Development of a Combined 2D-MGD TLC/HPTLC Method for the Separation of Terpinen-4-ol and α-Terpineol from Tea Tree, Melaleuca alternifolia, Essential Oil †
Abstract
1. Introduction
2. Materials and Methods
2.1. HPTLC Fingerprint/1D TLC
2.1.1. Sample and Plate Preparation
2.1.2. Development and Detection
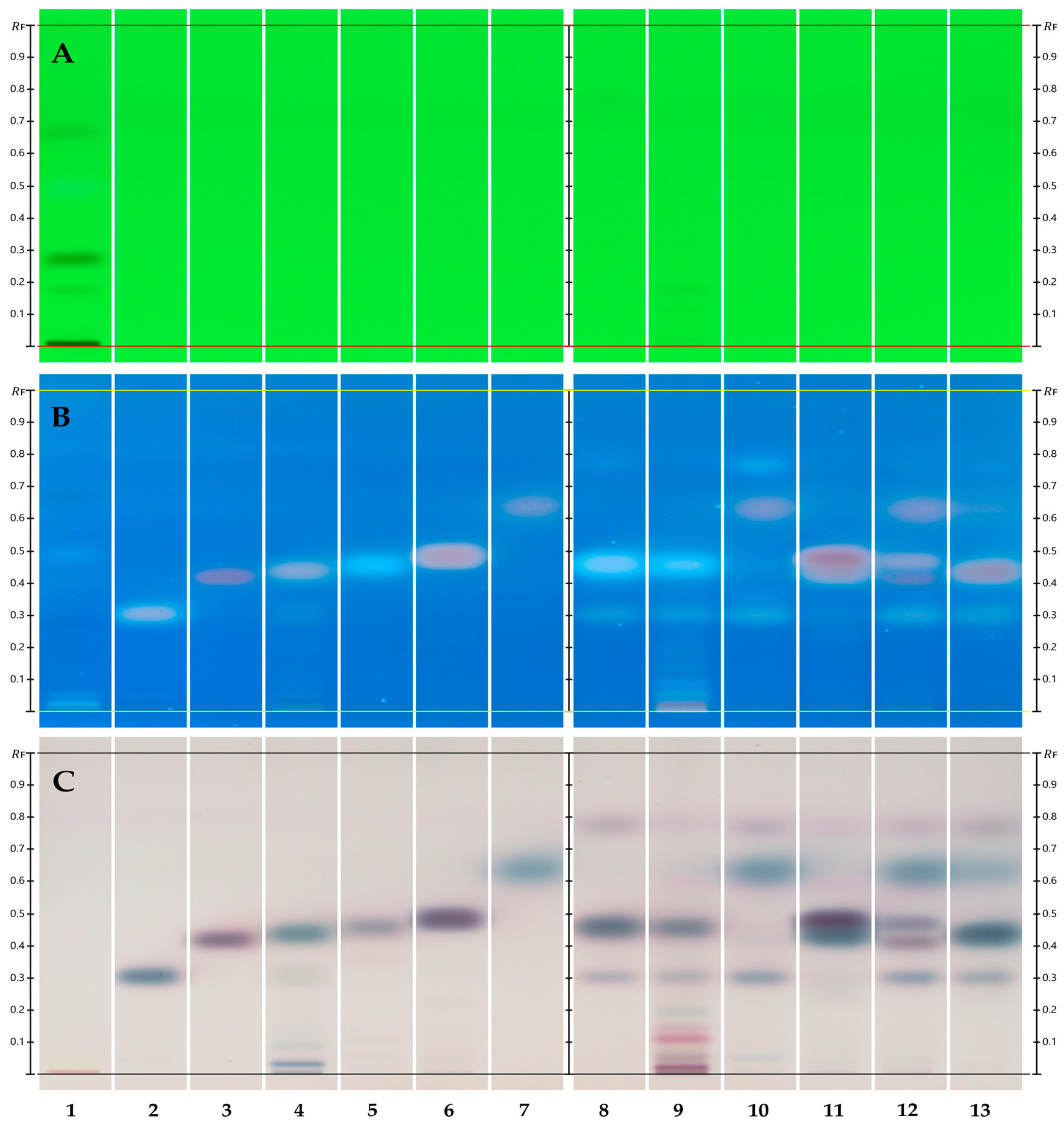
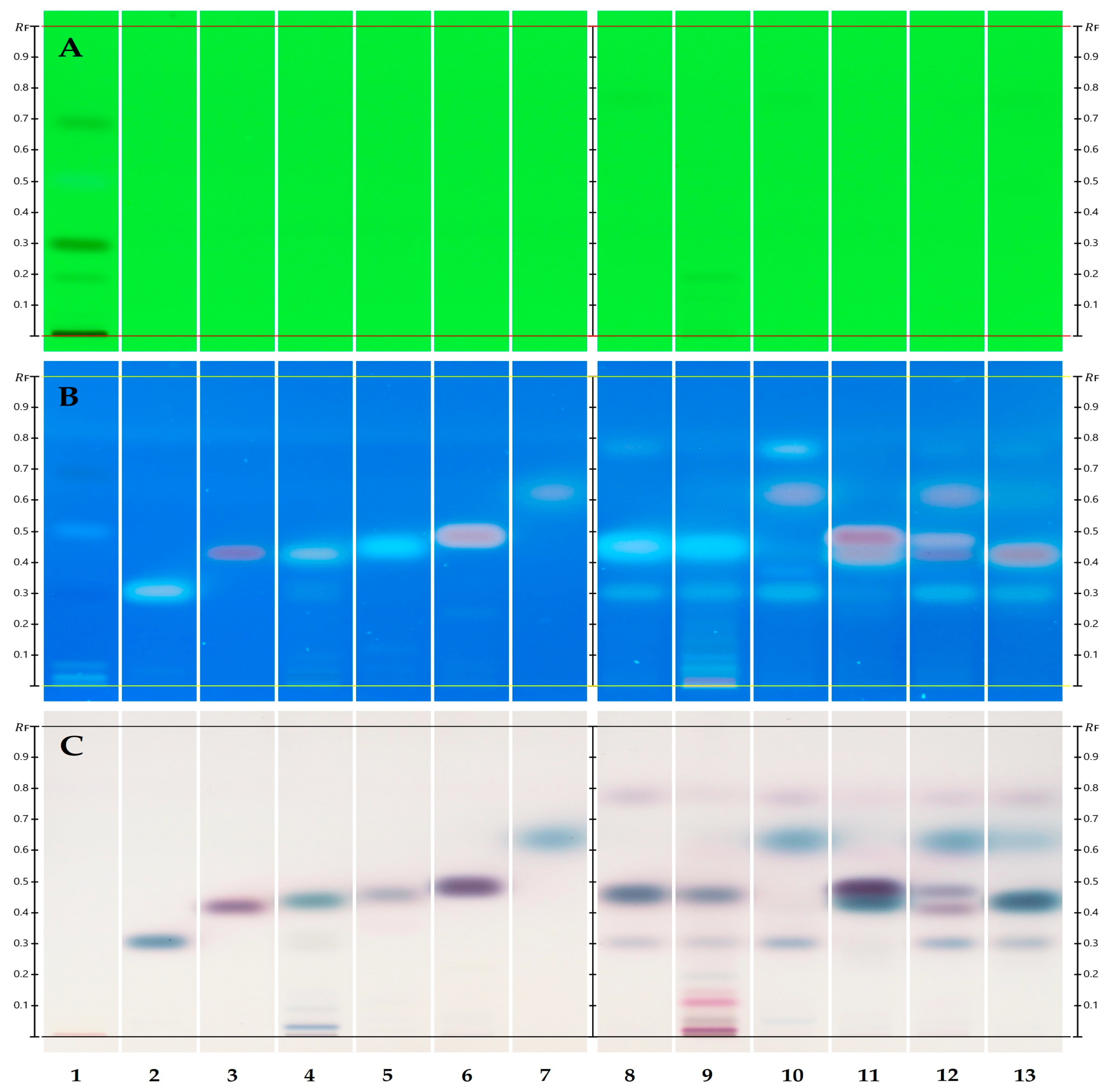
2.2. Two-Dimensional TLC/HPTLC
2.2.1. Sample and Plate Preparation
2.2.2. Development and Detection
2.3. 2D-MGD TLC/HPTLC
2.3.1. Sample and Plate Preparation
2.3.2. Development and Detection
2.4. Gas Chromatography–Mass Spectrometry (GC/MS)
2.4.1. Sample Preparation and Fractions
2.4.2. Identification of Components
2.5. Chiral Separation of Terpinen-4-ol and α-Terpineol (GC-FID)
2.5.1. Sample Preparation
2.5.2. Analysis
3. Results
3.1. HPTLC Fingerprint/1D TLC
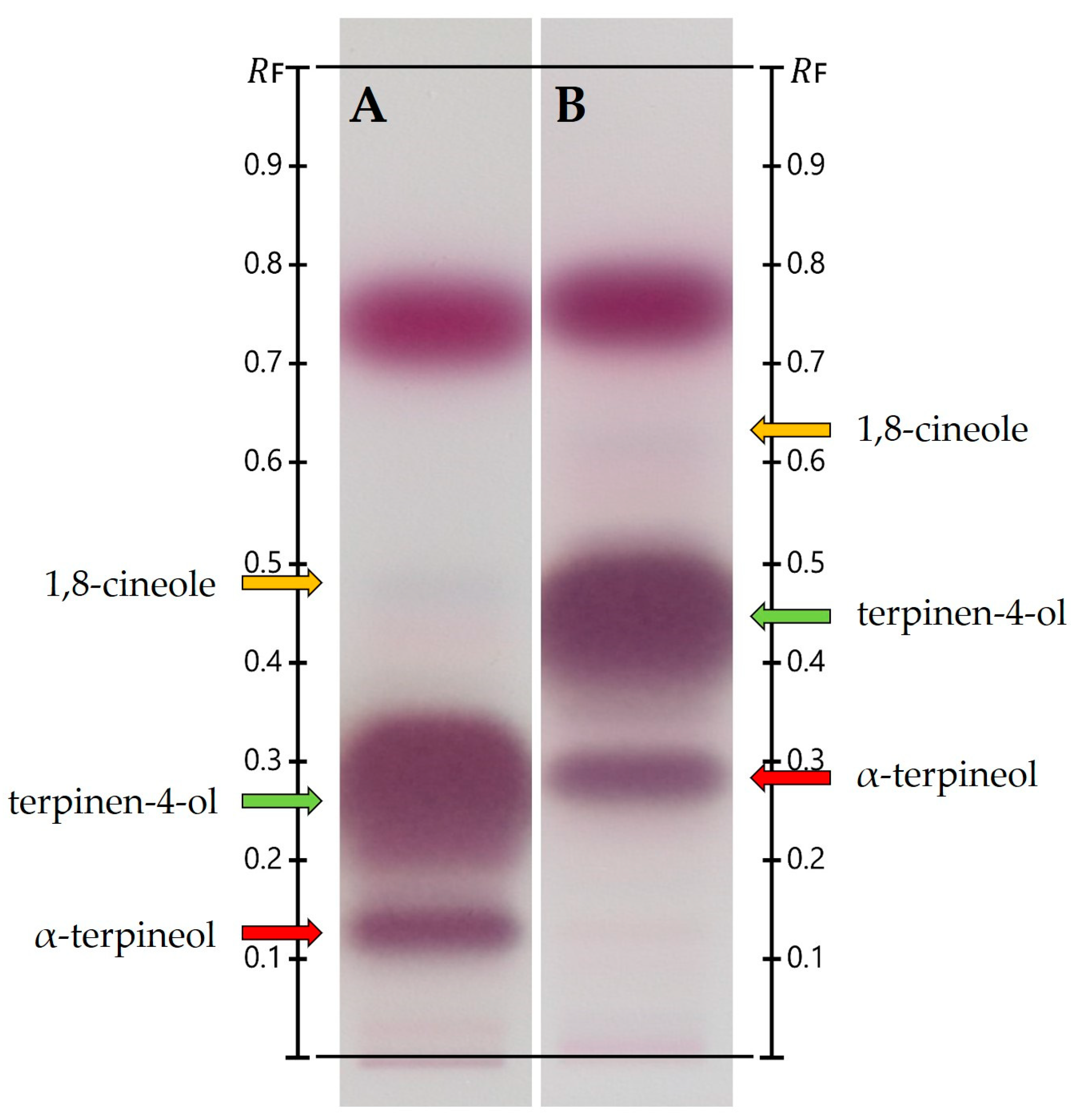
3.2. Two-Dimensional TLC/HPTLC
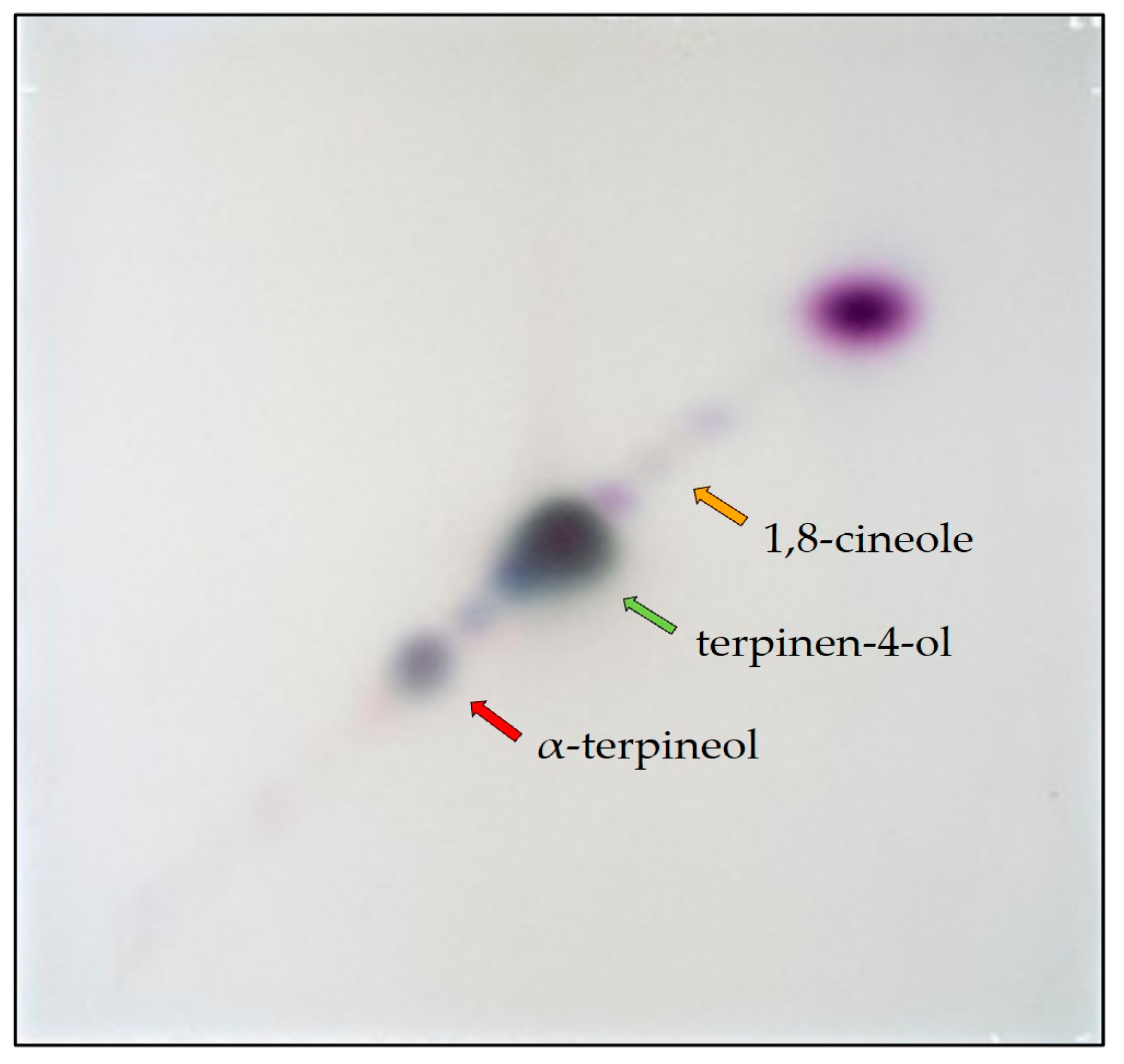
3.3. 2D-MGD TLC/HPTLC and Characterization of Fractions
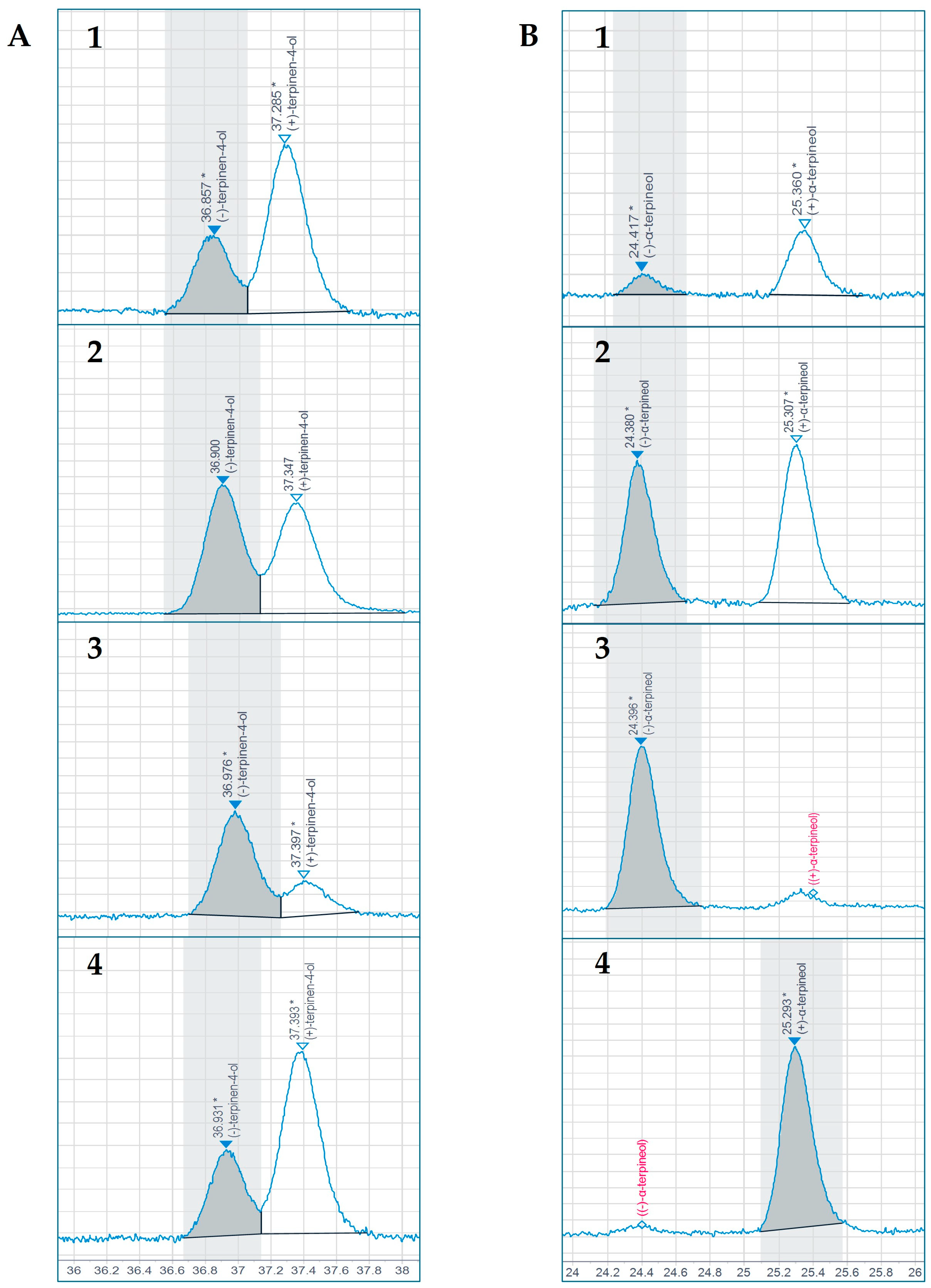
3.4. Enantiomeric Distribution of Terpinen-4-ol and α-Terpineol
4. Discussion
4.1. HPTLC Fingerprint/1D TLC
4.2. Two-Dimensional TLC/HPTLC
4.3. 2D-MGD TLC/HPTLC and Characterization of Fractions
4.4. Gas Chromatography–Mass Spectrometry
