Glycan Signatures on Neutrophils in an Equine Model for Autoimmune Uveitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Cell Preparation
2.2. Analysis of Surface Glycosylation by Flow Cytometry
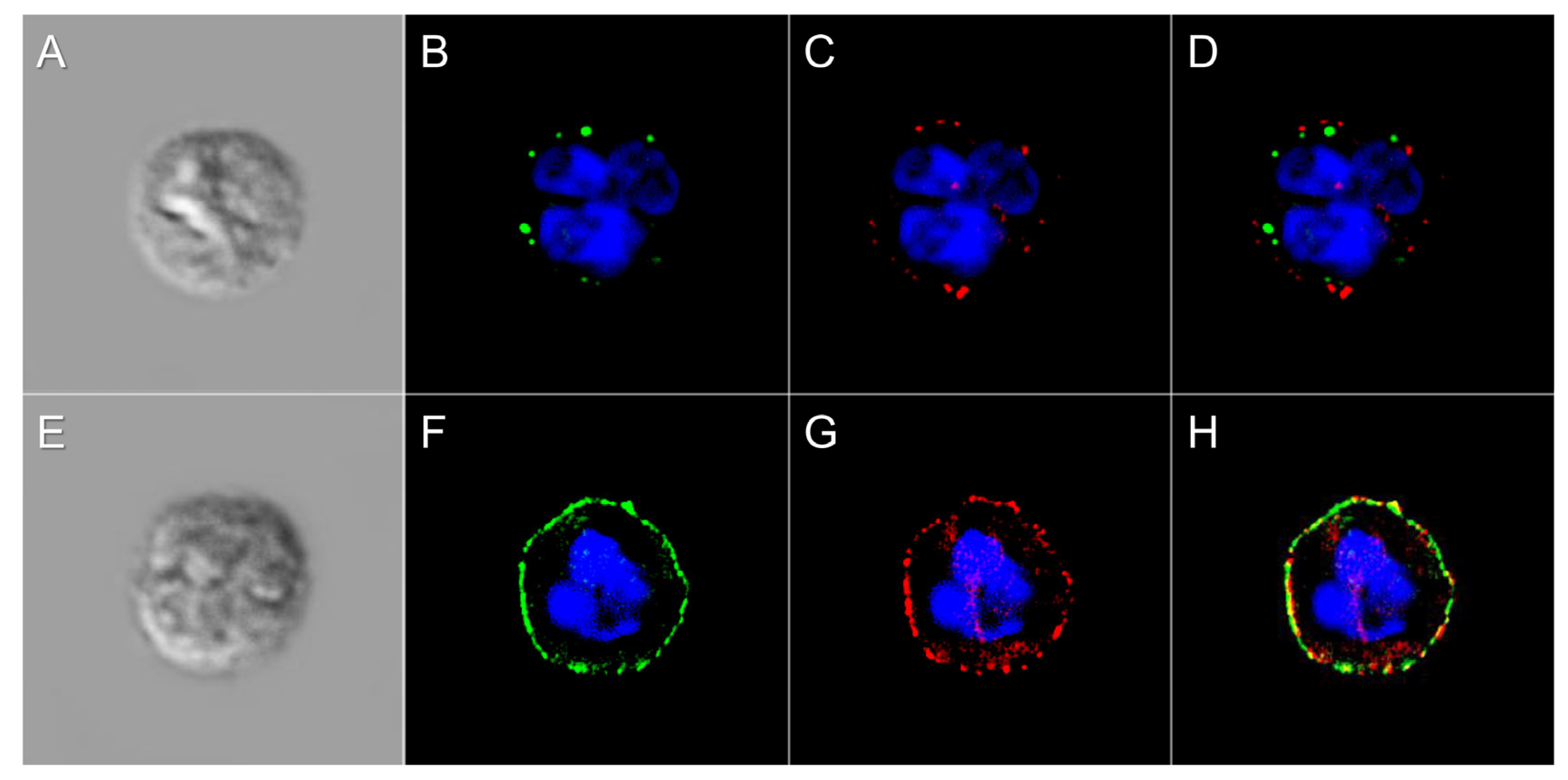
2.3. Immunocytochemistry
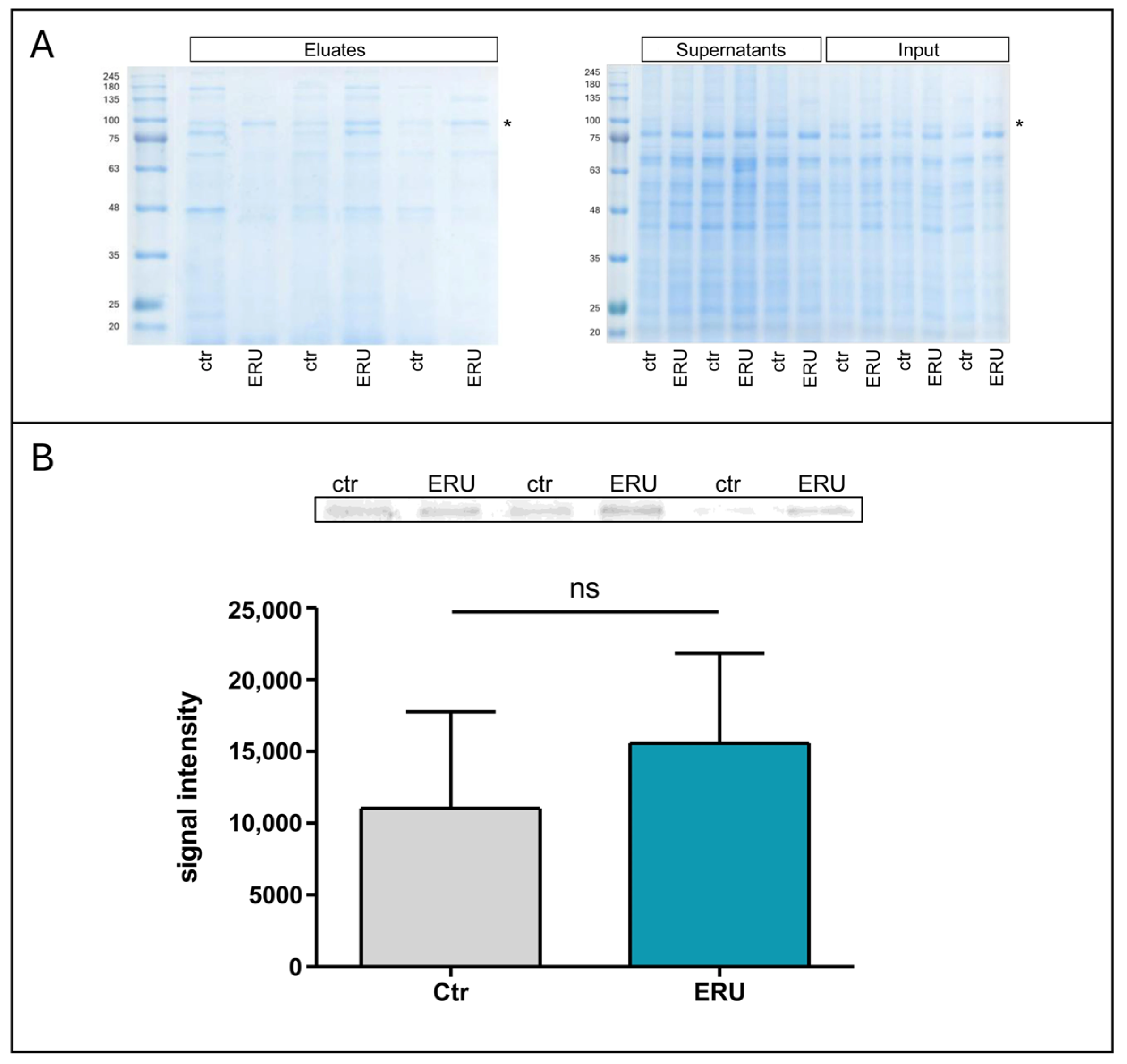
2.4. JAC Pull-Down
2.5. JAC Inhibition
2.6. Prediction of O-GalNAc Glycosylation and Evaluation of Neutrophil Proteome Data
2.7. Statistical Analysis
3. Results
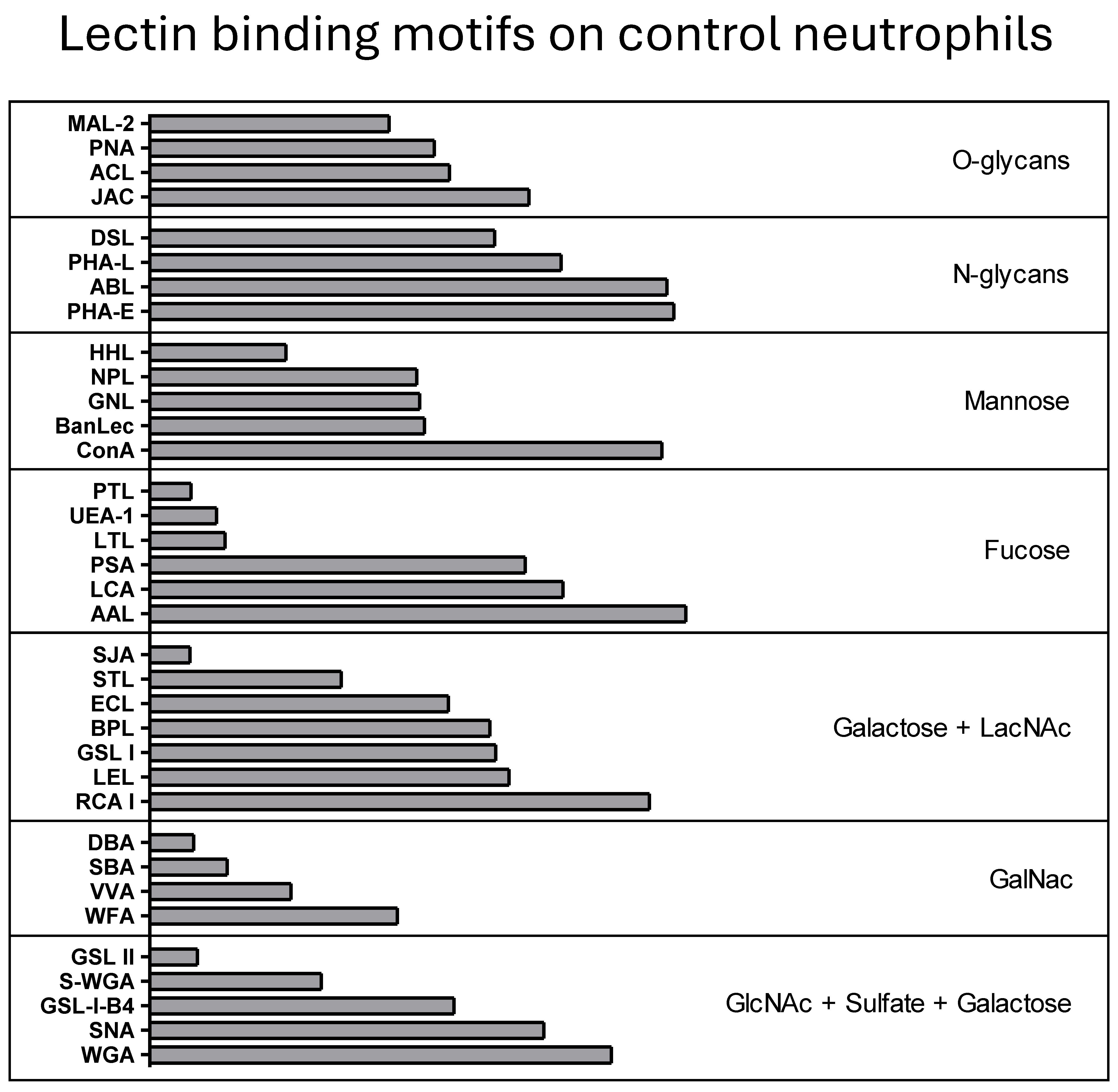
3.1. Defining the Surface Glycome of Equine Neutrophils
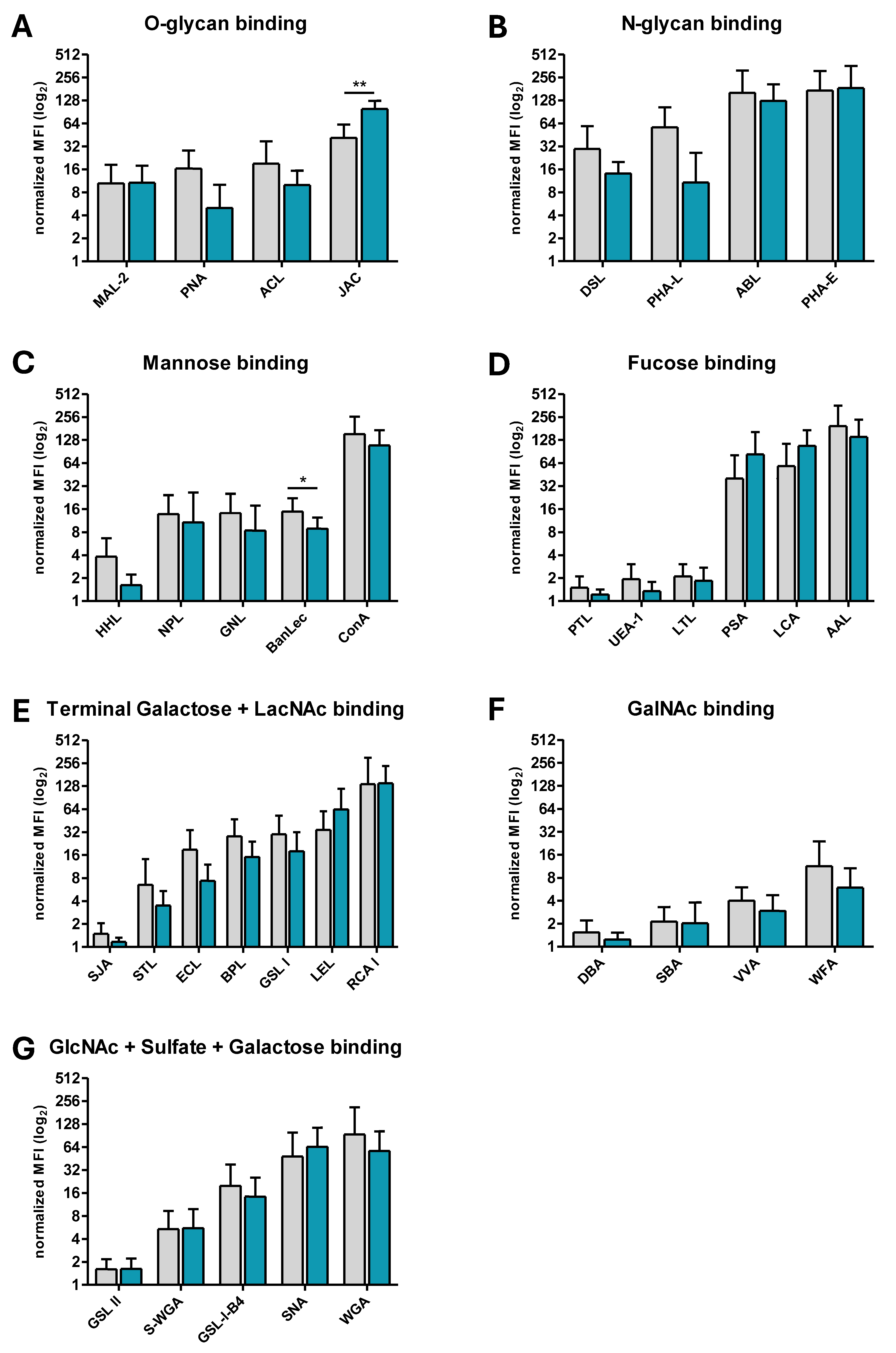
3.2. Differentially Expressed Glycans on ERU Neutrophils
| Acronym a | Specificity b | Norm. MFI ± SD c | p-Value Ctr vs. ERU d | |
|---|---|---|---|---|
| Controls | ERU | |||
| PHA-E | bisecting GlcNac preferred with type 2 LacNAc | 172.6 ± 136.9 | 185.4 ± 176.2 | 1.000 |
| RCA I | terminal ß-galactose residues, with a preference for type 2 LacNAc | 135.6 ± 164.8 | 139.4 ± 95.0 | 0.9638 |
| S-WGA | Oligosaccarides containing terminal GlcNAc | 5.4 ± 3.9 | 5.5 ± 4.2 | 0.9396 |
| AAL | α-linked fucose; preferring (α-1,2)-linked fucose terminated structures on type 2 LacNAc | 135.6 ± 71.8 | 139.8 ± 95.2 | 0.8802 |
| STL | internal type 2 LacNAc as linear glycans; type 2 LacdiNAc; Chitin oligomers | 3.9 ± 3.1 | 3.5 ± 1.9 | 0.8802 |
| ABL | biantennary N-glycans terminating with ß-GlcNAc or LacNAc; core 2 O-glycans | 108.7 ± 73.0 | 126.0 ± 82.3 | 0.6764 |
| MAL-2 | (α-2,3) sialylated O-glycans | 10.5 ± 7.9 | 8.7 ± 3.5 | 0.6100 |
| GSL II | α- or β-linked GlcNAc, preferring GlcNac capped LacNAc in multiantennary N-glycans | 1.6 ± 0.6 | 1.5 ± 0.3 | 0.5944 |
| SNA | (α-2,6) sialylated LacNAc or LacdiNAc | 48.0 ± 50.6 | 64.5 ± 49.9 | 0.5746 |
| SBA | Oligosaccharide structures with terminal α- or β-linked GalNAc | 2.1 ± 1.2 | 2.2 ± 1.9 | 0.5220 |
| WGA | multiple terminal N-acetylstructures; oligosaccharides containing terminal α- or β-linked GlcNac | 52.8 ± 54.1 | 57.1 ± 45.2 | 0.5136 |
| LTL | (α-1,3) linked fucose containing oligosaccharides | 2.1 ± 0.9 | 1.6 ± 0.4 | 0.4927 |
| GSL-I-B4 | terminal α-galactose residues | 19.9 ± 17.8 | 14.3 ± 11.1 | 0.4777 |
| PTL | Carbohydrate structures containing (α-1,3) linked galactose and (α-1,2) linked fucose | 1.5 ± 0.6 | 1.2 ± 0.2 | 0.4482 |
| DSL | (β-1,4) and (β-1,6) linked GlcNAc oligomers, preferring chitobiose or chitotriose; branching structures with several type 2 LacNac repeats | 18.4 ± 10.2 | 14.1 ± 5.8 | 0.4271 |
| ConA | terminal α-linked mannose, biantennary N-glycans with multiple extensions | 153.1 ± 105.8 | 108.6 ± 64.1 | 0.3450 |
| DBA | terminal GalNAc-α1,3-GalNAc | 1.5 ± 0.7 | 1.2 ± 0.2 | 0.3166 |
| SJA | terminal poly- or multiantennary type 2 LacNAc or LacdiNAc; bloodgroup B | 1.5 ± 0.6 | 1.2 ± 0.2 | 0.2804 |
