53: A potential axis for cancer therapy. Biochem. Pharmacol. 2023, 212, 115543. [Google Scholar] [CrossRef]
- Anderson, K.A.; Green, M.F.; Huynh, F.K.; Wagner, G.R.; Hirschey, M.D. SnapShot: Mammalian Sirtuins. Cell 2014, 159, 956–956.e1. [Google Scholar] [CrossRef]
- He, L.; Liu, Q.; Cheng, J.; Cao, M.; Zhang, S.; Wan, X.; Li, J.; Tu, H. SIRT4 in ageing. Biogerontology 2023, 24, 347–362. [Google Scholar] [CrossRef]
- Fiorentino, F.; Castiello, C.; Mai, A.; Rotili, D. Therapeutic Potential and Activity Modulation of the Protein Lysine Deacylase Sirtuin 5. J. Med. Chem. 2022, 65, 9580–9606. [Google Scholar] [CrossRef] [PubMed]
- Lombard, D.B.; Schwer, B.; Alt, F.W.; Mostoslavsky, R. SIRT6 in DNA repair, metabolism and ageing. J. Intern. Med. 2008, 263, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.F.; Michishita-Kioi, E.; Xi, Y.; Tasselli, L.; Kioi, M.; Moqtaderi, Z.; Tennen, R.I.; Paredes, S.; Young, N.L.; Chen, K.; et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 2012, 487, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wakino, S.; Yoshioka, K.; Tatematsu, S.; Hara, Y.; Minakuchi, H.; Sueyasu, K.; Washida, N.; Tokuyama, H.; Tzukerman, M.; et al. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J. Biol. Chem. 2010, 285, 13045–13056. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, J.; Kim, K.; An, H.J.; Gwon, M.G.; Gu, H.; Kim, H.J.; Yang, A.Y.; Kim, S.W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef]
- Fan, H.; Yang, H.C.; You, L.; Wang, Y.Y.; He, W.J.; Hao, C.M. The histone deacetylase, SIRT1, contributes to the resistance of young mice to ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2013, 83, 404–413. [Google Scholar] [CrossRef]
- Qiongyue, Z.; Xin, Y.; Meng, P.; Sulin, M.; Yanlin, W.; Xinyi, L.; Xuemin, S. Post-treatment With Irisin Attenuates Acute Kidney Injury in Sepsis Mice Through Anti-Ferroptosis via the SIRT1/Nrf2 Pathway. Front. Pharmacol. 2022, 13, 857067. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H. The Overexpression of Sirtuin1 (SIRT1) Alleviated Lipopolysaccharide (LPS)-Induced Acute Kidney Injury (AKI) via Inhibiting the Activation of Nucleotide-Binding Oligomerization Domain-Like Receptors (NLR) Family Pyrin Domain Containing 3 (NLRP3) Inflammasome. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2718–2726. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Mao, L.; Wu, J.; Deng, Z.; He, M.; An, S.; Zeng, Z.; Huang, Q.; Chen, Z. p53 Deacetylation Alleviates Sepsis-Induced Acute Kidney Injury by Promoting Autophagy. Front. Immunol. 2021, 12, 685523. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Wang, B.; Wu, Z.; Wu, S.; Jiang, G.; Wang, H.; Huang, Y. Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis via regulation of SIRT1/p53 acetylation. Chem. Biol. Interact. 2023, 382, 110607. [Google Scholar] [CrossRef]
