ET-1, MMPs, ZAG, and APN Link Reduced Ocular Perfusion to Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Examinations
2.4. Glaucoma Staging
2.5. Peripheral Blood and Intraocular Samples
2.6. Protein Quantification
2.7. Statistical Analysis
- (1)
- Selection of variables: Constant variables, variables with less than 66% data, and attributed variables with less than seven samples per category were discarded.
- (2)
- Data imputation: For model selection the data were imputed by means of the MICE algorithm [34]. After computing the pseudo-R2 matrix of all independent variables, we applied hierarchical clustering to separate these variables to k = max (3, (n − 50)/8) clusters. To avoid overfitting, only one variable per cluster was used to predict missing values for each independent variable.
- (3)
- Selection of covariates: We used 100 times of fivefold cross-validation of Lasso variable selection and discarded the variables which were selected in fewer than 5% of runs. Finally, using the median penalty weight factor lambda, the variables were selected by a concluding Lasso process using glmnet [35,36] from the complete data. For the Lasso approach, we allowed a slightly overfitted model (n ≥ 35 + 6.5∙m, with sample size m and model size m) to reduce the risk of missing important variables.
- (4)
- Model reduction: Since the Lasso approach does not account for p-values but selects variables to maximize a weighted sum of log-likelihood and model penalty, we performed iterative backward selection to discard the most insignificant variable of the multivariable model, and thus fulfill the predefined model size condition (n ≥ 50 + 8∙m) [37]. Model size limitation is important to avoid overfitting of models, which reduces the statistical power and increases risk of finding random correlations.
- (5)
- Application of the model to original data: Finally, the model resulting from step 4 was applied on the original, non-imputed data. The most insignificant variable was iteratively dropped until the model size condition was fulfilled on these data.
3. Results
3.1. Participants
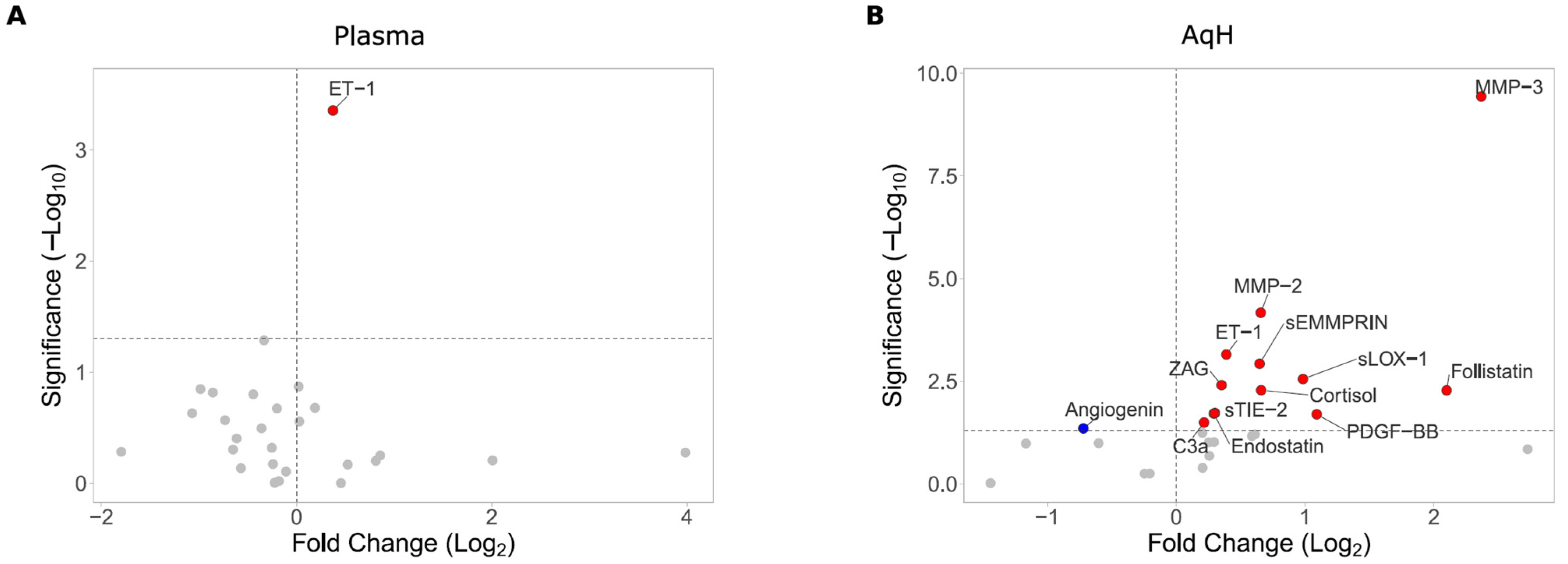
3.2. Peripheral and Intraocular Cytokine Profile
3.3. Correlation of Peripheral and Intraocular Cytokines with Clinical Parameters
3.4. Multivariable Analysis (MVA)
4. Discussion
4.1. ET-1
4.2. Matrix Metalloproteinases
4.3. Additional Significant Biomarkers
4.4. Metabolic Regulators: APN, ZAG, and Resistin
4.5. Plasma Biomarkers and Systemic Involvement
4.6. Integration of Biomarkers into Clinical Practice
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACG | angle-closure glaucoma |
| AI | acircularity index |
| APN | adiponectin |
| AqH | aqueous humor |
| DVP | deep vascular plexus |
| ET-1 | endothelin-1 |
| FAZ | foveal avascular zone |
| GCC | ganglion cell complex |
| GLV | global loss volume |
| ILM | internal limiting membrane |
| IOP | intraocular pressure |
| IPL | inner plexiform layer |