| WFA | Carbohydrate structures terminating in α- or β-linked GalNAc, terminal LacNAc | 11.4 ± 12.5 | 5.9 ± 4.7 | 0.2766 |
| VVA | terminal α- or β-linked GalNac, terminal ß-LacdiNAc | 4.0 ± 2.0 | 2.9 ± 1.8 | 0.2716 |
| ACL | core 1 and core 2 O-glycans; poly Lewis structures | 12.6 ± 7.2 | 10.0 ± 5.4 | 0.2272 |
| PSA | core fucose structures | 40.0 ± 40.5 | 82.7 ± 80.0 | 0.2123 |
| LEL | type 2 polyLacNac; type 2 LacdiNAc; Chitin oligomers | 34.1 ± 25.8 | 63.6 ± 54.5 | 0.1978 |
| LCA | Core fucose; terminal (α-1,2) mannose | 58.0 ± 55.8 | 106.9 ± 64.3 | 0.1714 |
| HHL | terminal α-mannose structures | 3.8 ± 2.9 | 2.0 ± 0.6 | 0.1711 |
| GSL I | α-GalNac residues and α-galactose residues | 29.9 ± 22.7 | 18.0 ± 14.0 | 0.1600 |
| UEA-1 | (α-1,2) linked fucose to (β-1,4) linked galactose | 1.9 ± 1.1 | 1.3 ± 0.4 | 0.1521 |
| BPL | oligosaccharides with terminal β-linked galactose or β-linked GalNac | 28.2 ± 18.8 | 15.1 ± 8.9 | 0.1282 |
| ECL | terminal type 2 LacNAc or LacdiNAc | 18.8 ± 15.1 | 7.3 ± 4.6 | 0.0993 |
| NPL | terminal (α-1,6) and (α-1,3) mannose residues; N-glycans with terminated short LacNAc | 13.8 ± 10.5 | 6.3 ± 4.6 | 0.0878 |
| PHA-L | (ß-1,6)-branched N-glycans | 56.9 ± 47.0 | 27.2 ± 19.6 | 0.0853 |
| GNL | terminal (α-1,6) and (α-1,3) mannose residues; N-glycans with terminated short LacNAc | 14.2 ± 11.2 | 8.2 ± 8.9 | 0.0700 |
| PNA | core 1 and core 2 O-glycans | 15.4 ± 11.1 | 5.0 ± 5.0 | 0.0636 |
| BanLec | Internal (α-1,3) glucosyl- and mannosyl residues; reducing terminal (α-1,3) and (α-1,6) glucosyl residues | 21.1 ± 17.8 | 8.8 ± 3.6 | 0.0163 |
| JAC | core 1 and core 3 O-glycans; 3-substituents α-GalNAc | 41.5 ± 20.3 | 98.9 ± 27.2 | 0.0012 |
3.3. BanLec-Bound Glycans Show Significantly Decreased Abundance on the Surface of ERU Neutrophils, Whereas JAC-Bound Glycans Are Significanty More Abundant
3.4. JAC Lectin Pull-Down Reveals ERU-Associated O-Glycosylated Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTM | Post-translational Modifications |
| AU | Autoimmune Uveitis |
| ERU | Equine Recurrent Uveitis |
| RT | Room Temperature |
| PBS | Phosphate-buffered Saline |
| NaCl | Sodium Chloride |
| AAL | Aleuria Aurantia Lectin |
| ABL | Agaricus Bisporus Lectin |
| ACL | Amaranthus Caudatus Lectin |
| BanLec | Banana Lectin |
| BPL | Bauhinia Purpurea Lectin |
| ConA | Concanavalin A |
| DBA | Dolichos Biflorus Agglutinin |
| DSL | Datura Stramonium Lectin |
| ECL | Erythrina Cristagalli Lectin |
| GNL | Galanthus Nivalis Lectin |
| GSL I | Griffonia Simplicifolia Lectin I |
| GSL-I-B4 | Griffonia Simplicifolia Lectin I Isolectin B4 |
| GSL II | Griffonia Simplicifolia Lectin II |
| HHL | Hippeastrum Hybrid Lectin |
| JAC | Jacalin |
