Dysregulated Sialylation in Cancer: From Immunosuppressive Microenvironment to Siglec-Targeted Therapeutics
Abstract
1. Introduction
2. The Normal Synthetic Pathway of Sialic Acid
3. Abnormal Sialic Acid Synthesis
3.1. The Impact of Sialyltransferases in Cancer Progression and Therapy
| Sialyltransferase Family | Sialyltransferase | Preferred Acceptor Saccharide | Glycan Specificity | Type of Cancer | Regulation | References |
|---|---|---|---|---|---|---|
| ST3Gal | ST3Gal-I | Galβ1,3GalNAc | O-glycan | BLCA, LIHC, BRCA | Uplation | [40,41,42] |
| ST3Gal-II | Galβ1,3GalNAc | O-glycan | OV | Uplation | [43] | |
| ST3Gal-III | Galβ1,3(4)GlcNAc | O-glycan, N-glycan | PAAD | Uplation | [44] | |
| ST3Gal-IV | Galβ1,4(3)GlcNAc | N-glycan, O-glycan | PAAD | Uplation | [44] | |
| ST3Gal-V | Galβ1,4Glc-ceramide | Glycolipid | BLCA | Downlation | [45] | |
| ST3Gal-VI | Galβ1,4GlcNAc | N-glycan, Glycolipid | COAD, BLCA, STAD, LUAD, MMLIHC | Downlation | [46,47,48,49,50] | |
| Uplation | [51] | |||||
| ST6Gal | ST6Gal-I | Galβ1,4GlcNAc | N-glycan | COAD, READ, OV, PRAD, LIHC, NSCLC | Uplation | [38,52,53,54,55] |
| ST6Gal-II | Galβ1,4GlcNAc | N-glycan | THCA | Uplation | [56] | |
| ST6GalNAc | ST6GalNAc-I | GalβNAcα1,O-Ser/Thr Galβ1,3GalNAcα1, O-Ser/Thr | O-glycan | LIHC, OV, COAD, BRCA, PRAD, ESCA | Uplation | [25,28,57,58,59] |
| Downlation | [60] | |||||
| ST6GalNAc-II | Galβ1,3GalNAcα1, O-Ser/Thr | O-glycans | COAD, READ, BRCA | Uplation | [61] | |
| Downlation | [62] | |||||
| ST6GalNAc-III | Siaα2,3Galβ1,3GalNAc | O-glycans | NSCLC | Downlation | [54] | |
| ST6GalNAc-IV | Siaα2,3Galβ1,3GalNAc | O-glycans | THCA, LIHC | Uplation | [63,64] | |
| ST6GalNAc-V | GM1b | Glycolipid | PRAD | Downlation | [65] | |
| ST6GalNAc-VI | All α-series gangliosides | Glycolipid | BLCA | Downlation | [66] | |
| ST8Sia | ST8Sia-I | Siaα2,3Galβ1,4Glc-ceramide | Glycolipid | BRCA | Uplation | [67] |
| ST8Sia-II | Siaα2,3Galβ1,4GlcNAc | N-glycan on NCAMa | SCLC | Uplation | [68] | |
| ST8Sia-III | Siaα2,3Galβ1,4GlcNAc | N-glycan on NCAMa | --- | --- | --- | |
| ST8Sia-IV | (Siaα2,8)nSiaα2,3Galβ1-R | N-glycan on NCAM | --- | --- | --- | |
| ST8Sia-V | GM1b, GT1b, GD1a, GD3 | Glycolipid | --- | --- | --- | |
| ST8Sia-VI | Siaα2,3(6)Gal | Sialic acid on O-glycan | NBL | Uplation | [69] |
3.2. Abnormal Sialidase Activity in Disease
4. Significance of Targeting the Sialic Acid–Siglec Axis
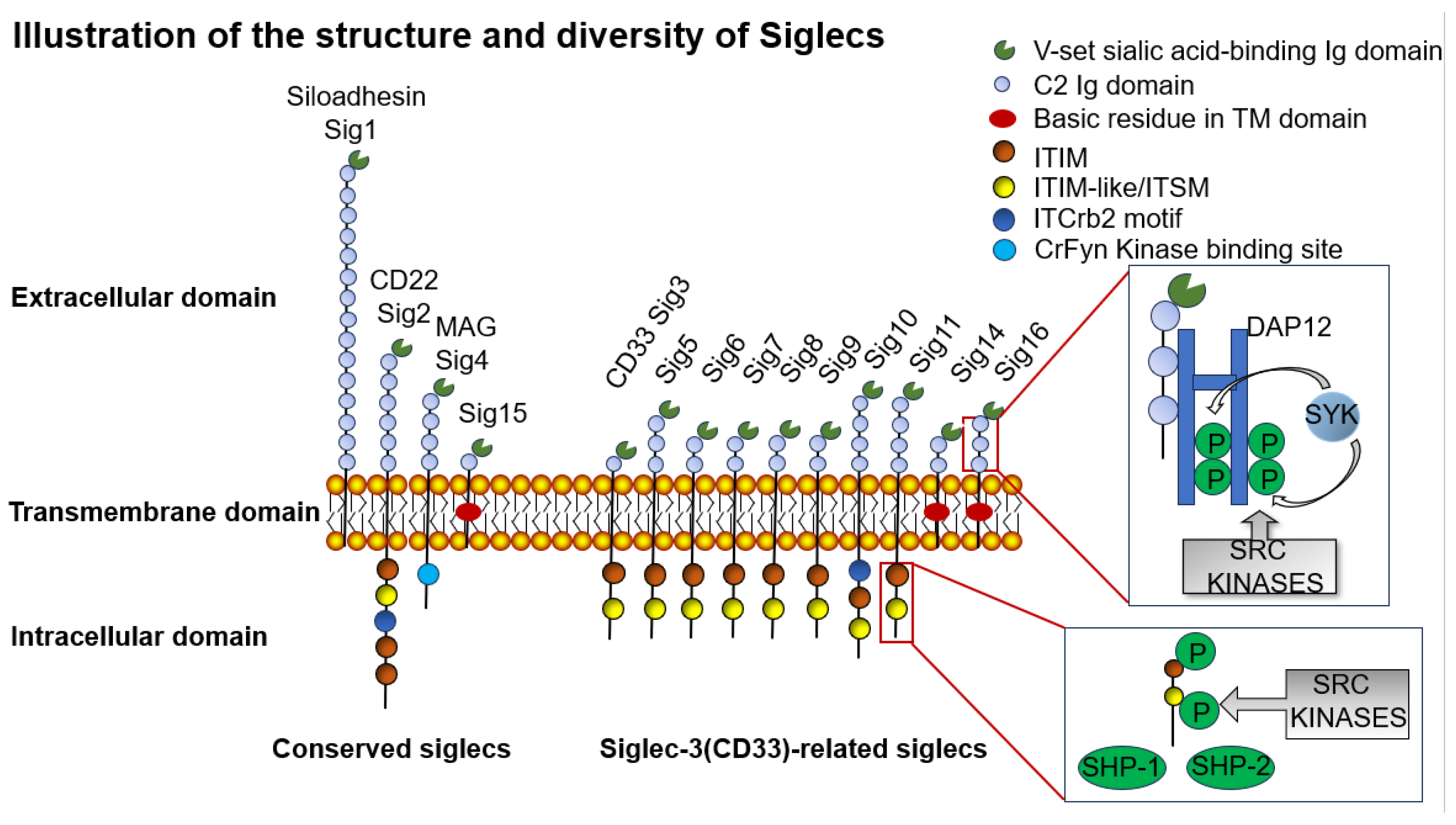
4.1. Siglec Family Classification and Function
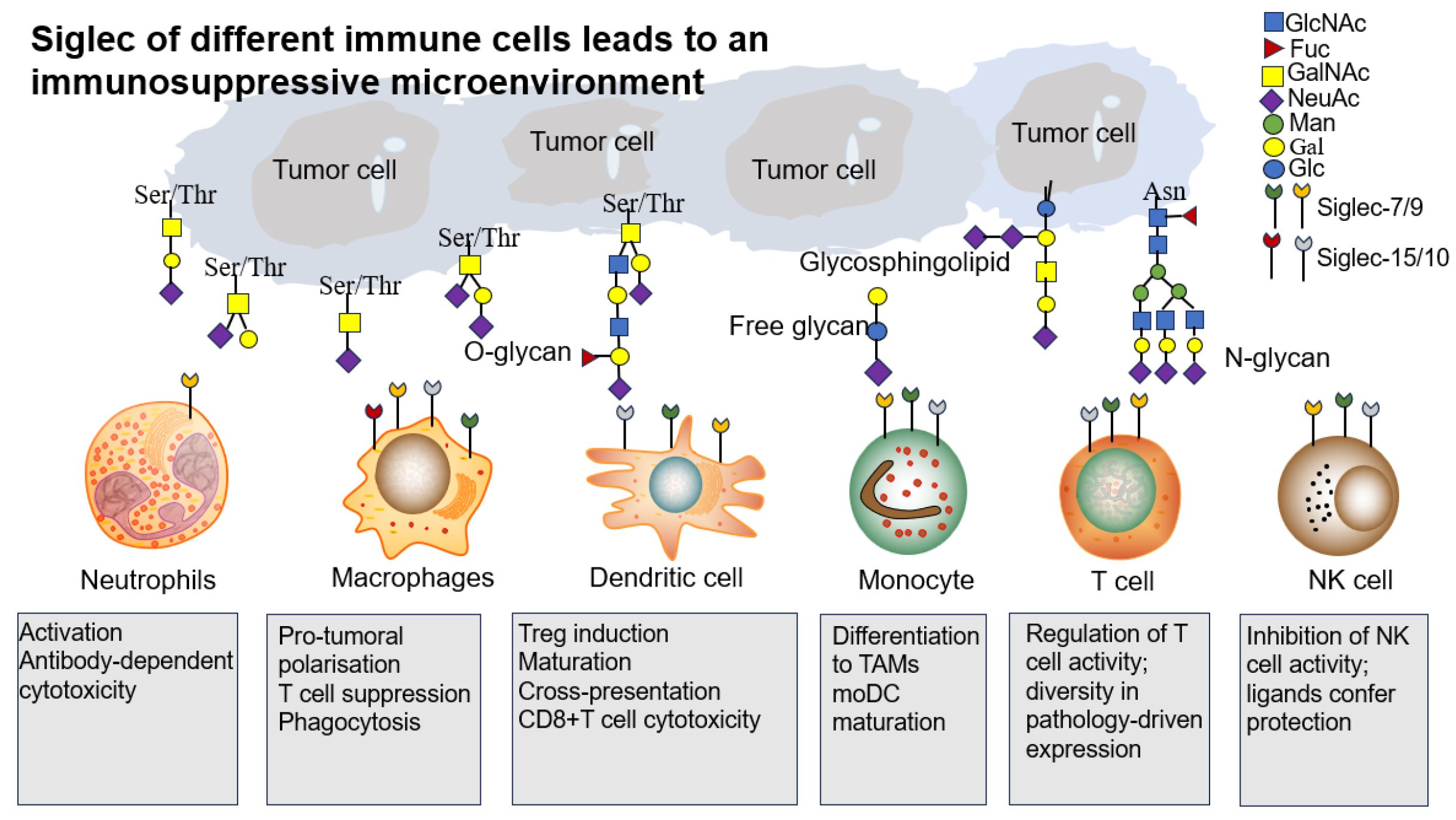
4.2. Generation of Immunosuppressive Microenvironment
4.2.1. Tumor-Associated Macrophages (TAMs)
4.2.2. Neutrophils
4.2.3. Dendritic Cells (DCs)
4.2.4. Natural Killer Cells (NK Cells)
4.2.5. Lymphocyte
4.3. Feasibility of Early Malignant Tumor Detection Using Sialic Acid and Siglecs
5. The Impact of Sialylation on Cancer Development
6. Methods of Targeting Siglec–Sialylation Therapy
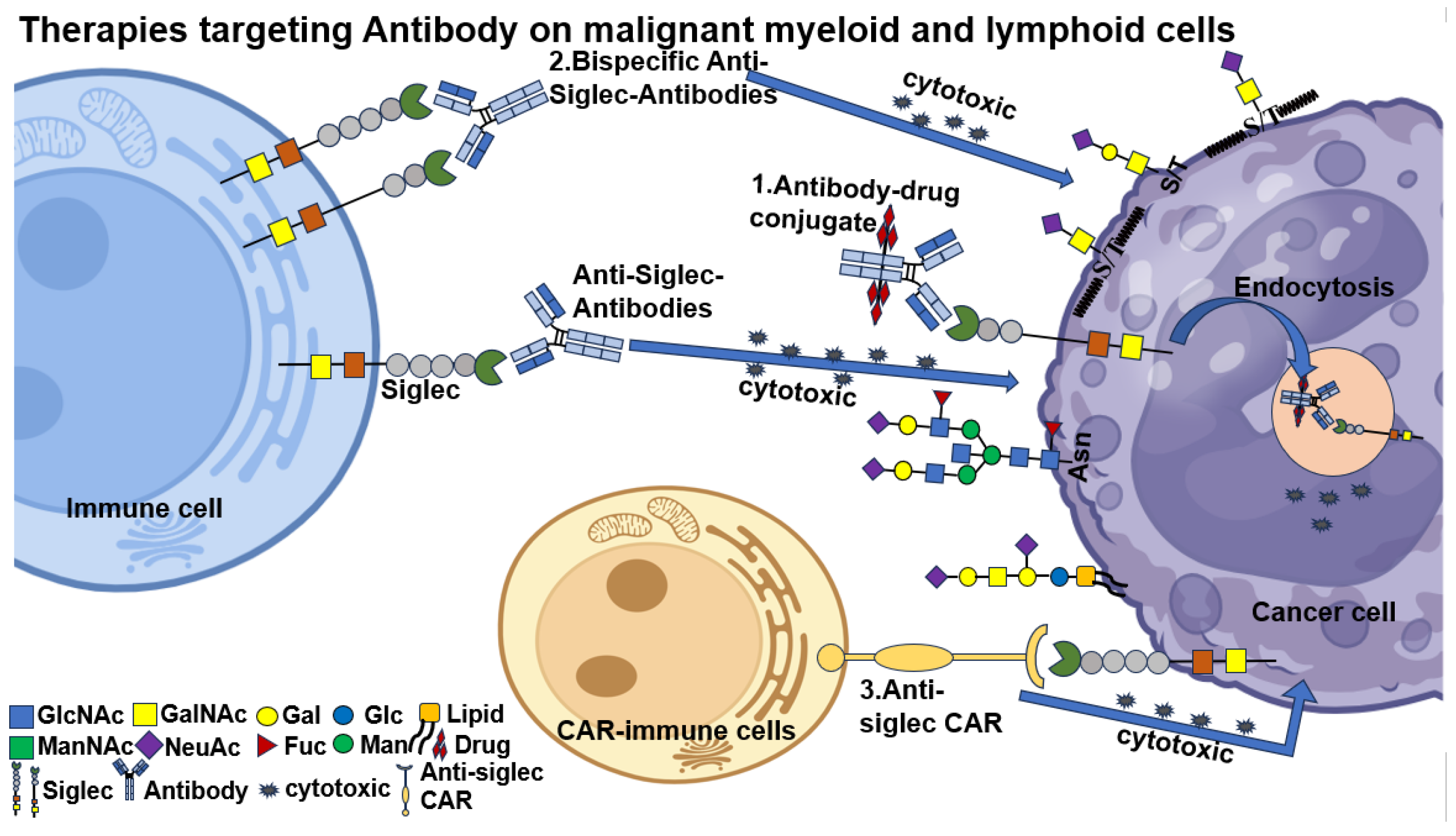
6.1. Antibody Therapy
6.1.1. Monoclonal Antibody (mAb)
6.1.2. Bispecific Antibody (BsAbs)
6.1.3. Chimeric Antigen Receptor T-Cell (CAR-T) Therapeutic Approaches
6.2. Siglec Inhibitor
6.3. Sialylation Inhibitor
6.3.1. Sialtransferase Inhibitors
6.3.2. Sialic Acid Mimics (SAMs)
6.3.3. Development of Novel Sialylation Materials
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhang, R.; Hei, H. Advances in post-translational modifications of proteins and cancer immunotherapy. Front. Immunol. 2023, 14, 1229397. [Google Scholar] [CrossRef] [PubMed]
- Eichler, J. Protein glycosylation. Curr. Biol. 2019, 29, R229–R231. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell. Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Kannagi, R.; Yin, J.; Miyazaki, K.; Izawa, M. Current relevance of incomplete synthesis and neo-synthesis for cancer-associated alteration of carbohydrate determinants—Hakomori’s concepts revisited. Biochim. Biophys. Acta. 2008, 1780, 525–531. [Google Scholar] [CrossRef]
- Varki, A.; Gagneux, P. Multifarious roles of sialic acids in immunity. Ann. N. Y. Acad. Sci. 2012, 1253, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.; Weigle, B.; Crocker, P.R. Siglec and anti-Siglec therapies. Curr. Opin. Chem. Biol. 2021, 62, 34–42. [Google Scholar] [CrossRef]
- Daly, J.; Sarkar, S.; Natoni, A.; Stark, J.C.; Riley, N.M.; Bertozzi, C.R.; Carlsten, M.; O’Dwyer, M.E. Targeting hypersialylation in multiple myeloma represents a novel approach to enhance NK cell-mediated tumor responses. Blood Adv. 2022, 6, 3352–3366. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Rodrigues Mantuano, N.; Kirchhammer, N.; Sanin, D.E.; Jacob, F.; Coelho, R.; Everest-Dass, A.V.; Wang, J.; Trefny, M.P.; Monaco, G.; et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci. Transl. Med. 2022, 14, eabj1270. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lou, Y.; Zhang, Y.; Liu, S.; Li, J.; Tao, J. Sialylated immunoglobulin G: A promising diagnostic and therapeutic strategy for autoimmune diseases. Theranostics 2021, 11, 5430–5446. [Google Scholar] [CrossRef]
- Marcos, N.T.; Bennett, E.P.; Gomes, J.; Magalhaes, A.; Gomes, C.; David, L.; Dar, I.; Jeanneau, C.; DeFrees, S.; Krustrup, D.; et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front. Biosci. (Elite Ed.) 2011, 3, 1443–1455. [Google Scholar] [CrossRef]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.S.; Hanisch, F.G.; Delannoy, P.; Le Bourhis, X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2006, 16, 54–64. [Google Scholar] [CrossRef]
