The Effects and Mechanisms of Low-Intensity Pulsed Ultrasound on Bone Remodeling: From Laboratory to Clinic
Abstract
1. Introduction
2. Discovery and Development of Ultrasound in Bone Remodeling Disorders
3. Classification and Parameter Studies of Ultrasound
3.1. Classification of Ultrasound
3.2. Research on Ultrasound Parameters
3.2.1. Intensity
3.2.2. Frequency
3.2.3. Pulse Repetition Frequency
3.2.4. Pulse Width
3.2.5. Pulse Duty Cycle
3.2.6. Exposure Time
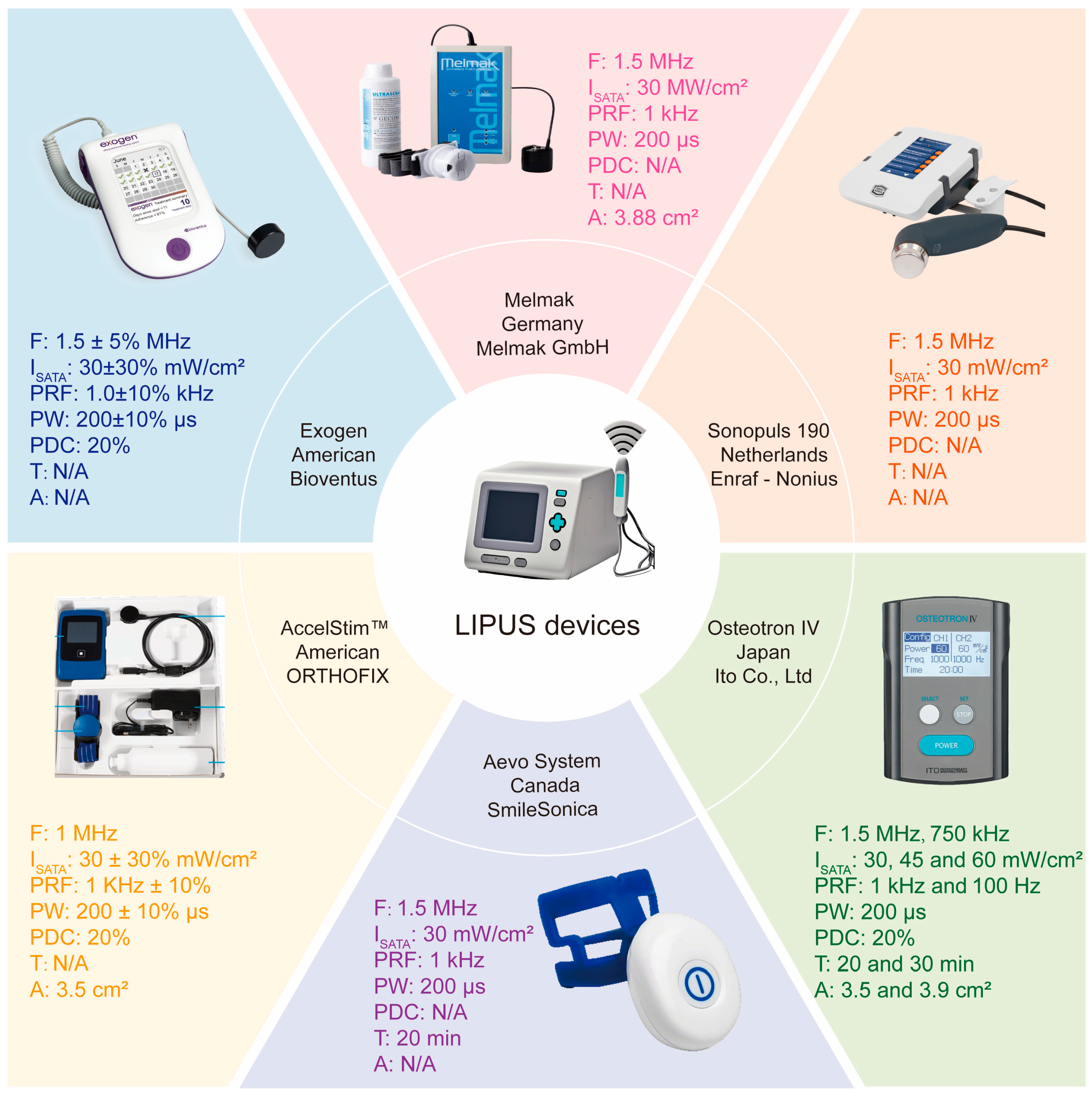
4. LIPUS Devices for Bone Remodeling Disorders
5. Clinical Applications of LIPUS in Bone Remodeling Disorders
5.1. Fractures and Nonunion
5.2. Osteoporosis
6. Laboratory Studies on LIPUS in Bone Remodeling Disorders
6.1. Animal Studies
6.1.1. Fracture and Nonunion Models
6.1.2. Osteoporosis Models
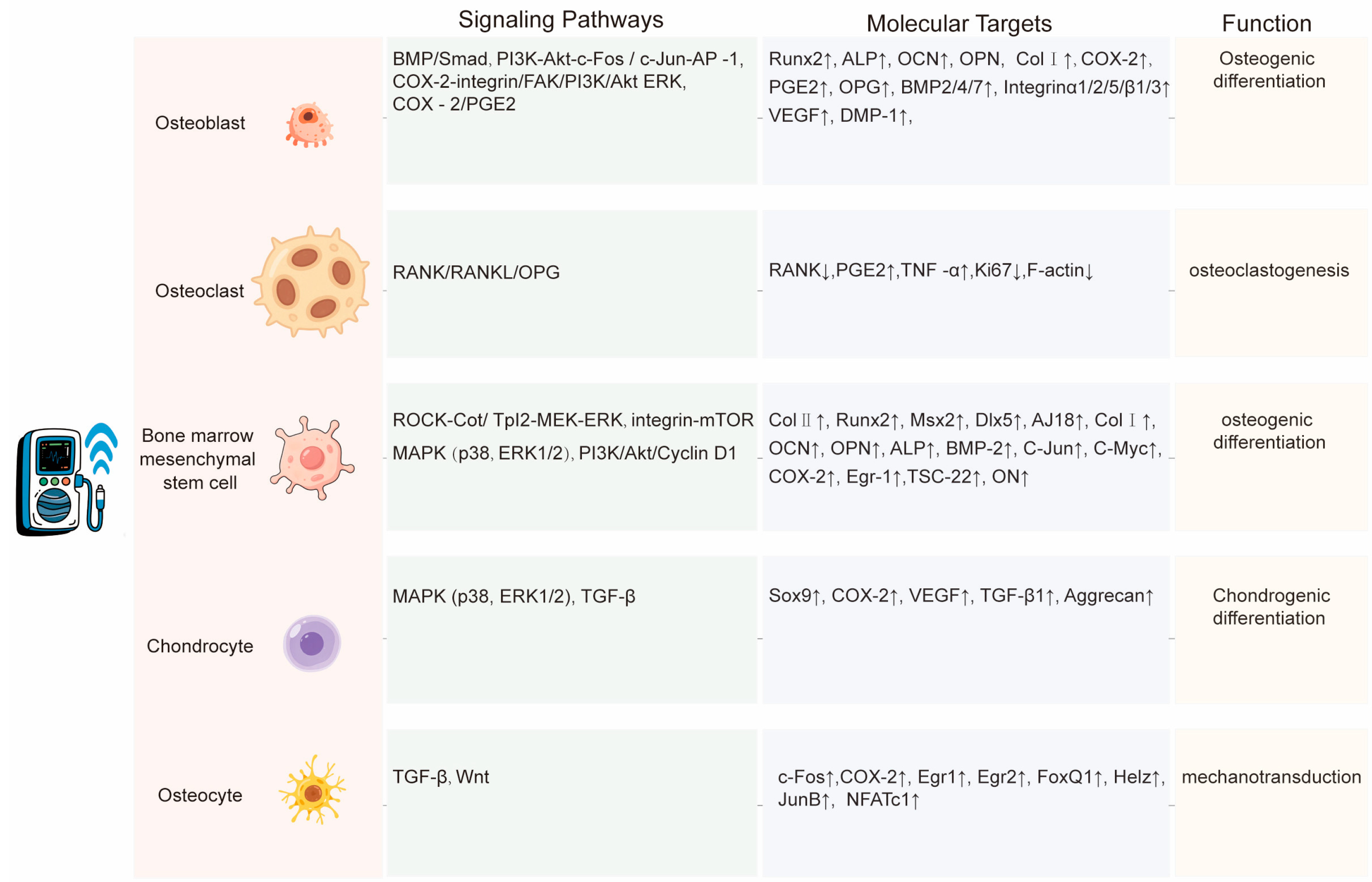
6.2. Cellular Studies
6.2.1. Osteoblasts
6.2.2. Osteoclasts
6.2.3. Bone Marrow Mesenchymal Stem Cells
6.2.4. Chondrocytes
6.2.5. Osteocytes
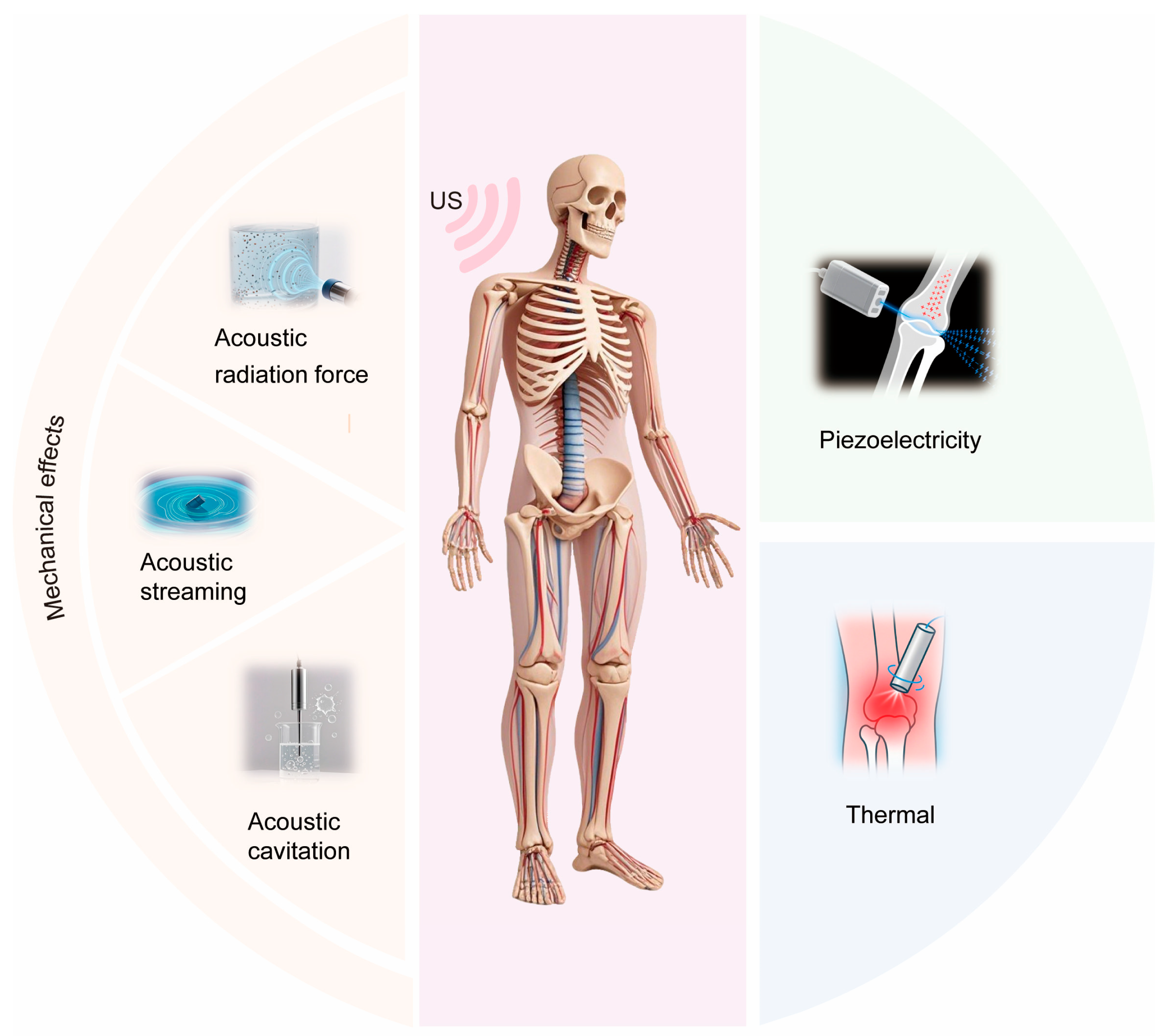
7. Physical Mechanisms of LIPUS on Bone
7.1. Mechanical Effects
7.1.1. Acoustic Radiation Force
7.1.2. Acoustic Streaming
7.1.3. Acoustic Cavitation
7.2. Piezoelectric Effect
7.3. Thermal Effects
7.4. Interactions Between LIPUS-Generated Secondary Physical Quantities and Biological Tissues
8. Conclusions and Future Perspectives
- Mechanistic Elucidation: Multi-omics platforms (transcriptomics, proteomics) should be integrated with multi-physical field modeling (e.g., finite element simulations) to establish multi-scale systems biology frameworks. These models should quantify the synergistic interplay of acoustic pressure, mechanical stress, piezoelectric potentials, and localized thermal changes induced by LIPUS. For example, 3D bone organoid systems replicating in vivo mechanical microenvironments, coupled with single-cell sequencing and validated through biomolecular experiments, could delineate dose–effect relationships between LIPUS parameters and repair outcomes.