| IR | insulin resistance |
| MAP | mean arterial pressure |
| MVA | multivariable analysis |
| NO | nitric oxide |
| NTG | normal-tension glaucoma |
| OCT | optical coherence tomography |
| OCT-A | optical coherence tomography angiography |
| ONH | optic nerve head |
| OP | ocular perfusion |
| OPL | outer plexiform layer |
| PG | pigmentary glaucoma |
| PGA | prostaglandin analogous |
| POAG | primary open-angle glaucoma |
| RGC | retinal ganglion cell |
| RNFL | retinal nerve fiber layer thickness |
| SVP | superficial vascular plexus |
| VCDR | vertical cup-to-disc ratio |
| VD | vessel density |
| XFG | pseudoexfoliation glaucoma |
References
- Vajaranant, T.S.; Wu, S.; Torres, M.; Varma, R. The Changing Face of Primary Open-Angle Glaucoma in the United States: Demographic and Geographic Changes from 2011 to 2050. Am. J. Ophthalmol. 2012, 154, 303–314.e3. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Kardon, R.H. A Framework for Comparing Structural and Functional Measures of Glaucomatous Damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Mansouri, K.; Weinreb, R.N. The Structure and Function Relationship in Glaucoma: Implications for Detection of Progression and Measurement of Rates of Change. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive Loss of Retinal Ganglion Cell Function Precedes Structural Loss by Several Years in Glaucoma Suspects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef]
- Gardiner, S.K.; Swanson, W.H.; Mansberger, S.L. Long-and Short-Term Variability of Perimetry in Glaucoma. Transl. Vis. Sci. Technol. 2022, 11, 3. [Google Scholar] [CrossRef]
- Guedes, V.; Schuman, J.S.; Hertzmark, E.; Wollstein, G.; Correnti, A.; Mancini, R.; Lederer, D.; Voskanian, S.; Velazquez, L.; Pakter, H.M.; et al. Optical Coherence Tomography Measurement of Macular and Nerve Fiber Layer Thickness in Normal and Glaucomatous Human Eyes. Ophthalmology 2003, 110, 177–189. [Google Scholar] [CrossRef]
- Mwanza, J.-C.; Kim, H.Y.; Budenz, D.L.; Warren, J.L.; Margolis, M.; Lawrence, S.D.; Jani, P.D.; Thompson, G.S.; Lee, R.K. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6344–6351. [Google Scholar] [CrossRef]
- Haefliger, I.O.; Flammer, J.; Lüscher, T.F. Nitric Oxide and Endothelin-1 Are Important Regulators of Human Ophthalmic Artery. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2340–2343. [Google Scholar]
- Wey, S.; Amanullah, S.; Spaeth, G.L.; Ustaoglu, M.; Rahmatnejad, K.; Katz, L.J. Is Primary Open-Angle Glaucoma an Ocular Manifestation of Systemic Disease? Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2019, 257, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Morrison, J.C.; Tokayer, J.; Tan, O.; Lombardi, L.; Baumann, B.; Lu, C.D.; Choi, W.; Fujimoto, J.G.; Huang, D. Quantitative OCT Angiography of Optic Nerve Head Blood Flow. Biomed. Opt. Express 2012, 3, 3127–3137. [Google Scholar] [CrossRef] [PubMed]
