Cecytb-2, a Cytochrome b561 Homolog, Functions as an Ascorbate-Specific Transmembrane Ferric Reductase at Intestinal Lumens of Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Strains and Maintenance
2.2. DNA Preparations
2.3. Protein Expression, Purification, and Basic Measurements
2.4. Diethylpyrocarbonate (DEPC) Treatment of Purified Cecytb-2-H6
2.5. EPR Measurement
2.6. Redox Titration
2.7. Stopped-Flow Analysis
2.8. Pulse Radiolysis
2.9. Protein Digestion and MALDI-TOF-MS and Online HPLC-Orbitrap MS/MS Measurements
2.10. Structural Studies of the Cecytb-2 Protein
2.11. Ferrozine-Based Colorimetric Assay for Transmembrane Electron Transfer Activity
2.12. Production and Purification of Anti-Cecytb-2C-Terminal Peptide Antibodies
2.13. Western Blot Analysis
2.14. In Situ Hybridization Analysis
2.15. Immunostaining
2.16. RNAi
3. Results
3.1. Seven Cytochrome b561 Homologues in C. elegans
3.2. Biochemical and Biophysical Properties of Cecytb-2
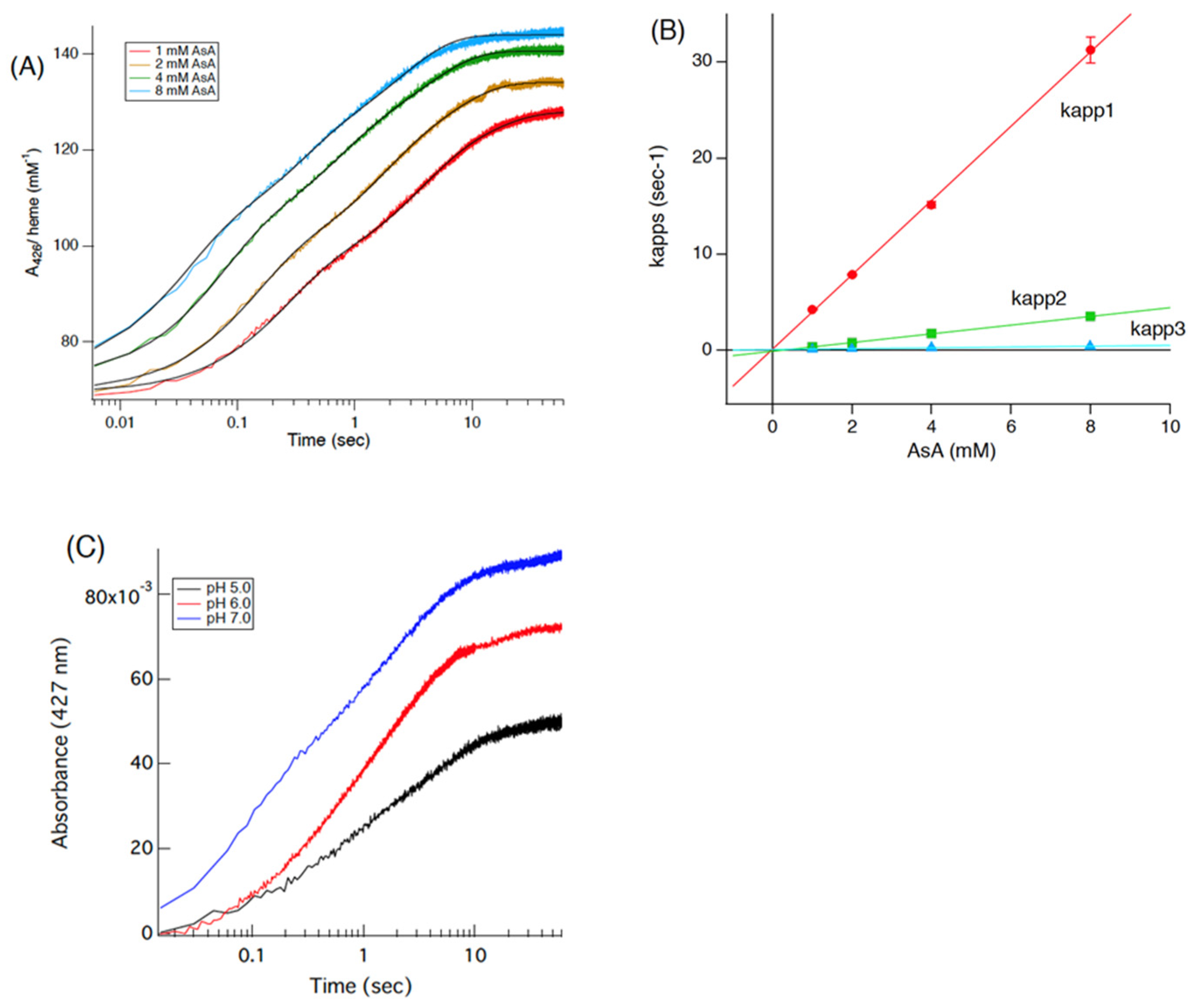
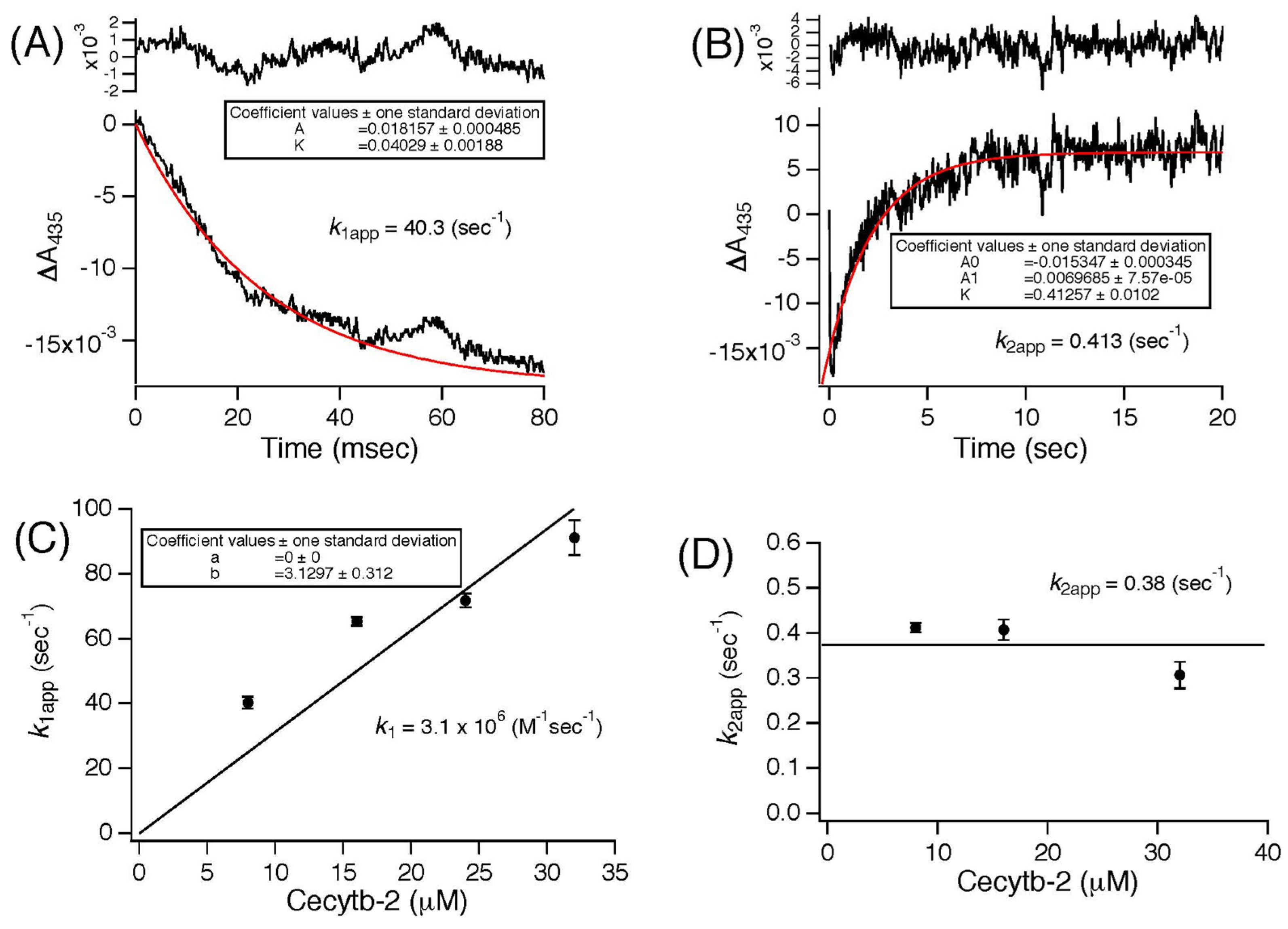
3.3. Stopped-Flow Studies of the Reaction of Cecytb-2 with AsA
3.4. Inhibition of Electron Transfer from AsA by DEPC Treatment of Cecytb-2
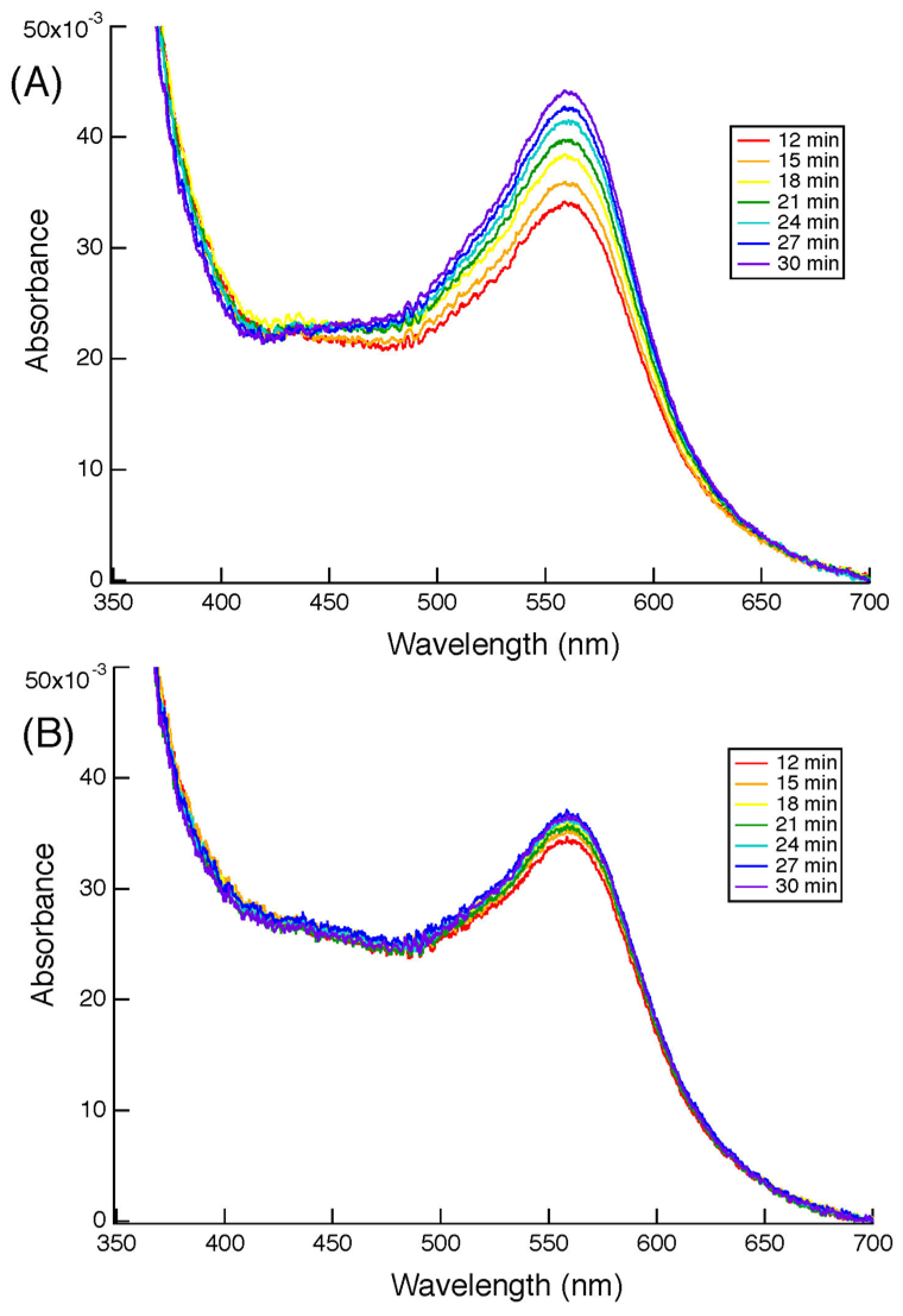
3.5. Pulse Radiolysis Study of Cecytb-2
3.6. AsA-Dependent Transmembrane Ferric Reductase Activity of Cecytb-2
3.7. Structural Study of the Cecytb-2 Protein
3.8. Localization of the Cecytb-2 Protein
3.9. RNAi Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AsA | Ascorbate |
| MDA | monodehydroascorbate |
| NGM | nematode growth medium |
| DEPC | diethylpyrocarbonate |
| PDTS | 3-(2-pyridyl)-5,6-bis(4-sulfophenyl)-1,2,4-triazine (ferrozine) |
| DMT-1 | divalent metal transporter-1 |
| SHE | standard hydrogen electrode |
| DHFR | dihydrofolate reductase |
| HALS | highly anisotropic low-spin |
| BP | 2,2′-dipyridyl |
| OG | octyl-β-D-glucoside |
| DDM | n-dodecyl-β-D-maltoside |
| HIF-1 | hypoxia-inducible factor 1 |
| HO | heme oxygenase |
References
- Tsubaki, M.; Nakayama, M.; Okuyama, E.; Ichikawa, Y.; Hori, H. Existence of two heme B centers in cytochrome b561 from bovine adrenal chromaffin vesicles as revealed by a new purification procedure and EPR spectroscopy. J. Biol. Chem. 1997, 272, 23206–23210. [Google Scholar] [CrossRef]
- Terland, O.; Flatmark, T. Oxidoreductase activities of chromaffin granule ghosts isolated from the bovine adrenal medulla. Biochim. Biophys. Acta 1980, 597, 318–330. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M. The secretory-vesicle ascorbate-regenerating system: A chain of concerted H+/e--transfer reactions. Biochim. Biophys. Acta 1993, 1144, 235–248. [Google Scholar] [CrossRef]