- Technological Innovation: Artificial intelligence (AI) and advanced ultrasound engineering should be leveraged to develop precision therapeutic strategies. Machine learning-driven analysis of clinical datasets could optimize high-frequency, narrow-pulse protocols (e.g., 3 MHz, 50 μs) to mitigate thermal risks. Hybrid therapies combining LIPUS with pharmacological agents or electromagnetic fields may overcome the inherent limitations of single-modality interventions.
- Clinical Translation: The development of miniaturized transducers and energy-efficient systems should be accelerated to enable intelligent wearable devices. Integration of biosensors (e.g., impedance monitoring, optical coherence tomography) will facilitate real-time biofeedback and adaptive parameter modulation. Concurrently, telemedicine-compatible home-use devices could revolutionize chronic bone disease management, bridging the gap between laboratory innovation and community healthcare delivery.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and Molecular Regulation of Bone Remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The Cell Biology of Bone Metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Kurenkova, A.D.; Medvedeva, E.V.; Newton, P.T.; Chagin, A.S. Niches for Skeletal Stem Cells of Mesenchymal Origin. Front. Cell Dev. Biol. 2020, 8, 592. [Google Scholar] [CrossRef]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The Role of Collagen in Bone Strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive Calcium Phosphate Materials and Applications in Bone Regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M. An Overview of Bone Cells and Their Regulating Factors of Differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Le, B.; Nurcombe, V.; Cool, S.; Van Blitterswijk, C.; De Boer, J.; LaPointe, V. The Components of Bone and What They Can Teach Us about Regeneration. Materials 2017, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Vallibhakara, S.A.-O.; Nakpalat, K.; Sophonsritsuk, A.; Tantitham, C.; Vallibhakara, O. Effect of Vitamin E Supplement on Bone Turnover Markers in Postmenopausal Osteopenic Women: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2021, 13, 4226. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, J.; Qiu, M.; Zhang, L.; Yang, K.; Chang, L.; Jia, P.; Qi, J.; Deng, L.; Li, C. Osteocytic HIF-1α Pathway Manipulates Bone Micro-Structure and Remodeling via Regulating Osteocyte Terminal Differentiation. Front. Cell Dev. Biol. 2022, 9, 721561. [Google Scholar] [CrossRef]
- Papachristou, D.J.; Georgopoulos, S.; Giannoudis, P.V.; Panagiotopoulos, E. Insights into the Cellular and Molecular Mechanisms That Govern the Fracture-Healing Process: A Narrative Review. J. Clin. Med. 2021, 10, 3554. [Google Scholar] [CrossRef] [PubMed]
- Bianco Prevot, L.; Nannini, A.; Mangiavini, L.; Bobba, A.; Buzzi, S.; Sinigaglia, F.; Peretti, G. What Is the Best Treatment of the Femoral Shaft Nonunion after Intramedullary Nailing? A Systematic Review. Life 2023, 13, 1508. [Google Scholar] [CrossRef]
- Kanis, J.A.; Kanis, J.A. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Synopsis of a WHO Report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef]
- Phromnoi, K.; Yodkeeree, S.; Pintha, K.; Mapoung, S.; Suttajit, M.; Saenjum, C.; Dejkriengkraikul, P. Anti-Osteoporosis Effect of Perilla Frutescens Leaf Hexane Fraction through Regulating Osteoclast and Osteoblast Differentiation. Molecules 2022, 27, 824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Zhu, D.; Cao, Z.; Liu, Y.; Sun, J.; Tan, L. Primary Cilia Respond to Intermittent Low-Magnitude, High-Frequency Vibration and Mediate Vibration-Induced Effects in Osteoblasts. Am. J. Physiol.-Cell Physiol. 2020, 318, C73–C82. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Ullah, M.; Concepcion, W.; Dahl, J.J.; Thakor, A.S. The Role of Ultrasound in Enhancing Mesenchymal Stromal Cell-Based Therapies. Stem Cells Transl. Med. 2020, 9, 850–866. [Google Scholar] [CrossRef]
- Ferreri, S.L.; Talish, R.; Trandafir, T.; Qin, Y.-X. Mitigation of Bone Loss with Ultrasound Induced Dynamic Mechanical Signals in an OVX Induced Rat Model of Osteopenia. Bone 2011, 48, 1095–1102. [Google Scholar] [CrossRef][Green Version]
- Miyasaka, M.; Nakata, H.; Hao, J.; Kim, Y.-K.; Kasugai, S.; Kuroda, S. Low-Intensity Pulsed Ultrasound Stimulation Enhances Heat-Shock Protein 90 and Mineralized Nodule Formation in Mouse Calvaria-Derived Osteoblasts. Tissue Eng. Part A 2015, 21, 2829–2839. [Google Scholar] [CrossRef]
- Yang, R.-S.; Lin, W.-L.; Chen, Y.-Z.; Tang, C.-H.; Huang, T.-H.; Lu, B.-Y.; Fu, W.-M. Regulation by Ultrasound Treatment on the Integrin Expression and Differentiation of Osteoblasts. Bone 2005, 36, 276–283. [Google Scholar] [CrossRef]
- An, Y.; Song, Y.; Wang, Z.; Wang, J.; Wu, G.; Zhu, G.; Chen, L. Effect of Low-Intensity Pulsed Ultrasound on the Biological Behaviors of Bone Marrow Mesenchymal Stem Cells on Titanium with Different Surface Topographies. Am. J. Transl. Res. 2018, 10, 67–76. [Google Scholar] [PubMed]
- Siebenmorgen, C.; Poortinga, A.; Van Rijn, P. Sono-Processes: Emerging Systems and Their Applicability within the (Bio-)Medical Field. Ultrason. Sonochem. 2023, 100, 106630. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Gich, M.; Carretero-Genevrier, A.; Puig, T.; Obradors, X. Piezo-Generated Charge Mapping Revealed through Direct Piezoelectric Force Microscopy. Nat. Commun. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Iula, A. Ultrasound Systems for Biometric Recognition. Sensors 2019, 19, 2317. [Google Scholar] [CrossRef]
- Harvey, E.N.; Loomis, A.L. The Destruction of Luminous Bacteria by High Frequency Sound Waves. J. Bacteriol. 1929, 17, 373–376. [Google Scholar] [CrossRef]
- Pohlman, R.; Richter, R.; Parow, E. Über die Ausbreitung und Absorption des Ultraschalls im menschlichen Gewebe und seine therapeutische Wirkung an Ischias und Plexusneuralgie. Dtsch. Med Wochenschr. 1939, 65, 251–254. [Google Scholar] [CrossRef]
- Kang, T.; Horton, L.; Emery, P.; Wakefield, R.J. Value of Ultrasound in Rheumatologic Diseases. J. Korean Med. Sci. 2013, 28, 497. [Google Scholar] [CrossRef]
- Barth, G.; Bülow, H. Zur Frage Der Ultraschallschädigung Jugendlicher Knochen. Strahlentherapie 1949, 79, 98. [Google Scholar]
- Maintz, G. Animal Experiments in the Study of the Effect of Ultrasonic Waves on Bone Regeneration. Strahlentherapie 1950, 82, 631–638. [Google Scholar] [PubMed]
- Corradi, C.; Cozzolino, A. Effect of Ultrasonics on the Development of Osseous Callus in Fractures. Arch. Ortop. 1953, 66, 77–98. [Google Scholar]
- Ardan, N.I.; Janes, J.M.; Herrick, J.F. Ultrasonic Energy and Surgically Produced Defects in Bone. J. Bone Jt. Surg. Am. 1957, 39, 394–402. [Google Scholar] [CrossRef]
- Shiro, I. Study on the Ultrasonic Irradiation in Orthopedic Surgery. Hirosaki Med. 1964, 16, 242–253. [Google Scholar]
- Xavier, C.; Duarte, L. Estimulaca Ultra-Sonica de Calo Osseo: Applicaca Clinica. Rev. Bras. Ortop. 1983, 18, 73–80. [Google Scholar]
- Heckman, J.D.; Ryaby, J.P.; McCabe, J.; Frey, J.J.; Kilcoyne, R.F. Acceleration of Tibial Fracture-Healing by Non-Invasive, Low-Intensity Pulsed Ultrasound. J. Bone Jt. Surg. 1994, 76, 26–34. [Google Scholar] [CrossRef]
- Rubin, C.; Bolander, M.; Ryaby, J.P.; Hadjiargyrou, M. The Use of Low-Intensity Ultrasound to Accelerate the Healing of Fractures. J. Bone Jt. Surg.-Am. Vol. 2001, 83, 259–270. [Google Scholar] [CrossRef]
- Kristiansen, T.K.; Ryaby, J.P.; McCABE, J.; Frey, J.J.; Roe, L.R. Accelerated Healing of Distal Radial Fractures with the Use of Specific, Low-Intensity Ultrasound. J. Bone Jt. Surg. 1997, 79, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.; Glover, M.; Yang, Y.; Bayliss, S.; Meads, C.; Lord, J. EXOGEN Ultrasound Bone Healing System for Long Bone Fractures with Non-Union or Delayed Healing: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2014, 12, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Poolman, R.W.; Agoritsas, T.; Siemieniuk, R.A.C.; Harris, I.A.; Schipper, I.B.; Mollon, B.; Smith, M.; Albin, A.; Nador, S.; Sasges, W.; et al. Low Intensity Pulsed Ultrasound (LIPUS) for Bone Healing: A Clinical Practice Guideline. BMJ 2017, 356, j576. [Google Scholar] [CrossRef]
- Huber, M.; Prantl, L.; Gehmert, S. Successful Treatment of Nonunion in Severe Finger Injury with Low-Intensity Pulsed Ultrasound (LIPUS): A Case Report. J. Med. Case Rep. 2012, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Lu, Y.; Zhang, H.; Kang, Y.; Mu, B.; Qian, Y.; Wang, A. Scale-up Disaggregation of Palygorskite Crystal Bundles via Ultrasonic Process for Using as Potential Drilling Fluid. Ultrason. Sonochem. 2022, 89, 106128. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, R.; Tang, P.; Sun, Y.; Johnson, C.; Saredy, J.; Wu, S.; Wang, J.; Lu, Y.; Saaoud, F.; et al. 37-Ultrasound May Suppress Tumor Growth, Inhibit Inflammation, and Establish Tolerogenesis by Remodeling Innatome via Pathways of ROS, Immune Checkpoints, Cytokines, and Trained Immunity/Tolerance. J. Immunol. Res. 2021, 2021, 6664453. [Google Scholar] [CrossRef]
- Xin, Z.; Lin, G.; Lei, H.; Lue, T.F.; Guo, Y. Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Potential Role in Urology. Transl. Androl. Urol. 2016, 5, 255–266. [Google Scholar] [CrossRef]
- Ahmadi, F.; McLoughlin, I.V.; Chauhan, S.; ter-Haar, G. Bio-Effects and Safety of Low-Intensity, Low-Frequency Ultrasonic Exposure. Prog. Biophys. Mol. Biol. 2012, 108, 119–138. [Google Scholar] [CrossRef]
- Lu, H.; Qin, L.; Lee, K.; Cheung, W.; Chan, K.; Leung, K. Identification of Genes Responsive to Low-Intensity Pulsed Ultrasound Stimulations. Biochem. Biophys. Res. Commun. 2009, 378, 569–573. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of Bone Repair with Ultrasound: A Review of the Possible Mechanic Effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Y.; Zhou, J.; Li, J.; Deng, F.; Wang, Z.; Song, J. Low-Intensity Pulsed Ultrasound Stimulation Facilitates Osteogenic Differentiation of Human Periodontal Ligament Cells. PLoS ONE 2014, 9, e95168. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, P.; Chen, G.; Sha, Y.; Yang, L. Effects of Low-Intensity Pulsed Ultrasound on Cell Viability, Proliferation and Neural Differentiation of Induced Pluripotent Stem Cells-Derived Neural Crest Stem Cells. Biotechnol. Lett. 2013, 35, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Reher, P.; Harris, M.; Whiteman, M.; Hai, H.K.; Meghji, S. Ultrasound Stimulates Nitric Oxide and Prostaglandin e 2 Production by Human Osteoblasts. Bone 2002, 31, 236–241. [Google Scholar] [CrossRef]