- Lommatzsch, C.; Rothaus, K.; Schopmeyer, L.; Feldmann, M.; Bauer, D.; Grisanti, S.; Heinz, C.; Kasper, M. Elevated Endothelin-1 Levels as Risk Factor for an Impaired Ocular Blood Flow Measured by OCT-A in Glaucoma. Sci. Rep. 2022, 12, 11801. [Google Scholar] [CrossRef]
- Lommatzsch, C.; Rothaus, K.; Koch, J.M.; Heinz, C.; Grisanti, S. OCTA Vessel Density Changes in the Macular Zone in Glaucomatous Eyes. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2018, 256, 1499–1508. [Google Scholar] [CrossRef]
- Rao, H.L.; Pradhan, Z.S.; Weinreb, R.N.; Riyazuddin, M.; Dasari, S.; Venugopal, J.P.; Puttaiah, N.K.; Rao, D.A.S.; Devi, S.; Mansouri, K.; et al. A Comparison of the Diagnostic Ability of Vessel Density and Structural Measurements of Optical Coherence Tomography in Primary Open Angle Glaucoma. PLoS ONE 2017, 12, e0173930. [Google Scholar] [CrossRef]
- Lommatzsch, C.; Rothaus, K.; Koch, J.M.; Heinz, C.; Grisanti, S. Vessel Density in OCT Angiography Permits Differentiation between Normal and Glaucomatous Optic Nerve Heads. Int. J. Ophthalmol. 2018, 11, 835–843. [Google Scholar] [CrossRef]
- Emre, M.; Orgül, S.; Haufschild, T.; Shaw, S.G.; Flammer, J. Increased Plasma Endothelin-1 Levels in Patients with Progressive Open Angle Glaucoma. Br. J. Ophthalmol. 2005, 89, 60–63. [Google Scholar] [CrossRef]
- Cellini, M.; Strobbe, E.; Gizzi, C.; Balducci, N.; Toschi, P.G.; Campos, E.C. Endothelin-1 Plasma Levels and Vascular Endothelial Dysfunction in Primary Open Angle Glaucoma. Life Sci. 2012, 91, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Cellini, M.; Strobbe, E.; Gizzi, C.; Campos, E.C. ET-1 Plasma Levels and Ocular Blood Flow in Retinitis Pigmentosa. Can. J. Physiol. Pharmacol. 2010, 88, 630–635. [Google Scholar] [CrossRef]
- Khuu, L.-A.; Tayyari, F.; Sivak, J.M.; Flanagan, J.G.; Singer, S.; Brent, M.H.; Huang, D.; Tan, O.; Hudson, C. Aqueous Humor Endothelin-1 and Total Retinal Blood Flow in Patients with Non-Proliferative Diabetic Retinopathy. Eye 2017, 31, 1443–1450. [Google Scholar] [CrossRef]
- Koukoula, S.C.; Katsanos, A.; Tentes, I.K.; Labiris, G.; Kozobolis, V.P. Retrobulbar Hemodynamics and Aqueous Humor Levels of Endothelin-1 in Exfoliation Syndrome and Exfoliation Glaucoma. Clin. Ophthalmol. 2018, 12, 1199–1204. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Li, J.; Chan, W.F.; Tripathi, B.J. Aqueous Humor in Glaucomatous Eyes Contains an Increased Level of TGF-Beta 2. Exp. Eye Res. 1994, 59, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Nga, A.D.C.; Yap, S.-L.; Samsudin, A.; Abdul-Rahman, P.S.; Hashim, O.H.; Mimiwati, Z. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in the Aqueous Humour of Patients with Primary Angle Closure Glaucoma—A Quantitative Study. BMC Ophthalmol. 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Lommatzsch, J.; Küchle, M.; Konstas, A.G.P.; Naumann, G.O.H. Matrix Metalloproteinases and Their Inhibitors in Aqueous Humor of Patients with Pseudoexfoliation Syndrome/Glaucoma and Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1117–1125. [Google Scholar] [CrossRef]