| LCA | Lens Culinaris Agglutinin |
| LEL | Lycopersicon Esculentum Lectin |
| LTL | Lotus Tetragonolobus Lectin |
| MAL-2 | Maackia Amurensis Lectin II |
| NPL | Narcissus Pseudonarcissus Lectin |
| PHA-E | Phaseolus Vulgaris Erythroagglutin |
| PHA-L | Phaseolus Vulgaris Leucoagglutinin |
| PNA | Peanut Agglutinin |
| PSA | Pisum Sativum Agglutinin |
| PTL | Psophocarpus Tetragonolobus Lectin |
| RCA I | Ricinus Communis Agglutitin I |
| SBA | Soybean Agglutinin |
| SJA | Sophora Japonica Agglutinin |
| SNA | Sambucus Nigra Lectin |
| STL | Solanum Tuberosum Lectin |
| S-WGA | Succinylated Wheat Germ Agglutinin |
| UEA-1 | Ulex Europaeus Agglutinin I |
| VVA | Vicia Villosa Lectin |
| WFA | Wisteria Floribunda Lectin |
| WGA | Wheat Germ Agglutinin |
| PFA | Paraform aldehyde |
| MFI | Mean Fluorescence Intensity |
| DAPI | 4′, 6-Diamidin-2-phenylindol |
| KS | Kolmogorov–Smirnov Test |
| CDCP1 | CUB Domain-containing Protein 1 |
| ITGB2 | Integrin β2 |
| RA | Rheumatoid Arthritis |
| RPE | Retinal Pigment Epithelial Cells |
References
- de Haan, N.; Pucic-Bakovic, M.; Novokmet, M.; Falck, D.; Lageveen-Kammeijer, G.; Razdorov, G.; Vuckovic, F.; Trbojevic-Akmacic, I.; Gornik, O.; Hanic, M.; et al. Developments and perspectives in high-throughput protein glycomics: Enabling the analysis of thousands of samples. Glycobiology 2022, 32, 651–663. [Google Scholar] [CrossRef]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef]
- Marrero Roche, D.E.; Chandler, K.B. Clinical glycoprotein mass spectrometry: The future of disease detection and monitoring. J. Mass Spectrom. 2024, 59, e5083. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Kissel, T.; Toes, R.E.M.; Huizinga, T.W.J.; Wuhrer, M. Glycobiology of rheumatic diseases. Nat. Rev. Rheumatol. 2023, 19, 28–43. [Google Scholar] [CrossRef]
- Szabo, E.; Farago, A.; Bodor, G.; Gemes, N.; Puskas, L.G.; Kovacs, L.; Szebeni, G.J. Identification of immune subsets with distinct lectin binding signatures using multi-parameter flow cytometry: Correlations with disease activity in systemic lupus erythematosus. Front. Immunol. 2024, 15, 1380481. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Kaya, Z.Z.; Serteser, M.; Ozturk, H.U.; Baykal, A.T. Glycan profiling of multiple sclerosis oligoclonal bands with MALDI-TOF. Anal. Methods 2025, 17, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Li, N.; Fan, W.; Li, X.; Zhou, Q.; Liu, J.; Li, W.; Zhang, Z.; Liu, X.; et al. YY1 Lactylation Aggravates Autoimmune Uveitis by Enhancing Microglial Functions via Inflammatory Genes. Adv. Sci. 2024, 11, e2308031. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, C.; Amann, B.; Degroote, R.L.; Witte, T.; Deeg, C.A. Aberrant Migratory Behavior of Immune Cells in Recurrent Autoimmune Uveitis in Horses. Front. Cell Dev. Biol. 2020, 8, 101. [Google Scholar] [CrossRef]