- Iliadi, K.G.; Avivi, A.; Iliadi, N.N.; Knight, D.; Korol, A.; Nevo, E.; Taylor, P.; Moran, M.F.; Kamyshev, N.G.; Boulianne, G.L. nemy encodes a cytochrome b561 that is required for Drosophila learning and memory. Proc. Natl. Acad. Sci. USA 2008, 105, 19986–19991. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Takeuchi, F.; Nakanishi, N. Cytochrome b561 protein family: Expanding roles and versatile transmembrane electron transfer abilities as predicted by a new classification system and protein sequence motif analyses. Biochim. Biophys. Acta 2005, 1753, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; An, Z.; Lei, H.; Liao, H.; Guo, X. Role of the human cytochrome b561 family in iron metabolism and tumors (Review). Oncol. Lett. 2025, 29, 111. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.P.; Almomani, R.; Biaggioni, I.; van Faassen, M.; van der Harst, P.; Sillje, H.H.W.; Mateo Leach, I.; Hemmelder, M.H.; Navis, G.; Luijckx, G.J.; et al. Mutations in CYB561 Causing a Novel Orthostatic Hypotension Syndrome. Circ. Res. 2018, 122, 846–854. [Google Scholar] [CrossRef]
- Shibao, C.A.; Garland, E.M.; Black, B.K.; Mathias, C.J.; Grant, M.B.; Root, A.W.; Robertson, D.; Biaggioni, I. Congenital absence of norepinephrine due to CYB561 mutations. Neurology 2020, 94, e200–e204. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, X.; Han, J.; Wang, M.; Liu, Z.; Ren, D.; Zhao, J.; Li, Z. Cytochrome b561 regulates iron metabolism by activating the Akt/mTOR pathway to promote Breast Cancer Cells proliferation. Exp. Cell. Res. 2023, 431, 113760. [Google Scholar] [CrossRef]
- Zhuo, J.; Zhao, Y.; Hao, R.; Li, H.; Zheng, Z.; Dai, L.; Sheng, A.; Yao, H.; Tang, Y.; Wang, R.; et al. CYB561 is a potential therapeutic target for breast cancer and is associated with immune cell infiltration. Eur. J. Med. Res. 2024, 29, 414. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhuo, J.; Zhang, Q.; Dai, L.; Tang, Y.; Wang, Y.; Sheng, A.; Yao, H.; Liu, W. CYB561 a potential prognostic biomarker for liver hepatocellular carcinoma. Clin. Exp. Med. 2024, 25, 23. [Google Scholar] [CrossRef] [PubMed]
- Boult, J.; Roberts, K.; Brookes, M.J.; Hughes, S.; Bury, J.P.; Cross, S.S.; Anderson, G.J.; Spychal, R.; Iqbal, T.; Tselepis, C. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin. Cancer Res. 2008, 14, 379–387. [Google Scholar] [CrossRef]
- Ma, J.; Huang, W.; Zhu, C.; Sun, X.; Zhang, Q.; Zhang, L.; Qi, Q.; Bai, X.; Feng, Y.; Wang, C. miR-423-3p activates FAK signaling pathway to drive EMT process and tumor growth in lung adenocarcinoma through targeting CYBRD1. J. Clin. Lab. Anal. 2021, 35, e24044. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, R.; Trudeau, S.J.; Wolinsky, E.; Ast, T.; Liang, J.H.; Jiang, C.; Ma, Y.; Teng, M.; Mootha, V.K.; et al. CYB561A3 is the key lysosomal iron reductase required for Burkitt B-cell growth and survival. Blood 2021, 138, 2216–2230. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Liu, Q.; He, F.; Zhu, H. LncRNA HOXD-AS1 affects proliferation and apoptosis of cervical cancer cells by promoting FRRS1 expression via transcription factor ELF1. Cell Cycle 2022, 21, 416–426. [Google Scholar] [CrossRef]