- Faiq, M.A.; Sengupta, T.; Nath, M.; Velpandian, T.; Saluja, D.; Dada, R.; Dada, T.; Chan, K.C. Ocular Manifestations of Central Insulin Resistance. Neural Regen. Res. 2023, 18, 1139–1146. [Google Scholar] [CrossRef]
- Faiq, M.A.; Sofi, R.A. A Glimpse into the Mysteries of Glaucoma: From Theories to Clinics. Oman J. Ophthalmol. 2019, 12, 1–3. [Google Scholar] [CrossRef]
- Faiq, M.A.; Dada, R.; Saluja, D.; Dada, T. Glaucoma–Diabetes of the Brain: A Radical Hypothesis about Its Nature and Pathogenesis. Med. Hypotheses 2014, 82, 535–546. [Google Scholar] [CrossRef]
- Faiq, M.A.; Dada, T. Diabetes Type 4: A Paradigm Shift in the Understanding of Glaucoma, the Brain Specific Diabetes and the Candidature of Insulin as a Therapeutic Agent. Curr. Mol. Med. 2017, 17, 46–59. [Google Scholar] [CrossRef]
- Munk, M.R.; Turgut, F.; Faes, L.; Jaggi, D.; Freund, K.B.; Sadda, S.R.; Peto, T.; Wang, R.K.; Pircher, M.; Curcio, C.A.; et al. Standardization of OCT Angiography Nomenclature in Retinal Vascular Diseases: Consensus-Based Recommendations. Ophthalmol. Retina 2025, 9, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Brusini, P. OCT Glaucoma Staging System: A New Method for Retinal Nerve Fiber Layer Damage Classification Using Spectral-Domain OCT. Eye 2018, 32, 113–119. [Google Scholar] [CrossRef]
- McFadden, D. Conditional Logit Analysis of Qualitative Choice Behavior. In Frontiers in Econometrics; Academic Press: New York, NY, USA, 1974; pp. 105–142. ISBN 0-12-776150-0. [Google Scholar]
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Tay, J.K.; Narasimhan, B.; Hastie, T. Elastic Net Regularization Paths for All Generalized Linear Models. J. Stat. Softw. 2023, 106, 1–31. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Grieshaber, M.C.; Flammer, J. Does the Blood-Brain Barrier Play a Role in Glaucoma? Surv. Ophthalmol. 2007, 52 (Suppl. S2), S115–S121. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, Y.-L.; Zheng, Y.; Biswal, M.; Zhao, P.-Q.; Wang, Z.-Y. Analyzing Cytokines as Biomarkers to Evaluate Severity of Glaucoma. Int. J. Ophthalmol. 2017, 10, 925–930. [Google Scholar] [CrossRef]
- Khalef, N.; Labib, H.; Helmy, H.; El Hamid, M.A.; Moemen, L.; Fahmy, I. Levels of Cytokines in the Aqueous Humor of Eyes with Primary Open Angle Glaucoma, Pseudoexfoliation Glaucoma and Cataract. Electron. Physician 2017, 9, 3833–3837. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, M.C.; Mozaffarieh, M.; Flammer, J. What Is the Link between Vascular Dysregulation and Glaucoma? Surv. Ophthalmol. 2007, 52 (Suppl. S2), S144–S154. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.A.; Elewa, A.M.; Arafa, L.F. Endothelin-1 and Nitric Oxide Levels in Patients with Glaucoma. Ophthalmic Res. 2011, 46, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Yorio, T.; Krishnamoorthy, R.; Prasanna, G. Endothelin: Is It a Contributor to Glaucoma Pathophysiology? J. Glaucoma 2002, 11, 259–270. [Google Scholar] [CrossRef]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The Nitric Oxide-Guanylate Cyclase Pathway and Glaucoma. Nitric Oxide Biol. Chem. 2018, 77, 75–87. [Google Scholar] [CrossRef]