- Nakamura, T.; Fujihara, S.; Yamamoto-Nagata, K.; Katsura, T.; Inubushi, T.; Tanaka, E. Low-Intensity Pulsed Ultrasound Reduces the Inflammatory Activity of Synovitis. Ann. Biomed. Eng. 2011, 39, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.-H.; Leung, K.-S.; Cheung, W.-H. Ultrasound as a Stimulus for Musculoskeletal Disorders. J. Orthop. Transl. 2017, 9, 52–59. [Google Scholar] [CrossRef]
- Li, J.G.-R.; Chang, W.H.-S.; Lin, J.C.-A.; Sun, J.-S. Optimum Intensities of Ultrasound for Pge 2 Secretion and Growth of Osteoblasts. Ultrasound Med. Biol. 2002, 28, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.; Ter Haar, G. Recommendations for Reporting Therapeutic Ultrasound Treatment Parameters. Ultrasound Med. Biol. 2022, 48, 1299–1308. [Google Scholar] [CrossRef]
- Martin, E.; Aubry, J.-F.; Schafer, M.; Verhagen, L.; Treeby, B.; Pauly, K.B. ITRUSST Consensus on Standardised Reporting for Transcranial Ultrasound Stimulation. Brain Stimul. 2024, 17, 607–615. [Google Scholar] [CrossRef]
- Lyon, R.; Liu, X.C.; Meier, J. The Effects of Therapeutic vs. High-intensity Ultrasound on the Rabbit Growth Plate. J. Orthop. Res. 2003, 21, 865–871. [Google Scholar] [CrossRef]
- Dalla-Bona, D.A.; Tanaka, E.; Inubushi, T.; Oka, H.; Ohta, A.; Okada, H.; Miyauchi, M.; Takata, T.; Tanne, K. Cementoblast Response to Low- and High-Intensity Ultrasound. Arch. Oral Biol. 2008, 53, 318–323. [Google Scholar] [CrossRef]
- Sun, S.; Sun, L.; Kang, Y.; Tang, L.; Qin, Y.-X.; Ta, D. Therapeutic Effects of Low-Intensity Pulsed Ultrasound on Osteoporosis in Ovariectomized Rats: Intensity-Dependent Study. Ultrasound Med. Biol. 2020, 46, 108–121. [Google Scholar] [CrossRef]
- Janes, J.M.; Kelly, P.J.; Herrick, J.F.; Peterson, L.F. The Effect of High-Dosage Ultrasonic Energy on Femora of the Dog: A Roentgenographic, Histological, and Microangiographic Study. J. Bone Jt. Surg. Am. 1962, 44, 1299–1307. [Google Scholar] [CrossRef]
- Spadaro, J.A.; Albanese, S.A. Application of Low-Intensity Ultrasound to Growing Bone in Rats. Ultrasound Med. Biol. 1998, 24, 567–573. [Google Scholar] [CrossRef]
- Warden, S.J.; Bennell, K.L.; Forwood, M.R.; McMeeken, J.M.; Wark, J.D. Skeletal Effects of Low-Intensity Pulsed Ultrasound on the Ovariectomized Rodent. Ultrasound Med. Biol. 2001, 27, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, S.; Chen, Y.; Liu, C.; Li, D.; Cheng, Q.; He, M.; Li, Y.; Xu, K.; Ta, D. Pulsed Frequency Modulated Ultrasound Promotes Therapeutic Effects of Osteoporosis Induced by Ovarian Failure in Mice. Ultrasonics 2023, 132, 106973. [Google Scholar] [CrossRef]
- Stoffels, I.; Morscher, S.; Helfrich, I.; Hillen, U.; Leyh, J.; Burton, N.C.; Sardella, T.C.P.; Claussen, J.; Poeppel, T.D.; Bachmann, H.S.; et al. Metastatic Status of Sentinel Lymph Nodes in Melanoma Determined Noninvasively with Multispectral Optoacoustic Imaging. Sci. Transl. Med. 2015, 7, 317ra199. [Google Scholar] [CrossRef]
- Ford, S.J.; Bigliardi, P.L.; Sardella, T.C.P.; Urich, A.; Burton, N.C.; Kacprowicz, M.; Bigliardi, M.; Olivo, M.; Razansky, D. Structural and Functional Analysis of Intact Hair Follicles and Pilosebaceous Units by Volumetric Multispectral Optoacoustic Tomography. J. Investig. Dermatol. 2016, 136, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Needles, A.; Heinmiller, A.; Sun, J.; Theodoropoulos, C.; Bates, D.; Hirson, D.; Yin, M.; Foster, F.S. Development and Initial Application of a Fully Integrated Photoacoustic Micro-Ultrasound System. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2013, 60, 888–897. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Nicoletti, G.; Rossano, F.; D’Andrea, F. Histologic Effects of External Ultrasound-Assisted Lipectomy on Adipose Tissue. Aesth Plast. Surg. 2008, 32, 111–115. [Google Scholar] [CrossRef]
- Moy, W.J.; Su, E.; Chen, J.J.; Oh, C.; Jing, J.C.; Qu, Y.; He, Y.; Chen, Z.; Wong, B.J.F. Association of Electrochemical Therapy With Optical, Mechanical, and Acoustic Impedance Properties of Porcine Skin. JAMA Facial Plast. Surg. 2017, 19, 502–509. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karri, R.R.; Koduru, J.R.; Manickam, S.; Tyagi, I.; Mubarak, N.M. Suhas Recent Trends in the Applications of Sonochemical Reactors as an Advanced Oxidation Process for the Remediation of Microbial Hazards Associated with Water and Wastewater: A Critical Review. Ultrason. Sonochem. 2023, 94, 106302. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.M.Z.; Cheng, J.; Lin, W.; Qin, Y.-X. Low-Intensity Amplitude Modulated Ultrasound Increases Osteoblastic Mineralization. Cell. Mol. Bioeng. 2011, 4, 81–90. [Google Scholar] [CrossRef]
- Tassinary, J.A.F.; Lunardelli, A.; Basso, B.D.S.; Dias, H.B.; Catarina, A.V.; Stülp, S.; Haute, G.V.; Martha, B.A.; Melo, D.A.D.S.; Nunes, F.B.; et al. Low-Intensity Pulsed Ultrasound (LIPUS) Stimulates Mineralization of MC3T3-E1 Cells through Calcium and Phosphate Uptake. Ultrasonics 2018, 84, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Costa Alvarenga, É.; Rodrigues, R.; Caricati-Neto, A.; Silva-Filho, F.C.; Paredes-Gamero, E.J.; Ferreira, A.T. Low-Intensity Pulsed Ultrasound-Dependent Osteoblast Proliferation Occurs by via Activation of the P2Y Receptor: Role of the P2Y1 Receptor. Bone 2010, 46, 355–362. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Guo, S.; He, Y.; Bai, G.; Zhang, W. Low-Intensity Pulsed Ultrasound Stimulation Facilitates in Vitro Osteogenic Differentiation of Human Adipose-Derived Stem Cells via up-Regulation of Heat Shock Protein (HSP)70, HSP90, and Bone Morphogenetic Protein (BMP) Signaling Pathway. Biosci. Rep. 2018, 38, BSR20180087. [Google Scholar] [CrossRef]
- Samuel, S.; Miller, D.L.; Fowlkes, J.B. The Relationship of Acoustic Emission and Pulse-Repetition Frequency in the Detection of Gas Body Stability and Cell Death. Ultrasound Med. Biol. 2006, 32, 439–447. [Google Scholar] [CrossRef]
- Monici, M.; Bernabei, P.A.; Basile, V.; Romano, G.; Conti, A.; Breschi, L.; Masotti, L.; Cogoli, A. Can Ultrasound Counteract Bone Loss? Effect of Low-Intensity Ultrasound Stimulation on a Model of Osteoclastic Precursor. Acta Astronaut. 2007, 60, 383–390. [Google Scholar] [CrossRef]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S. Bioeffects Committee of the American Institute of Ultrasound in Medicine Overview of Therapeutic Ultrasound Applications and Safety Considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef]
- O’Brien, W.D. Ultrasound–Biophysics Mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. [Google Scholar] [CrossRef]
- Ter Haar, G.R. Therapeutic and Surgical Applications. In Physical Principles of Medical Ultrasonics; Hill, C.R., Bamber, J.C., Ter Haar, G.R., Eds.; Wiley: Hoboken, NJ, USA, 2004; pp. 407–456. ISBN 978-0-471-97002-6. [Google Scholar]
- Samuel, S.; Fowlkes, J.; Miller, D. An in Vitro Study of the Correlation between Bubble Distribution, Acoustic Emission, and Cell Damage by Contrast Ultrasound. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2009, 56, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.F.; Fontes-Pereira, A.J.; De Albuquerque Pereira, W.C. Influence of Low-Intensity Pulsed Ultrasound Parameters on the Bone Mineral Density in Rat Model: A Systematic Review. Ultrasound Med. Biol. 2023, 49, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, J.; Chen, X.; Huang, B.; Lu, D.; Zhang, T. Mediation of Long-Pulsed Ultrasound Enhanced Microbubble Recombinant Tissue Plasminogen Activator Thrombolysis in a Rat Model of Platelet-Rich Thrombus. Cardiovasc. Diagn. Ther. 2024, 14, 51–58. [Google Scholar] [CrossRef]
- Nakahara, R.; Ito, A.; Nagai-Tanima, M.; Tai, C.; Zhao, Z.; Xu, S.; Miyamoto, F.; Abiko, S.; Aoyama, T.; Kuroki, H. Effects of Different Low-Intensity Pulsed Ultrasound Intensities and Durations on a Post-Traumatic Knee Joint Contracture Model in Rats. Ultrasound Med. Biol. 2025, 51, 396–401. [Google Scholar] [CrossRef]
- Warden, S.J.; Fuchs, R.K.; Kessler, C.K.; Avin, K.G.; Cardinal, R.E.; Stewart, R.L. Ultrasound Produced by a Conventional Therapeutic Ultrasound Unit Accelerates Fracture Repair. Phys. Ther. 2006, 86, 1118–1127. [Google Scholar] [CrossRef]
- Zhou, S.; Bachem, M.G.; Seufferlein, T.; Li, Y.; Gross, H.J.; Schmelz, A. Low Intensity Pulsed Ultrasound Accelerates Macrophage Phagocytosis by a Pathway That Requires Actin Polymerization, Rho, and Src/MAPKs Activity. Cell. Signal. 2008, 20, 695–704. [Google Scholar] [CrossRef]
- Nandi, T.; Kop, B.R.; Naftchi-Ardebili, K.; Stagg, C.J.; Pauly, K.B.; Verhagen, L. Biophysical Effects and Neuromodulatory Dose of Transcranial Ultrasonic Stimulation. Brain Stimul. 2025, 18, 659–664. [Google Scholar] [CrossRef]
- Quarato, C.M.I.; Lacedonia, D.; Salvemini, M.; Tuccari, G.; Mastrodonato, G.; Villani, R.; Fiore, L.A.; Scioscia, G.; Mirijello, A.; Saponara, A.; et al. A Review on Biological Effects of Ultrasounds: Key Messages for Clinicians. Diagnostics 2023, 13, 855. [Google Scholar] [CrossRef]
- Liu, J.; Ren, W.; Wang, S.; Yang, J.; Zhang, H.; Zeng, Y.; Yin, D.; Shang, P. The Effects and Mechanisms of Electromagnetic Fields on Bone Remodeling: From Clinical to Laboratory. J. Orthop. Transl. 2025, 52, 14–26. [Google Scholar] [CrossRef]
- Haglin, J.M.; Jain, S.; Eltorai, A.E.M.; Daniels, A.H. Bone Growth Stimulation: A Critical Analysis Review. JBJS Rev. 2017, 5, e8. [Google Scholar] [CrossRef]
- Jiang, X.; Savchenko, O.; Li, Y.; Qi, S.; Yang, T.; Zhang, W.; Chen, J. A Review of Low-Intensity Pulsed Ultrasound for Therapeutic Applications. IEEE Trans. Biomed. Eng. 2019, 66, 2704–2718. [Google Scholar] [CrossRef]