- Dhar, C.; Sasmal, A.; Varki, A. From “Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc. Front. Immunol. 2019, 10, 807. [Google Scholar] [CrossRef]
- van Karnebeek, C.D.; Bonafé, L.; Wen, X.Y.; Tarailo-Graovac, M.; Balzano, S.; Royer-Bertrand, B.; Ashikov, A.; Garavelli, L.; Mammi, I.; Turolla, L.; et al. NANS-mediated synthesis of sialic acid is required for brain and skeletal development. Nat. Genet. 2016, 48, 777–784. [Google Scholar] [CrossRef]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.A.; Moons, S.J.; Timmermans, S.; de Jong, H.; Boltje, T.J.; Büll, C. Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021, 297, 100906. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Chen, X.; Schnaar, R.L.; Varki, A. Sialic Acids and Other Nonulosonic Acids. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022; pp. 185–204. [Google Scholar]
- Willems, A.P.; Sun, L.; Schulz, M.A.; Tian, W.; Ashikov, A.; van Scherpenzeel, M.; Hermans, E.; Clausen, H.; Yang, Z.; Lefeber, D.J. Activity of N-acylneuraminate-9-phosphatase (NANP) is not essential for de novo sialic acid biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1471–1479. [Google Scholar] [CrossRef]
- Tangvoranuntakul, P.; Gagneux, P.; Diaz, S.; Bardor, M.; Varki, N.; Varki, A.; Muchmore, E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. USA 2003, 100, 12045–12050. [Google Scholar] [CrossRef]
- Bergfeld, A.K.; Pearce, O.M.; Diaz, S.L.; Pham, T.; Varki, A. Metabolism of vertebrate amino sugars with N-glycolyl groups: Elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J. Biol. Chem. 2012, 287, 28865–28881. [Google Scholar] [CrossRef]
- Samraj, A.N.; Pearce, O.M.; Läubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef]
- Spruit, C.M.; Nemanichvili, N.; Okamatsu, M.; Takematsu, H.; Boons, G.J.; de Vries, R.P. N-Glycolylneuraminic Acid in Animal Models for Human Influenza A Virus. Viruses 2021, 13, 815. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.; Bäckström, M.; Dalziel, M.; Gschmeissner, S.; Karlsson, H.; Noll, T.; Gätgens, J.; Clausen, H.; Hansson, G.C.; Burchell, J.; et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem. 2006, 281, 3586–3594. [Google Scholar] [CrossRef]
- Luo, Y.; Cao, H.; Lei, C.; Liu, J. ST6GALNAC1 promotes the invasion and migration of breast cancer cells via the EMT pathway. Genes Genom. 2023, 45, 1367–1376. [Google Scholar] [CrossRef]
- Munkley, J.; Oltean, S.; Vodák, D.; Wilson, B.T.; Livermore, K.E.; Zhou, Y.; Star, E.; Floros, V.I.; Johannessen, B.; Knight, B.; et al. The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget 2015, 6, 34358–34374. [Google Scholar] [CrossRef]
- Tamura, F.; Sato, Y.; Hirakawa, M.; Yoshida, M.; Ono, M.; Osuga, T.; Okagawa, Y.; Uemura, N.; Arihara, Y.; Murase, K.; et al. RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential in gastric cancer cells. Gastric Cancer 2016, 19, 85–97. [Google Scholar] [CrossRef]
- Wang, W.Y.; Cao, Y.X.; Zhou, X.; Wei, B.; Zhan, L.; Sun, S.Y. Stimulative role of ST6GALNAC1 in proliferation, migration and invasion of ovarian cancer stem cells via the Akt signaling pathway. Cancer Cell. Int. 2019, 19, 86. [Google Scholar] [CrossRef]
- Alabiad, M.A.; Harb, O.A.; Abozaid, M.; Embaby, A.; Mandour, D.; Hemeda, R.; Shalaby, A.M. The Diagnostic and Prognostic Roles of Combined Expression of Novel Biomarkers in Lung Adenocarcinoma and Lung Squamous Cell Carcinoma: An Immunohistochemical Study. Iran. J. Pathol. 2021, 16, 162–173. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Z.; Majdaeen, M.; Agbele, A.T.; Abedi-Firouzjah, R. Functions of Sialyltransferases in gynecological malignancies: A systematic review. Pathol. Res. Pract. 2024, 254, 155159. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell. 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Archer Goode, E.; Garnham, R.; Hodgson, K.; Orozco-Moreno, M.; Turner, H.; Livermore, K.; Putri Nangkana, K.; Frame, F.M.; Bermudez, A.; et al. ST6GAL1-mediated aberrant sialylation promotes prostate cancer progression. J. Pathol. 2023, 261, 71–84. [Google Scholar] [CrossRef]
- Bhalerao, N.; Chakraborty, A.; Marciel, M.P.; Hwang, J.; Britain, C.M.; Silva, A.D.; Eltoum, I.E.; Jones, R.B.; Alexander, K.L.; Smythies, L.E.; et al. ST6GAL1 sialyltransferase promotes acinar to ductal metaplasia and pancreatic cancer progression. JCI Insight 2023, 8, e161563. [Google Scholar] [CrossRef]
- Meng, Q.; Ren, C.; Wang, L.; Zhao, Y.; Wang, S. Knockdown of ST6Gal-I inhibits the growth and invasion of osteosarcoma MG-63 cells. Biomed. Pharmacother. 2015, 72, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Lu, J.; Deng, Y.; Liu, Q.; Yan, X.; Cui, Y.; Xiao, X.; Fang, M.; Yang, F.; Sawaki, H.; et al. ST6GAL1 inhibits metastasis of hepatocellular carcinoma via modulating sialylation of MCAM on cell surface. Oncogene 2023, 42, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Gc, S.; Tuy, K.; Rickenbacker, L.; Jones, R.; Chakraborty, A.; Miller, C.R.; Beierle, E.A.; Hanumanthu, V.S.; Tran, A.N.; Mobley, J.A.; et al. α2,6 Sialylation mediated by ST6GAL1 promotes glioblastoma growth. JCI Insight 2022, 7, 158799. [Google Scholar] [CrossRef]
- Smithson, M.; Irwin, R.; Williams, G.; Alexander, K.L.; Smythies, L.E.; Nearing, M.; McLeod, M.C.; Al Diffalha, S.; Bellis, S.L.; Hardiman, K.M. Sialyltransferase ST6GAL-1 mediates resistance to chemoradiation in rectal cancer. J. Biol. Chem. 2022, 298, 101594. [Google Scholar] [CrossRef]