- Shoshani, Y.Z.; Harris, A.; Shoja, M.M.; Rusia, D.; Siesky, B.; Arieli, Y.; Wirostko, B. Endothelin and Its Suspected Role in the Pathogenesis and Possible Treatment of Glaucoma. Curr. Eye Res. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef]
- Neufeld, A.H.; Sawada, A.; Becker, B. Inhibition of Nitric-Oxide Synthase 2 by Aminoguanidine Provides Neuroprotection of Retinal Ganglion Cells in a Rat Model of Chronic Glaucoma. Proc. Natl. Acad. Sci. USA 1999, 96, 9944–9948. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.R.; Rao, V.R.; Dauphin, R.; Prasanna, G.; Johnson, C.; Yorio, T. Role of the ETB Receptor in Retinal Ganglion Cell Death in Glaucoma. Can. J. Physiol. Pharmacol. 2008, 86, 380–393. [Google Scholar] [CrossRef]
- Huet, E.; Vallée, B.; Delbé, J.; Mourah, S.; Prulière-Escabasse, V.; Tremouilleres, M.; Kadomatsu, K.; Doan, S.; Baudouin, C.; Menashi, S.; et al. EMMPRIN Modulates Epithelial Barrier Function through a MMP-Mediated Occludin Cleavage: Implications in Dry Eye Disease. Am. J. Pathol. 2011, 179, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lim, S.-H. Matrix Metalloproteinases and Glaucoma. Biomolecules 2022, 12, 1368. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Moss, S.E.; Alexander, R.A.; Ali, R.R.; Fitzke, F.W.; Cordeiro, M.F. Retinal Ganglion Cell Apoptosis in Glaucoma Is Related to Intraocular Pressure and IOP-Induced Effects on Extracellular Matrix. Investig. Ophthalmol. Vis. Sci. 2005, 46, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Robinson, M.R.; Dibas, M.; Stamer, W.D. Matrix Metalloproteinases and Glaucoma Treatment. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2020, 36, 208–228. [Google Scholar] [CrossRef]
- Määttä, M.; Tervahartiala, T.; Harju, M.; Airaksinen, J.; Autio-Harmainen, H.; Sorsa, T. Matrix Metalloproteinases and Their Tissue Inhibitors in Aqueous Humor of Patients with Primary Open-Angle Glaucoma, Exfoliation Syndrome, and Exfoliation Glaucoma. J. Glaucoma 2005, 14, 64–69. [Google Scholar] [CrossRef]
- Molière, S.; Jaulin, A.; Tomasetto, C.-L.; Dali-Youcef, N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. Int. J. Mol. Sci. 2023, 24, 10649. [Google Scholar] [CrossRef]
- Inatani, M.; Tanihara, H.; Katsuta, H.; Honjo, M.; Kido, N.; Honda, Y. Transforming Growth Factor-Beta 2 Levels in Aqueous Humor of Glaucomatous Eyes. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 2001, 239, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.M.; Benz, C.; Clark, A.F.; Wordinger, R.J. The Effects of Transforming Growth Factor-Β2 on the Expression of Follistatin and Activin A in Normal and Glaucomatous Human Trabecular Meshwork Cells and Tissues. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7358–7369. [Google Scholar] [CrossRef]