- Gao, D.; Wang, H.; Xu, Y.; Zheng, D.; Zhang, Q.; Li, W. Protective effect of astaxanthin against contrast-induced acute kidney injury via SIRT1-p53 pathway in rats. Int. Urol. Nephrol. 2019, 51, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xie, W.; Yang, X.; Xia, N.; Yang, K. Inhibiting microRNA-449 Attenuates Cisplatin-Induced Injury in NRK-52E Cells Possibly via Regulating the SIRT1/P53/BAX Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 818–823. [Google Scholar] [CrossRef]
- Wei, S.; Gao, Y.; Dai, X.; Fu, W.; Cai, S.; Fang, H.; Zeng, Z.; Chen, Z. SIRT1-mediated HMGB1 deacetylation suppresses sepsis-associated acute kidney injury. Am. J. Physiol. Ren. Physiol. 2019, 316, F20–F31. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.J.; Du, Y.; Liu, Y.Q.; Cai, J.R.; Wu, X.Q.; Hu, S.Q. SIRT2 tyrosine nitration by peroxynitrite in response to renal ischemia/reperfusion injury. Free Radic. Res. 2021, 55, 1104–1118. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhu, L.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; Peng, T.; Lu, Y. S-Sulfhydration of SIRT3 by Hydrogen Sulfide Attenuates Mitochondrial Dysfunction in Cisplatin-Induced Acute Kidney Injury. Antioxid. Redox Signal. 2019, 31, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective effects of sirtuin 3 in a murine model of sepsis-induced acute kidney injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Chen, R.; Lu, H.; Sui, M.; Zhu, Y.; Zeng, L. SIRT3 Protects Against Acute Kidney Injury via AMPK/mTOR-Regulated Autophagy. Front. Physiol. 2018, 9, 1526. [Google Scholar] [CrossRef]
- Ouyang, J.; Zeng, Z.; Fang, H.; Li, F.; Zhang, X.; Tan, W. SIRT3 Inactivation Promotes Acute Kidney Injury Through Elevated Acetylation of SOD2 and p53. J. Surg. Res. 2019, 233, 221–230. [Google Scholar] [CrossRef]
- Jian, Y.; Yang, Y.; Cheng, L.; Yang, X.; Liu, H.; Li, W.; Wan, Y.; Yang, D. Sirt3 mitigates LPS-induced mitochondrial damage in renal tubular epithelial cells by deacetylating YME1L1. Cell Prolif. 2023, 56, e13362. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, J.; Li, Y.; Yuan, L.; Liu, F.; Yuan, Y.; Tang, X. Matrine alleviates cisplatin-induced acute kidney injury by inhibiting mitochondrial dysfunction and inflammation via SIRT3/OPA1 pathway. J. Cell. Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef]
- Li, M.; Li, C.M.; Ye, Z.C.; Huang, J.; Li, Y.; Lai, W.; Peng, H.; Lou, T.Q. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J. Cell. Mol. Med. 2020, 24, 5109–5121. [Google Scholar] [CrossRef]
- Chiba, T.; Peasley, K.D.; Cargill, K.R.; Maringer, K.V.; Bharathi, S.S.; Mukherjee, E.; Zhang, Y.; Holtz, A.; Basisty, N.; Yagobian, S.D.; et al. Sirtuin 5 Regulates Proximal Tubule Fatty Acid Oxidation to Protect against AKI. J. Am. Soc. Nephrol. JASN 2019, 30, 2384–2398. [Google Scholar] [CrossRef]