- Cui, H.; Yang, S.; Jiang, Y.; Li, C.; Zhao, Y.; Shi, Y.; Hao, Y.; Qian, F.; Tang, B.; Yu, P. The glycosyltransferase ST6Gal-I is enriched in cancer stem-like cells in colorectal carcinoma and contributes to their chemo-resistance. Clin. Transl. Oncol. 2018, 20, 1175–1184. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dorsett, K.A.; Trummell, H.Q.; Yang, E.S.; Oliver, P.G.; Bonner, J.A.; Buchsbaum, D.J.; Bellis, S.L. ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J. Biol. Chem. 2018, 293, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Videira, P.A.; Correia, M.; Malagolini, N.; Crespo, H.J.; Ligeiro, D.; Calais, F.M.; Trindade, H.; Dall′Olio, F. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 2009, 9, 357. [Google Scholar] [CrossRef]
- Wu, H.; Shi, X.L.; Zhang, H.J.; Song, Q.J.; Yang, X.B.; Hu, W.D.; Mei, G.L.; Chen, X.; Mao, Q.S.; Chen, Z. Overexpression of ST3Gal-I promotes migration and invasion of HCCLM3 in vitro and poor prognosis in human hepatocellular carcinoma. Onco Targets Ther. 2016, 9, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Picco, G.; Julien, S.; Brockhausen, I.; Beatson, R.; Antonopoulos, A.; Haslam, S.; Mandel, U.; Dell, A.; Pinder, S.; Taylor-Papadimitriou, J.; et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 2010, 20, 1241–1250. [Google Scholar] [CrossRef]
- Wen, K.C.; Sung, P.L.; Hsieh, S.L.; Chou, Y.T.; Lee, O.K.; Wu, C.W.; Wang, P.H. α2,3-sialyltransferase type I regulates migration and peritoneal dissemination of ovarian cancer cells. Oncotarget 2017, 8, 29013–29027. [Google Scholar] [CrossRef]
- Pérez-Garay, M.; Arteta, B.; Llop, E.; Cobler, L.; Pagès, L.; Ortiz, R.; Ferri, M.J.; de Bolós, C.; Figueras, J.; de Llorens, R.; et al. α2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell. Biol. 2013, 45, 1748–1757. [Google Scholar] [CrossRef]
- Ouyang, S.; Liu, J.H.; Ni, Z.; Ding, G.F.; Wang, Q.Z. Downregulation of ST3GAL5 is associated with muscle invasion, high grade and a poor prognosis in patients with bladder cancer. Oncol. Lett. 2020, 20, 828–840. [Google Scholar] [CrossRef]
- Hu, J.; Shan, Y.; Ma, J.; Pan, Y.; Zhou, H.; Jiang, L.; Jia, L. LncRNA ST3Gal6-AS1/ST3Gal6 axis mediates colorectal cancer progression by regulating α-2,3 sialylation via PI3K/Akt signaling. Int. J. Cancer 2019, 145, 450–460. [Google Scholar] [CrossRef]
- Dalangood, S.; Zhu, Z.; Ma, Z.; Li, J.; Zeng, Q.; Yan, Y.; Shen, B.; Yan, J.; Huang, R. Identification of glycogene-type and validation of ST3GAL6 as a biomarker predicts clinical outcome and cancer cell invasion in urinary bladder cancer. Theranostics 2020, 10, 10078–10091. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Cao, J.; Yang, J.; Chen, Z.; Wang, W.; Wang, S.; Zhang, L.; Xie, L.; Fang, L.; et al. The novel role of circular RNA ST3GAL6 on blocking gastric cancer malignant behaviours through autophagy regulated by the FOXP2/MET/mTOR axis. Clin. Transl. Med. 2022, 12, e707. [Google Scholar] [CrossRef]
- Glavey, S.V.; Manier, S.; Natoni, A.; Sacco, A.; Moschetta, M.; Reagan, M.R.; Murillo, L.S.; Sahin, I.; Wu, P.; Mishima, Y.; et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood 2014, 124, 1765–1776. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Sun, J.; Wu, J.; He, X.; Wang, S.; Wang, X.; Miao, X.; Huang, R.; Yan, J. Comprehensive landscape of the ST3GAL family reveals the significance of ST3GAL6-AS1/ST3GAL6 axis on EGFR signaling in lung adenocarcinoma cell invasion. Front. Cell. Dev. Biol. 2022, 10, 931132. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, X.; Liang, L.; Pan, X.; Lv, H.; Zhao, Y. Sialyltransferase ST3GAL6 mediates the effect of microRNA-26a on cell growth, migration, and invasion in hepatocellular carcinoma through the protein kinase B/mammalian target of rapamycin pathway. Cancer Sci. 2017, 108, 267–276. [Google Scholar] [CrossRef]
- Christie, D.R.; Shaikh, F.M.; Lucas, J.A.t.; Lucas, J.A., 3rd; Bellis, S.L. ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J. Ovarian Res. 2008, 1, 3. [Google Scholar] [CrossRef]
- Wei, A.; Fan, B.; Zhao, Y.; Zhang, H.; Wang, L.; Yu, X.; Yuan, Q.; Yang, D.; Wang, S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin signaling pathway. Oncotarget 2016, 7, 65374–65388. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, X.; Han, Y.; Lei, T.; Wu, Q.; Yu, X.; Wang, L.; Fan, Z.; Wang, S. Modification of α2,6-sialylation mediates the invasiveness and tumorigenicity of non-small cell lung cancer cells in vitro and in vivo via Notch1/Hes1/MMPs pathway. Int. J. Cancer 2018, 143, 2319–2330. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Meng, F.; Huang, T.; Wang, S.; Zheng, Z.; Zheng, G.; Li, W.; Zhang, J.; Liu, Y. α2,6-Sialylation promotes hepatocellular carcinoma cells migration and invasion via enhancement of nSmase2-mediated exosomal miRNA sorting. J. Physiol. Biochem. 2023, 79, 19–34. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Wang, G.; Xiao, J.; Zhang, N.; Chen, Y.; Yu, H.; Wang, G.; Zhao, Y. Resveratrol Inhibits the Tumorigenesis of Follicular Thyroid Cancer via ST6GAL2-Regulated Activation of the Hippo Signaling Pathway. Mol. Ther. Oncolytics 2020, 16, 124–133. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Q.; Wang, L.; Zhao, Y.; Zhang, Q.; Meng, Q.; Pawan; Wang, S. Silencing of ST6GalNAc I suppresses the proliferation, migration and invasion of hepatocarcinoma cells through PI3K/AKT/NF-κB pathway. Tumour Biol. 2016, 37, 12213–12221. [Google Scholar] [CrossRef]