- Peng, X.; Li, H.; Zhu, L.; Zhao, S.; Li, Z.; Li, S.; Wu, D.; Chen, J.; Zheng, S.; Su, W. Single-cell sequencing of the retina shows that LDHA regulates pathogenesis of autoimmune uveitis. J. Autoimmun. 2024, 143, 103160. [Google Scholar] [CrossRef]
- Deeg, C.A.; Hauck, S.M.; Amann, B.; Pompetzki, D.; Altmann, F.; Raith, A.; Schmalzl, T.; Stangassinger, M.; Ueffing, M. Equine recurrent uveitis--a spontaneous horse model of uveitis. Ophthalmic Res. 2008, 40, 151–153. [Google Scholar] [CrossRef]
- Gilger, B.C. Immune Relevant Models for Ocular Inflammatory Diseases. ILAR J. 2018, 59, 352–362. [Google Scholar] [CrossRef]
- Soth, R.; Hoffmann, A.L.C.; Deeg, C.A. Enhanced ROS Production and Mitochondrial Metabolic Shifts in CD4(+) T Cells of an Autoimmune Uveitis Model. Int. J. Mol. Sci. 2024, 25, 11513. [Google Scholar] [CrossRef]
- Malalana, F.; Stylianides, A.; McGowan, C. Equine recurrent uveitis: Human and equine perspectives. Vet. J. 2015, 206, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.C.; Gilger, B.C. Prognosis and impact of equine recurrent uveitis. Equine Vet. J. 2016, 48, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Li, Z.; Xiao, Z.; Chen, G.; Li, Y.; Huang, J.; Hu, Y.; Huang, H.; Zhu, W.; et al. STING Deficiency Promotes Th17-Like Tfh to Aggravate the Experimental Autoimmune Uveitis. Investig. Ophthalmol. Vis. Sci. 2025, 66, 8. [Google Scholar] [CrossRef]
- Barfusser, C.; Wiedemann, C.; Hoffmann, A.L.C.; Hirmer, S.; Deeg, C.A. Altered Metabolic Phenotype of Immune Cells in a Spontaneous Autoimmune Uveitis Model. Front. Immunol. 2021, 12, 601619. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R.; Chan, C.C.; Fujino, Y.; Najafian, F.; Grover, S.; Hansen, C.T.; Wilder, R.L. Recruitment of antigen-nonspecific cells plays a pivotal role in the pathogenesis of a T cell-mediated organ-specific autoimmune disease, experimental autoimmune uveoretinitis. J. Neuroimmunol. 1993, 47, 177–188. [Google Scholar] [CrossRef]
- Kerr, E.C.; Copland, D.A.; Dick, A.D.; Nicholson, L.B. The dynamics of leukocyte infiltration in experimental autoimmune uveoretinitis. Prog. Retin. Eye Res. 2008, 27, 527–535. [Google Scholar] [CrossRef]
- Kerr, E.C.; Raveney, B.J.; Copland, D.A.; Dick, A.D.; Nicholson, L.B. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 2008, 31, 354–361. [Google Scholar] [CrossRef]
- Deeg, C.A.; Kaspers, B.; Gerhards, H.; Thurau, S.R.; Wollanke, B.; Wildner, G. Immune responses to retinal autoantigens and peptides in equine recurrent uveitis. Investig. Ophthalmol. Vis. Sci. 2001, 42, 393–398. [Google Scholar]