- Wordinger, R.J.; Sharma, T.; Clark, A.F. The Role of TGF-Β2 and Bone Morphogenetic Proteins in the Trabecular Meshwork and Glaucoma. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2014, 30, 154–162. [Google Scholar] [CrossRef]
- O’Reilly, M.S.; Boehm, T.; Shing, Y.; Fukai, N.; Vasios, G.; Lane, W.S.; Flynn, E.; Birkhead, J.R.; Olsen, B.R.; Folkman, J. Endostatin: An Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell 1997, 88, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Mekada, A.; Sasahara, M.; Yamada, E.; Kani, K.; Hazama, F. Platelet-Derived Growth Factor B-Chain Expression in the Rat Retina and Optic Nerve: Distribution and Changes after Transection of the Optic Nerve. Vis. Res. 1998, 38, 3031–3039. [Google Scholar] [CrossRef]
- Lorbeer, R.; Baumeister, S.E.; Dörr, M.; Nauck, M.; Grotevendt, A.; Völzke, H.; Vasan, R.S.; Wallaschofski, H.; Lieb, W. Circulating Angiopoietin-2, Its Soluble Receptor Tie-2, and Mortality in the General Population. Eur. J. Heart Fail. 2013, 15, 1327–1334. [Google Scholar] [CrossRef]
- Guo, X.; Xiang, Y.; Yang, H.; Yu, L.; Peng, X.; Guo, R. Association of the LOX-1 Rs1050283 Polymorphism with Risk for Atherosclerotic Cerebral Infarction and Its Effect on sLOX-1 and LOX-1 Expression in a Chinese Population. J. Atheroscler. Thromb. 2017, 24, 572–582. [Google Scholar] [CrossRef]
- Kott, K.A.; Genetzakis, E.; Gray, M.P.; Hansen, P.; McGuire, H.M.; Yang, J.Y.; Grieve, S.M.; Vernon, S.T.; Figtree, G.A. Serum Soluble Lectin-like Oxidized Low-Density Lipoprotein Receptor-1 (sLOX-1) Is Associated with Atherosclerosis Severity in Coronary Artery Disease. Biomolecules 2023, 13, 1187. [Google Scholar] [CrossRef]
- Hubens, W.H.G.; Beckers, H.J.M.; Gorgels, T.G.M.F.; Webers, C.A.B. Increased Ratios of Complement Factors C3a to C3 in Aqueous Humor and Serum Mark Glaucoma Progression. Exp. Eye Res. 2021, 204, 108460. [Google Scholar] [CrossRef] [PubMed]
- Rozsíval, P.; Hampl, R.; Obenberger, J.; Stárka, L.; Rehák, S. Aqueous Humour and Plasma Cortisol Levels in Glaucoma and Cataract Patients. Curr. Eye Res. 1981, 1, 391–396. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Guo, B.; Li, Y.; Jin, X.; Liu, S.; Miao, C. Nitric Oxide/Cyclic GMP Pathway Mediates the Endothelin-1-Upregulation of Adiponectin Expression in Rat Cardiomyocytes. Biomed. Rep. 2017, 7, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liang, Z.; Liu, R.; Li, K.; Tan, X.; Luo, Y.; Yang, M.; Gu, H.F.; Liu, H.; Li, L.; et al. Effects of Sitagliptin on Circulating Zinc-A2-Glycoprotein Levels in Newly Diagnosed Type 2 Diabetes Patients: A Randomized Trial. Eur. J. Endocrinol. 2016, 174, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Górna, I.; Przysławski, J. Zinc and the Innovative Zinc-A2-Glycoprotein Adipokine Play an Important Role in Lipid Metabolism: A Critical Review. Nutrients 2021, 13, 2023. [Google Scholar] [CrossRef]