4.5. Enantiomeric Distribution of Terpinen-4-ol and α-Terpineol
4.6. Discussion Summary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Plant List. Available online: https://wfoplantlist.org/taxon/wfo-0000239396-2024-12?page=1 (accessed on 14 January 2025).
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the antimicrobial actions of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Satchell, A.C.; Saurajen, A.; Bell, C.; Barnetson, R. StC. Treatment of dandruff with 5% tea tree oil shampoo. J. Am. Acad. Dermatol. 2002, 47, 852–855. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- Blackwood, B.; Thompson, G.; McMullan, R.; Stevenson, M.; Riley, T.V.; Alderdice, F.A.; Trinder, T.J.; Lavery, G.G.; McAuley, D.F. Tea tree oil (5%) body wash versus standard care (Johnson’s Baby Softwash) to prevent colonization with methicillin-resistant Staphylococcus aureus in critically ill adults: A randomized controlled trial. J. Antimicrob. Chemother. 2013, 68, 1193–1199. [Google Scholar] [CrossRef]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2017, 29, 201–213. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Robacker, D.C. Attractiveness to Anastrepha ludens (Diptera: Tephritidae) of plant essential oils and a synthetic food-odour lure. J. Appl. Entomol. 2007, 131, 202–208. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J. Evaluation of seven essential oils identifies cubeb oil as most effective attractant for detection of Xyleborus glabratus. J. Pest Sci. 2014, 87, 681–689. [Google Scholar] [CrossRef]
- Buteler, M.; Alma, A.M.; Herrera, M.L.; Gorosito, N.B.; Fernández, P.C. Novel organic repellent for leaf-cutting ants: Tea tree oil and its potential use as a management tool. Int. J. Pest Manag. 2021, 67, 1–9. [Google Scholar] [CrossRef]
- Benelli, G.; Canale, A.; Flamini, G.; Cioni, P.L.; Demi, F.; Ceccarini, L.; Macchia, M.; Conti, B. Biotoxicity of Melaleuca alternifolia (Myrtaceae) essential oil against the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), and its parasitoid Psyttalia concolor (Hymenoptera: Braconidae). Ind. Crops Prod. 2013, 50, 596–603. [Google Scholar] [CrossRef]
- Shelly, T.E.; Epsky, N.D. Exposure to tea tree oil enhances the mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Fla Entomol. 2015, 98, 1127–1133. [Google Scholar] [CrossRef][Green Version]
- Epsky, N.D.; Niogret, J. Short-range attraction of Ceratitis capitata (Diptera: Tephritidae) sterile males to six commercially available plant essential oils. Nat. Volatiles Essent. Oils 2017, 4, 1–7. Available online: https://www.nveo.org/index.php/journal/article/view/43 (accessed on 14 January 2025).