- Weigand, M.; Hauck, S.M.; Deeg, C.A.; Degroote, R.L. Deviant proteome profile of equine granulocytes associates to latent activation status in organ specific autoimmune disease. J. Proteom. 2021, 230, 103989. [Google Scholar] [CrossRef]
- Reno, F.; Pagano, C.A.; Bignotto, M.; Sabbatini, M. Neutrophil Heterogeneity in Wound Healing. Biomedicines 2025, 13, 694. [Google Scholar] [CrossRef]
- Sheahan, B.J.; Schubert, A.G.; Schubert, W.; Sheats, M.K.; Schnabel, L.V.; Gilbertie, J.M. Equine neutrophils selectively release neutrophil extracellular traps in response to chemical and bacterial agonists. Front. Vet. Sci. 2025, 12, 1512343. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, L.; Ohnesorge, B.; von Borstel, M.; Schumski, A.; Strutzberg-Minder, K.; Morgelin, M.; Deeg, C.A.; Haagsman, H.P.; Beineke, A.; von Kockritz-Blickwede, M.; et al. Neutrophil Extracellular Traps in the Pathogenesis of Equine Recurrent Uveitis (ERU). Cells 2019, 8, 1528. [Google Scholar] [CrossRef] [PubMed]
- Maier-Begandt, D.; Alonso-Gonzalez, N.; Klotz, L.; Erpenbeck, L.; Jablonska, J.; Immler, R.; Hasenberg, A.; Mueller, T.T.; Herrero-Cervera, A.; Aranda-Pardos, I.; et al. Neutrophils-biology and diversity. Nephrol. Dial. Transplant. 2024, 39, 1551–1564. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Michau, T.M. Equine recurrent uveitis: New methods of management. Vet. Clin. N. Am. Equine Pract. 2004, 20, 417–427. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kang, D.H.; Gho, Y.S.; Suh, M.K.; Kang, C.H. Highly sensitive and fast protein detection with coomassie brilliant blue in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. B Korean Chem. Soc. 2002, 23, 1511–1512. [Google Scholar]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Bojar, D.; Meche, L.; Meng, G.; Eng, W.; Smith, D.F.; Cummings, R.D.; Mahal, L.K. A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem. Biol. 2022, 17, 2993–3012. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Nakamura, S.; Wang, H.; Iwasaki, H.; Tachibana, K.; Maebara, K.; Cheng, L.; Hirabayashi, J.; Narimatsu, H. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: Quantitative analysis by frontal affinity chromatography. Glycobiology 2006, 16, 46–53. [Google Scholar] [CrossRef]
- Lescar, J.; Loris, R.; Mitchell, E.; Gautier, C.; Chazalet, V.; Cox, V.; Wyns, L.; Pérez, S.; Breton, C.; Imberty, A. Isolectins I-A and I-B of Griffonia(Bandeiraea) simplicifolia: Crystal structure of metal-free GS I-B4 and molecular basis for metal binding and monosaccharide specificity. J. Biol. Chem. 2002, 277, 6608–6614. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Devi, S.K.; Ng, T.B. Banana lectin: A brief review. Molecules 2014, 19, 18817–18827. [Google Scholar] [CrossRef]