- Santana-Rodríguez, N.; Clavo, B.; Llontop, P.; Fiuza, M.D.; Calatayud-Gastardi, J.; López, D.; López-Fernández, D.; Aguiar-Santana, I.A.; Ayub, A.; Alshehri, K.; et al. Pulsed Ultrasounds Reduce Pain and Disability, Increasing Rib Fracture Healing, in a Randomized Controlled Trial. Pain Med. 2019, 20, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E.; Rudzki, M.-M.; Rudzki, M.; Borchardt, B.; Häusser, H.; Rüter, A. Beschleunigt niedrig intensiver, gepulster Ultraschall die Heilung von Skaphoidfrakturen? Handchir. Mikrochir. Plast. Chir. 2000, 32, 115–122. [Google Scholar] [CrossRef]
- Schofer, M.D.; Block, J.E.; Aigner, J.; Schmelz, A. Improved Healing Response in Delayed Unions of the Tibia with Low-Intensity Pulsed Ultrasound: Results of a Randomized Sham-Controlled Trial. BMC Musculoskelet. Disord. 2010, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.-S.; Lee, W.-S.; Tsui, H.-F.; Liu, P.P.-L.; Cheung, W.-H. Complex Tibial Fracture Outcomes Following Treatment with Low-Intensity Pulsed Ultrasound. Ultrasound Med. Biol. 2004, 30, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Handolin, L.; Kiljunen, V.; Arnala, I.; Pajarinen, J.; Partio, E.K.; Rokkanen, P. The Effect of Low Intensity Ultrasound and Bioabsorbable Self-Reinforced Poly-L-Lactide Screw Fixation on Bone in Lateral Malleolar Fractures. Arch. Orthop. Trauma. Surg. 2005, 125, 317–321. [Google Scholar] [CrossRef]
- Rutten, S.; Nolte, P.A.; Korstjens, C.M.; Van Duin, M.A.; Klein-Nulend, J. Low-Intensity Pulsed Ultrasound Increases Bone Volume, Osteoid Thickness and Mineral Apposition Rate in the Area of Fracture Healing in Patients with a Delayed Union of the Osteotomized Fibula. Bone 2008, 43, 348–354. [Google Scholar] [CrossRef]
- Urita, A.; Iwasaki, N.; Kondo, M.; Nishio, Y.; Kamishima, T.; Minami, A. Effect of Low-Intensity Pulsed Ultrasound on Bone Healing at Osteotomy Sites After Forearm Bone Shortening. J. Hand Surg. 2013, 38, 498–503. [Google Scholar] [CrossRef]
- Arimoto, S.; Hasegawa, T.; Takeda, D.; Tateishi, C.; Akashi, M.; Furudoi, S.; Komori, T. Effect of Low-Intensity Pulsed Ultrasound after Intraoral Vertical Ramus Osteotomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Korstjens, C.M.; Rutten, S.; Nolte, P.A.; van Duin, M.A.; Klein-Nulend, J. Low-Intensity Pulsed Ultrasound Increases Blood Vessel Size during Fracture Healing in Patients with a Delayed-Union of the Osteotomized Fibula. Histol. Histopathol. 2018, 33, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Nolte, P.A.; Van Der Krans, A.; Patka, P.; Janssen, I.M.C.; Ryaby, J.P.; Albers, G.H.R. Low-Intensity Pulsed Ultrasound in the Treatment of Nonunions. J. Trauma Acute Care Surg. 2001, 51, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Dudda, M.; Hauser, J.; Muhr, G.; Esenwein, S.A. Low-Intensity Pulsed Ultrasound as a Useful Adjuvant During Distraction Osteogenesis: A Prospective, Randomized Controlled Trial. J. Trauma Acute Care Surg. 2011, 71, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.H.; Schmelz, A. Low-Intensity Pulsed Ultrasound Shortens the Treatment Time in Tibial Distraction Osteogenesis. Int. Orthop. (SICOT) 2014, 38, 1477–1482. [Google Scholar] [CrossRef][Green Version]
- Song, M.H.; Kim, T.-J.; Kang, S.H.; Song, H.-R. Low-Intensity Pulsed Ultrasound Enhances Callus Consolidation in Distraction Osteogenesis of the Tibia by the Technique of Lengthening over the Nail Procedure. BMC Musculoskelet. Disord. 2019, 20, 108. [Google Scholar] [CrossRef]
- Gan, T.Y.; Kuah, D.E.; Graham, K.S.; Markson, G. Low-Intensity Pulsed Ultrasound in Lower Limb Bone Stress Injuries: A Randomized Controlled Trial. Clin. J. Sport. Med. 2014, 24, 457–460. [Google Scholar] [CrossRef]
- Zacherl, M.; Gruber, G.; Radl, R.; Rehak, P.H.; Windhager, R. No Midterm Benefit from Low Intensity Pulsed Ultrasound after Chevron Osteotomy for Hallux Valgus. Ultrasound Med. Biol. 2009, 35, 1290–1297. [Google Scholar] [CrossRef]
- White, N.J.; Patterson, E.D.; Dhaliwal, G.S.; Hildebrand, K.A. WECAN Low-Intensity Pulsed Ultrasound Versus Sham in the Treatment of Operatively Managed Scaphoid Nonunions: The SNAPU Randomized Controlled Trial. J. Bone Jt. Surg. 2024, 106, 1573–1582. [Google Scholar] [CrossRef]
- Leung, K.-S.; Lee, W.-S.; Cheung, W.-H.; Qin, L. Lack of Efficacy of Low-Intensity Pulsed Ultrasound on Prevention of Postmenopausal Bone Loss Evaluated at the Distal Radius in Older Chinese Women. Clin. Orthop. Relat. Res. 2004, 427, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Lv, H.; Li, Z.; Zhang, L.; Tang, P. The Effects of Low-Intensity Pulsed Ultrasound on Fresh Fracture: A Meta-Analysis. Medicine 2017, 96, e8181. [Google Scholar] [CrossRef]
- Zura, R.; Mehta, S.; Rocca, G.J.D.; Jones, J.; Steen, R.G. A Cohort Study of 4,190 Patients Treated with Low-Intensity Pulsed Ultrasound (LIPUS): Findings in the Elderly versus All Patients. BMC Musculoskelet. Disord. 2015, 16, 45. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.C.; Pacchiana, P.D. Common Complications of Fracture Repair. Clin. Tech. Small Anim. Pract. 2004, 19, 168–179. [Google Scholar] [CrossRef]
- Borole, A.; Trubiano, J.; Viqueira, M.; Kalahasti, K.; Stamos, T.; Kirschenbaum, D.; Katt, B.M. Intrinsic and Extrinsic Methods for Augmenting Fracture Healing in the Hand and Wrist. Cureus 2024, 16, e74972. [Google Scholar] [CrossRef]
- Wilson, H.M. Modulation of Macrophages by Biophysical Cues in Health and Beyond. Discov. Immunol. 2023, 2, kyad013. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.H.R.W.; Keenan, G.; Nayagam, S.; Atkins, R.M.; Marsh, D.; Clement, N.D. Low-Intensity Pulsed Ultrasound Does Not Influence Bone Healing by Distraction Osteogenesis: A Multicentre Double-Blind Randomised Control Trial. Bone Jt. J. 2017, 99, 494–502. [Google Scholar] [CrossRef]
- Ali, Y.; Samaneh, R. Therapeutic Applications of Low-Intensity Pulsed Ultrasound in Osteoporosis. Asian J. Pharm. 2017, 11, S1–S6. [Google Scholar]
- Leung, K.S.; Cheung, W.H.; Zhang, C.; Lee, K.M.; Lo, H.K. Low Intensity Pulsed Ultrasound Stimulates Osteogenic Activity of Human Periosteal Cells. Clin. Orthop. Relat. Res. 2004, 418, 253–259. [Google Scholar] [CrossRef]
- Sugaya, H.; Yoshioka, T.; Tomaru, Y.; Kumagai, H.; Yamazaki, M.; Mishima, H. An Exploratory Clinical Trial for Concentrated Autologous Bone Marrow Aspirate Transplantation in the Treatment of Osteonecrosis of the Femoral Head. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 441–447. [Google Scholar] [CrossRef]
- Warden, S.J.; Bennell, K.L.; Matthews, B.; Brown, D.J.; McMeeken, J.M.; Wark, J.D. Efficacy of Low-Intensity Pulsed Ultrasound in the Prevention of Osteoporosis Following Spinal Cord Injury. Bone 2001, 29, 431–436. [Google Scholar] [CrossRef]
- Klug, W.; Franke, W.-G.; Knoch, H.-G. Scintigraphic Control of Bone-Fracture Healing under Ultrasonic Stimulation: An Animal Experimental Study. Eur. J. Nucl. Med. 1986, 11, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Heybeli, N.; Yeşildağ, A.; Oyar, O.; Gülsoy, U.K.; Tekinsoy, M.A.; Mumcu, E.F. Diagnostic Ultrasound Treatment Increases the Bone Fracture-Healing Rate in an Internally Fixed Rat Femoral Osteotomy Model. J. Ultrasound Med. 2002, 21, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, A.; Inui, K.; Azuma, Y.; Nishimura, N.; Yamano, Y. Low-Intensity Pulsed Ultrasound Accelerates Bone Maturation in Distraction Osteogenesis in Rabbits. J. Bone Jt. Surgery. Br. Vol. 2000, 82, 1077–1082. [Google Scholar] [CrossRef]
- Tis, J.E.; Meffert, R.H.; Inoue, N.; McCarthy, E.F.; Shaun Machen, M.; McHale, K.A.; Chao, E.Y.S. The Effect of Low Intensity Pulsed Ultrasound Applied to Rabbit Tibiae during the Consolidation Phase of Distraction Osteogenesis. J. Orthop. Res. 2002, 20, 793–800. [Google Scholar] [CrossRef]
- Hantes, M.E.; Mavrodontidis, A.N.; Zalavras, C.G.; Karantanas, A.H.; Karachalios, T.; Malizos, K.N. Low-Intensity Transosseous Ultrasound Accelerates Osteotomy Healing in a Sheep Fracture Model. J. Bone Jt. Surg. 2004, 86, 2275–2282. [Google Scholar] [CrossRef]
- Kieves, N.R.; Canapp, S.O.; Lotsikas, P.J.; Christopher, S.A.; Leasure, C.S.; Canapp, D.; Gavin, P.R. Effects of Low-intensity Pulsed Ultrasound on Radiographic Healing of Tibial Plateau Leveling Osteotomies in Dogs: A Prospective, Randomized, Double-blinded Study. Vet. Surg. 2018, 47, 614–622. [Google Scholar] [CrossRef]
- McClure, S.R.; Miles, K.; VanSickle, D.; South, T. The Effect of Variable Waveform Low-Intensity Pulsed Ultrasound in a Fourth Metacarpal Osteotomy Gap Model in Horses. Ultrasound Med. Biol. 2010, 36, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.-H.; Chow, S.K.; Sun, M.-H.; Qin, L.; Leung, K.-S. Low-Intensity Pulsed Ultrasound Accelerated Callus Formation, Angiogenesis and Callus Remodeling in Osteoporotic Fracture Healing. Ultrasound Med. Biol. 2011, 37, 231–238. [Google Scholar] [CrossRef]
- Naruse, K.; Sekiya, H.; Harada, Y.; Iwabuchi, S.; Kozai, Y.; Kawamata, R.; Kashima, I.; Uchida, K.; Urabe, K.; Seto, K.; et al. Prolonged Endochondral Bone Healing in Senescence Is Shortened by Low-Intensity Pulsed Ultrasound in a Manner Dependent on COX-2. Ultrasound Med. Biol. 2010, 36, 1098–1108. [Google Scholar] [CrossRef]
- Inoue, S.; Hatakeyama, J.; Aoki, H.; Kuroki, H.; Niikura, T.; Oe, K.; Fukui, T.; Kuroda, R.; Akisue, T.; Moriyama, H. Utilization of Mechanical Stress to Treat Osteoporosis: The Effects of Electrical Stimulation, Radial Extracorporeal Shock Wave, and Ultrasound on Experimental Osteoporosis in Ovariectomized Rats. Calcif. Tissue Int. 2021, 109, 215–229. [Google Scholar] [CrossRef]
- Wu, S.; Kawahara, Y.; Manabe, T.; Ogawa, K.; Matsumoto, M.; Sasaki, A.; Yuge, L. Low-Intensity Pulsed Ultrasound Accelerates Osteoblast Differentiation and Promotes Bone Formation in an Osteoporosis Rat Model. Pathobiology 2009, 76, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Tang, L.; Zhao, T.; Kang, Y.; Sun, L.; Liu, C.; Li, Y.; Xu, F.; Qin, Y.-X.; Ta, D. Longitudinal Effects of Low-Intensity Pulsed Ultrasound on Osteoporosis and Osteoporotic Bone Defect in Ovariectomized Rats. Ultrasonics 2021, 113, 106360. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Ko, C.; Seo, D.H.; Woo, D.G.; Kim, J.M.; Chun, K.J.; Kim, H.S. Low-intensity Ultrasound Stimulation Prevents Osteoporotic Bone Loss in Young Adult Ovariectomized Mice. J. Orthop. Res. 2011, 29, 116–125. [Google Scholar] [CrossRef]