- Ohtani, S.; Iwamura, A.; Deng, W.; Ueda, K.; Wu, G.; Jayachandran, G.; Kondo, S.; Atkinson, E.N.; Minna, J.D.; Roth, J.A.; et al. Tumor suppressor 101F6 and ascorbate synergistically and selectively inhibit non-small cell lung cancer growth by caspase-independent apoptosis and autophagy. Cancer Res. 2007, 67, 6293–6303. [Google Scholar] [CrossRef]
- El Behery, M.; Fujimura, M.; Kimura, T.; Tsubaki, M. Direct measurements of ferric reductase activity of human 101F6 and its enhancement upon reconstitution into phospholipid bilayer nanodisc. Biochem Biophys. Rep. 2020, 21, 100730. [Google Scholar] [CrossRef] [PubMed]
- Recuenco, M.C.; Rahman, M.M.; Takeuchi, F.; Kobayashi, K.; Tsubaki, M. Electron transfer reactions of candidate tumor suppressor 101F6 protein, a cytochrome b561 homologue, with ascorbate and monodehydroascorbate radical. Biochemistry 2013, 52, 3660–3668. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Mckie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Oakhill, J.S.; Marritt, S.J.; Gareta, E.G.; Cammack, R.; McKie, A.T. Functional characterization of human duodenal cytochrome b (Cybrd1): Redox properties in relation to iron and ascorbate metabolism. Biochim. Biophys. Acta 2008, 1777, 260–268. [Google Scholar] [CrossRef]
- Ganasen, M.; Togashi, H.; Takeda, H.; Asakura, H.; Tosha, T.; Yamashita, K.; Hirata, K.; Nariai, Y.; Urano, T.; Yuan, X.; et al. Structural basis for promotion of duodenal iron absorption by enteric ferric reductase with ascorbate. Commun. Biol. 2018, 1, 1–12. [Google Scholar] [CrossRef]
- Gunshin, H.; Starr, C.N.; DiRenzo, C.; Fleming, M.D.; Jin, J.; Greer, E.L.; Sellers, V.M.; Galica, S.M. Cybrd1 (duodenal cytochrome b) is not necessary for dietary iron absorption in mice. Blood 2005, 106, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Latunde-Dada, G.O.; Simpson, R.J.; McKie, A.T. Duodenal cytochrome b expression stimulates iron uptake by human intestinal epithelial cells. J. Nutr. 2008, 138, 991–995. [Google Scholar] [CrossRef]
- Choi, J.; Masaratana, P.; Latunde-Dada, G.O.; Arno, M.; Simpson, R.J.; McKie, A.T. Duodenal reductase activity and spleen iron stores are reduced and erythropoiesis is abnormal in Dcytb knockout mice exposed to hypoxic conditions. J. Nutr. 2012, 142, 1929–1934. [Google Scholar] [CrossRef]