- Niogret, J.; Epsky, N.D.; Gill, M.A.; Espinoza, H.R.; Kendra, P.E.; Heath, R. Attraction and electroantennographic responses of male Mediterranean fruit fly (Diptera: Tephritidae) to six plant essential oils. J. Entomol. Zool. 2017, 5, 958–964. Available online: https://www.entomoljournal.com/archives/2017/vol5issue3/PartN/5-1-173-636.pdf (accessed on 14 January 2025).
- Food and Agriculture Organization of the United Nations; International Atomic Energy Agency. Trapping Guidelines for Area-Wide Fruit Fly Programmes, 2nd ed.; Enkerlin, W.R., Reyes-Flores, J., Eds.; License: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; p. 65. Available online: https://www.iaea.org/sites/default/files/trapping-guideline.pdf (accessed on 14 January 2025).
- Tisserand, R.; Young, R. Essential Oil Safety, 2nd ed.; Churchill Livingston Elsevier: London, UK, 2014; p. 440. [Google Scholar]
- Bejar, E. Adulteration of Tea Tree Oil (Melaleuca alternifolia and M. linariifolia). In Botanical Adulterants Prevention Bulletin; ABC-AHP-NCNPR Botanical Adulterants Prevention Program: Austin, TX, USA, 2017; pp. 1–8. [Google Scholar]
- International Standard ISO-4730; Essential Oil of Melaleuca, Terpinen-4-ol Type (Tea Tree oil). ISO: Geneva, Switzerland, 2017.