- Herrick, J.F.; Janes, J.M.; Ardan, N.I. Experimental Studies Relative to the Therapeutic Use of Ultrasound. J. Am. Vet. Med. Assoc. 1956, 128, 571–577. [Google Scholar]
- Corradi, C.; Cozzolino, A. Azione Degli Ultrasuoni Sulla Evoluzione Delle Fratture Sperimentali Dei Conigli. Minerva Ortop. 1952, 3, 44–45. [Google Scholar]
- Murolo, C.; Claudio, F. Effect of ultrasonics on repair of fractures. G. Ital. Chir. 1952, 8, 897–903. [Google Scholar]
- Ying, Z.; Lin, T.; Yan, S. Low-Intensity Pulsed Ultrasound Therapy: A Potential Strategy to Stimulate Tendon-Bone Junction Healing. J. Zhejiang Univ. Sci. B 2012, 13, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.C.L.; Cliquet, A. The Action of Low-intensity Pulsed Ultrasound in Bones of Osteopenic Rats. Artif. Organs 2004, 28, 114–118. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, E.; Park, K.; Han, M.; Kim, K.; Park, J. OPEN Ultrasound Stimulation Improves Inflammatory Resolution, Neuroprotection, and Functional Recovery after Spinal Cord Injury. Sci. Rep. 2022, 12, 3636. [Google Scholar]
- Tang, L.; An, S.; Zhang, Z.; Fan, X.; Guo, J.; Sun, L.; Ta, D. MSTN Is a Key Mediator for Low-Intensity Pulsed Ultrasound Preventing Bone Loss in Hindlimb-Suspended Rats. Bone 2021, 143, 115610. [Google Scholar] [CrossRef]
- Yoshida, A.; Sasaki, H.; Furuya, Y.; Yoshinari, M.; Yajima, Y. Effect of Low-Intensity Pulsed Ultrasound on Bone-Healing Process in Murine Low-Turnover Osteoporosis Model. J. Hard Tissue Biol. 2013, 22, 301–310. [Google Scholar] [CrossRef][Green Version]
- Harrison, A.; Lin, S.; Pounder, N.; Mikuni-Takagaki, Y. Mode & Mechanism of Low Intensity Pulsed Ultrasound (LIPUS) in Fracture Repair. Ultrasonics 2016, 70, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, A. Experimental Observation of Piezoelectric Effect in Cancellous Bone Generated by Ultrasound Irradiation. J. Acoust. Soc. Am. 2016, 140, EL441–EL445. [Google Scholar] [CrossRef]
- Pinton, G.; Aubry, J.-F.; Bossy, E.; Muller, M.; Pernot, M.; Tanter, M. Attenuation, Scattering, and Absorption of Ultrasound in the Skull Bone: Absorption of Ultrasound in the Skull Bone. Med. Phys. 2011, 39, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, L.; Yan, S.; He, Y.; Zhu, H.; Li, Y.; Wang, D.; Yang, K. Low-Intensity Pulsed Ultrasound/Nanomechanical Force Generators Enhance Osteogenesis of BMSCs through Microfilaments and TRPM7. J. Nanobiotechnol. 2022, 20, 378. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-H.; Chen, S.-C.; Chiu, L.-H.; Yang, C.-B.; Tsai, Y.-H.; Zuo, C.S.; Chang, W.H.-S.; Lai, W.-F. Effects of Low-Intensity Pulsed Ultrasound, Dexamethasone/TGF-Β1 and/or BMP-2 on the Transcriptional Expression of Genes in Human Mesenchymal Stem Cells: Chondrogenic vs. Osteogenic Differentiation. Ultrasound Med. Biol. 2010, 36, 1022–1033. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, S.; Lv, H.; Wei, M.; Fang, Y.; Shang, P. Static Magnetic Field of 0.2–0.4 T Promotes the Recovery of Hindlimb Unloading-Induced Bone Loss in Mice. Int. J. Radiat. Biol. 2021, 97, 746–754. [Google Scholar] [CrossRef]
- Wang, J.; Zhen, C.; Zhang, G.; Yang, Z.; Shang, P. A 0.2 T–0.4 T Static Magnetic Field Improves the Bone Quality of Mice Subjected to Hindlimb Unloading and Reloading Through the Dual Regulation of BMSCs via Iron Metabolism. Int. J. Mol. Sci. 2024, 25, 13136. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Zhang, G.; Fang, Y.; Fang, Z.; Shang, P.; Zhang, H. Static Magnetic Field (2–4 T) Improves Bone Microstructure and Mechanical Properties by Coordinating Osteoblast/Osteoclast Differentiation in Mice. Bioelectromagnetics 2021, 42, 200–211. [Google Scholar] [CrossRef]
- Zhen, C.; Wang, S.; Yang, J.; Zhang, G.; Cai, C.; Wang, J.; Wang, A.; Xu, Y.; Fang, Y.; Wei, M.; et al. Moderate Static Magnetic Field Regulates Iron Metabolism and Salvage Bone Loss Caused by Iron Accumulation. J. Orthop. Transl. 2025, 50, 144–157. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lou, C.; Cai, C.; Ren, W.; Liu, J.; Gong, M.; Shang, P.; Zhang, H. Moderate Static Magnetic Field Promotes Fracture Healing and Regulates Iron Metabolism in Mice. BioMed Eng. OnLine 2023, 22, 107. [Google Scholar] [CrossRef]
- Zhang, G.; Zhen, C.; Yang, J.; Zhang, Z.; Wu, Y.; Che, J.; Shang, P. 1–2 T Static Magnetic Field Combined with Ferumoxytol Prevent Unloading-Induced Bone Loss by Regulating Iron Metabolism in Osteoclastogenesis. J. Orthop. Transl. 2023, 38, 126–140. [Google Scholar] [CrossRef]
- Montalibet, A.; Jossinet, J.; Matias, A.; Cathignol, D. Electric Current Generated by Ultrasonically Induced Lorentz Force in Biological Media. Med. Biol. Eng. Comput. 2001, 39, 15–20. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Liu, S.; Zhou, X.; Yin, T.; Liu, Z.; Yang, Z. Transcranial Magneto-Acoustic Stimulation Improves Neuroplasticity in Hippocampus of Parkinson’s Disease Model Mice. Neurotherapeutics 2019, 16, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Khatua, C.; Balla, V.K. In Vitro Carcinoma Treatment Using Magnetic Nanocarriers under Ultrasound and Magnetic Fields. ACS Omega 2018, 3, 5459–5469. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The Role of Osteoblasts in Energy Homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of Bone Development and Repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, Function, and Phosphorylation in Osteoblast Differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, C.; Rosen, C.J.; Sato, T.; Xu, R.; Li, P.; Wei, X.; Bi, R.; Yuan, Q.; Zhou, C. Klotho in Osx+-Mesenchymal Progenitors Exerts pro-Osteogenic and Anti-Inflammatory Effects during Mandibular Alveolar Bone Formation and Repair. Signal Transduct. Target. Ther. 2022, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Recent Advances in Osteoclast Biological Behavior. Front. Cell Dev. Biol. 2021, 9, 788680. [Google Scholar] [CrossRef]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Mostafa, S.; Pakvasa, M.; Coalson, E.; Zhu, A.; Alverdy, A.; Castillo, H.; Fan, J.; Li, A.; Feng, Y.; Wu, D.; et al. The Wonders of BMP9: From Mesenchymal Stem Cell Differentiation, Angiogenesis, Neurogenesis, Tumorigenesis, and Metabolism to Regenerative Medicine. Genes Dis. 2019, 6, 201–223. [Google Scholar] [CrossRef]
- Kodama, J.; Wilkinson, K.J.; Iwamoto, M.; Otsuru, S.; Enomoto-Iwamoto, M. The Role of Hypertrophic Chondrocytes in Regulation of the Cartilage-to-Bone Transition in Fracture Healing. Bone Rep. 2022, 17, 101616. [Google Scholar] [CrossRef]
- Warden, S.J.; Favaloro, J.M.; Bennell, K.L.; McMeeken, J.M.; Ng, K.-W.; Zajac, J.D.; Wark, J.D. Low-Intensity Pulsed Ultrasound Stimulates a Bone-Forming Response in UMR-106 Cells. Biochem. Biophys. Res. Commun. 2001, 286, 443–450. [Google Scholar] [CrossRef]
- Gleizal, A.; Li, S.; Pialat, J.-B.; Beziat, J.-L. Transcriptional Expression of Calvarial Bone after Treatment with Low-Intensity Ultrasound: An in Vitro Study. Ultrasound Med. Biol. 2006, 32, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Hou, S.; Tang, C. Ultrasound Increased BMP-2 Expression via PI3K, Akt, c-Fos/c-Jun, and AP-1 Pathways in Cultured Osteoblasts. J. Cell. Biochem. 2009, 106, 7–15. [Google Scholar] [CrossRef]
- Saito, M.; Soshi, S.; Tanaka, T.; Fujii, K. Intensity-Related Differences in Collagen Post-Translational Modification in MC3T3-E1 Osteoblasts after Exposure to Low- and High-Intensity Pulsed Ultrasound. Bone 2004, 35, 644–655. [Google Scholar] [CrossRef]
- Kuan-Jung Li, J.; Cheng-An Lin, J.; Liu, H.-C.; Sun, J.-S.; Ruaan, R.-C.; Shih, C.; Hong-Shong Chang, W. Comparison of Ultrasound and Electromagnetic Field Effects on Osteoblast Growth. Ultrasound Med. Biol. 2006, 32, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Miyauchi, A.; Itoman, M.; Mikuni-Takagaki, Y. Distinct Anabolic Response of Osteoblast to Low-Intensity Pulsed Ultrasound. J. Bone Miner. Res. 2003, 18, 360–369. [Google Scholar] [CrossRef]
- Borsje, M.A.; Ren, Y.; De Haan-Visser, H.W.; Kuijer, R. Comparison of Low-Intensity Pulsed Ultrasound and Pulsed Electromagnetic Field Treatments on OPG and RANKL Expression in Human Osteoblast-like Cells. Angle Orthod. 2010, 80, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.A.; Xie, C.; Zuscik, M.J.; Kingsley, P.; Schwarz, E.M.; Awad, H.; Guldberg, R.; Drissi, H.; Puzas, J.E.; Boyce, B.; et al. Reduced COX-2 Expression in Aged Mice Is Associated With Impaired Fracture Healing. J. Bone Miner. Res. 2009, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, T.; Matsui, N.; Fujioka, H.; Tsunoda, M.; Mizuno, K. Low Intensity Pulsed Ultrasound Exposure Increases Prostaglandin E2Production via the Induction of Cyclooxygenase-2 mRNA in Mouse Osteoblasts. Biochem. Biophys. Res. Commun. 1999, 256, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-H.; Yang, R.-S.; Huang, T.-H.; Lu, D.-Y.; Chuang, W.-J.; Huang, T.-F.; Fu, W.-M. Ultrasound Stimulates Cyclooxygenase-2 Expression and Increases Bone Formation through Integrin, Focal Adhesion Kinase, Phosphatidylinositol 3-Kinase, and Akt Pathway in Osteoblasts. Mol. Pharmacol. 2006, 69, 2047–2057. [Google Scholar] [CrossRef]
- Sun, J.-S.; Hong, R.-C.; Chang, W.H.-S.; Chen, L.-T.; Lin, F.-H.; Liu, H.-C. In Vitro Effects of Low-Intensity Ultrasound Stimulation on the Bone Cells. J. Biomed. Mater. Res. 2001, 57, 449–456. [Google Scholar] [CrossRef]
- Lu, H.; Qin, L.; Cheung, W.; Lee, K.; Wong, W.; Leung, K. Low-Intensity Pulsed Ultrasound Accelerated Bone-Tendon Junction Healing Through Regulation of Vascular Endothelial Growth Factor Expression and Cartilage Formation. Ultrasound Med. Biol. 2008, 34, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, P.; Alam, M.; Li, S.; Chow, S.K.H.; Zheng, Y. Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing: A Review. J. Ultrasound Med. 2022, 41, 547–563. [Google Scholar] [CrossRef]