- Haschler, T.N.; Horsley, H.; Balys, M.; Anderson, G.; Taanman, J.W.; Unwin, R.J.; Norman, J.T. Sirtuin 5 depletion impairs mitochondrial function in human proximal tubular epithelial cells. Sci. Rep. 2021, 11, 15510. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Li, Y.; Zhao, Y.; Jiang, H. Sirt5 Attenuates Cisplatin-Induced Acute Kidney Injury through Regulation of Nrf2/HO-1 and Bcl-2. BioMed Res. Int. 2019, 2019, 4745132. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Meng, L.; Cao, G.; Wu, Y. Sirtuin 6 overexpression relieves sepsis-induced acute kidney injury by promoting autophagy. Cell Cycle 2019, 18, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, L.; Du, Y.; Li, H.; Feng, L.; Li, X.; Han, X.; Liu, H. Polydatin Attenuates Cisplatin-Induced Acute Kidney Injury via SIRT6-Mediated Autophagy Activation. Oxidative Med. Cell. Longev. 2022, 2022, 9035547. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Y.; Yang, N.; Liu, J.; Lu, L.; Gao, Z. SIRT6 mitigates acute kidney injury by enhancing lipid metabolism and reducing tubular epithelial cell apoptosis via suppression of the ACMSD signaling pathway. Cell. Signal. 2025, 131, 111757. [Google Scholar] [CrossRef] [PubMed]
- Miyasato, Y.; Yoshizawa, T.; Sato, Y.; Nakagawa, T.; Miyasato, Y.; Kakizoe, Y.; Kuwabara, T.; Adachi, M.; Ianni, A.; Braun, T.; et al. Sirtuin 7 Deficiency Ameliorates Cisplatin-induced Acute Kidney Injury Through Regulation of the Inflammatory Response. Sci. Rep. 2018, 8, 5927. [Google Scholar] [CrossRef]
- Sánchez-Navarro, A.; Martínez-Rojas, M.; Albarrán-Godinez, A.; Pérez-Villalva, R.; Auwerx, J.; de la Cruz, A.; Noriega, L.G.; Rosetti, F.; Bobadilla, N.A. Sirtuin 7 Deficiency Reduces Inflammation and Tubular Damage Induced by an Episode of Acute Kidney Injury. Int. J. Mol. Sci. 2022, 23, 2573. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.Q.; Fan, S.J.; Xu, D.Y.; Lin, L.M.; Luo, W.; Ye, B.Z.; Zou, C.P.; Zhu, H.; Zhuang, Z.S.; et al. JOSD2 alleviates acute kidney injury through deubiquitinating SIRT7 and negativity regulating SIRT7-NF-κB inflammatory pathway in renal tubular epithelial cells. Acta Pharmacol. Sin. 2025. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Z.; Wang, R. Zn2+ improves sepsis-induced acute kidney injury by upregulating SIRT7-mediated Parkin acetylation. Am. J. Physiol. Ren. Physiol. 2024, 327, F184–F197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.Q.; Cai, J.R.; Ji, H.X.; Xu, T. SIRT7 silencing by miR-152-3p confers cell apoptosis and renal functional impairment induced by renal ischaemia/reperfusion injury. Int. Urol. Nephrol. 2023, 55, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Wu, S.W.; Shi, Z.M.; Hu, B. Traditional Chinese medicine for colorectal cancer treatment: Potential targets and mechanisms of action. Chin. Med. 2023, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lan, Y.; Li, J.; Wang, P.; Xiong, X. The role of Chinese herbal medicine in the regulation of oxidative stress in treating hypertension: From therapeutics to mechanisms. Chin. Med. 2024, 19, 150. [Google Scholar] [CrossRef]