- Monsigny, M.; Sene, C.; Obrenovitch, A.; Roche, A.C.; Delmotte, F.; Boschetti, E. Properties of succinylated wheat-germ agglutinin. Eur. J. Biochem. 1979, 98, 39–45. [Google Scholar] [CrossRef]
- van Kooyk, Y.; Rabinovich, G.A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008, 9, 593–601. [Google Scholar] [CrossRef]
- Wolfert, M.A.; Boons, G.J. Adaptive immune activation: Glycosylation does matter. Nat. Chem. Biol. 2013, 9, 776–784. [Google Scholar] [CrossRef]
- Ohnishi, T.; Muroi, M.; Tanamoto, K. MD-2 is necessary for the toll-like receptor 4 protein to undergo glycosylation essential for its translocation to the cell surface. Clin. Diagn. Lab. Immunol. 2003, 10, 405–410. [Google Scholar] [CrossRef]
- da Silva Correia, J.; Ulevitch, R.J. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 2002, 277, 1845–1854. [Google Scholar] [CrossRef]
- Kawahara, R.; Ugonotti, J.; Chatterjee, S.; Tjondro, H.C.; Loke, I.; Parker, B.L.; Venkatakrishnan, V.; Dieckmann, R.; Sumer-Bayraktar, Z.; Karlsson-Bengtsson, A.; et al. Glycoproteome remodeling and organelle-specific N-glycosylation accompany neutrophil granulopoiesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2303867120. [Google Scholar] [CrossRef]
- Hoffmann, A.L.C.; Hauck, S.M.; Deeg, C.A.; Degroote, R.L. Pre-Activated Granulocytes from an Autoimmune Uveitis Model Show Divergent Pathway Activation Profiles upon IL8 Stimulation In Vitro. Int. J. Mol. Sci. 2022, 23, 9555. [Google Scholar] [CrossRef] [PubMed]
- Ugonotti, J.; Chatterjee, S.; Thaysen-Andersen, M. Structural and functional diversity of neutrophil glycosylation in innate immunity and related disorders. Mol. Asp. Med. 2021, 79, 100882. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.; Humphreys, J.M.; Edwards, S.W. Protein synthesis is activated in primed neutrophils: A possible role in inflammation. Biosci. Rep. 1987, 7, 881–890. [Google Scholar] [CrossRef]
- McMullen, R.J., Jr.; Fischer, B.M. Medical and Surgical Management of Equine Recurrent Uveitis. Vet. Clin. N. Am. Equine Pract. 2017, 33, 465–481. [Google Scholar] [CrossRef]
- Gilger, B.C.; Malok, E.; Cutter, K.V.; Stewart, T.; Horohov, D.W.; Allen, J.B. Characterization of T-lymphocytes in the anterior uvea of eyes with chronic equine recurrent uveitis. Vet. Immunol. Immunopathol. 1999, 71, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Ehrenhofer, M.; Thurau, S.R.; Reese, S.; Wildner, G.; Kaspers, B. Immunopathology of recurrent uveitis in spontaneously diseased horses. Exp. Eye Res. 2002, 75, 127–133. [Google Scholar] [CrossRef]
- Deeg, C.A.; Thurau, S.R.; Gerhards, H.; Ehrenhofer, M.; Wildner, G.; Kaspers, B. Uveitis in horses induced by interphotoreceptor retinoid-binding protein is similar to the spontaneous disease. Eur. J. Immunol. 2002, 32, 2598–2606. [Google Scholar] [CrossRef]