- Hulme, S.E.; Whitesides, G.M. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew. Chem. Int. Ed. 2011, 50, 4774–4807. [Google Scholar] [CrossRef] [PubMed]
- Patananan, A.N.; Budenholzer, L.M.; Pedraza, M.E.; Torres, E.R.; Adler, L.N.; Clarke, S.G. The invertebrate Caenorhabditis elegans biosynthesizes ascorbate. Arch. Biochem. Biophys. 2015, 569, 32–44. [Google Scholar] [CrossRef]
- Yabuta, Y.; Nagata, R.; Aoki, Y.; Kariya, A.; Wada, K.; Yanagimoto, A.; Hara, H.; Bito, T.; Okamoto, N.; Yoshida, S.; et al. L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans. Metabolites 2020, 10, 334. [Google Scholar] [CrossRef]
- Abosharaf, H.A.; Sakamoto, Y.; Radwan, A.M.; Yuzu, K.; Fujimura, M.; Diab, T.; Mohamed, T.M.; Chatani, E.; Kimura, T.; Tsubaki, M. Functional assembly of Caenorhabditis elegans cytochrome b-2 (Cecytb-2) into phospholipid bilayer nanodisc with enhanced iron reductase activity. Biomolecules 2021, 11, 96. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Nakanishi, N.; Rahman, M.M.; Sakamoto, Y.; Takigami, T.; Kobayashi, K.; Hori, H.; Hase, T.; Park, S.-Y.; Tsubaki, M. Importance of conserved Lys83 residue of Zea mays cytochrome b561 for ascorbate-specific transmembrane electron transfer as revealed by site-directed mutagenesis studies. Biochemistry 2009, 48, 10665–10678. [Google Scholar] [CrossRef]
- Nakanishi, N.; Rahman, M.M.; Sakamoto, Y.; Miura, M.; Takeuchi, F.; Park, S.-Y.; Tsubaki, M. Inhibition of electron acceptance from ascorbate by the specific N-carbethoxylations of maize cytochrome b561: A common mechanism for the transmembrane electron transfer in cytochrome b561 protein family. J. Biochem. 2009, 146, 857–866. [Google Scholar] [CrossRef]
- Recuenco, M.C.; Fujito, M.; Rahman, M.M.; Sakamoto, Y.; Takeuchi, F.; Tsubaki, M. Functional expression and characterization of human 101F6 protein, a homologue of cytochrome b561 and a candidate tumor suppressor gene product. BioFactors 2009, 34, 219–230. [Google Scholar] [CrossRef]
- Sunga, A.J.; Tolstorukov, I.; Cregg, J.M. Posttransformational vector amplification in the yeast Pichia pastoris. FEMS Yeast Res. 2008, 8, 870–876. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Tolbert, N.E.; Bieber, L.L. Protein determination in membrane and lipoprotein sampled: Manual and automated procedures. Methods Enzymol. 1981, 72, 296–303. [Google Scholar]
- Fuhrhop, J.-H.; Smith, K.M. Laboratory Methods. In Porphyrins and Metalloporphyrins; Smith, K.M., Ed.; Elsevier Scientific Publishing Co.: Amsterdam, The Netherlands, 1975; pp. 755–869. [Google Scholar]
- Recuenco, M.C.; Rahman, M.M.; Sakamoto, Y.; Takeuchi, F.; Hori, H.; Tsubaki, M. Functional characterization of the recombinant human tumor suppressor 101F6 protein, a cytochrome b561 homologue. J. Biochem. 2013, 153, 233–242. [Google Scholar] [CrossRef]