- Kvorjak, M.; Ahmed, Y.; Miller, M.L.; Sriram, R.; Coronnello, C.; Hashash, J.G.; Hartman, D.J.; Telmer, C.A.; Miskov-Zivanov, N.; Finn, O.J.; et al. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2020, 8, 167–178. [Google Scholar] [CrossRef]
- Munkley, J.; Livermore, K.; Rajan, P.; Elliott, D.J. RNA splicing and splicing regulator changes in prostate cancer pathology. Hum. Genet. 2017, 136, 1143–1154. [Google Scholar] [CrossRef]
- Iwaya, T.; Sawada, G.; Amano, S.; Kume, K.; Ito, C.; Endo, F.; Konosu, M.; Shioi, Y.; Akiyama, Y.; Takahara, T.; et al. Downregulation of ST6GALNAC1 is associated with esophageal squamous cell carcinoma development. Int. J. Oncol. 2017, 50, 441–447. [Google Scholar] [CrossRef]
- Jia, L.; Luo, S.; Ren, X.; Li, Y.; Hu, J.; Liu, B.; Zhao, L.; Shan, Y.; Zhou, H. miR-182 and miR-135b Mediate the Tumorigenesis and Invasiveness of Colorectal Cancer Cells via Targeting ST6GALNAC2 and PI3K/AKT Pathway. Dig. Dis. Sci. 2017, 62, 3447–3459. [Google Scholar] [CrossRef]
- Ferrer, C.M.; Reginato, M.J. Sticking to sugars at the metastatic site: Sialyltransferase ST6GalNAc2 acts as a breast cancer metastasis suppressor. Cancer Discov. 2014, 4, 275–277. [Google Scholar] [CrossRef]
- Miao, X.; Jia, L.; Zhou, H.; Song, X.; Zhou, M.; Xu, J.; Zhao, L.; Feng, X.; Zhao, Y. miR-4299 mediates the invasive properties and tumorigenicity of human follicular thyroid carcinoma by targeting ST6GALNAC4. IUBMB Life 2016, 68, 136–144. [Google Scholar] [CrossRef]
- Dai, T.; Li, J.; Liang, R.B.; Yu, H.; Lu, X.; Wang, G. Identification and Experimental Validation of the Prognostic Significance and Immunological Correlation of Glycosylation-Related Signature and ST6GALNAC4 in Hepatocellular Carcinoma. J. Hepatocell Carcinoma 2023, 10, 531–551. [Google Scholar] [CrossRef]
- Bai, L.; Luo, L.; Gao, W.; Bu, C.; Huang, J. miR-182 modulates cell proliferation and invasion in prostate cancer via targeting ST6GALNAC5. Braz. J. Med. Biol. Res. 2021, 54, e9695. [Google Scholar] [CrossRef]
- Wang, L.; Wu, S.; He, H.; Ai, K.; Xu, R.; Zhang, L.; Zhu, X. CircRNA-ST6GALNAC6 increases the sensitivity of bladder cancer cells to erastin-induced ferroptosis by regulating the HSPB1/P38 axis. Lab. Investig. 2022, 102, 1323–1334. [Google Scholar] [CrossRef]
- Steenackers, A.; Vanbeselaere, J.; Cazet, A.; Bobowski, M.; Rombouts, Y.; Colomb, F.; Le Bourhis, X.; Guérardel, Y.; Delannoy, P. Accumulation of unusual gangliosides G(Q3) and G(P3) in breast cancer cells expressing the G(D3) synthase. Molecules 2012, 17, 9559–9572. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, X.; Yang, J.; Jiang, Y.; Yang, H. Effects of the regulation of polysialyltransferase ST8SiaII on the invasiveness and metastasis of small cell lung cancer cells. Oncol. Rep. 2017, 37, 131–138. [Google Scholar] [CrossRef]
- Yoon, H.K.; An, H.K.; Ko, M.J.; Kim, K.S.; Mun, S.W.; Kim, D.H.; Kim, C.M.; Kim, C.H.; Choi, Y.W.; Lee, Y.C. Upregulation of Human ST8Sia VI (α2,8-Sialyltransferase) Gene Expression by Physcion in SK-N-BE(2)-C Human Neuroblastoma Cells. Int. J. Mol. Sci. 2016, 2, 1246. [Google Scholar] [CrossRef]
- Gorelik, A.; Illes, K.; Mazhab-Jafari, M.T.; Nagar, B. Structure of the immunoregulatory sialidase NEU1. Sci. Adv. 2023, 9, eadf8169. [Google Scholar] [CrossRef]
- Ren, L.R.; Zhang, L.P.; Huang, S.Y.; Zhu, Y.F.; Li, W.J.; Fang, S.Y.; Shen, L.; Gao, Y.L. Effects of sialidase NEU1 siRNA on proliferation, apoptosis, and invasion in human ovarian cancer. Mol. Cell. Biochem. 2016, 411, 213–219. [Google Scholar] [CrossRef]
- Peng, Q.; Gao, L.; Cheng, H.B.; Wang, J.S.; Wang, J. Sialidase NEU1 May Serve as a Potential Biomarker of Proliferation, Migration and Prognosis in Melanoma. World J. Oncol. 2022, 13, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, Y.; He, W.; Kuo, F.; Zhang, Y.; Wang, D.; He, L.; Yang, Y.; Wang, H.; Chen, Y. Correlation Between Sialidase NEU1 mRNA Expression Changes in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 870374. [Google Scholar] [CrossRef]
- Mei, S.; Li, D.; Wang, A.; Zhu, G.; Zhou, B.; Li, N.; Qin, Y.; Zhang, Y.; Jiang, S. The role of sialidase Neu1 in respiratory diseases. Respir. Res. 2024, 25, 134. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mondal, S.; Butti, R.; Prasanna Gunasekaran, V.; Chatterjee, U.; Halder, A.; Kundu, G.C.; Mandal, C. Desialylation of Sonic-Hedgehog by Neu2 Inhibits Its Association with Patched1 Reducing Stemness-Like Properties in Pancreatic Cancer Sphere-forming Cells. Cells 2020, 9, 1512. [Google Scholar] [CrossRef]
- Tatsuta, T.; Ito, J.; Yamamoto, K.; Sugawara, S.; Hosono, M.; Sato, M.; Miyagi, T. Sialidase NEU3 Contributes to the Invasiveness of Bladder Cancer. Biomedicines 2024, 12, 192. [Google Scholar] [CrossRef]
- Kakugawa, Y.; Wada, T.; Yamaguchi, K.; Yamanami, H.; Ouchi, K.; Sato, I.; Miyagi, T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl. Acad. Sci. USA 2002, 99, 10718–10723. [Google Scholar] [CrossRef]
- Zhang, X.; Dou, P.; Akhtar, M.L.; Liu, F.; Hu, X.; Yang, L.; Yang, D.; Zhang, X.; Li, Y.; Qiao, S.; et al. NEU4 inhibits motility of HCC cells by cleaving sialic acids on CD44. Oncogene 2021, 40, 5427–5440. [Google Scholar] [CrossRef]
- Brockhausen, I.; Wandall, H.H.; Hagen, K.G.T.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; pp. 117–128. [Google Scholar]
- Crocker, P.R.; Varki, A. Siglecs, sialic acids and innate immunity. Trends Immunol. 2001, 22, 337–342. [Google Scholar] [CrossRef]