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of essential oils: A multitask issue for quality control. Three case studies: Lavandula angustifolia Mill., Citrus limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef]
- Bhushan, R. TLC separation and identification of essential oil constituents on silica gel plates. J. Liq. Chromatogr. 1982, 5, 1097–1102. [Google Scholar] [CrossRef]
- Heimler, D.; Veriano, V. Thin-layer chromatographic characterization of essential oils. J. Chromatogr. A 1998, 448, 301–305. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin, Germany, 2001; pp. 151–152. [Google Scholar]
- Golkiewicz, W.; Jaroniec, M. Continuous TLC as a pilot technique for optimization of gradient HPLC. 1. Theoretical considerations for stepwise gradient TLC with binary mobile phase. J. High Resolut. Chromatogr. 1978, 1, 245–249. [Google Scholar] [CrossRef]
- Fecka, I.; Cisowski, W. Multiple gradient development TLC in analysis of complex phenolic acids from Lycopus europeaus L. Chromatographia 1999, 49, 256–260. [Google Scholar] [CrossRef]
- Krecisz, P.; Czarnecka, K.; Szymanski, P. Thin-layer chromatography gradient optimization strategy for wet load adsorption flash chromatography. J. Chromatogr. Sci. 2022, 60, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, F.H. Two-step, two-dimensional development thin-layer chromatography of lipids on a micro-scale. J. Chromatogr. A 1973, 86, 197–199. [Google Scholar] [CrossRef]
- Wedge, D.E.; Nagle, D.G. A new 2D-TLC bioautography method for the discovery of novel antifungal agents to control plant pathogens. J. Nat. Prod. 2000, 63, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Cid-Hernández, M.; López Dellamary-Toral, F.A.; González-Ortiz, L.J.; Sánchez-Peña, M.J.; Pacheco-Moisés, F.P. Two-dimensional thin layer chromatography-bioautography designed to separate and locate metabolites with antioxidant activity contained on Spirulina platensis. Int. J. Anal. Chem. 2018, 2018, 4605373. [Google Scholar] [CrossRef] [PubMed]
- Glensk, M.; Bialy, Z.; Jurzysta, M.; Cisowski, W. Two-dimensional TLC with a sorbent gradient for the analysis of alfalfa root saponins. Chromatographia 2001, 54, 669–672. [Google Scholar] [CrossRef]
- Rabel, F.; Sherma, J. A review of advances in two-dimensional thin-layer chromatography. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 627–639. [Google Scholar] [CrossRef]
- Matysik, G.; Soczewiński, E. Stepwise gradient development in thin-layer chromatography: II. Two-dimensional gradients for complex mixtures. J. Chromatogr. A 1986, 369, 19–25. [Google Scholar] [CrossRef]
- Matysik, E.; Woźniak, A.; Paduch, R.; Rejdak, R.; Polak, B.; Donica, H. The new TLC method for separation and determination of multicomponent mixtures of plant extracts. J. Anal. Methods Chem. 2016, 2016, 1813581. [Google Scholar] [CrossRef]
- Tabanca, N.; Wedge, D.E.; Li, X.C.; Gao, Z.; Ozek, T.; Bernier, U.R.; Epsky, N.D.; Baser, K.H.C.; Ozek, G. Biological evaluation, overpressured layer chromatography separation, and isolation of a new acetylenic derivative compound from Prangos platychlaena ssp. platychlaena fruit essential oils. J. Planar Chromatogr. Mod. TLC 2018, 31, 61–71. [Google Scholar] [CrossRef]
- Łuczkiewicz, M.; Migas, P.; Kokotkiewicz, A.; Walijewska, M.; Cisowski, W. Two-dimensional TLC with adsorbent gradient for separation of quinolizidine alkaloids in the herb and in-vitro cultures of several Genista species. J. Planar Chromatogr. Mod. TLC 2004, 17, 89–94. [Google Scholar] [CrossRef]
- Valle, D.L., Jr.; Puzon, J.J.; Cabrera, E.C.; Rivera, W.L. Thin layer chromatography-bioautography and gas chromatography-mass spectrometry of antimicrobial leaf extracts from Philippine Piper betle L. against multidrug-resistant bacteria. J. Evid. Based Complement. Altern. Med. 2016, 2016, 4976791. [Google Scholar] [CrossRef]
- Rocamora, C.R.; Ramasamy, K.; Lim, S.M.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC based approach for bioassay-guided evaluation of antidiabetic and neuroprotective effects of eight essential oils of the Lamiaceae family plants. J. Pharm. Biomed. Anal. 2020, 178, 112909. [Google Scholar] [CrossRef]
- Perera, W.H.; Frommenwiler, D.A.; Sharaf, M.H.M.; Reich, E. An improved high-performance thin-layer chromatographic method to unambiguously assess Ginkgo biloba leaf finished products. J. Planar Chromatogr. Mod. TLC 2021, 34, 559–560. [Google Scholar] [CrossRef]
- Migacz, I.P.; Wang, M.; Faoro, J.A.M.; dos Santos, S.M.; Formagio, A.S.N.; Kassuya, C.A.L.; Rehman, J.U.; Perera, W.H.; Gonçalves, V.C.; de Almeida Chaves, D.S.; et al. Bioprospecting and repurposing of leaf biomass to support sustainable biopharmacy: Evaluation of seasonal chemical variations and biological activities of six Eucalyptus essential oils. Int. J. Environ. Res. 2024, 18, 74. [Google Scholar] [CrossRef]
- Reich, E. Instrumental Thin-Layer Chromatography (Planar Chromatography). In Handbook of Thin-Layer Chromatography, 3rd ed.; Sherma, J., Fried, B., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 135–151. [Google Scholar]
- Reich, E.; Schibli, A.; Debatt, A. Validation of high-performance thin-layer chromatographic methods for the identification of botanicals in a cGMP environment. J. AOAC Int. 2008, 91, 13–20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2662610/ (accessed on 2 December 2024). [CrossRef]