- Takeuchi, F.; Kobayashi, K.; Tagawa, S.; Tsubaki, M. Ascorbate inhibits the carbethoxylation of two histidyl and one tyrosyl residues indispensable for the transmembrane electron transfer reaction of cytochrome b561. Biochemistry 2001, 40, 4067–4076. [Google Scholar] [CrossRef]
- Berczi, A.; Marton, Z.; Laskay, K.; Toth, A.; Rakhely, G.; Duzs, A.; Sebok-Nagy, K.; Pali, T.; Zimanyi, L. Spectral and Redox Properties of a Recombinant Mouse Cytochrome b561 Protein Suggest Transmembrane Electron Transfer Function. Molecules 2023, 28, 2261. [Google Scholar] [CrossRef]
- Takigami, T.; Takeuchi, F.; Nakagawa, M.; Hase, T.; Tsubaki, M. Stopped-flow analyses on the reaction of ascorbate with cytochrome b561 purified from bovine chromaffin vesicle membanes. Biochemistry 2003, 42, 8110–8118. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Nakanishi, N.; Sakamoto, Y.; Hori, H.; Hase, T.; Park, S.-Y.; Tsubaki, M. Roles of conserved Arg72 and Tyr71 in the ascorbate-specific transmembrane electron transfer catalyzed by Zea mays cytochrome b561. J. Biosci. Bioeng. 2013, 115, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, G.; Tsai, A.-L.; Kulmacz, R.J. High-yield production, purification and characterization of functional human duodenal cytochrome b in an Escherichia coli system. Protein Expr. Purif. 2011, 79, 115–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, K.; Tsubaki, M.; Tagawa, S. Distinct roles of two heme centers for transmembrane electron transfer in cytochrome b561 from bovine adrenal chromaffin vesicles as revealed by pulse radiolysis. J. Biol. Chem. 1998, 273, 16038–16042. [Google Scholar] [CrossRef]
- Seike, Y.; Takeuchi, F.; Tsubaki, M. Reversely-oriented cytochrome b561 in reconstituted vesicles catalyzes transmembrane electron transfer and supports the extravesicular dopamine β-hydroxylase activity. J. Biochem. 2003, 134, 859–867. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Miura, M.; Takeuchi, F.; Park, S.-Y.; Tsubaki, M. Interaction of modified tail-anchored proteins with liposomes: Effect of extensions of hydrophilic segment at the COOH-terminus of holo-cytochromes b5. J. Biosci. Bioeng. 2012, 113, 322–331. [Google Scholar] [CrossRef]
- Pountney, D.J.; Konijn, A.M.; McKie, A.T.; Peters, T.J.; Raja, K.B.; Salisbury, J.R.; Simpson, R.J. Iron proteins of duodenal enterocytes isolated form mice wtih genetically and experimentally altered iron metabolism. British J. Haematol. 1999, 105, 1066–1073. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Mitani, S.; Du, H.; Hall, D.H.; Driscoll, M.; Chalfie, M. Combinatorial control of touch receptor neuron expression in Caenorhabditis elegans. Development 1993, 119, 773–783. [Google Scholar] [CrossRef]