- Liu, J.; Yao, C.; Wang, Y.; Zhao, J.; Luo, H. Non-drug interventions of traditional Chinese medicine in preventing type 2 diabetes: A review. Chin. Med. 2023, 18, 151. [Google Scholar] [CrossRef]
- Tang, J.; Li, L.; Chen, Z.; Liao, C.; Hu, K.; Yang, Y.; Huang, J.; Tang, L.; Zhang, L.; Li, L. Agrimol B alleviates cisplatin-induced acute kidney injury by activating the Sirt1/Nrf2 signaling pathway in mice. Acta Biochim. Biophys. Sin. 2024, 56, 551–563. [Google Scholar] [CrossRef]
- Qiu, C.W.; Chen, B.; Zhu, H.F.; Liang, Y.L.; Mao, L.S. Gastrodin alleviates cisplatin nephrotoxicity by inhibiting ferroptosis via the SIRT1/FOXO3A/GPX4 signaling pathway. J. Ethnopharmacol. 2024, 319, 117282. [Google Scholar] [CrossRef]
- Zha, C.; Qi, Y.; Xing, F.; Li, J. Astragaloside IV Inhibits the Pyroptosis in the Acute Kidney Injury through Targeting the SIRT1/FOXO3a Axis. Chem. Pharm. Bull. 2024, 72, 923–931. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Gao, D.; Li, W.; Zheng, D. Astaxanthin attenuates contrast agent-induced acute kidney injury in vitro and in vivo via the regulation of SIRT1/FOXO3a expression. Int. Urol. Nephrol. 2018, 50, 1171–1180. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Chen, F.; Li, H.; Chen, B. Ginkgetin aglycone ameliorates LPS-induced acute kidney injury by activating SIRT1 via inhibiting the NF-κB signaling pathway. Cell Biosci. 2017, 7, 44. [Google Scholar] [CrossRef]
- Khajevand-Khazaei, M.R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wei, W.; Huang, J.; Liu, X.; Ci, X. Isoorientin Attenuates Cisplatin-Induced Nephrotoxicity Through the Inhibition of Oxidative Stress and Apoptosis via Activating the SIRT1/SIRT6/Nrf-2 Pathway. Front. Pharmacol. 2020, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, L.; Tao, X.; Han, X.; Yin, L.; Qi, Y.; Peng, J. Protective Effect of the Total Flavonoids from Rosa laevigata Michx Fruit on Renal Ischemia-Reperfusion Injury through Suppression of Oxidative Stress and Inflammation. Molecules 2016, 21, 952. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, Z.; Lai, W.; Rao, J.; Huang, W.; Zhang, X.; Yao, Z.; Lou, T. Activation of Sirtuin 3 by Silybin Attenuates Mitochondrial Dysfunction in Cisplatin-induced Acute Kidney Injury. Front. Pharmacol. 2017, 8, 178. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Y.; Ma, Y.; Yan, Y.; Hua, M.; Gao, Q.; Geng, X.; Zhou, Q. Protective Effects of Liquiritigenin against Cisplatin-Induced Nephrotoxicity via NRF2/SIRT3-Mediated Improvement of Mitochondrial Function. Molecules 2022, 27, 3823. [Google Scholar] [CrossRef]