- Do, T.K.T.; Trettin, I.; De Vaumas, R.; Cañigueral, S.; Valder, C.; Reich, E. Proposal for a standardized method for the identification of essential oils by HPTLC. Pharmeuropa Bio Sci. Notes 2021, 2021, 157–166. [Google Scholar]
- Cieśla, L.; Bogucka-Kocka, A.; Hajnos, M.; Petruczynik, A.; Waksmundzka-Hajnos, M. Two-dimensional thin-layer chromatography with adsorbent gradient as a method of chromatographic fingerprinting of furanocoumarins for distinguishing selected varieties and forms of Heracleum spp. J. Chromatogr. A 2008, 1207, 160–168. [Google Scholar] [CrossRef]
- Cieśla, Ł.; Skalicka-Woźniak, K.; Hajnos, M.; Hawrył, M.; Waksmundzka-Hajnos, M. Multidimensional TLC procedure for separation of complex natural mixtures spanning a wide polarity range; Application for fingerprint construction and for investigation of systematic relationships within the Peucedanum genus. Acta Chromatogr. 2009, 21, 641–657. [Google Scholar] [CrossRef]
- Hałka-Grysińska, A.; Dzido, T.H.; Sitarczyk, E.; Klimek-Turek, A.; Chomicki, A. A new semiautomatic device with horizontal developing chamber for gradient thin-layer chromatography. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 257–263. [Google Scholar] [CrossRef]
- Duncan, J.D.; Armstrong, D.W.; Stalcup, A.M. Normal phase TLC separation of enantiomers using chiral ion interaction agents. J. Liq. Chromatogr. 1990, 13, 1091–1103. [Google Scholar] [CrossRef]
- Del Bubba, M.; Checchini, L.; Lepri, L. Thin-layer chromatography enantioseparations on chiral stationary phases: A review. Anal. Bioanal. Chem. 2013, 405, 533–554. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2017, 96, 151–171. [Google Scholar] [CrossRef]
- Tarafder, A.; Miller, L. Chiral chromatography method screening strategies: Past, present and future. J. Chromatogr. A 2021, 1638, 461878. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Avula, B.; Wang, Y.H.; Chittiboyina, A.G.; Parcher, J.F.; Khan, I.A. Quality evaluation of terpinen-4-ol-type Australian tea tree oils and commercial products: An integrated approach using conventional and chiral GC/MS combined with chemometrics. J. Agric. Food Chem. 2015, 63, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.F.; Davies, N.W.; Chin, S.T.; Larkman, T.; Marriott, P.J. Enantiomeric distribution of selected terpenes for authenticity assessment of Australian Melaleuca alternifolia oil. Ind. Crops Prod. 2015, 67, 475–483. [Google Scholar] [CrossRef]
- Linge, K.L.; Cooper, L.; Downey, A. Comparison of approaches for authentication of commercial terpinen-4-ol-type tea tree oils using chiral GC/MS. J. Agric. Food Chem. 2024, 72, 8389–8400. [Google Scholar] [CrossRef] [PubMed]
- Ozek, T.; Tabanca, N.; Demirci, F.; Wedge, D.E.; Baser, K.H.C. Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod. 2010, 4, 180–192. [Google Scholar]
- Allenspach, M.; Valder, C.; Flamm, D.; Steuer, C. Authenticity control of pine sylvestris essential oil by chiral gas chromatographic analysis of α-pinene. Sci. Rep. 2021, 11, 16923. [Google Scholar] [CrossRef]
- Davies, N.W.; Larkman, T.; Marriott, P.J.; Khan, I.A. Determination of enantiomeric distribution of terpenes for quality assessment of Australian tea tree oil. J. Agric. Food Chem. 2016, 64, 4817–4819. [Google Scholar] [CrossRef]
- Tabanca, N.; Niogret, J.; Kendra, P.K.; Epsky, N.D. TLC-based bioassay to isolate kairomones from tea tree essential oil that attract male Mediterranean fruit flies, Ceratitis capitata (Wiedemann). Biomolecules 2020, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Tabanca, N.; Kendra, P.E. Identification of Melaleuca alternifolia essential oil USDA. In HPTLC Association Methods for Identification of Complex Mixtures: Essential Oils; HPTLC Association: Rheinfelden, Switzerland, 2024; Available online: https://www.hptlc-association.org/methods/method_for_identification_of_complex_mixtures.cfm (accessed on 25 November 2024).
- Do, T.K.T.; Schmid, M.; Phanse, M.; Charegaonkar, A.; Sprecher, H.; Obkircher, M.; Reich, E. Development of the first universal mixture for use in system suitability tests for high-performance thin layer chromatography. J. Chromatogr. A 2021, 1638, 461830. [Google Scholar] [CrossRef] [PubMed]
- MassFinder. MassFinder Software, version 3; Dr. Hochmuth Scientific Consulting: Hamburg, Germany, 2004. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp: Carol Stream, IL, USA, 2017; pp. 1–804. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Wiley Science Solution. Wiley Registry 12th Edition/NIST 2020 Mass Spectral Library; Solutions; Scientific Instrument Services Inc.: Ringoes, NJ, USA, 2020. [Google Scholar]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Dowell, A. Tea Tree Oil Laboratory Guidance Document. In Botanical Adulterants Prevention Bulletin; ABC-AHP-NCNP Botanical Adulterants Prevention Program: Austin, TX, USA, 2018; pp. 1–13. [Google Scholar]
- Niaouli oil (Melaleuca quinquenervia, Melaleuca viridiflora, Melaleuca leucadendra) HPTLC Assoc. In HPTLC Association Methods for Identification of Herbal Drugs and Herbal Drug Preparations: Melaleuca sp.; HPTLC Association: Rheinfelden, Switzerland, 2012; Available online: https://www.hptlc-association.org/methods/methods_for_identification_of_herbals.cfm (accessed on 9 January 2025).