- Crocker, P.R. Siglecs: Sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 2002, 12, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R. Siglecs in innate immunity. Curr. Opin. Pharmacol. 2005, 5, 431–437. [Google Scholar] [CrossRef]
- Angata, T.; Margulies, E.H.; Green, E.D.; Varki, A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl. Acad. Sci. USA 2004, 101, 13251–13256. [Google Scholar] [CrossRef]
- Siddiqui, S.S. Non-canonical roles of Siglecs: Beyond sialic acid-binding and immune cell modulation. Mol. Aspects Med. 2023, 90, 101145. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, W.; Wan, T.; Zhang, J.; Chen, T.; Yu, Y.; Wang, J.; Cao, X. Cloning and characterization of Siglec-10, a novel sialic acid binding member of the Ig superfamily, from human dendritic cells. J. Biol. Chem. 2001, 276, 28106–28112. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Bärenwaldt, A.; Läubli, H. The sialoglycan-Siglec glyco-immune checkpoint-a target for improving innate and adaptive anti-cancer immunity. Expert Opin. Ther. Targets 2019, 23, 839–853. [Google Scholar] [CrossRef]
- Gonzalez-Gil, A.; Li, T.A.; Kim, J.; Schnaar, R.L. Human sialoglycan ligands for immune inhibitory Siglecs. Mol. Aspects Med. 2023, 90, 101110. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Stanczak, M.A.; Siddiqui, S.S.; Trefny, M.P.; Thommen, D.S.; Boligan, K.F.; von Gunten, S.; Tzankov, A.; Tietze, L.; Lardinois, D.; Heinzelmann-Schwarz, V.; et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J. Clin. Investig. 2018, 128, 4912–4923. [Google Scholar] [CrossRef]
- van de Wall, S.; Santegoets, K.C.M.; van Houtum, E.J.H.; Büll, C.; Adema, G.J. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends Immunol. 2020, 41, 274–285. [Google Scholar] [CrossRef]
- Läubli, H.; Pearce, O.M.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Ibarlucea-Benitez, I.; Weitzenfeld, P.; Smith, P.; Ravetch, J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2107424118. [Google Scholar] [CrossRef]
- Rodriguez, E.; Boelaars, K.; Brown, K.; Eveline Li, R.J.; Kruijssen, L.; Bruijns, S.C.M.; van Ee, T.; Schetters, S.T.T.; Crommentuijn, M.H.W.; van der Horst, J.C.; et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat. Commun. 2021, 12, 1270. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, Z.; Liu, Y.; Li, X.; Zhang, Q.; Xu, X.; Gu, Y.; Zhang, Y.; Zhao, D.; Cao, X. The lectin Siglec-G inhibits dendritic cell cross-presentation by impairing MHC class I-peptide complex formation. Nat. Immunol. 2016, 17, 1167–1175. [Google Scholar] [CrossRef]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.; Schetters, S.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; den Haan, J.M.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef]
- Wang, J.; Manni, M.; Bärenwaldt, A.; Wieboldt, R.; Kirchhammer, N.; Ivanek, R.; Stanczak, M.; Zippelius, A.; König, D.; Rodrigues Manutano, N.; et al. Siglec Receptors Modulate Dendritic Cell Activation and Antigen Presentation to T Cells in Cancer. Front. Cell. Dev. Biol. 2022, 10, 828916. [Google Scholar] [CrossRef]
- Vuchkovska, A.; Glanville, D.G.; Scurti, G.M.; Nishimura, M.I.; White, P.; Ulijasz, A.T.; Iwashima, M. Siglec-5 is an inhibitory immune checkpoint molecule for human T cells. Immunology 2022, 166, 238–248. [Google Scholar] [CrossRef]
- Hong, S.; Yu, C.; Rodrigues, E.; Shi, Y.; Chen, H.; Wang, P.; Chapla, D.G.; Gao, T.; Zhuang, R.; Moremen, K.W.; et al. Modulation of Siglec-7 Signaling Via In Situ-Created High-Affinity cis-Ligands. ACS Cent. Sci. 2021, 7, 1338–1346. [Google Scholar] [CrossRef]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Démoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Gao, R.; Xing, B.; Gao, Y.; Yang, Y.; Qin, S.; Zhang, L.; Ouyang, H.; Du, P.; et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell 2021, 184, 792–809.e723. [Google Scholar] [CrossRef]
- Mulder, K.; Patel, A.A.; Kong, W.T.; Piot, C.; Halitzki, E.; Dunsmore, G.; Khalilnezhad, S.; Irac, S.E.; Dubuisson, A.; Chevrier, M.; et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity 2021, 54, 1883–1900.e1885. [Google Scholar] [CrossRef]
- Varchetta, S.; Brunetta, E.; Roberto, A.; Mikulak, J.; Hudspeth, K.L.; Mondelli, M.U.; Mavilio, D. Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes. PLoS ONE 2012, 7, e45821. [Google Scholar] [CrossRef]
- Bandala-Sanchez, E.; Zhang, Y.; Reinwald, S.; Dromey, J.A.; Lee, B.H.; Qian, J.; Böhmer, R.M.; Harrison, L.C. T cell regulation mediated by interaction of soluble CD52 with the inhibitory receptor Siglec-10. Nat. Immunol. 2013, 14, 741–748. [Google Scholar] [CrossRef]
- Beatson, R.; Graham, R.; Grundland Freile, F.; Cozzetto, D.; Kannambath, S.; Pfeifer, E.; Woodman, N.; Owen, J.; Nuamah, R.; Mandel, U.; et al. Cancer-associated hypersialylated MUC1 drives the differentiation of human monocytes into macrophages with a pathogenic phenotype. Commun. Biol. 2020, 3, 644. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Chan, C.; Jansen, J.H.M.; Bräutigam, M.; Kölling, M.A.; Gehlert, C.L.; Baumann, N.; Mester, S.; Foss, S.; Andersen, J.T.; et al. Disruption of the sialic acid/Siglec-9 axis improves antibody-mediated neutrophil cytotoxicity towards tumor cells. Front. Immunol. 2023, 14, 1178817. [Google Scholar] [CrossRef]
- Liao, C.; Luo, S.; Liu, X.; Zhang, L.; Xie, P.; Zhou, W.; Lu, Y.; Zhong, H.; Zhang, X.; Xiong, Z.; et al. Siglec-F(+) neutrophils in the spleen induce immunosuppression following acute infection. Theranostics 2024, 14, 2589–2604. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef]