- Ali, M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv. Colloid Interface Sci. 2023, 314, 102881. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, X.; Liu, F.; Tang, J.; Zhang, Y.; Zhang, X.; Zhang, L.; Zhang, T.; Wang, Y.; Su, M.; et al. Ultrasmall Polyphenol-NAD(+) Nanoparticle-Mediated Renal Delivery for Mitochondrial Repair and Anti-Inflammatory Treatment of AKI-to-CKD Progression. Adv. Mater. 2024, 36, e2310731. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Deng, G.; Liu, Y.; Wei, B.; Liu, X.; Bao, W.; Wang, Q.; Wu, S. Porous Se@SiO2 nanospheres attenuate cisplatin-induced acute kidney injury via activation of Sirt1. Toxicol. Appl. Pharmacol. 2019, 380, 114704. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, X.; Yang, G.; Tan, H.; Cheng, X.; Tang, Q.; Yang, H.; Zhao, Y.; Ding, X.; Li, S.; et al. Dexmedetomidine ameliorates acute kidney injury by regulating mitochondrial dynamics via the α2-AR/SIRT1/PGC-1α pathway activation in rats. Mol. Med. 2024, 30, 184. [Google Scholar] [CrossRef]
- Barati, A.; Rahbar Saadat, Y.; Meybodi, S.M.; Nouraei, S.; Moradi, K.; Kamrani Moghaddam, F.; Malekinejad, Z.; Hosseiniyan Khatibi, S.M.; Zununi Vahed, S.; Bagheri, Y. Eplerenone reduces renal ischaemia/reperfusion injury by modulating Klotho, NF-κB and SIRT1/SIRT3/PGC-1α signalling pathways. J. Pharm. Pharmacol. 2023, 75, 819–827. [Google Scholar] [CrossRef]
- Bishr, A.; Atwa, A.M.; El-Mokadem, B.M.; El-Din, M.N. Canagliflozin potentially promotes renal protection against glycerol-induced acute kidney injury by activating the AMPK/SIRT1/FOXO-3a/PGC-1α and Nrf2/HO-1 pathways. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Zha, H.; Zhang, Y.; Shi, L.; Wang, J.; Huang, H.; Yue, R.; Hu, B.; Zhu, J.; et al. Dapagliflozin attenuates AKI to CKD transition in diabetes by activating SIRT3/PGC1-α signaling and alleviating aberrant metabolic reprogramming. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167433. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Z.; He, T.; Gao, J.X.; Liu, Y.; Liu, J.Q.; Han, S.C.; Li, Y.; Shi, J.H.; Han, J.T.; Tao, K.; et al. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci. Rep. 2016, 6, 32199. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Li, H.; Yan, Y. Melatonin attenuates ischemia-reperfusion-induced acute kidney injury by regulating abnormal autophagy and pyroptosis through SIRT1-mediated p53 deacetylation. Int. Immunopharmacol. 2025, 162, 115092. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Lei, S.; Tang, C.; Wang, K.; Xia, Z. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Hu, H.; Zhang, W.; Zhang, Y.; Ge, Y.; Ma, T.; Wu, J.; Li, L.; Sun, M.; et al. Melatonin attenuates sepsis-induced acute kidney injury by promoting mitophagy through SIRT3-mediated TFAM deacetylation. Autophagy 2024, 20, 151–165. [Google Scholar] [CrossRef]