- Vázquez, A.; Tabanca, N.; Kendra, P.E. HPTLC analysis and chemical composition of selected Melaleuca essential oils. Molecules 2023, 28, 3925. [Google Scholar] [CrossRef] [PubMed]

| Track | Name | CAS # | Source |
|---|---|---|---|
| 1 | UHM | --- | Sigma- Aldrich, St. Louis, MO, USA |
| 2 | (−)-α-terpineol | 10482-56-1 | Sigma- Aldrich, St. Louis, MO, USA |
| 3 | viridiflorol | 0552-02-03 | Sigma- Aldrich, St. Louis, MO, USA |
| 4 | (−)-linalool | 1126-91-0 | Sigma- Aldrich, St. Louis, MO, USA |
| 5 | (−)-terpinen-4-ol | 20126-76-5 | Sigma- Aldrich, St. Louis, MO, USA |
| 6 | nerolidol | 7212-44-4 | Sigma- Aldrich, St. Louis, MO, USA |
| 7 | 1,8-cineole | 470-82-6 | Sigma- Aldrich, St. Louis, MO, USA |
| 8 | M. alternifolia (Maiden & Betche) Cheel (tea tree oil) | --- | Apothecary Shoppe, Portland, OR, USA |
| 9 | M. alternifolia (Maiden & Betche) Cheel (tea tree oil) | --- | SAT Group, Kannauj, India |
| 10 | M. cajuputi Powell (Cajeput oil) | --- | Nature’s Gift, Madison, TN, USA |
| 11 | M. quinquenervia (Cav.) S.T.Blake linalool CT (Nerolina oil) | --- | Nature’s Gift, Madison, TN, USA |
| 12 | M. quinquenervia (Cav.) S.T.Blake cineole CT (Niaouli oil) | --- | Nature’s Gift, Madison, TN, USA |
| 13 | M. ericifolia Sm. (Rosalina oil) | --- | Nature’s Gift, Madison, TN, USA |
| Development Number | Solvent System | Vol, mL | Ratio | Solvent Front, cm | Direction |
|---|---|---|---|---|---|
| 1 | n-hexane/ethyl acetate | 50 | 82:18 | 18 | 0° (traditional position) |
| 2 | n-hexane/ethyl acetate | 50 | 82:18 | 18 | 90° clockwise |
| Development Number | Solvent System | Vol, mL | Ratio | Solvent Front, cm | Direction |
|---|---|---|---|---|---|
| 1 | n-hexane/ethyl acetate | 50 | 85:15 | 18 | 0° (traditional position) |
| 2 | n-hexane/ethyl acetate | 50 | 99:1 | 18 | 90° clockwise |
| 3 | n-hexane/ethyl acetate | 50 | 97:3 | 17 | Remain at 90° |
| 4 | n-hexane/ethyl acetate | 50 | 95:5 | 15 | Remain at 90° |
| * RI Exp | ** RI Lit | Compounds | Total TTO | Fr-1 | Fr-2 | Fr-3 | Fr-4 | Fr-5 | Fr-6 |
|---|---|---|---|---|---|---|---|---|---|
| 938 | 930 | α-Thujene RI, MS | 0.09 | - | - | - | - | - | - |
| 946 | 939 | α-Pinene RI, MS, Std | 3.37 | 0.11 | - | - | - | - | - |
| 970 | 975 | Sabinene | 0.05 | - | - | - | - | - | - |
| 989 | 979 | β-Pinene RI, MS, Std | 0.17 | 0.44 | 0.26 | - | - | - | - |
| 998 | 990 | Myrcene RI, MS, Std | 0.64 | 0.24 | 0.13 | - | - | - | - |
| 1010 | 1002 | α-Phellandrene RI, MS, Std | 0.58 | 0.54 | 0.21 | 0.03 | - | - | - |
| 1022 | 1017 | α-Terpinene RI, MS, Std | 10.7 | 27.39 | 6.57 | 1.39 | - | - | - |
| 1030 | 1024 | p-Cymene RI, MS, Std | 1.0 | 3.24 | 1.19 | 0.12 | - | - | - |
| 1039 | 1029 | Limonene RI, MS, Std | 0.5 | 0.71 | 0.06 | - | - | - | - |
| 1039 | 1029 | β-Phellandrene RI, MS, Std | 0.38 | - | 0.04 | - | - | - | - |
| 1040 | 1031 | 1,8-Cineole RI, MS, Std | 2.29 | - | - | 1.87 | - | - | - |
| 1071 | 1059 | γ-Terpinene RI, MS, Std | 19.79 | 57.94 | 17.75 | 3.20 | - | - | - |
| 1081 | 1070 | cis-Sabinene hydrate RI, MS | 0.01 | - | - | - | - | 3.09 | - |
| 1084 | 1072 | cis-Linalool oxide RI, MS, Std | - | - | - | - | - | - | - |
| 1098 | 1088 | Terpinolene RI, MS, Std | 3.63 | 9.39 | 5.12 | 0.76 | - | - | - |
| 1107 | 1096 | Linalool RI, MS, Std | 0.12 | - | - | 0 | - | - | 0.57 |
| 1123 | 1121 | cis-p-Menth-2-en-1-ol | 0.28 | - | - | 0 | - | - | 8.15 |
| 1125 | 1122 | trans-p-Menth-2-en-1-ol | 0.19 | - | - | 0 | - | - | 4.93 |
| 1183 | 1177 | Terpinen-4-ol RI, MS, Std | 37.95 | - | - | 91.53 | 100 | - | - |
| 1188 | 1182 | p-Cymen-8-ol RI, MS | - | - | - | - | - | - | 5.65 |
| 1198 | 1188 | α-Terpineol RI, MS, Std | 3.12 | - | - | 0.98 | - | 94.06 | 1.38 |
| 1206 | 1196 | cis-Piperitol RI, MS, Std | 0.07 | - | - | - | - | - | 0.68 |
| 1218 | 1208 | trans-Piperitol RI, MS | 0.1 | - | - | - | - | - | 4.94 |
| 1273 | 1269 | trans-Ascaridol glycol RI, MS | 0.02 | - | - | - | - | - | 24.11 |
| 1291 | 1288 | cis-Ascaridol glycol RI, MS | 0.01 | - | - | - | - | - | - |
| 1356 | 1348 | α-Cubebene RI, MS | 0.05 | - | 0.50 | - | - | - | - |
| 1378 | 1376 | Isoledene RI, MS | 0.09 | - | 0.48 | - | - | - | - |