- Stewart, N.; Daly, J.; Drummond-Guy, O.; Krishnamoorthy, V.; Stark, J.C.; Riley, N.M.; Williams, K.C.; Bertozzi, C.R.; Wisnovsky, S. The glycoimmune checkpoint receptor Siglec-7 interacts with T-cell ligands and regulates T-cell activation. J. Biol. Chem. 2024, 300, 105579. [Google Scholar] [CrossRef]
- Nerreter, T.; Köchel, C.; Jesper, D.; Eichelbrönner, I.; Putz, E.; Einsele, H.; Seggewiss-Bernhardt, R. Dasatinib enhances migration of monocyte-derived dendritic cells by reducing phosphorylation of inhibitory immune receptors Siglec-9 and Siglec-3. Exp. Hematol. 2014, 42, e771–e773. [Google Scholar] [CrossRef]
- Belisle, J.A.; Horibata, S.; Jennifer, G.A.; Petrie, S.; Kapur, A.; André, S.; Gabius, H.J.; Rancourt, C.; Connor, J.; Paulson, J.C.; et al. Identification of Siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Mol. Cancer 2010, 9, 118. [Google Scholar] [CrossRef]
- Meyer, S.J.; Linder, A.T.; Brandl, C.; Nitschke, L. B Cell Siglecs-News on Signaling and Its Interplay with Ligand Binding. Front. Immunol. 2018, 9, 2820. [Google Scholar] [CrossRef]
- Wong, K.L.; Li, Z.; Ma, F.; Wang, D.; Song, N.; Chong, C.H.; Luk, K.K.; Leung, S.O. SM03, an Anti-CD22 Antibody, Converts Cis-to-Trans Ligand Binding of CD22 against α2,6-Linked Sialic Acid Glycans and Immunomodulates Systemic Autoimmune Diseases. J. Immunol. 2022, 208, 2726–2737. [Google Scholar] [CrossRef]
- Liu, S.; Deng, B.; Yin, Z.; Lin, Y.; An, L.; Liu, D.; Pan, J.; Yu, X.; Chen, B.; Wu, T.; et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am. J. Hematol. 2021, 96, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wang, X.; Zhang, J.; Liu, D.; He, J.; Huang, C.; Liao, J.; Wang, Y.; Feng, Y.; Li, H.; et al. Siglec-9 acts as an immune-checkpoint molecule on macrophages in glioblastoma, restricting T-cell priming and immunotherapy response. Nat. Cancer 2023, 4, 1273–1291. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.W.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Akita, K.; Ishida, A.; Mori, Y.; Toda, M.; Inoue, M.; Ohta, M.; Yashiro, M.; Sawada, T.; Hirakawa, K.; et al. Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth. J. Biol. Chem. 2013, 288, 31842–31852. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, T.; Teranishi, T.; Alphey, M.S.; Crocker, P.R.; Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 2002, 277, 6324–6332. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Guo, R.; Bian, Z.; Song, Y. Targeting macrophages in hematological malignancies: Recent advances and future directions. J. Hematol. Oncol. 2022, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Siddiqui, B.A.; Anandhan, S.; Yadav, S.S.; Subudhi, S.K.; Gao, J.; Goswami, S.; Allison, J.P. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021, 11, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2022, 21, 509–528. [Google Scholar] [CrossRef]
- Friedrich, M.; Jasinski-Bergner, S.; Lazaridou, M.F.; Subbarayan, K.; Massa, C.; Tretbar, S.; Mueller, A.; Handke, D.; Biehl, K.; Bukur, J.; et al. Tumor-induced escape mechanisms and their association with resistance to checkpoint inhibitor therapy. Cancer Immunol. Immunother. 2019, 68, 1689–1700. [Google Scholar] [CrossRef]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2022, 55, 1749. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Patel, S.; Muffly, L.; Hossain, N.M.; Oak, J.; Baird, J.H.; Frank, M.J.; Shiraz, P.; Sahaf, B.; Craig, J.; et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat. Med. 2021, 27, 1419–1431. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Song, D.; Yuan, J.; Mullin, B.H.; Xu, J. Molecular structure, expression, and the emerging role of Siglec-15 in skeletal biology and cancer. J. Cell. Physiol. 2022, 237, 1711–1719. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell. Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Büll, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Holdbrooks, A.T.; Britain, C.M.; Bellis, S.L. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem. 2018, 293, 1610–1622. [Google Scholar] [CrossRef]
- Almaraz, R.T.; Tian, Y.; Bhattarcharya, R.; Tan, E.; Chen, S.H.; Dallas, M.R.; Chen, L.; Zhang, Z.; Zhang, H.; Konstantopoulos, K.; et al. Metabolic flux increases glycoprotein sialylation: Implications for cell adhesion and cancer metastasis. Mol. Cell. Proteom. 2012, 11, M112.017558. [Google Scholar] [CrossRef]
- Rodrigues, J.G.; Balmaña, M.; Macedo, J.A.; Poças, J.; Fernandes, Â.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhães, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell. Immunol. 2018, 333, 46–57. [Google Scholar] [CrossRef]
- Scott, D.A.; Drake, R.R. Glycosylation and its implications in breast cancer. Expert Rev. Proteom. 2019, 16, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.; Cho, W.; Li, B.; Prakobphol, A.; Johansen, E.; Anderson, N.L.; Regnier, F.E.; Gibson, B.W.; Fisher, S.J. Sweetening the pot: Adding glycosylation to the biomarker discovery equation. Clin. Chem. 2010, 56, 223–236. [Google Scholar] [CrossRef]