- Romney, S.J.; Newman, B.S.; Thacker, C.; Leibold, E.A. HIF-1 regulates iron homeostasis in Caenorhabditis elegans by activation and inhibition of genes involved in iron uptake and storage. PLoS Genet. 2011, 7, e1002394. [Google Scholar] [CrossRef]
- Okuyama, E.; Yamamoto, R.; Ichikawa, Y.; Tsubaki, M. Structural basis for the electron transfer across the chromaffin vesicle membranes catalyzed by cytochrome b561: Analyses of cDNA nucleotide sequences and visible absorption spectra. Biochim. Biophys. Acta 1998, 1383, 269–278. [Google Scholar] [CrossRef]
- Ludwiczek, S.; Rosell, F.I.; Ludwiczek, M.L.; Mauk, A.G. Recombinant expression and initial characterization of the putative human enteric ferric reductase Dcytb. Biochemistry 2008, 47, 753–761. [Google Scholar] [CrossRef]
- Desmet, F.; Bérczi, A.; Zimányi, L.; Asard, H.; Van Doorslaer, S. Axial ligation of the high-potential heme center in an Arabidopsis cytochrome b561. FEBS Lett. 2011, 585, 545–548. [Google Scholar] [CrossRef]
- Liu, W.; Rogge, C.E.; Kamensky, Y.; Tsai, A.-L.; Kulmacz, R.J. Development of a bacterial system for high yield expression of fully functional adrenal cytochrome b561. Protein Expr. Purif. 2007, 56, 145–152. [Google Scholar] [CrossRef]
- Bérczi, A.; Desmet, F.; Van Doorslaer, S.; Asard, H. Spectral characterization of the recombinant mouse tumor suppressor 101F6 protein. Eur. Biophys. J. 2010, 39, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Kamensky, Y.; Liu, W.; Tsai, A.-L.; Kulmacz, R.J.; Palmer, G. The axial ligation and stoichiometry of heme centers in adrenal cytochrome b561. Biochemistry 2007, 46, 8647–8658. [Google Scholar] [CrossRef]
- Takeuchi, F.; Hori, H.; Obayashi, E.; Shiro, Y.; Tsubaki, M. Properties of two distinct heme centers of cytochrome b561 from bovine chromaffin vesicles studied by EPR, resonance Raman, and ascorbate reduction assay. J. Biochem. 2004, 135, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Takeuchi, F.; Tsubaki, M. Histidine cycle mechanism for the concerted proton/electron transfer from ascorbate to the cytosolic heme b center of cytochrome b561: A unique machinery for the biological transmembrane electron transfer. J. Biochem. 2007, 142, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ma, D.; Yan, C.; Gong, X.; Du, M.; Shi, Y. Structure and mechanism of a eukaryotic transmembrane ascorbate-dependent oxidoreductase. Proc. Natl. Acad. Sci. USA 2014, 111, 1813–1818. [Google Scholar] [CrossRef]
- Au, C.; Benedetto, A.; Anderson, J.; Labrousse, A.; Erikson, K.; Ewbank, J.J.; Aschner, M. SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans. PLoS ONE 2009, 4, e7792. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Song, H.-O.; Park, B.-J.; Singaravelu, G.; Sun, J.L.; Ahnn, J.; Cho, J.H. Functional assessment of Nramp-like metal transporters and manganese in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2009, 390, 136–141. [Google Scholar] [CrossRef]
