Circulating, Extracellular Vesicle-Associated Tissue Factor in Cancer Patients with and without Venous Thromboembolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject and Study Design
2.2. Diagnosis of VTE
2.3. EV Isolation from Patients Blood and Analysis of EV-TF, Plasmatic TF, and Cytokine Concentration
2.4. Statistical Analysis
3. Results
3.1. Study Population
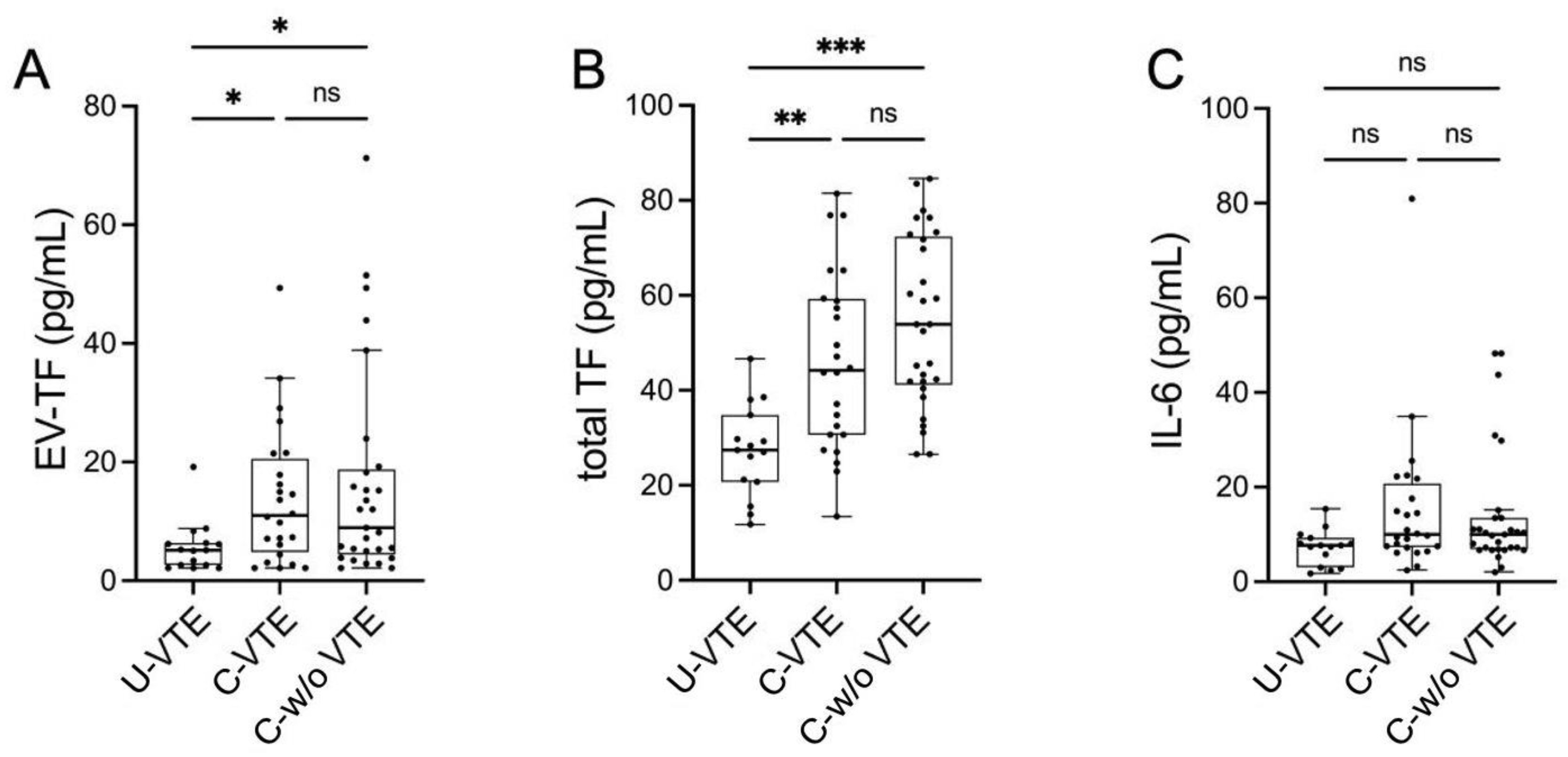
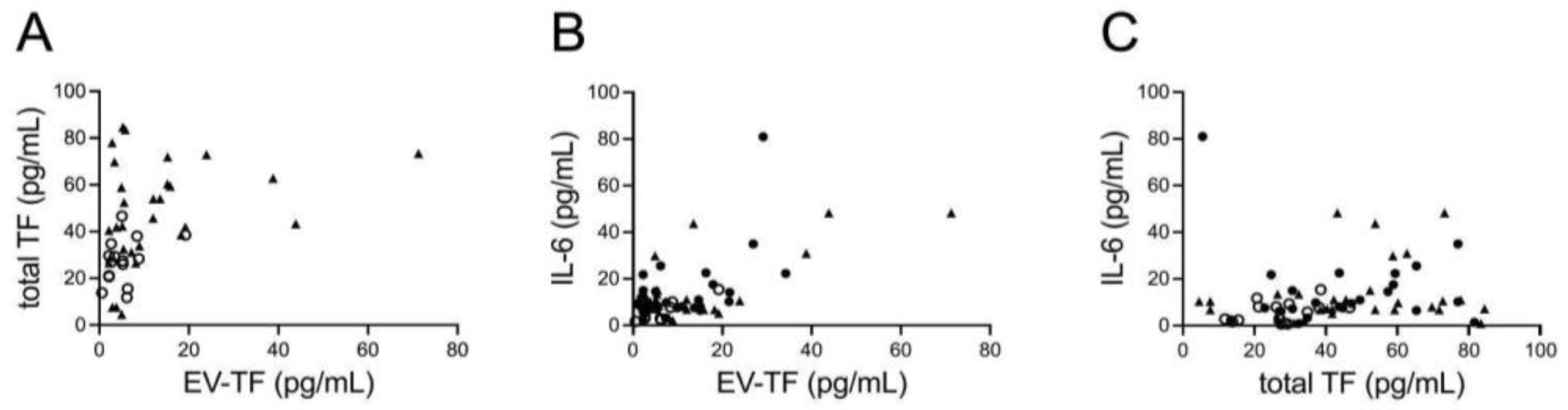
3.2. Prothrombotic Activity
3.3. Circulating IL-6 Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Brunson, A.; Adesina, O.; Keegan, T.H.M.; Wun, T. The incidence of cancer-associated thrombosis is increasing over time. Blood Adv. 2022, 6, 307–320. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mackman, N.; Falanga, A.; Pabinger, I.; Noble, S.; Ageno, W.; Moik, F.; Lee, A.Y.Y. Cancer-associated venous thromboembolism. Nat. Rev. Dis. Primers 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Geddings, J.E.; Mackman, N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 2013, 122, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Nieri, D.; Neri, T.; Petrini, S.; Vagaggini, B.; Paggiaro, P.; Celi, A. Cell-derived microparticles and the lung. Eur. Respir. Rev. 2016, 25, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Cancer: Regulation, Tumor Growth, and Metastasis. Semin. Thromb. Hemost. 2019, 45, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Mackman, N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers 2021, 13, 3839. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Acedo, A.L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Platelets, Thrombo-Inflammation, and Cancer: Collaborating With the Enemy. Front. Immunol. 2019, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Mack, A.; Vanden Hoek, T.; Du, X. Thromboinflammation and the Role of Platelets. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1175–1180. [Google Scholar] [CrossRef]
- Wang, Y.; Golden, J.B.; Fritz, Y.; Zhang, X.; Diaconu, D.; Camhi, M.I.; Gao, H.; Dawes, S.M.; Xing, X.; Ganesh, S.K.; et al. Interleukin 6 regulates psoriasiform inflammation-associated thrombosis. JCI Insight 2016, 1, e89384. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Nieri, D.; Morani, C.; De Francesco, M.; Gaeta, R.; Niceforo, M.; De Santis, M.; Giusti, I.; Dolo, V.; Daniele, M.; Papi, A.; et al. Enhanced prothrombotic and proinflammatory activity of circulating extracellular vesicles in acute exacerbations of chronic obstructive pulmonary disease. Respir. Med. 2024, 223, 107563. [Google Scholar] [CrossRef] [PubMed]