- Wichert, B.; Milde-Langosch, K.; Galatenko, V.; Schmalfeldt, B.; Oliveira-Ferrer, L. Prognostic role of the sialyltransferase ST6GAL1 in ovarian cancer. Glycobiology 2018, 28, 898–903. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; He, J.; Li, M.; Yao, X.; Yang, H.; Luo, Z.; Luo, P.; Su, M. Nanointegrative Glycoengineering-Activated Necroptosis of Triple Negative Breast Cancer Stem Cells Enables Self-Amplifiable Immunotherapy for Systemic Tumor Rejection. Adv. Healthc. Mater. 2024, 13, e2303337. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Vickers, A.; Cheung, N.K. Sialyltransferase STX (ST8SiaII): A novel molecular marker of metastatic neuroblastoma. Int. J. Cancer 2006, 119, 152–156. [Google Scholar] [CrossRef]
- Bhat, A.M.; Mohapatra, B.C.; Luan, H.; Mushtaq, I.; Chakraborty, S.; Kumar, S.; Wu, W.; Nolan, B.; Dutta, S.; Storck, M.D.; et al. GD2 and its biosynthetic enzyme GD3 synthase promote tumorigenesis in prostate cancer by regulating cancer stem cell behavior. Sci. Rep. 2024, 14, 13523. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- O’Sullivan, J.A.; Carroll, D.J.; Cao, Y.; Salicru, A.N.; Bochner, B.S. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J. Allergy Clin. Immunol. 2018, 141, 1774–1785.e1777. [Google Scholar] [CrossRef]
- Delputte, P.L.; Van Gorp, H.; Favoreel, H.W.; Hoebeke, I.; Delrue, I.; Dewerchin, H.; Verdonck, F.; Verhasselt, B.; Cox, E.; Nauwynck, H.J. Porcine sialoadhesin (CD169/Siglec-1) is an endocytic receptor that allows targeted delivery of toxins and antigens to macrophages. PLoS ONE 2011, 6, e16827. [Google Scholar] [CrossRef]
- Shan, D.; Press, O.W. Constitutive endocytosis and degradation of CD22 by human B cells. J. Immunol. 1995, 154, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, F.; Cao, J.; Lu, F.; Zhang, Y.; Ma, Y.; Zeng, K.; Zhang, L.; Zhao, H. Anticancer bispecific antibody R&D advances: A study focusing on research trend worldwide and in China. J. Hematol. Oncol. 2021, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef]
- Suurs, F.V.; Lub-de Hooge, M.N.; de Vries, E.G.E.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef]
- Wang, T.T.; Ravetch, J.V. Functional diversification of IgGs through Fc glycosylation. J. Clin. Investig. 2019, 129, 3492–3498. [Google Scholar] [CrossRef]
- Löffler, A.; Kufer, P.; Lutterbüse, R.; Zettl, F.; Daniel, P.T.; Schwenkenbecher, J.M.; Riethmüller, G.; Dörken, B.; Bargou, R.C. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000, 95, 2098–2103. [Google Scholar] [CrossRef]
- Wolf, E.; Hofmeister, R.; Kufer, P.; Schlereth, B.; Baeuerle, P.A. BiTEs: Bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 2005, 10, 1237–1244. [Google Scholar] [CrossRef]
- Aigner, M.; Feulner, J.; Schaffer, S.; Kischel, R.; Kufer, P.; Schneider, K.; Henn, A.; Rattel, B.; Friedrich, M.; Baeuerle, P.A.; et al. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia 2013, 27, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Frankel, A.E.; Cao, Q.; Lewis, D.; Grzywacz, B.; Verneris, M.R.; Ustun, C.; Lazaryan, A.; McClune, B.; Warlick, E.D.; et al. Phase I study of a bispecific ligand-directed toxin targeting CD22 and CD19 (DT2219) for refractory B-cell malignancies. Clin. Cancer Res. 2015, 21, 1267–1272. [Google Scholar] [CrossRef]
- Rossi, E.A.; Chang, C.H.; Goldenberg, D.M. Anti-CD22/CD20 Bispecific antibody with enhanced trogocytosis for treatment of Lupus. PLoS ONE 2014, 9, e98315. [Google Scholar] [CrossRef]
- Ellis, G.I.; Sheppard, N.C.; Riley, J.L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 2021, 22, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Jogalekar, M.P.; Rajendran, R.L.; Khan, F.; Dmello, C.; Gangadaran, P.; Ahn, B.C. CAR T-Cell-Based gene therapy for cancers: New perspectives, challenges, and clinical developments. Front. Immunol. 2022, 13, 925985. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Dudley, M.E.; Carpenter, R.O.; Kassim, S.H.; Rose, J.J.; Telford, W.G.; Hakim, F.T.; Halverson, D.C.; Fowler, D.H.; Hardy, N.M.; et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013, 122, 4129–4139. [Google Scholar] [CrossRef]
- Kalos, M.; Levine, B.L.; Porter, D.L.; Katz, S.; Grupp, S.A.; Bagg, A.; June, C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011, 3, 95ra73. [Google Scholar] [CrossRef]
- Boyiadzis, M.M.; Dhodapkar, M.V.; Brentjens, R.J.; Kochenderfer, J.N.; Neelapu, S.S.; Maus, M.V.; Porter, D.L.; Maloney, D.G.; Grupp, S.A.; Mackall, C.L.; et al. Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: Clinical perspective and significance. J. Immuno. Ther. Cancer 2018, 6, 137. [Google Scholar] [CrossRef]
- Pan, J.; Niu, Q.; Deng, B.; Liu, S.; Wu, T.; Gao, Z.; Liu, Z.; Zhang, Y.; Qu, X.; Zhang, Y.; et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 2019, 33, 2854–2866. [Google Scholar] [CrossRef]
- Li, S.; Tao, Z.; Xu, Y.; Liu, J.; An, N.; Wang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; et al. CD33-Specific Chimeric Antigen Receptor T Cells with Different Co-Stimulators Showed Potent Anti-Leukemia Efficacy and Different Phenotype. Hum. Gene Ther. 2018, 29, 626–639. [Google Scholar] [CrossRef]
- Roddie, C.; Lekakis, L.J.; Marzolini, M.A.V.; Ramakrishnan, A.; Zhang, Y.; Hu, Y.; Peddareddigari, V.G.R.; Khokhar, N.; Chen, R.; Basilico, S.; et al. Dual targeting of CD19 and CD22 with bicistronic CAR-T cells in patients with relapsed/refractory large B-cell lymphoma. Blood 2023, 141, 2470–2482. [Google Scholar] [CrossRef] [PubMed]
- Jetani, H.; Navarro-Bailón, A.; Maucher, M.; Frenz, S.; Verbruggen, C.; Yeguas, A.; Vidriales, M.B.; González, M.; Rial Saborido, J.; Kraus, S.; et al. Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia. Blood 2021, 138, 1830–1842. [Google Scholar] [CrossRef]