- Breuer, W.; Epsztejn, S.; Cabantchik, Z.I. Iron acquired from transferrin by K562 cells is delivered into a cytoplasmic pool of chelatable iron(II). J. Biol. Chem. 1995, 270, 24209–24215. [Google Scholar] [CrossRef]
- Takeuchi, F.; Hori, H.; Tsubaki, M. Selective perturbation of the intravesicular heme center of cytochrome b561 by cysteinyl modification with 4,4′-dithiodipyridine. J. Biochem. 2005, 138, 751–762. [Google Scholar] [CrossRef]
- Liu, W.; Rogge, C.E.; da Silva, G.F.Z.; Shinkarev, V.P.; Tsai, A.-L.; Kamensky, Y.; Palmer, G.; Kulmacz, R.J. His92 and His110 selectively affect different heme centers of adrenal cytochrome b561. Biochim. Biophys. Acta 2008, 1777, 1218–1228. [Google Scholar] [CrossRef]
- Liu, W.; da Silva, G.F.Z.; Wu, G.; Palmer, G.; Tsai, A.-L.; Kulmacz, R.J. Functional and structural roles of residues in the third extramembrane segment of adrenal cytochrome b561. Biochemistry 2011, 50, 3149–3160. [Google Scholar] [CrossRef][Green Version]
- da Silva, G.F.Z.; Shinkarev, V.P.; Kamensky, Y.A.; Palmer, G. Spectroscopic evidence of the role of an axial ligand histidinate in the mechanism of adrenal cytochrome b561. Biochemistry 2012, 51, 8730–8742. [Google Scholar] [CrossRef] [PubMed]
- Latunde-Dada, G.O.; Simpson, R.J.; McKie, A.T. Recent advances in mammalian haem transport. Trends Biochem. Sci. 2006, 31, 182–188. [Google Scholar] [CrossRef]
- Yuan, X.; Fleming, M.D.; Hamza, I. Heme transport and erythropoiesis. Curr. Op. Chem. Biol. 2013, 17, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Rao, A.U.; Amigo, J.; Tian, M.; Upadhyay, S.K.; Hall, C.; Uhm, S.; Mathew, M.K.; Fleming, M.D.; Paw, B.H.; et al. Haem homeostasis is regulated by the conserved and concerted funtions of HRG-1 proteins. Nature 2008, 453, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Samuel, T.K.; Sinclair, J.; Dailey, H.A.; Hamza, I. An intercellular heme-trafficking protein delivers maternal heme to the embryo during development in C. elegans. Cell. 2011, 145, 720–731. [Google Scholar] [CrossRef]
- Chen, C.; Samuel, T.K.; Krause, M.; Dailey, H.A.; Hamza, I. Heme utilization in the Caenorhabditis elegans hypodermal cells is facilitated by heme-responsive gene-2. J. Biol. Chem. 2012, 287, 9601–9612. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.U.; Carta, L.K.; Lesuisse, E.; Hamza, I. Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. USA 2005, 102, 4270–4275. [Google Scholar] [CrossRef]
- Perally, S.; LaCourse, E.J.; Campbell, A.M.; Brophy, P.M. Heme transport and detoxification in nematodes: Subproteomics evidence of differential role of glutathione transferases. J. Proteome Res. 2008, 7, 4557–4565. [Google Scholar] [CrossRef] [PubMed]