- Schatten, H.; Lewis, M.L.; Chakrabarti, A. Spaceflight and Clinorotation Cause Cytoskeleton and Mitochondria Changes and Increases in Apoptosis in Cultured Cells. Acta Astronaut. 2001, 49, 399–418. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity: The Architectural Basis of Cellular Mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599. [Google Scholar] [CrossRef]
- Hughes-Fulford, M. Function of the Cytoskeleton in Gravisensing during Spaceflight. Adv. Space Res. 2003, 32, 1585–1593. [Google Scholar] [CrossRef]
- Charbord, P. Bone Marrow Mesenchymal Stem Cells: Historical Overview and Concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Li, J.; Yin, W.; An, Y.; Wang, Y.; Wang, M.; Wu, Q.; Qu, Z.; Ning, G.; et al. A Pilot Study of Parameter-Optimized Low-Intensity Pulsed Ultrasound Stimulation for the Bone Marrow Mesenchymal Stem Cells Viability Improvement. Comput. Math. Methods Med. 2019, 2019, 8386024. [Google Scholar] [CrossRef]
- Xie, S.; Jiang, X.; Wang, R.; Xie, S.; Hua, Y.; Zhou, S.; Yang, Y.; Zhang, J. Low-intensity Pulsed Ultrasound Promotes the Proliferation of Human Bone Mesenchymal Stem Cells by Activating PI3K/AKt Signaling Pathways. J. Cell. Biochem. 2019, 120, 15823–15833. [Google Scholar] [CrossRef] [PubMed]
- Aliabouzar, M.; Lee, S.; Zhou, X.; Zhang, G.L.; Sarkar, K. Effects of Scaffold Microstructure and Low Intensity Pulsed Ultrasound on Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Biotechnol. Bioeng. 2018, 115, 495–506. [Google Scholar] [CrossRef]
- Zhi, Z.; Na, T.; Jue, W.; Zhihe, Z.; Lijun, T. Effects of Pulsed Ultrasound and Pulsed Electromagnetic Field on the Extracellular Matrix Secretion of Rat Bone Marrow Mesenchymal Stem Cell Pellets in Chondrogenesis. Hua Xi Kou Qiang Yi Xue Za Zhi 2016, 34, 291–294. [Google Scholar] [CrossRef]
- Sena, K.; Leven, R.M.; Mazhar, K.; Sumner, D.R.; Virdi, A.S. Early Gene Response to Low-Intensity Pulsed Ultrasound in Rat Osteoblastic Cells. Ultrasound Med. Biol. 2005, 31, 703–708. [Google Scholar] [CrossRef]
- Sena, K.; Angle, S.R.; Kanaji, A.; Aher, C.; Karwo, D.G.; Sumner, D.R.; Virdi, A.S. Low-Intensity Pulsed Ultrasound (LIPUS) and Cell-to-Cell Communication in Bone Marrow Stromal Cells. Ultrasonics 2011, 51, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Angle, S.R.; Sena, K.; Sumner, D.R.; Virdi, A.S. Osteogenic Differentiation of Rat Bone Marrow Stromal Cells by Various Intensities of Low-Intensity Pulsed Ultrasound. Ultrasonics 2011, 51, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, X.; Qu, Y.; Lin, Q.; Cheng, K.; Gao, M.; Ren, S.; Zhang, T.; Li, X. TGF-Β1-Induced Chondrogenesis of Bone Marrow Mesenchymal Stem Cells Is Promoted by Low-Intensity Pulsed Ultrasound through the Integrin-mTOR Signaling Pathway. Stem Cell Res. Ther. 2017, 8, 281. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Q.; Zhang, T.; Wang, X.; Cheng, K.; Gao, M.; Xia, P.; Li, X. Low-Intensity Pulsed Ultrasound Promotes Chondrogenesis of Mesenchymal Stem Cells via Regulation of Autophagy. Stem Cell Res. Ther. 2019, 10, 41. [Google Scholar] [CrossRef]
- Ikeda, K.; Takayama, T.; Suzuki, N.; Shimada, K.; Otsuka, K.; Ito, K. Effects of Low-Intensity Pulsed Ultrasound on the Differentiation of C2C12 Cells. Life Sci. 2006, 79, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Kusuyama, J.; Bandow, K.; Shamoto, M.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. Low Intensity Pulsed Ultrasound (LIPUS) Influences the Multilineage Differentiation of Mesenchymal Stem and Progenitor Cell Lines through ROCK-Cot/Tpl2-MEK-ERK Signaling Pathway. J. Biol. Chem. 2014, 289, 10330–10344. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Cai, Y.; Li, X.; Bai, M.; Sun, T.; Du, L. Ultrasound Irradiation Combined with Hepatocyte Growth Factor Accelerate the Hepatic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Ultrasound Med. Biol. 2018, 44, 1044–1052. [Google Scholar] [CrossRef]
- Naruse, K.; Mikuni-Takagaki, Y.; Azuma, Y.; Ito, M.; Oota, T.; Kameyama, K.; Itoman, M. Anabolic Response of Mouse Bone-Marrow-Derived Stromal Cell Clone ST2 Cells to Low-Intensity Pulsed Ultrasound. Biochem. Biophys. Res. Commun. 2000, 268, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Wiltink, A.; Nijweide, P.J.; Hekkenberg, R.T.; Helders, P.J.M. Effect of Therapeutic Ultrasound on Endochondral Ossification. Ultrasound Med. Biol. 1995, 21, 121–127. [Google Scholar] [CrossRef]
- Mukai, S.; Ito, H.; Nakagawa, Y.; Akiyama, H.; Miyamoto, M.; Nakamura, T. Transforming Growth Factor-Β1 Mediates the Effects of Low-Intensity Pulsed Ultrasound in Chondrocytes. Ultrasound Med. Biol. 2005, 31, 1713–1721. [Google Scholar] [CrossRef]
- Yang, K.; Parvizi, J.; Wang, S.; Lewallen, D.G.; Kinnick, R.R.; Greenleaf, J.F.; Bolander, M.E. Exposure to Low-intensity Ultrasound Increases Aggrecan Gene Expression in a Rat Femur Fracture Model. J. Orthop. Res. 1996, 14, 802–809. [Google Scholar] [CrossRef]
- Fung, C.-H.; Cheung, W.-H.; Pounder, N.M.; Harrison, A.; Leung, K.-S. Osteocytes Exposed to Far Field of Therapeutic Ultrasound Promotes Osteogenic Cellular Activities in Pre-Osteoblasts through Soluble Factors. Ultrasonics 2014, 54, 1358–1365. [Google Scholar] [CrossRef]
- Shimizu, T.; Fujita, N.; Tsuji-Tamura, K.; Kitagawa, Y.; Fujisawa, T.; Tamura, M.; Sato, M. Osteocytes as Main Responders to Low-Intensity Pulsed Ultrasound Treatment during Fracture Healing. Sci. Rep. 2021, 11, 10298. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.; Kim, S.Y.; Pahk, K.J. Increased Intracellular Diffusivity of Macromolecules within a Mammalian Cell by Low-Intensity Pulsed Ultrasound. Ultrason. Sonochem. 2023, 100, 106644. [Google Scholar] [CrossRef]
- Udroiu, I.; Todaro, F.; Vitaliti, A.; Palmieri, D.; Guida, E.; Perilli, G.; Duranti, L.; D’Ottavi, C.; Mattei, M.; Dolci, S.; et al. Low-Intensity Pulsed Ultrasound Induces Multifaced Alterations in Chromosome Segregation, Cytoskeletal Filaments and Cell Junctions. Sci. Rep. 2025, 15, 4964. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines 2020, 11, 921. [Google Scholar] [CrossRef]
- Humphrey, V.F. Ultrasound and Matter—Physical Interactions. Prog. Biophys. Mol. Biol. 2007, 93, 195–211. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Z.; Xu, Y.; Li, H.; Li, Y.; Peng, L.; Shan, H.; Liu, X.; Wu, H.; Wu, L.; et al. Low-Frequency Ultrasound Sensitive Piezo1 Channels Regulate Keloid-Related Characteristics of Fibroblasts. Adv. Sci. 2024, 11, 2305489. [Google Scholar] [CrossRef]
- Menz, M.D.; Ye, P.; Firouzi, K.; Nikoozadeh, A.; Pauly, K.B.; Khuri-Yakub, P.; Baccus, S.A. Radiation Force as a Physical Mechanism for Ultrasonic Neurostimulation of the Ex Vivo Retina. J. Neurosci. 2019, 39, 6251–6264. [Google Scholar] [CrossRef]
- Wright, C.C.; Hynynen, K.; Goertz, D.E. Pulsed Focused Ultrasound-Induced Displacements in Confined In Vitro Blood Clots. IEEE Trans. Biomed. Eng. 2012, 59, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Destgeer, G.; Park, J.; Ahmed, H.; Park, K.; Sung, H.J. Microfluidic Flow Switching via Localized Acoustic Streaming Controlled by Surface Acoustic Waves. RSC Adv. 2018, 8, 3206–3212. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rivas, D.; Kuhn, S. Synergy of Microfluidics and Ultrasound: Process Intensification Challenges and Opportunities. Top. Curr. Chem. (Z) 2016, 374, 70. [Google Scholar] [CrossRef]
- Jourdain De Thieulloy, M.; Dorward, M.; Old, C.; Gabl, R.; Davey, T.; Ingram, D.M.; Sellar, B.G. On the Use of a Single Beam Acoustic Current Profiler for Multi-Point Velocity Measurement in a Wave and Current Basin. Sensors 2020, 20, 3881. [Google Scholar] [CrossRef] [PubMed]
- Satake, S. Micro- and Nanoscale Imaging of Fluids in Water Using Refractive-Index-Matched Materials. Nanomaterials 2022, 12, 3203. [Google Scholar] [CrossRef]
- Li, W.; Ma, H.; He, R.; Ren, X.; Zhou, C. Prospects and Application of Ultrasound and Magnetic Fields in the Fermentation of Rare Edible Fungi. Ultrason. Sonochem. 2021, 76, 105613. [Google Scholar] [CrossRef]
- Guo, T.; Li, H.; Lv, Y.; Lu, H.; Niu, J.; Sun, J.; Yang, G.-Y.; Ren, C.; Tong, S. Pulsed Transcranial Ultrasound Stimulation Immediately After The Ischemic Brain Injury Is Neuroprotective. IEEE Trans. Biomed. Eng. 2015, 62, 2352–2357. [Google Scholar] [CrossRef]
- Cohen, G.; Natsheh, H.; Sunny, Y.; Bawiec, C.R.; Touitou, E.; Lerman, M.A.; Lazarovici, P.; Lewin, P.A. Enhanced Therapeutic Anti-Inflammatory Effect of Betamethasone on Topical Administration with Low-Frequency, Low-Intensity (20 kHz, 100 mW/cm2) Ultrasound Exposure on Carrageenan-Induced Arthritis in a Mouse Model. Ultrasound Med. Biol. 2015, 41, 2449–2457. [Google Scholar] [CrossRef]
- Yang, N.; Li, J.; Yu, S.; Xia, G.; Li, D.; Yuan, L.; Wang, Q.; Ding, L.; Fan, Z.; Li, J. Application of Nanomaterial-Based Sonodynamic Therapy in Tumor Therapy. Pharmaceutics 2024, 16, 603. [Google Scholar] [CrossRef]
- Tu, B.; Li, Y.; Wen, W.; Liu, J. Bibliometric and Visualized Analysis of Ultrasound Combined with Microbubble Therapy Technology from 2009 to 2023. Front. Pharmacol. 2024, 15, 1418142. [Google Scholar] [CrossRef] [PubMed]
- Poncet, C.; Ferrouillat, S.; Vignal, L.; Memponteil, A.; Bulliard-Sauret, O.; Gondrexon, N. Enhancement of Heat Transfer in Forced Convection by Using Dual Low-High Frequency Ultrasound. Ultrason. Sonochem. 2021, 71, 105351. [Google Scholar] [CrossRef]
- Zhong, P.; Cioanta, I.; Cocks, F.H.; Preminger, G.M. Inertial Cavitation and Associated Acoustic Emission Produced during Electrohydraulic Shock Wave Lithotripsy. J. Acoust. Soc. Am. 1997, 101, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Komarov, S.V. Enhancement of Oscillation Amplitude of Cavitation Bubble Due to Acoustic Wake Effect in Multibubble Environment. Ultrason. Sonochem. 2021, 78, 105734. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Liu, H.; Wu, N.; Zhang, G.; Tu, Y.; Zhao, Y. Improving the Gel Properties of Duck Egg White by Synergetic Phosphorylation/Ultrasound: Gel Properties, Crystalline Structures, and Protein Structure. Ultrason. Sonochem. 2022, 89, 106149. [Google Scholar] [CrossRef]