- Degroote, R.L.; Schmalen, A.; Hauck, S.M.; Deeg, C.A. Unveiling Differential Responses of Granulocytes to Distinct Immunostimulants with Implications in Autoimmune Uveitis. Biomedicines 2023, 12, 19. [Google Scholar] [CrossRef]
- Fingerhut, L.; Yucel, L.; Strutzberg-Minder, K.; von Kockritz-Blickwede, M.; Ohnesorge, B.; de Buhr, N. Ex Vivo and In Vitro Analysis Identify a Detrimental Impact of Neutrophil Extracellular Traps on Eye Structures in Equine Recurrent Uveitis. Front. Immunol. 2022, 13, 830871. [Google Scholar] [CrossRef]
- Horohov, D.W. The equine immune responses to infectious and allergic disease: A model for humans? Mol. Immunol. 2015, 66, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Deeg, C.A.; Raith, A.J.; Amann, B.; Crabb, J.W.; Thurau, S.R.; Hauck, S.M.; Ueffing, M.; Wildner, G.; Stangassinger, M. CRALBP is a highly prevalent autoantigen for human autoimmune uveitis. Clin. Dev. Immunol. 2007, 2007, 39245. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Hu, Y.; Xie, Y.; Shi, Y.; Chen, G.; Huang, J.; Xiao, Z.; Zhu, W.; Huang, H.; et al. Neutrophil extracellular traps potentiate effector T cells via endothelial senescence in uveitis. JCI Insight 2025, 10, e180248. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Andre, S.; Kaltner, H.; Kayser, K.; Murphy, P.V.; Gabius, H.J. Merging carbohydrate chemistry with lectin histochemistry to study inhibition of lectin binding by glycoclusters in the natural tissue context. Histochem. Cell Biol. 2016, 145, 185–199. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Wang, T.; Li, W. Core Fucosylation Regulates the Function of Pre-BCR, BCR and IgG in Humoral Immunity. Front. Immunol. 2022, 13, 844427. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, V.; Dieckmann, R.; Loke, I.; Tjondro, H.C.; Chatterjee, S.; Bylund, J.; Thaysen-Andersen, M.; Karlsson, N.G.; Karlsson-Bengtsson, A. Glycan analysis of human neutrophil granules implicates a maturation-dependent glycosylation machinery. J. Biol. Chem. 2020, 295, 12648–12660. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, L.; Amann, B.; Hirmer, S.; Degroote, R.L.; Hauck, S.M.; Deeg, C.A. NEU1 is more abundant in uveitic retina with concomitant desialylation of retinal cells. Glycobiology 2021, 31, 873–883. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Jeyaprakash, A.A.; Katiyar, S.; Swaminathan, C.P.; Sekar, K.; Surolia, A.; Vijayan, M. Structural basis of the carbohydrate specificities of jacalin: An X-ray and modeling study. J. Mol. Biol. 2003, 332, 217–228. [Google Scholar] [CrossRef]
- Rubin, J.R.; Taylor, S.K.; Rudchenko, S.; Stojanovic, M.N.; Hess, H. Single Molecule Kinetic Fingerprinting of Glycans on IgA1 Antibodies. Anal. Chem. 2025, 97, 14388–14396. [Google Scholar] [CrossRef] [PubMed]