- Scalise, V.; Lombardi, S.; Sanguinetti, C.; Nieri, D.; Pedrinelli, R.; Celi, A.; Neri, T. A novel prothrombotic role of proprotein convertase subtilisin kexin 9: The generation of procoagulant extracellular vesicles by human mononuclear cells. Mol. Biol. Rep. 2022, 49, 4129–4134. [Google Scholar] [CrossRef] [PubMed]
- Tesselaar, M.E.T.; Romijn, F.P.H.T.M.; Van Der Linden, I.K.; Prins, F.A.; Bertina, R.M.; Osanto, S. Microparticle-associated tissue factor activity: A link between cancer and thrombosis? J. Thromb. Haemost. 2007, 5, 520–527. [Google Scholar] [CrossRef]
- Tesselaar, M.E.T.; Romijn, F.P.H.T.M.; van der Linden, I.K.; Bertina, R.M.; Osanto, S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J. Thromb. Haemost. 2009, 7, 1421–1423. [Google Scholar] [CrossRef]
- Woei-A-Jin, F.J.S.H.; Tesselaar, M.E.T.; Garcia Rodriguez, P.; Romijn, F.P.H.T.M.; Bertina, R.M.; Osanto, S. Tissue factor-bearing microparticles and CA19.9: Two players in pancreatic cancer-associated thrombosis? Br. J. Cancer 2016, 115, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Thålin, C.; Lundström, S.; Wallén, H.; Mackman, N. Comparison of microvesicle tissue factor activity in non-cancer severely ill patients and cancer patients. Thromb. Res. 2018, 165, 1–5. [Google Scholar] [CrossRef]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bulato, C.; Castelli, M.; Gavasso, S.; Simioni, P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb. Res. 2011, 127, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I.; Liebman, H.A.; Neuberg, D.; Lacroix, R.; Bauer, K.A.; Furie, B.C.; Furie, B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin. Cancer Res. 2009, 15, 6830–6840. [Google Scholar] [CrossRef] [PubMed]
- Koizume, S.; Miyagi, Y. Tissue factor in cancer-associated thromboembolism: Possible mechanisms and clinical applications. Br. J. Cancer 2022, 127, 2099–2107. [Google Scholar] [CrossRef]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]