- Spörri, L.; Uldry, A.-C.; Kreuzer, M.; Herzog, E.L.; Zinkernagel, M.S.; Unterlauft, J.D.; Zysset-Burri, D.C. Exploring the Ocular Surface Microbiome and Tear Proteome in Glaucoma. Int. J. Mol. Sci. 2024, 25, 6257. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.; George, T. Circulating Resistin Levels in Relation with Insulin Resistance, Inflammatory and Endothelial Dysfunction Markers in Patients with Type 2 Diabetes and Impaired Fasting Glucose. Endocr. Metab. Sci. 2020, 1, 100059. [Google Scholar] [CrossRef]
- Bauer, D.; Kasper, M.; Walscheid, K.; Koch, J.M.; Müther, P.S.; Kirchhof, B.; Heiligenhaus, A.; Heinz, C. Alteration of MCP-1 and MMP-9 in Aqueous Humor Is Associated with Secondary Glaucoma in Fuchs Uveitis Syndrome. Ocul. Immunol. Inflamm. 2020, 28, 688–698. [Google Scholar] [CrossRef]
- Weisser, B.; Erb, C. Neuroprotective Effects of Anti-Diabetic Drugs in the Treatment of Patients with Diabetes and Glaucoma or at High Risk for Glaucoma. Klin. Monatsbl. Augenheilkd. 2024, 241, 302–307. [Google Scholar] [CrossRef]
- Bojikian, K.D.; Chen, C.L.; Wen, J.C.; Zhang, Q.; Xin, C.; Gupta, D.; Mudumbai, R.C.; Johnstone, M.A.; Wang, R.K.; Chen, P.P. Optic Disc Perfusion in Primary Open Angle and Normal Tension Glaucoma Eyes Using Optical Coherence Tomography-Based Microangiography. PLoS ONE 2016, 11, e0154691. [Google Scholar] [CrossRef]
| Control | Glaucoma | Adj. p-Value | ||
|---|---|---|---|---|
| Clinical Data | ||||
| Glaucoma Entity (n) | POAG:0| XFG:0| NTG:0| PG:0| ACG:0 | POAG:63| XFG:11| NTG:6| PG:2| ACG:3| NA:2 | N.A. | |
| 1st Diagnosis of Glaucoma (Mo) | N.A. | 62.00 [27.50–120.00] | N.A. | |
| Age at Operation | 66.77 ± 9.01 | 67.00 [57.00–73.00] | 0.8395 | 2 |
| Sex (n) | Female:20| Male:10 | Female:48| Male:39 | 0.4622 | 1 |
| IOP (mmHg) | 14.00 [12.00–14.00] | 18.00 [15.00–23.50] | <0.00001 * | 3 |
| Refractive Error (D) | 0.07 ± 2.88 | 0.00 [−1.50–0.75] | 0.3477 | 3 |
| Visual Acuity (LogMAR) | 0.40 [0.30–0.60] | 0.20 [0.02–0.30] | <0.00001 * | 3 |
| MAP (mmHg) | 95.00 [92.50–99.50] | 95.00 [91.67–101.17] | 0.88884 | 3 |
| Topical Antiglaucoma | 0 [0–0] | 3.00 [1.00–3.50] | N.A. | |
| Beta-blocker, n (%) | (0%) | 53 (60.9%) | N.A. | |
| Carbonic Anhydrase Inhibitor, n (%) | (0%) | 58 (66.7%) | N.A. | |
| Prostaglandin Analog. n (%) | (0%) | 64 (73.6%) | N.A. | |
| Alpha-adrenergic Agonist, n (%) | (0%) | 28 (32.2%) | N.A. | |
| Pilocarpine, n (%) | (0%) | 2 (2.3%) | N.A. | |
| Systemic Carbonic Anhydrase Inhibitor, n (%) | (0%) | 6 (8.6%) | N.A. | |
| OCT and OCT-A Parameters | ||||
| Ganglion Cell Complex (µm) | 96.55 ± 8.41 | 77.00 [69.25–86.00] | <0.00001 * | 3 |
| Focal Loss Volume (%) | 0.41 [0.21–0.80] | 5.48 [2.17–9.12] | <0.00001 * | 3 |
| Global Loss Volume (%) | 2.10 [0.50–4.25] | 17.97 ± 10.73 | <0.00001 * | 3 |
| RNFL Thickness (µm) | 96.79 ± 8.72 | 74.32 ± 13.87 | <0.00001 * | 2 |
| Cup/Disc Ratio Total | 0.31 ± 0.15 | 0.65 [0.55–0.76] | <0.00001 * | 3 |
| Rim Area (mm2) | 1.37 ± 0.35 | 0.73 [0.52–0.96] | <0.00001 * | 3 |