| 1380 | 1376 | α-Copaene RI, MS, Std | 0.16 | - | 1.00 | - | - | - | - |
| 1395 | 1390 | β-Elemene RI, MS, Std | 0.02 | - | 0.23 | - | - | - | - |
| 1410 | 1409 | α-Gurjunene RI, MS | 0.61 | - | 2.87 | - | - | - | - |
| 1417 | 1416 | β-Maaliene RI, MS | 0.03 | - | 0.25 | - | - | - | - |
| 1421 | 1419 | β-Caryophyllene RI, MS, Std | 0.77 | - | 4.33 | - | - | - | - |
| 1429 | 1425 | γ-Maaliene RI, MS | 0.09 | - | 0.68 | - | - | - | - |
| 1435 | 1433 | α-Maaliene RI, MS, Std | 0.05 | - | 0.71 | - | - | - | - |
| 1439 | 1441 | Aromadendrene RI, MS, Std | 1.90 | - | 10.24 | - | - | - | - |
| 1443 | 1443 | Selina-5,11-diene RI, MS | 0.23 | - | 1.42 | - | - | - | - |
| 1450 | 1453 | trans-Muurola-3.5-diene RI, MS | 0.21 | - | 1.30 | - | - | - | - |
| 1453 | 1454 | α-Humulene RI, MS, Std | 0.13 | - | 0.85 | - | - | - | - |
| 1459 | 1460 | Alloaromadendrene RI, MS, Std | 0.99 | - | 5.73 | - | - | - | - |
| 1473 | 1476 | trans-Cadina-1(6),4-diene RI, MS | 0.58 | - | 3.55 | - | - | - | - |
| 1476 | 1479 | γ-Muurolene RI, MS | 0.05 | - | 0.34 | - | - | - | - |
| 1486 | 1490 | β-Selinene RI, MS | 0.12 | - | 0.90 | - | - | - | - |
| 1488 | 1490 | Alloaromadendr-9-ene RI, MS | 0.30 | - | 0.90 | - | - | - | - |
| 1492 | 1493 | cis-β-Guaiene RI, MS | 0.12 | - | 1.76 | - | - | - | - |
| 1494 | 1496 | Ledene RI, MS | 2.02 | - | 11.69 | - | - | - | - |
| 1497 | 1500 | Bicyclogermacrene RI, MS | 0.43 | - | 1.98 | - | - | - | - |
| 1498 | 1500 | α-Muurolene RI, MS | 0.05 | - | 0.65 | - | - | - | - |
| 1501 | 1501 | Epizonarene RI, MS | 0.04 | - | 0.20 | - | - | - | - |
| 1512 | 1513 | γ-Cadinene RI, MS | 0.02 | - | 0.37 | - | - | - | - |
| 1518 | 1522 | trans-Calamene RI, MS | 0.01 | - | 0.25 | - | - | - | - |
| 1521 | 1523 | δ-Cadinene RI, MS | 2.27 | - | 11.74 | - | - | - | - |
| 1527 | 1529 | Zonarene RI, MS | 0.06 | - | 1.02 | - | - | - | - |
| 1531 | 1534 | trans-Cadina-1,4-diene | 0.29 | - | 1.85 | - | - | - | - |
| 1535 | 1538 | α-Cadinene RI, MS | 0.01 | - | 0.23 | - | - | - | - |
| 1560 | 1563 | (E)-Nerolidol RI, MS, Std | 0.07 | - | - | - | - | - | 1.31 |
| 1562 | 1567 | Maaliol RI, MS | 0.02 | - | - | - | - | - | 0.96 |
| 1563 | 1568 | Palustrol RI, MS | 0.09 | - | - | - | - | - | 0.92 |
| 1572 | 1578 | Spathulenol RI, MS | 0.09 | - | - | - | - | - | 18.85 |
| 1585 | 1590 | Globulol RI, MS, Std | 0.47 | - | - | - | - | - | 6.90 |
| 1586 | 1592 | Viridiflorol RI, MS, Std | 0.18 | - | - | - | - | - | 1.81 |
| 1588 | 1595 | Cubeban-11-ol RI, MS | 0.18 | - | - | - | - | - | 1.63 |
| 1594 | 1600 | Rosiflorol RI, MS | 0.14 | - | - | - | - | - | 5.15 |
| 1597 | 1600 | Guaiol RI, MS | 0.15 | - | - | - | - | - | 1.58 |
| 1599 | 1602 | Ledol RI, MS | 0.24 | - | - | - | - | - | 1.58 |
| 1609 | 1607 | 5-epi-7-epi-α-Eudesmol RI, MS | 0.12 | - | - | - | - | - | 4.79 |
| Total | 98.51 | 100 | 99.35 | 99.88 | 100 | 97.15 | 95.89 |
| Chemical | (+) Enantiomer (n = 3) | (−) Enantiomer (n = 3) | (+)/(−) Ratio (n = 3) |
|---|---|---|---|
| terpinen-4-ol | 69.4 ± 0.6 | 30.6 ± 0.6 | 2.27 ± 0.06 |
| α-terpineol | 77.4 ± 0.2 | 22.6 ± 0.2 | 3.42 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, A.; Tabanca, N. Development of a Combined 2D-MGD TLC/HPTLC Method for the Separation of Terpinen-4-ol and α-Terpineol from Tea Tree, Melaleuca alternifolia, Essential Oil. Biomolecules 2025, 15, 147. https://doi.org/10.3390/biom15010147
Vázquez A, Tabanca N. Development of a Combined 2D-MGD TLC/HPTLC Method for the Separation of Terpinen-4-ol and α-Terpineol from Tea Tree, Melaleuca alternifolia, Essential Oil. Biomolecules. 2025; 15(1):147. https://doi.org/10.3390/biom15010147
Chicago/Turabian StyleVázquez, Aimé, and Nurhayat Tabanca. 2025. "Development of a Combined 2D-MGD TLC/HPTLC Method for the Separation of Terpinen-4-ol and α-Terpineol from Tea Tree, Melaleuca alternifolia, Essential Oil" Biomolecules 15, no. 1: 147. https://doi.org/10.3390/biom15010147
APA StyleVázquez, A., & Tabanca, N. (2025). Development of a Combined 2D-MGD TLC/HPTLC Method for the Separation of Terpinen-4-ol and α-Terpineol from Tea Tree, Melaleuca alternifolia, Essential Oil. Biomolecules, 15(1), 147. https://doi.org/10.3390/biom15010147


_Kwok.png)