- Sánchez-López, V.; Gao, L.; Ferrer-Galván, M.; Arellano-Orden, E.; Elías-Hernández, T.; Jara-Palomares, L.; Asensio-Cruz, M.I.; Castro-Pérez, M.J.; Rodríguez-Martorell, F.J.; Lobo-Beristain, J.L.; et al. Differential biomarker profiles between unprovoked venous thromboembolism and cancer. Ann. Med. 2020, 52, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Koizume, S.; Takahashi, T.; Ueno, M.; Oishi, R.; Nagashima, S.; Sano, Y.; Fukushima, T.; Tezuka, S.; Morimoto, M.; et al. Tissue factor and its procoagulant activity on cancer-associated thromboembolism in pancreatic cancer. Cancer Sci. 2021, 112, 4679–4691. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Menzies, K.E.; Wang, J.-G.; Hyrien, O.; Hathcock, J.; Mackman, N.; Taubman, M.B. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J. Thromb. Haemost. 2008, 6, 1983–1985. [Google Scholar] [CrossRef] [PubMed]
- Reddel, C.J.; Allen, J.D.; Ehteda, A.; Taylor, R.; Chen, V.M.Y.; Curnow, J.L.; Kritharides, L.; Robertson, G. Increased thrombin generation in a mouse model of cancer cachexia is partially interleukin-6 dependent. J. Thromb. Haemost. 2017, 15, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Wei, R.; Miao, X.; Sun, S.; Liang, G.; Chu, C.; Zhao, L.; Zhu, X.; Guo, Q.; et al. IL (Interleukin)-6 Contributes to Deep Vein Thrombosis and Is Negatively Regulated by miR-338-5p. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 323–334. [Google Scholar] [CrossRef]
- Salemi, R.; Gattuso, G.; Tomasello, B.; Lavoro, A.; Gaudio, A.; Libra, M.; Signorelli, S.S.; Candido, S. Co-Occurrence of Interleukin-6 Receptor Asp358Ala Variant and High Plasma Levels of IL-6: An Evidence of IL-6 Trans-Signaling Activation in Deep Vein Thrombosis (DVT) Patients. Biomolecules 2022, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Salet, D.M.; Bekkering, S.; Middeldorp, S.; van den Hoogen, L. Targeting thromboinflammation in antiphospholipid syndrome. J. Thromb. Haemost. 2023, 21, 744–757. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer cell-derived tissue factor-positive extracellular vesicles: Biomarkers of thrombosis and survival. Curr. Opin. Hematol. 2019, 26, 349–356. [Google Scholar] [CrossRef]

| U-VTE (n = 15) | C-VTE (n = 24) | C-w/o VTE (n = 29) | p | |
|---|---|---|---|---|
| Sex (M/F) | 9/6 | 11/13 | 16/13 | NS |
| Age, years [missing values] | 70.5 ± 13.8 [0] | 66.9 ± 12.0 [0] | 69.6 ± 9.6 [0] | NS |
| BMI, kg/m2 [missing values] | 27.7 ± 3.8 [5] | 24.6 ± 4.6 [3] | 24.7 ± 4.8 [0] | NS |
| Total WBC, mL−1 [missing values] | 8010 (2640) [0] | 7745 (6150) [0] | 9520 (5140) [0] | NS |
| Neutrophils, mL−1 [missing values] | 5594 (2843) [0] | 5519 (5304) [0] | 6120 (4170) [0] | NS |
| Lymphocytes, mL−1 [missing values] | 1800 (1154) [0] | 1309 (777) [0] | 1320 (1010) [0] | NS |