| Disc Area (mm2) | 2.03 ± 0.32 | 2.08 ± 0.36 | 0.562 | 2 |
| VD ONH Whole (%) | 47.00 ± 2.52 | 35.97 ± 6.86 | <0.00001 * | 3 |
| VD Macula SVP Whole (%) | 41.90 ± 4.15 | 37.39 ± 4.40 | <0.00001 * | 2 |
| VD Fovea SVP (%) | 19.99 ± 6.69 | 17.85 [11.22–21.95] | 0.128 | 3 |
| VD Macula DVP Whole (%) | 39.96 ± 4.37 | 41.79 ± 4.75 | 0.124 | 2 |
| VD Fovea DVP (%) | 34.28 ± 8.49 | 32.39 ± 8.08 | 0.380 | 2 |
| FAZ (mm2) | 0.26 ± 0.10 | 0.25 [0.19–0.35] | 0.549 | 3 |
| Dependent Variables | Group | n | Residual Deviance | Null Deviance | Pseudo R2 | Independent Variables |
|---|---|---|---|---|---|---|
| VD PeriONH Average | Glaucoma | 63 | 1590.6 | 4603.1 | 0.65 | RNFL (+) APN AqH (−) VCDR (−) |
| All | 86 | 1593.8 | 7799.1 | 0.80 | RNFL (+) APN AqH (−) ZAG Plasma (+) VCDR (−) MMP-2 AqH (−) Topical Beta-Blocker (−) | |
| OCT GSS Score | Glaucoma | 68 | 136.88 | 205.78 | 0.33 | Ganglion Cell Complex (−) Topical Beta-Blocker (+) |
| All | 94 | 79.707 | 319.29 | 0.75 | Global Loss Volume (+) Age at Operation (−) MICB plasma (−) Topical Beta-Blocker (+) Pseudophakia (+) MMP-9 Plasma (+) | |
| RNFL Thickness | Glaucoma | 69 | 3053.2 | 13,412.6 | 0.77 | Ganglion Cell Complex (+) sE-Selectin Plasma (+) sTIE-2 AqH (−) |
| All | 93 | 4524.6 | 23,651.2 | 0.81 | Global Loss Volume (−) Ganglion Cell Complex (+) sE-Selectin Plasma (+) Group MMP-3 AqH | |
| Ganglion Cell Complex | Glaucoma | 69 | 3496.5 | 13,212.6 | 0.74 | RNFL (+) Endoglin AqH (+) Follistatin Plasma Total Cup/Disc Ratio |
| All | 86 | 3296.4 | 17,593.6 | 0.81 | RNFL (+) VCDR (−) Angiostatin AqH (−) ZAG AqH (+) MMP-9 Plasma (+) FAZ (−) PIGF-1 Plasma | |
| VD Macula SVP Whole | Glaucoma | 65 | 784.54 | 1179.39 | 0.33 | RNFL (+) VEGF-A Plasma (+) Rim Area |
| All | 86 | 742.19 | 1926.89 | 0.61 | Global Loss Volume (−) Rim Area (+) Sex = Male (−) FAZ-AI (−) VEGF-A Plasma APN AqH MMP-9 Plasma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasper, M.; Rothaus, K.; Schopmeyer, L.; Bauer, D.; Grisanti, S.; Heinz, C.; Loser, K.; Lommatzsch, C. ET-1, MMPs, ZAG, and APN Link Reduced Ocular Perfusion to Glaucoma. Biomolecules 2025, 15, 1364. https://doi.org/10.3390/biom15101364
Kasper M, Rothaus K, Schopmeyer L, Bauer D, Grisanti S, Heinz C, Loser K, Lommatzsch C. ET-1, MMPs, ZAG, and APN Link Reduced Ocular Perfusion to Glaucoma. Biomolecules. 2025; 15(10):1364. https://doi.org/10.3390/biom15101364
Chicago/Turabian StyleKasper, Maren, Kai Rothaus, Lasse Schopmeyer, Dirk Bauer, Swaantje Grisanti, Carsten Heinz, Karin Loser, and Claudia Lommatzsch. 2025. "ET-1, MMPs, ZAG, and APN Link Reduced Ocular Perfusion to Glaucoma" Biomolecules 15, no. 10: 1364. https://doi.org/10.3390/biom15101364
APA StyleKasper, M., Rothaus, K., Schopmeyer, L., Bauer, D., Grisanti, S., Heinz, C., Loser, K., & Lommatzsch, C. (2025). ET-1, MMPs, ZAG, and APN Link Reduced Ocular Perfusion to Glaucoma. Biomolecules, 15(10), 1364. https://doi.org/10.3390/biom15101364