- Kobroob, A.; Kongkaew, A.; Wongmekiat, O. Melatonin Reduces Aggravation of Renal Ischemia-Reperfusion Injury in Obese Rats by Maintaining Mitochondrial Homeostasis and Integrity through AMPK/PGC-1α/SIRT3/SOD2 Activation. Curr. Issues Mol. Biol. 2023, 45, 8239–8254. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gao, L.; Zhou, S.; Ma, Y.R.; Xiao, X.; Jiang, Q.; Kang, Z.H.; Liu, M.L.; Liu, T.X. Erythropoietin promotes energy metabolism to improve LPS-induced injury in HK-2 cells via SIRT1/PGC1-α pathway. Mol. Cell. Biochem. 2023, 478, 651–663. [Google Scholar] [CrossRef]
- Guo, J.; Wang, R.; Liu, D. Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Sepsis-Induced Acute Kidney Injury by Promoting Mitophagy of Renal Tubular Epithelial Cells via the SIRT1/Parkin Axis. Front. Endocrinol. 2021, 12, 639165. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zuo, B.; Wang, Y.; Li, S.; Yang, J.; Sun, D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020, 255, 117719. [Google Scholar] [CrossRef] [PubMed]

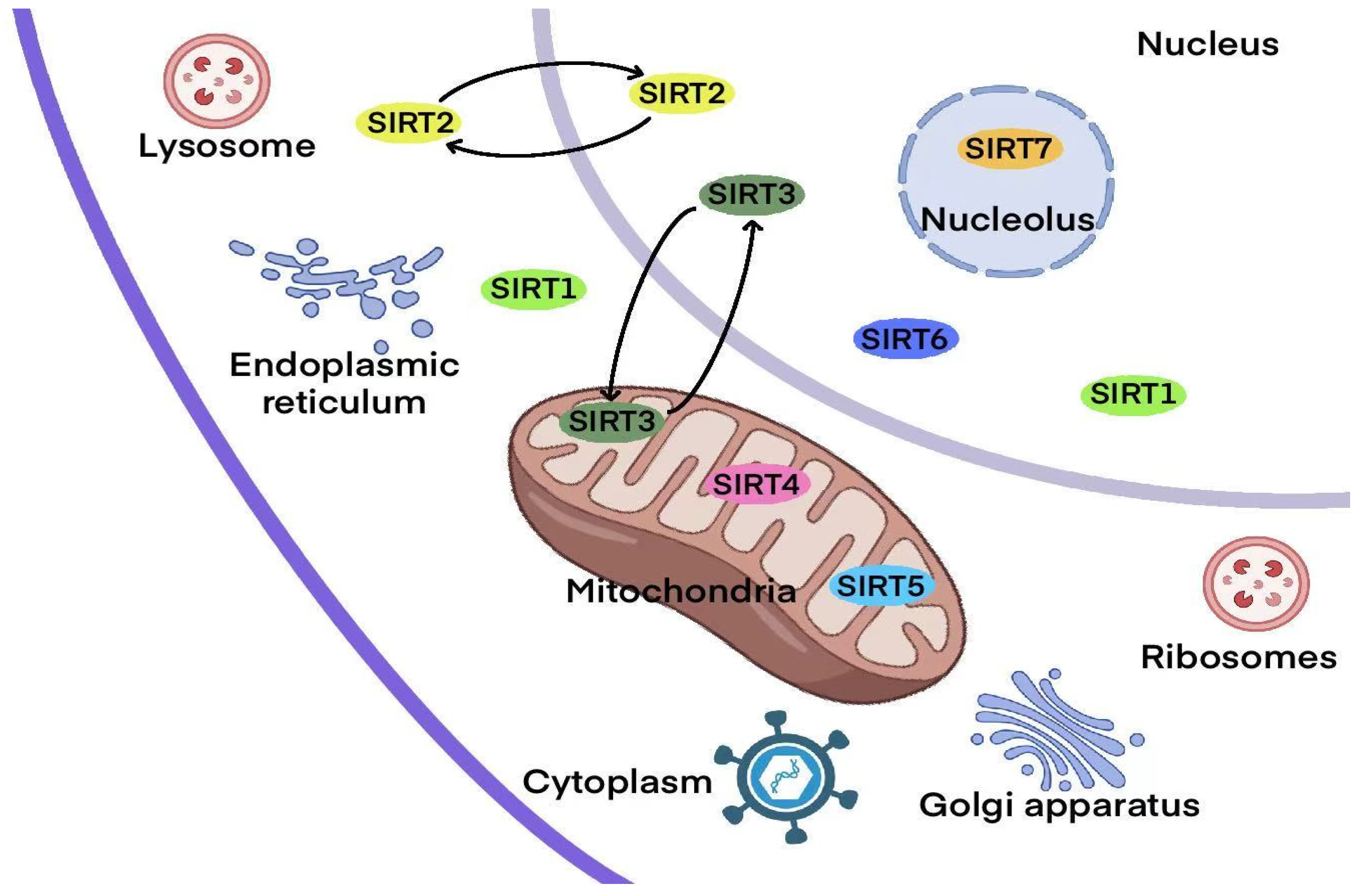
| SIRT | Class | Cellular Localization | Enzymatic Activity | Histone Deacetylation Target | Biological Function | Refs. |
|---|---|---|---|---|---|---|
| SIRT1 | I | Nucleus and cytoplasm | Deacetylase, Depropionylase | H1-K26Ac, H3-K9Ac, H4-K16Ac | Chromatin modification, DNA repair, cell cycle regulation, cell metabolism and survival | [59] |
| SIRT2 | I | Nucleus and cytoplasm | Deacetylase, Demyristoylase, Depropionylase | H3-K18Ac, H3-K56Ac, H4-K16Ac | Cell cycle regulation, microtubule dynamics, inflammation, differentiation | [60] |
| SIRT3 | I | Mitochondria | Deacetylase, Depropionylase | H3-K56Ac, H4-K14Ac | Apoptosis, nuclear gene expression, control of metabolism | [27] |
| SIRT4 | II | Mitochondria | Deacetylase, ADP ribosyltransferase, Biotinidase, Lipoamidase | H4-K16Ac | Resistance, genomic stability, energy metabolism | [61] |
| SIRT5 | III | Mitochondria | Deacetylase, Demethylase, Desuccinylase, Glutaminase | Unknown | Mitochondrial metabolism, amino acid degradation, cellular respiration, reactive oxygen species management | [62] |
| SIRT6 | IV | Nucleus | Deacetylase, ADP-ribosylation, Defattyacylation | H3-K9Ac, H3-K18Ac, H3-K56Ac | Cell proliferation, energy metabolism, DNA damage repair, stem cell differentiation | [63] |
| SIRT7 | IV | Nucleolus | Deacetylase, Desuccinylase | H3-K18Ac | DNA repair, RNA transcription, metabolism regulation | [64] |
| SIRT | Protection/Damage | Disease Model | Active Molecule | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| SIRT1 | Protection | Cisplatin-induced AKI, ischemia–reperfusion AKI, sepsis-induced AKI | Unclear | Unclear | [66,67,68] |
| Protection | Sepsis-induced AKI | Unclear | Overexpression of Sirtuin 1 (SIRT1) alleviates lipopolysaccharide (LPS)-induced AKI by inhibiting the activation of the nucleotide-binding oligomerization domain-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) inflammasome. | [69] | |