| Hemoglobin, g/dL [missing values] | 14.8 (2.1) [0] | 11.3 (2.2) [0] | 11.6 (1.4) [0] | <0.001 U-VTE vs. C-VTE <0.001 U-VTE vs. C-w/o VTE |
| Hematocrit, % [missing values] | 42.0 (5.3) | 33.7 (6.8) | 35.3 (4.6) | <0.001 U-VTE vs. C-VTE <0.001 U-VTE vs. C-w/o VTE |
| Platelets, ×103/mL [missing values] | 194 (65) [0] | 218 (100) [0] | 265 (125) [0] | NS |
| aPTT, s [missing values] | 29.4 (3.2) [1] | 28.1 (3.9) [2] | 27.6 (5.5) [2] | NS |
| INR [missing values] | 1.02 (0.08) [1] | 1.12 (0.20) [1] | 1.07 (0.20) [2] | NS |
| Creatinine, mg/dL [missing values] | 0.98 ± 0.24 [0] | 0.88 ± 0.31 [0] | 0.86 ± 0.31 [0] | NS |
| eGFR, mL/min/1.73 m2 [missing values] | 84.6 ± 29.8 [2] | 81.0 ± 29.5 [2] | 81.9 ± 35.6 [0] | NS |
| Site of Primary Cancer | C-VTE (n = 24) | C-w/o VTE (n = 29) |
|---|---|---|
| Blood, n | 2 | 0 |
| Prostate, n | 0 | 1 |
| Lung, n | 9 | 20 |
| Kidney, n | 5 | 0 |
| Pancreas, n | 3 | 2 |
| Colon, n | 2 | 1 |
| Ovary, n | 1 | 0 |
| Melanoma, n | 1 | 0 |
| Breast, n | 1 | 2 |
| Liver and biliary tract, n | 0 | 2 |
| Cardias, n | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lami, V.; Nieri, D.; Pagnini, M.; Gattini, M.; Donati, C.; De Santis, M.; Cipriano, A.; Bazzan, E.; Sbrana, A.; Celi, A.; et al. Circulating, Extracellular Vesicle-Associated Tissue Factor in Cancer Patients with and without Venous Thromboembolism. Biomolecules 2025, 15, 83. https://doi.org/10.3390/biom15010083
Lami V, Nieri D, Pagnini M, Gattini M, Donati C, De Santis M, Cipriano A, Bazzan E, Sbrana A, Celi A, et al. Circulating, Extracellular Vesicle-Associated Tissue Factor in Cancer Patients with and without Venous Thromboembolism. Biomolecules. 2025; 15(1):83. https://doi.org/10.3390/biom15010083
Chicago/Turabian StyleLami, Valentina, Dario Nieri, Marta Pagnini, Mario Gattini, Claudia Donati, Mariella De Santis, Alessandro Cipriano, Erica Bazzan, Andrea Sbrana, Alessandro Celi, and et al. 2025. "Circulating, Extracellular Vesicle-Associated Tissue Factor in Cancer Patients with and without Venous Thromboembolism" Biomolecules 15, no. 1: 83. https://doi.org/10.3390/biom15010083
APA StyleLami, V., Nieri, D., Pagnini, M., Gattini, M., Donati, C., De Santis, M., Cipriano, A., Bazzan, E., Sbrana, A., Celi, A., & Neri, T. (2025). Circulating, Extracellular Vesicle-Associated Tissue Factor in Cancer Patients with and without Venous Thromboembolism. Biomolecules, 15(1), 83. https://doi.org/10.3390/biom15010083