- Shen, X.; Huang, L.; Ma, D.; Zhao, J.; Xie, Y.; Li, Q.; Zeng, A.; Zeng, K.; Tian, R.; Wang, T.; et al. Ultrasound Microbubbles Enhance the Neuroprotective Effect of Mouse Nerve Growth Factor on Intraocular Hypertension-Induced Neuroretina Damage in Rabbits. J. Ophthalmol. 2016, 2016, 4235923. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Becker, R.O. Generation of Electric Potentials by Bone in Response to Mechanical Stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Pawluk, R.J.; Becker, R.O. Effects of Electric Currents on Bone In Vivo. Nature 1964, 204, 652–654. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Pawluk, R.J.; Pilla, A.A. Acceleration of Fracture Repair by Electromagnetic Fields. A Surgically Noninvasive Method. Ann. N. Y. Acad. Sci. 1974, 238, 242–262. [Google Scholar] [CrossRef]
- Suzuyama, H.; Tsubata, T.; Kitajima, S.; Maehara, K.; Hosokawa, A.; Tsuchiya, T.; Matsukawa, M. Simulation of Ultrasonically Induced Electrical Potentials in Bone. J. Acoust. Soc. Am. 2023, 154, 1315–1323. [Google Scholar]
- Hosokawa, A. Numerical Simulation of Piezoelectric Effect under Ultrasound Irradiation with Consideration of Conductivity. Jpn. J. Appl. Phys. 2016, 55, 07KF03. [Google Scholar] [CrossRef]
- Lin, C.-T.; Chen, Y.-W.; Su, J.; Wu, C.-T.; Hsiao, C.-N.; Shiao, M.-H.; Chang, M.-N. Facile Preparation of a Platinum Silicide Nanoparticle-Modified Tip Apex for Scanning Kelvin Probe Microscopy. Nanoscale Res. Lett. 2015, 10, 401. [Google Scholar] [CrossRef][Green Version]
- Dai, X.; Yao, X.; Zhang, W.; Cui, H.; Ren, Y.; Deng, J.; Zhang, X. The Osteogenic Role of Barium Titanate/Polylactic Acid Piezoelectric Composite Membranes as Guiding Membranes for Bone Tissue Regeneration. Int. J. Nanomed. 2022, 17, 4339–4353. [Google Scholar] [CrossRef] [PubMed]
- Shang, P.; Liu, J.; Wang, S.; Yang, J.; Zhang, Z.; Li, A.; Zhang, H.; Zeng, H. Translational Research of Electromagnetic Fields on Diseases Related With Bone Remodeling: Review and Prospects. Prog. Biochem. Biophys. 2024, 52, 439–455. [Google Scholar] [CrossRef]
- Chen, W.; Deng, Y.; Qiao, G.; Cai, W. Ultrasound Rejuvenation for Upper Facial Skin: A Randomized Blinded Prospective Study. J. Cosmet. Dermatol. 2024, 23, 3942–3949. [Google Scholar] [CrossRef]
- Song, M.; Zhang, M.; He, S.; Li, L.; Hu, H. Ultrasonic Neuromodulation Mediated by Mechanosensitive Ion Channels: Current and Future. Front. Neurosci. 2023, 17, 1232308. [Google Scholar] [CrossRef]
- Leskinen, J.J.; Olkku, A.; Mahonen, A.; Hynynen, K. Nonuniform Temperature Rise in In Vitro Osteoblast Ultrasound Exposures With Associated Bioeffect. IEEE Trans. Biomed. Eng. 2014, 61, 920–927. [Google Scholar] [CrossRef]
- Bender, L.F.; Janes, J.M.; Herrick, J.F. Histologic Studies Following Exposure of Bone to Ultrasound. Arch. Phys. Med. Rehabil. 1954, 35, 555–559. [Google Scholar] [PubMed]
- Smith, N.B.; Temkin, J.M.; Shapiro, F.; Hynynen, K. Thermal Effects of Focused Ultrasound Energy on Bone Tissue. Ultrasound Med. Biol. 2001, 27, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.A.; Kijas, A.W.; Lauko, J.; Rowan, A.E. The Mechanosensory Role of Osteocytes and Implications for Bone Health and Disease States. Front. Cell Dev. Biol. 2022, 9, 770143. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Krstić, J.; Mojsilović, S.; Mojsilović, S.S.; Santibanez, J.F. Regulation of the Mesenchymal Stem Cell Fate by Interleukin-17: Implications in Osteogenic Differentiation. J. Stem Cells 2021, 13, 1696–1713. [Google Scholar] [CrossRef]
- Marino, A.A.; Gross, B.D. Piezoelectricity in Cementum, Dentine and Bone. Arch. Oral Biol. 1989, 34, 507–509. [Google Scholar] [CrossRef]
- Brighton, C.T. The Treatment of Non-Unions with Electricity. J. Bone Jt. Surg. Am. 1981, 63, 847–851. [Google Scholar] [CrossRef]
- Kang, H.; Hou, Z.; Qin, Q.-H. Experimental Study of Time Response of Bending Deformation of Bone Cantilevers in an Electric Field. J. Mech. Behav. Biomed. Mater. 2018, 77, 192–198. [Google Scholar] [CrossRef]
- Ferioli, M.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Ultimo, S.; Capitani, S.; Neri, L.M. Impact of Physical Exercise in Cancer Survivors during and after Antineoplastic Treatments. Oncotarget 2018, 9, 14005–14034. [Google Scholar] [CrossRef]
- Xu, H.; Gu, S.; Riquelme, M.A.; Burra, S.; Callaway, D.; Cheng, H.; Guda, T.; Schmitz, J.; Fajardo, R.J.; Werner, S.L.; et al. Connexin 43 Channels Are Essential for Normal Bone Structure and Osteocyte Viability. J. Bone Miner. Res. 2015, 30, 436–448. [Google Scholar] [CrossRef]
- Voog, J.; Jones, D.L. Stem Cells and the Niche: A Dynamic Duo. Cell Stem Cell 2010, 6, 103–115. [Google Scholar] [CrossRef]
- Guo, X.; Lv, M.; Lin, J.; Guo, J.; Lin, J.; Li, S.; Sun, Y.; Zhang, X. Latest Progress of LIPUS in Fracture Healing: A Mini-Review. J. Ultrasound Med. 2024, 43, 643–655. [Google Scholar] [CrossRef]
- Peng, X.; He, W.; Xin, F.; Genin, G.M.; Lu, T.J. The Acoustic Radiation Force of a Focused Ultrasound Beam on a Suspended Eukaryotic Cell. Ultrasonics 2020, 108, 106205. [Google Scholar] [CrossRef]
- Sorum, B.; Rietmeijer, R.A.; Gopakumar, K.; Adesnik, H.; Brohawn, S.G. Ultrasound Activates Mechanosensitive TRAAK K+ Channels through the Lipid Membrane. Proc. Natl. Acad. Sci. USA 2021, 118, e2006980118. [Google Scholar] [CrossRef]
- Veronick, J.; Assanah, F.; Nair, L.S.; Vyas, V.; Huey, B.; Khan, Y. The Effect of Acoustic Radiation Force on Osteoblasts in Cell/Hydrogel Constructs for Bone Repair. Exp. Biol. Med. 2016, 241, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Clark, C.C.; Brighton, C.T. Up-Regulation of Bone Morphogenetic Proteins in Cultured Murine Bone Cells with Use of Specific Electric Fields. J. Bone Jt. Surg. 2006, 88, 1053–1065. [Google Scholar] [CrossRef]
- Qiu, Z.; Kala, S.; Guo, J.; Xian, Q.; Zhu, J.; Zhu, T.; Hou, X.; Wong, K.F.; Yang, M.; Wang, H.; et al. Targeted Neurostimulation in Mouse Brains with Non-Invasive Ultrasound. Cell Rep. 2020, 32, 108033. [Google Scholar] [CrossRef] [PubMed]
- Suarez Castellanos, I.; Singh, T.; Balteanu, B.; Bhowmick, D.C.; Jeremic, A.; Zderic, V. Calcium-Dependent Ultrasound Stimulation of Secretory Events from Pancreatic Beta Cells. J. Ther. Ultrasound 2017, 5, 30. [Google Scholar] [CrossRef]
- Haddad, O.; Hawse, J.R.; Subramaniam, M.; Spelsberg, T.C.; Bensamoun, S.F. Tieg1-Null Osteocytes Display Defects in Their Morphology, Density and Surrounding Bone Matrix. J. Musculoskelet. Res. 2009, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, J.; Zhou, C.; Lai, W. Substrate Stiffness Regulates the Differentiation Profile and Functions of Osteoclasts via Cytoskeletal Arrangement. Cell Prolif. 2022, 55, e13172. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Guo, J.; Kala, S.; Zhu, J.; Xian, Q.; Qiu, W.; Li, G.; Zhu, T.; Meng, L.; Zhang, R.; et al. The Mechanosensitive Ion Channel Piezo1 Significantly Mediates In Vitro Ultrasonic Stimulation of Neurons. iScience 2019, 21, 448–457. [Google Scholar] [CrossRef]
- Shen, X.; Song, Z.; Xu, E.; Zhou, J.; Yan, F. Sensitization of Nerve Cells to Ultrasound Stimulation through Piezo1-Targeted Microbubbles. Ultrason. Sonochem. 2021, 73, 105494. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Wu, L.; Qin, Y.-X. Piezo1 Channel Activation in Response to Mechanobiological Acoustic Radiation Force in Osteoblastic Cells. Bone Res. 2021, 9, 16. [Google Scholar] [CrossRef]
- Haddad, J.B.; Obolensky, A.G.; Shinnick, P. The Biologic Effects and the Therapeutic Mechanism of Action of Electric and Electromagnetic Field Stimulation on Bone and Cartilage: New Findings and a Review of Earlier Work. J. Altern. Complement. Med. 2007, 13, 485–490. [Google Scholar] [CrossRef]
- Hua, W.; Kai, R.; Wenchun, Z.; Baojian, G.; Songlin, P. Effect of Electromagnetic Fields on Proliferation and Differentiation of Cultured Mouse Bone Marrow Mesenchymal Stem Cells. Curr. Med. Sci. 2005, 25, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Aaron, R.K.; Ciombor, D.M.; Simon, B.J. Treatment of Nonunions with Electric and Electromagnetic Fields. Clin. Orthop. Relat. Res. 2004, 419, 21–29. [Google Scholar] [CrossRef]
- Kujawska, T.; Secomski, W.; Bilmin, K.; Nowicki, A.; Grieb, P. Impact of Thermal Effects Induced by Ultrasound on Viability of Rat C6 Glioma Cells. Ultrasonics 2014, 54, 1366–1372. [Google Scholar] [CrossRef]
- Prieto, M.L.; Firouzi, K.; Khuri-Yakub, B.T.; Madison, D.V.; Maduke, M. Spike Frequency–Dependent Inhibition and Excitation of Neural Activity by High-Frequency Ultrasound. J. Gen. Physiol. 2020, 152, e202012672. [Google Scholar] [CrossRef]
- Ling, L.; Feng, X.; Wei, T.; Wang, Y.; Wang, Y.; Zhang, W.; He, L.; Wang, Z.; Zeng, Q.; Xiong, Z. Effects of Low-Intensity Pulsed Ultrasound (LIPUS)-Pretreated Human Amnion-Derived Mesenchymal Stem Cell (hAD-MSC) Transplantation on Primary Ovarian Insufficiency in Rats. Stem Cell Res. Ther. 2017, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.B.; Padilla, F.; Haworth, K.J.; Ellens, N.; Dalecki, D.; Miller, D.L.; Wear, K.A.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of Therapeutic Ultrasound Applications and Safety Considerations: 2024 Update. J. Ultrasound Med. 2025, 44, 381–433. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.R. The Stimulation of Bone Growth by Ultrasound. Arch. Orth. Traum. Surg. 1983, 101, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Sun, J.; Chang, S.; Lin, J.C. Study of Thermal Effects of Ultrasound Stimulation on Fracture Healing. Bioelectromagnetics 2002, 23, 256–263. [Google Scholar] [CrossRef]


| Parameters | Intensity | Frequency | Pulse Repetition Frequency | Pulse Width | Pulse Duty Cycle | Exposure Time |
|---|---|---|---|---|---|---|
| Value | 30 mW/cm2 | 1.5 MHz | 1 kHz | 200 μs | 20% | 20 min |
| Acronym | Definition | Physical Meaning | Calculation Formula (Example) | Symbol and Description |
|---|---|---|---|---|
| Isppa | The average intensity at the strongest point in the beam within a single pulse. | Represents the instantaneous peak intensity of a pulse, directly related to the peak pressure amplitude. | psp: Spatial-peak pressure amplitude (Pa) Z: Characteristic acoustic impedance (Rayl) | |