- López, C.; Burkhardt, B.; Chan, J.K.C.; Leoncini, L.; Mbulaiteye, S.M.; Ogwang, M.D.; Orem, J.; Rochford, R.; Roschewski, M.; Siebert, R. Burkitt lymphoma. Nat. Rev. Dis. Primers 2022, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, Z.; Jia, H.; Tong, C.; Guo, Y.; Ti, D.; Han, X.; Liu, Y.; Zhang, W.; Wang, C.; et al. Correction to: Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J. Hematol. Oncol. 2020, 13, 53. [Google Scholar] [CrossRef]
- Albinger, N.; Pfeifer, R.; Nitsche, M.; Mertlitz, S.; Campe, J.; Stein, K.; Kreyenberg, H.; Schubert, R.; Quadflieg, M.; Schneider, D.; et al. Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J. 2022, 12, 61. [Google Scholar] [CrossRef]
- Schubert, M.L.; Dreger, P. CD22 CAR T-cell therapy: New hope for patients with large B-cell lymphoma. Lancet 2024, 404, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef]
- Gray, M.A.; Stanczak, M.A.; Mantuano, N.R.; Xiao, H.; Pijnenborg, J.F.A.; Malaker, S.A.; Miller, C.L.; Weidenbacher, P.A.; Tanzo, J.T.; Ahn, G.; et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 2020, 16, 1376–1384. [Google Scholar] [CrossRef]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef]
- Schweizer, A.; Wöhner, M.; Prescher, H.; Brossmer, R.; Nitschke, L. Targeting of CD22-positive B-cell lymphoma cells by synthetic divalent sialic acid analogues. Eur. J. Immunol. 2012, 42, 2792–2802. [Google Scholar] [CrossRef]
- Büll, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; van der Vlag, J.; Heise, T.; et al. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell-Mediated Tumor Immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Balneger, N.; Cornelissen, L.A.M.; Wassink, M.; Moons, S.J.; Boltje, T.J.; Bar-Ephraim, Y.E.; Das, K.K.; Søndergaard, J.N.; Büll, C.; Adema, G.J. Sialic acid blockade in dendritic cells enhances CD8(+) T cell responses by facilitating high-avidity interactions. Cell. Mol. Life Sci. 2022, 79, 98. [Google Scholar] [CrossRef]
- Chen, W.C.; Completo, G.C.; Sigal, D.S.; Crocker, P.R.; Saven, A.; Paulson, J.C. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010, 115, 4778–4786. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sui, D.; Liu, M.; Su, Y.; Wang, Y.; Liu, M.; Luo, X.; Liu, X.; Deng, Y.; Song, Y. Sialic acid conjugate-modified liposomes enable tumor homing of epirubicin via neutrophil/monocyte infiltration for tumor therapy. Acta Biomater. 2021, 134, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Boltje, T.J.; van Dinther, E.A.; Peters, T.; de Graaf, A.M.; Leusen, J.H.; Kreutz, M.; Figdor, C.G.; den Brok, M.H.; Adema, G.J. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Nycholat, C.M.; Macauley, M.S. Therapeutic Targeting of Siglecs using Antibody- and Glycan-Based Approaches. Trends Pharmacol. Sci. 2015, 36, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Sigal, D.S.; Saven, A.; Paulson, J.C. Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22. Leuk. Lymphoma 2012, 53, 208–210. [Google Scholar] [CrossRef]
- Nycholat, C.M.; Duan, S.; Knuplez, E.; Worth, C.; Elich, M.; Yao, A.; O’Sullivan, J.; McBride, R.; Wei, Y.; Fernandes, S.M.; et al. A Sulfonamide Sialoside Analogue for Targeting Siglec-8 and -F on Immune Cells. J. Am. Chem. Soc. 2019, 141, 14032–14037. [Google Scholar] [CrossRef]
- Macauley, M.S.; Pfrengle, F.; Rademacher, C.; Nycholat, C.M.; Gale, A.J.; von Drygalski, A.; Paulson, J.C. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J. Clin. Investig. 2013, 123, 3074–3083. [Google Scholar] [CrossRef] [PubMed]



| Source of Immune Cell | Immune Cells Involved | Siglecs Involved | Effect on Tumor Progression/Antitumor Immunity | Key References |
|---|---|---|---|---|
| myeloid cell | Tumor-associated Macrophages (TAMs) | Siglec-E, Siglec-1, Siglec-10, Siglec-9, Siglec-11, Siglec-3, Siglec-16, Siglec-15 | Polarization into pro-tumorigenic M2- like macrophages, inhibition of phagocytosis (‘don’t eat me signal’) | [92,93,94,95] |
| myeloid cell | Tumor-associated Neutrophils (TANs) | Siglec-9 (Siglec-E) | Inhibition of ROS production | [92] |
| myeloid cell | Dendritic cells | Siglec-E, Siglec-G, Siglec-7, Siglec-9, Siglec-10, Siglec-15 | Inhibition of antigen presentation | [96,97,98] |
| Lymphocyte cell | Tumor-infiltrating T cells | Siglec-7, Siglec-9, Siglec-5, Siglec-14 | Inhibition of T cell activation and | [99] |
| Lymphocyte cell | NK cells | Siglec-7, Siglec-9 | Inhibition of NK cell-mediated tumor cell killing | [9,100,101,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gao, Z.; Zhang, Y.; Ai, S.; Li, W.; Sun, L. Dysregulated Sialylation in Cancer: From Immunosuppressive Microenvironment to Siglec-Targeted Therapeutics. Biomolecules 2025, 15, 1375. https://doi.org/10.3390/biom15101375
Zhang Y, Gao Z, Zhang Y, Ai S, Li W, Sun L. Dysregulated Sialylation in Cancer: From Immunosuppressive Microenvironment to Siglec-Targeted Therapeutics. Biomolecules. 2025; 15(10):1375. https://doi.org/10.3390/biom15101375
Chicago/Turabian StyleZhang, Yuecheng, Zhengyao Gao, Yuhan Zhang, Siqin Ai, Wenyan Li, and Lingbo Sun. 2025. "Dysregulated Sialylation in Cancer: From Immunosuppressive Microenvironment to Siglec-Targeted Therapeutics" Biomolecules 15, no. 10: 1375. https://doi.org/10.3390/biom15101375
APA StyleZhang, Y., Gao, Z., Zhang, Y., Ai, S., Li, W., & Sun, L. (2025). Dysregulated Sialylation in Cancer: From Immunosuppressive Microenvironment to Siglec-Targeted Therapeutics. Biomolecules, 15(10), 1375. https://doi.org/10.3390/biom15101375