- Gurrea-Rubio, M.; Fox, D.A.; Castresana, J.S. CD6 in Human Disease. Cells 2025, 14, 272. [Google Scholar] [CrossRef]
- Zhang, L.; Borjini, N.; Lun, Y.; Parab, S.; Asonye, G.; Singh, R.; Bell, B.A.; Bonilha, V.L.; Ivanov, A.; Fox, D.A.; et al. CDCP1 regulates retinal pigmented epithelial barrier integrity for the development of experimental autoimmune uveitis. JCI Insight 2022, 7, e157038. [Google Scholar] [CrossRef]
- Wen, L.; Moser, M.; Ley, K. Molecular mechanisms of leukocyte β2 integrin activation. Blood 2022, 139, 3480–3492. [Google Scholar] [CrossRef]
- Brazil, J.C.; Sumagin, R.; Cummings, R.D.; Louis, N.A.; Parkos, C.A. Targeting of Neutrophil Lewis X Blocks Transepithelial Migration and Increases Phagocytosis and Degranulation. Am. J. Pathol. 2016, 186, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Brazil, J.C.; Kelm, M.; Lehoux, S.; Azcutia, V.; Cummings, R.D.; Nusrat, A.; Parkos, C.A. Regulation of Neutrophil Function by Selective Targeting of Glycan Epitopes Expressed on the Integrin CD11b/CD18. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Azcutia, V.; Kelm, M.; Fink, D.; Cummings, R.D.; Nusrat, A.; Parkos, C.A.; Brazil, J.C. Sialylation regulates neutrophil transepithelial migration, CD11b/CD18 activation, and intestinal mucosal inflammatory function. JCI Insight 2023, 8, e167151. [Google Scholar] [CrossRef]


| Protein a | Gene Name b | Accession Number c | Ratio ERU/Controls d | Molecular Weight e |
|---|---|---|---|---|
| CUB Domain Containing Protein 1 | CDCP1 | ENSECAP00000022716 | 2.2 | 93.0 |
| Integrin, Beta 2 | ITGB2 | ENSECAP00000021220 | 1.9 | 94.8 |
| Adhesion G Protein-Coupled Receptor E5 | ADRE5 | ENSECAP00000008739 | 1.4 | 90.7 |
| Oxysterol Binding Protein-Like 8 | OSBPL8 | ENSECAP00000013659 | 1.1 | 95.6 |
| Glucosidase, alpha | GANAB | ENSECAP00000012762 | 1.1 | 96.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprenzel, C.J.; Amann, B.; Deeg, C.A.; Degroote, R.L. Glycan Signatures on Neutrophils in an Equine Model for Autoimmune Uveitis. Biomolecules 2025, 15, 1444. https://doi.org/10.3390/biom15101444
Sprenzel CJ, Amann B, Deeg CA, Degroote RL. Glycan Signatures on Neutrophils in an Equine Model for Autoimmune Uveitis. Biomolecules. 2025; 15(10):1444. https://doi.org/10.3390/biom15101444
Chicago/Turabian StyleSprenzel, Carolin J., Barbara Amann, Cornelia A. Deeg, and Roxane L. Degroote. 2025. "Glycan Signatures on Neutrophils in an Equine Model for Autoimmune Uveitis" Biomolecules 15, no. 10: 1444. https://doi.org/10.3390/biom15101444
APA StyleSprenzel, C. J., Amann, B., Deeg, C. A., & Degroote, R. L. (2025). Glycan Signatures on Neutrophils in an Equine Model for Autoimmune Uveitis. Biomolecules, 15(10), 1444. https://doi.org/10.3390/biom15101444