| Protection | Sepsis-induced AKI | P53 | SIRT1 promotes deacetylation of p53, thereby promoting autophagy to alleviate AKI caused by sepsis. | [70] | |
| Protection | Cisplatin-induced AKI | P53 | The SIRT1/P53/BAX pathway alleviates cisplatin-induced apoptosis. | [73] | |
| Protection | Sepsis-induced AKI | HMGB1 | SIRT1-mediated deacetylation of HMGB1 inhibits sepsis-associated AKI. | [74] | |
| SIRT2 | Protection | Ischemia–reperfusion AKI | FOXO3a | SIRT2 deacetylates FOXO3a and inhibits cell apoptosis. | [75] |
| SIRT3 | Protection | Cisplatin-induced AKI | Unclear | SIRT3 can improve kidney injury by improving mitochondrial dynamics. | [76] |
| Protection | Sepsis-induced AKI | Unclear | SIRT3 prevents AKI through autophagy regulated by AMPK/mTOR. | [79] | |
| Protection | Ischemia–reperfusion AKI | P53 | SIRT3 alleviates AKI by inhibiting the acetylation of p53. | [80] | |
| Protection | Sepsis-induced AKI | YME1L1 | SIRT3 promotes OPA1-mediated mitochondrial fusion by deacetylating YME1L1, alleviating mitochondrial damage induced by LPS in renal tubular epithelial cells. | [81] | |
| Protection | Cisplatin-induced AKI | Hepatic kinase B1 | SIRT3 may regulate FAO by deacetylating hepatic kinase B1 and activating AMP-activated protein kinase, thereby alleviating AKI. | [83] | |
| SIRT5 | Protection | Cisplatin-induced AKI | Unclear | SIRT5 alleviates AKI by regulating Nrf2/HO-1. | [86] |
| SIRT6 | Protection | Sepsis-induced AKI | Unclear | SIRT6 alleviates AKI by inhibiting the ACMSD signaling pathway, enhancing lipid metabolism, and reducing renal tubular epithelial cell apoptosis. | [89] |
| Protection | Cisplatin-induced AKI | H4K9ac | SIRT6 alleviates ferroptosis in cisplatin-induced AKI by inhibiting the BAP1/xCT signaling axis. | [5] | |
| SIRT7 | Damage | Cisplatin-induced AKI | Unclear | Loss of SIRT7 reduces tumor necrosis factor-alpha (TNF-α) expression by regulating the nuclear expression of the transcription factor NF-kB. | [90,91] |
| SIRT7 | Protection | Ischemia–reperfusion AKI | Unclear | Silencing of SIRT7 leads to cell apoptosis and renal dysfunction caused by renal ischemia/reperfusion injury. | [94] |
| Drug/Compound | Sirtuin Pathway | Mechanism of Action | Potential Nephroprotective Effect | Reference |
|---|---|---|---|---|
| Irisin | SIRT1/Nrf2 | Activates SIRT1/Nrf2 pathway to inhibit ferroptosis | Alleviates AKI in septic mice | [98] |
| Agrimol B | SIRT1/Nrf2 | Activates SIRT1/Nrf2 signaling pathway | Relieves cisplatin-induced AKI | [99] |
| Gastrodin | SIRT1/FOXO3A/GPX4 | Inhibits ferroptosis via SIRT1/FOXO3A/GPX4 pathway | Reduces cisplatin nephrotoxicity | [100] |
| Astragaloside IV | SIRT1/FOXO3A | Intravenous injection targeting SIRT1/FOXO3a axis to suppress pyroptosis | Inhibits AKI pyroptosis | [101] |
| Astaxanthin | SIRT1/FOXO3A | Activates SIRT1/FOXO3a pathway, inhibits apoptosis | Alleviates drug-induced apoptosis | [102] |