- McGhee, J.D.; Fukushige, T.; Krause, M.W.; Minnema, S.E.; Goszczynski, B.; Gaudet, J.; Kohara, Y.; Bossinger, O.; Zhao, Y.; Khattra, J.; et al. ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev. Biol. 2009, 327, 551–565. [Google Scholar] [CrossRef]
- Sommermann, E.M.; Strohmaier, K.R.; Maduro, M.F.; Rothman, J.H. Endoderm development in Caenorhabditis elegans: The synergistic action of ELT-2 and -7 mediates the specification→differentiation transition. Dev. Biol. 2010, 347, 154–166. [Google Scholar] [CrossRef]
- Shah, Y.M.; Matsubara, T.; Ito, S.; Gonzalez, F.J. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009, 9, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiannaki, M.; Matak, P.; Keith, B.; Simon, M.C.; Vaulont, S.; Peyssonnaux, C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Investig. 2009, 119, 1159–1166. [Google Scholar] [CrossRef]
- Bishop, T.; Lau, K.W.; Epstein, A.C.R.; Kim, S.K.; Jiang, M.; O’Rourke, D.; Pugh, C.W.; Gleadle, J.M.; Taylor, M.S.; Hodgkin, J.; et al. Genetic analysis of pathways regulated by the von Hippel-Lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2004, 2, 1549–1560. [Google Scholar] [CrossRef]
- Shen, C.; Nettleton, D.; Jiang, M.; Kim, S.K.; Powell-Coffman, J.A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 2005, 280, 20580–20588. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, M.; Fukuzawa, M.; Hori, H.; Kobayashi, K.; Recuenco, M.C.; Tsubaki, M. Cecytb-2, a Cytochrome b561 Homolog, Functions as an Ascorbate-Specific Transmembrane Ferric Reductase at Intestinal Lumens of Caenorhabditis elegans. Biomolecules 2025, 15, 1385. https://doi.org/10.3390/biom15101385
Miura M, Fukuzawa M, Hori H, Kobayashi K, Recuenco MC, Tsubaki M. Cecytb-2, a Cytochrome b561 Homolog, Functions as an Ascorbate-Specific Transmembrane Ferric Reductase at Intestinal Lumens of Caenorhabditis elegans. Biomolecules. 2025; 15(10):1385. https://doi.org/10.3390/biom15101385
Chicago/Turabian StyleMiura, Masahiro, Misaki Fukuzawa, Hiroshi Hori, Kazuo Kobayashi, Mariam C. Recuenco, and Motonari Tsubaki. 2025. "Cecytb-2, a Cytochrome b561 Homolog, Functions as an Ascorbate-Specific Transmembrane Ferric Reductase at Intestinal Lumens of Caenorhabditis elegans" Biomolecules 15, no. 10: 1385. https://doi.org/10.3390/biom15101385
APA StyleMiura, M., Fukuzawa, M., Hori, H., Kobayashi, K., Recuenco, M. C., & Tsubaki, M. (2025). Cecytb-2, a Cytochrome b561 Homolog, Functions as an Ascorbate-Specific Transmembrane Ferric Reductase at Intestinal Lumens of Caenorhabditis elegans. Biomolecules, 15(10), 1385. https://doi.org/10.3390/biom15101385