| Ispta | The average intensity at the strongest point in the beam over the total exposure time (including pulse off periods). | Reflects the average energy level at the beam’s hotspot over prolonged exposure; the primary determinant for thermal effects. | DC: Duty cycle | |
| Isata | The average intensity over the entire beam cross-sectional area and the total exposure time. | Represents the average power density across the entire transducer output face. | W: Acoustic output power (W) a: Transducer surface area (m2) |
| Ref | Number of Cases | Target | Parameters (F, ISATA, PD, PRF, PDC, T) * | Treatment Period (days) | Positive Results | Negative Results |
|---|---|---|---|---|---|---|
| [87] | 51 | Rib fractures | 1.0 MHz, 500 mW/cm2, N/A, N/A, 10%, N/A | 180 | This study presents the first evidence that LIPUS treatment is capable of improving rib fracture outcome, significantly accelerating bone callus healing and decreasing pain. | N/A |
| [88] | 30 | Fresh scaphoid fractures | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 65 | Achieved a fracture healing time of 43.2 ± 10.9 days in the ultrasound group and 62 ± 19.2 days in the control group. | N/A |
| [89] | 51 | Tibial shaft fractures | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 112 | The group treated with LIPUS had significantly better bone healing after treatment than the control group. | N/A |
| [90] | 30 | Tibial fractures | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 90 | LIPUS-treated group was found to have the least complications and showed statistically better healing. | N/A |
| [91] | 30 | Lateral malleolus fractures | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 42 | N/A | No statistically significant effect of LIPUS on fracture healing of lateral malleolus was observed. |
| [92] | 13 | Delayed union of fibula after high tibial osteotomy | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 60–120 | LIPUS treatment accelerated the clinical healing of delayed fibular union by increasing the osteoid thickness, mineral deposition rate, and bone volume in the nascent bone formation area. | LIPUS treatment did not alter osteoid thickness or the mineral apposition rate in cancellous bone regions. |
| [93] | 27 | Osteotomy sites after forearm bone shortening osteotomies | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 84 | LIPUS treatment was found to reduce the cortical healing time by 27%; the healing time of endosteum was shortened by 18%. | N/A |
| [94] | 21 | Recovery after intraoral vertical ramus osteotomy | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 21 | LIPUS-treated patients had increased bone mineral density and faster bone healing than patients in the control group. | N/A |
| [95] | 13 | Delayed union after fibular osteotomy | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 150 | LIPUS treatment significantly increased the size of blood vessels. There was a significant correlation between vessel size and osteoid volume during delayed healing. | LIPUS treatment did not change the number of blood vessels. |
| [96] | 29 | Nonunion | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 154 | LIPUS can be used to treat challenging, established nonunions. | N/A |
| [97] | 36 | Underwent distraction osteogenesis (2 cm) | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 165 | LIPUS application during callus distraction served as a useful adjunct therapy for distraction osteogenesis. | N/A |
| [98] | 21 | Tibial distraction osteogenesis | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | N/A | LIPUS treatment accelerated callus maturation by 27%, increased the daily radiographic callus density by 33%, and reduced the fixation time by 95 days compared to the control group. | N/A |
| [99] | 30 | Distraction osteogenesis of the tibia | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | N/A | The healing indexes of the anterior and medial cortices were significantly improved in the LIPUS group, exhibiting a markedly faster healing rate than the control group. | No significant difference in the external fixation index was found between the LIPUS and control groups. |
| [100] | 23 | Lower limb bone stress injuries | 1.5 MHz, 117 mW/cm2, 1 kHz, 200 ms, N/A, 20 | 28 | N/A | No significant differences were found between the LIPUS treatment and placebo groups in changes to MRI grading and bone marrow edema size. |
| [101] | 44 | Chevron osteotomy | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 21 | The LIPUS group showed an increase in the distal metatarsal articular angle compared to the placebo group. | The hallux valgus angle, intermetatarsal angle, sesamoid position, and metatarsal index showed no statistically significant differences between the LIPUS treatment and placebo groups. |
| [102] | 142 | Scaphoid nonunion | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 56 | N/A | LIPUS therapy exerted no significant effect on healing time in patients with established scaphoid nonunions. |
| [103] | 20 | Postmenopause | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | 180 | N/A | LIPUS treatment showed no change in the overall or trabecular bone mineral density of the distal radius. |
| Ref. | Species | Model | Parameters (F, ISATA, PD, PRF, PDC, T) * | Positive Results | Negative Results |
|---|---|---|---|---|---|
| [59] | Rat | Ovariectomy | 1.0 Hz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | N/A | LIPUS treatment had no significant effect on bone loss in the distal femur or proximal tibia. |
| [114] | Rabbit | Leg fracture | N/A, 200 mW/cm2, N/A, N/A, N/A, 3 | Fractures treated with ultrasound healed 35 days faster than those in the control group. | N/A |
| [115] | Rat | Femoral osteotomy Model | 7.5 MHz, 11.8 mW/cm2, 1 Hz, 1 ms, N/A, 10 | Ultrasound treatment enhanced bone mineral density in the fracture region and promoted the acceleration of fracture repair. | N/A |
| [116] | Rabbit | Transverse osteotomy of the tibial diaphysis | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | Radiographic assessment revealed a substantial maturity in bone formation within the LIPUS group, while the control group exhibited only immature bone regeneration. | N/A |
| [117] | Rabbit | Mid-tibia1 osteotomy | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS treatment resulted in an enlarged distraction callus and decreased fibrous tissue in the tibia. | LIPUS treatment showed no significant effect on the mechanical properties and density of the regenerated bone. |
| [118] | Sheep | Midshaft osteotomy of the left tibia | 1.0 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | The LIPUS treatment group demonstrated a 24-day reduction in healing time compared to the control group, and also exhibited enhanced cortical bone density and ultimate strength. | N/A |
| [119] | Canine | Tibial plateau leveling osteotomy | 1.5 MHz, 30 mW/cm2, 1 kHz, N/A, 20%, 20 | N/A | The LIPUS and sham groups showed no significant difference in radiographic bone healing or limb function via objective gait analysis. |
| [120] | Horse | Fracture gap of the fourth metacarpal bone | 1.5 MHz, 44 mW/cm2, 1 kHz, 2 ms, 33%, 40 | N/A | No significant differences were observed in any radiographic or histological parameters between the LIPUS treatment group and the control group. |
| [121] | Rat | Ovariectomy-induced osteoporotic fracture | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS treatment accelerated fracture healing by enhancing callus formation, angiogenesis, and callus remodeling. | N/A |
| [122] | Mice | Fracture of femur | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS treatment halved the endochondral bone remodeling period to 10 days and accelerated femoral fracture healing. | N/A |
| [123] | Rat | Ovariectomized | 1.0 MHz, 1500 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS treatment mitigated estrogen deficiency-induced bone loss and fragility by enhancing bone formation. | N/A |
| [124] | Rat | Ovariectomy | 1.0 MHz, 30 mW/cm2, 1 kHz, N/A, N/A, 20 | LIPUS stimulation promoted an increase in femoral wet weight in ovariectomized rats. | N/A |
| [125] | Rat | Ovariectomy and osteoporotic bone defect | 1.5 MHz, 30 and 150 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS at 150 mW/cm2 showed higher drill-hole healing after 3 weeks and greater improvements in bone density, microstructure, and biomechanical properties by 6 weeks compared to 30 mW/cm2. | N/A |
| [126] | Mice | Ovariectomy | 1.5 MHz, 30 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS treatment significantly enhanced bone microarchitecture parameters compared to the control group, as evidenced by increased bone volume/tissue volume, trabecular number, trabecular pattern factor, and polar moment of inertia. | N/A |
| [18] | Rat | Ovariectomy | 1.5 MHz, 5, 30 and 100 mW/cm2, 1 kHz, 200 μs, N/A, 20 | LIPUS treatment improved bone microarchitecture, increasing the bone volume/total volume ratio, the apparent level elastic modulus, and the mechanical strength of trabeculae. | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, B.; Sun, W.; Cai, C.; Shang, P. The Effects and Mechanisms of Low-Intensity Pulsed Ultrasound on Bone Remodeling: From Laboratory to Clinic. Biomolecules 2025, 15, 1351. https://doi.org/10.3390/biom15101351
Zong B, Sun W, Cai C, Shang P. The Effects and Mechanisms of Low-Intensity Pulsed Ultrasound on Bone Remodeling: From Laboratory to Clinic. Biomolecules. 2025; 15(10):1351. https://doi.org/10.3390/biom15101351
Chicago/Turabian StyleZong, Bo, Weikang Sun, Chao Cai, and Peng Shang. 2025. "The Effects and Mechanisms of Low-Intensity Pulsed Ultrasound on Bone Remodeling: From Laboratory to Clinic" Biomolecules 15, no. 10: 1351. https://doi.org/10.3390/biom15101351
APA StyleZong, B., Sun, W., Cai, C., & Shang, P. (2025). The Effects and Mechanisms of Low-Intensity Pulsed Ultrasound on Bone Remodeling: From Laboratory to Clinic. Biomolecules, 15(10), 1351. https://doi.org/10.3390/biom15101351