| Ginkgolide/Rutin/Isoflavone/Flavonoids | SIRT1 | Activates SIRT1 | Relieves AKI | [103,104,105] |
| Silymarin | SIRT1/SIRT3 | Activates SIRT3 | Relieves AKI | [106,107] |
| Glycyrrhizin | SIRT3 | Activates SIRT3 | Relieves AKI | [108] |
| Ultrasmall Polyphenol-NAD Nanoparticles | SIRT1 | Restores AKI mouse kidney function and immune microenvironment via NAD-Sirtuin-1 axis | Effectively alleviates or prevents AKI | [109] |
| Porous Se@SiO2 Nanoballs | SIRT1 | Activates SIRT1 | Reduces cisplatin-induced AKI | [110] |
| Dexmedetomidine (DEX) | SIRT1 | Upregulates α2-AR/SIRT1/PGC-1α pathway, protecting mitochondria structure and function | Reduces septic AKI | [111] |
| Eplerenone | SIRT1/SIRT3/PGC-1α | Regulates SIRT1/SIRT3/PGC-1α signaling pathway | Reduces renal ischemia/reperfusion injury | [112] |
| Canagliflozin (Cana) | SIRT1/FOXO-3a/PGC-1α | Activates SIRT1/FOXO-3a/PGC-1α pathway | Promotes kidney protection from glycerol-induced AKI | [113] |
| Dapagliflozin | SIRT3/PGC-1α | Activates SIRT3/PGC1-α signaling, reduces metabolic reprogramming | Alleviates AKI in diabetic patients | [114] |
| Melatonin | SIRT1 | Activates SIRT1, regulating p53 deacetylation, autophagy, and pyroptosis | Prevents severe burn-induced AKI in rats | [115] |
| Melatonin | SIRT1/Nrf2/HO-1 | Through SIRT1/Nrf2/HO-1 pathway, alleviates diabetic rat acute kidney ischemia/reperfusion injury | Reduces AKI | [116] |
| Melatonin | SIRT3 | Mediates TFAM deacetylation via SIRT3, promotes mitochondrial autophagy | Alleviates sepsis-induced AKI | [117] |
| Melatonin | SIRT3/SOD2 | Activates SIRT3/SOD2, maintaining mitochondrial stability and integrity | Reduces renal ischemia/reperfusion injury | [118] |
| Erythropoietin | SIRT1/PGC1-α | Promotes energy metabolism via SIRT1/PGC1-α pathway | Improves cell injury | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chen, W.; Li, S.; Zhao, S.; Cheng, F. Sirtuin Family in Acute Kidney Injury: Insights into Cellular Mechanisms and Potential Targets for Treatment. Biomolecules 2025, 15, 1445. https://doi.org/10.3390/biom15101445
Yang S, Chen W, Li S, Zhao S, Cheng F. Sirtuin Family in Acute Kidney Injury: Insights into Cellular Mechanisms and Potential Targets for Treatment. Biomolecules. 2025; 15(10):1445. https://doi.org/10.3390/biom15101445
Chicago/Turabian StyleYang, Songyuan, Wu Chen, Siqi Li, Sheng Zhao, and Fan Cheng. 2025. "Sirtuin Family in Acute Kidney Injury: Insights into Cellular Mechanisms and Potential Targets for Treatment" Biomolecules 15, no. 10: 1445. https://doi.org/10.3390/biom15101445
APA StyleYang, S., Chen, W., Li, S., Zhao, S., & Cheng, F. (2025). Sirtuin Family in Acute Kidney Injury: Insights into Cellular Mechanisms and Potential Targets for Treatment. Biomolecules, 15(10), 1445. https://doi.org/10.3390/biom15101445







