Anti-Inflammatory and Anti-Migratory Effects of Morin on Non-Small-Cell Lung Cancer Metastasis via Inhibition of NLRP3/MAPK Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cell Cultures
2.3. The Sulforhodamine B (SRB) Assay
2.4. Measurement of Pro-Inflammatory Cytokines Released by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Wound–Scratch Assay
2.6. Cell Invasion Assay
2.7. Gelatin Zymography Assay
2.8. Measurement of Pro-Inflammatory Cytokines and NLRP3 mRNA Levels by Reverse Transcription–Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of Morin on NSCLC Cell Viability
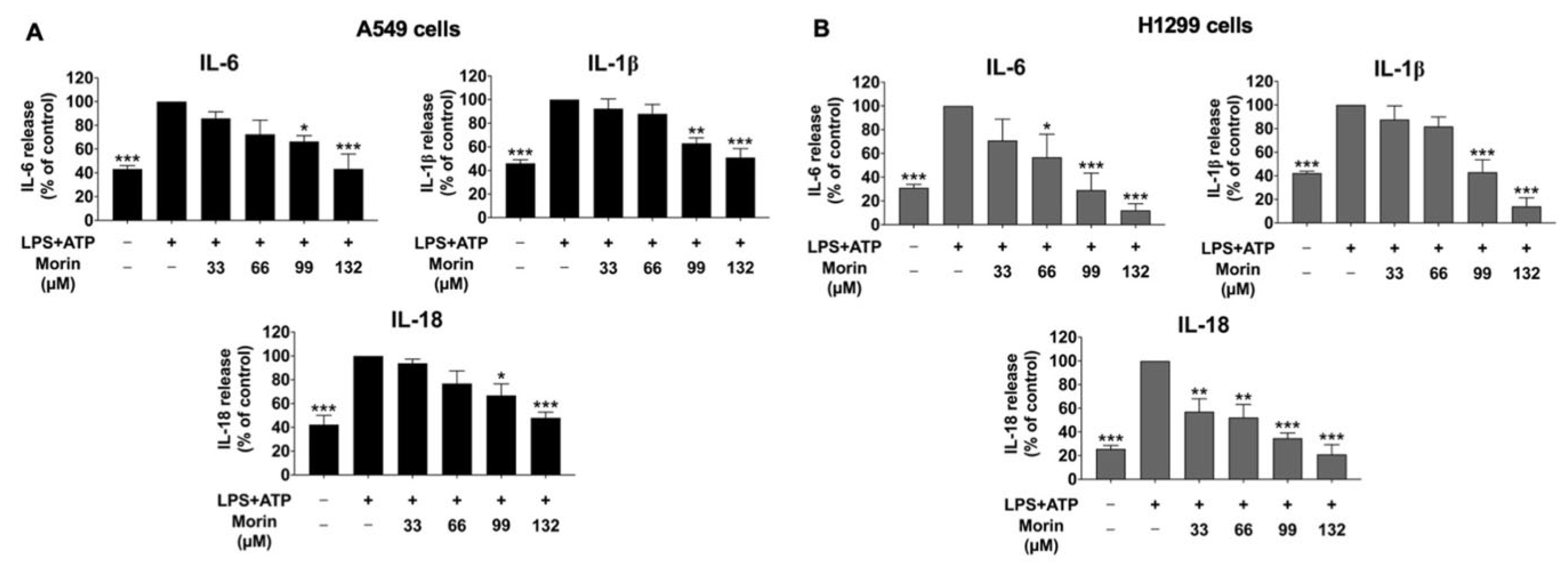
3.2. Effect of Morin on Anti-Inflammatory Properties in LPS+ATP-Stimulated Cytokine Secretion of NSCLC Cells
3.3. Effects of Morin on Anti-Migration Properties in LPS+ATP-Stimulated NSCLC Cells
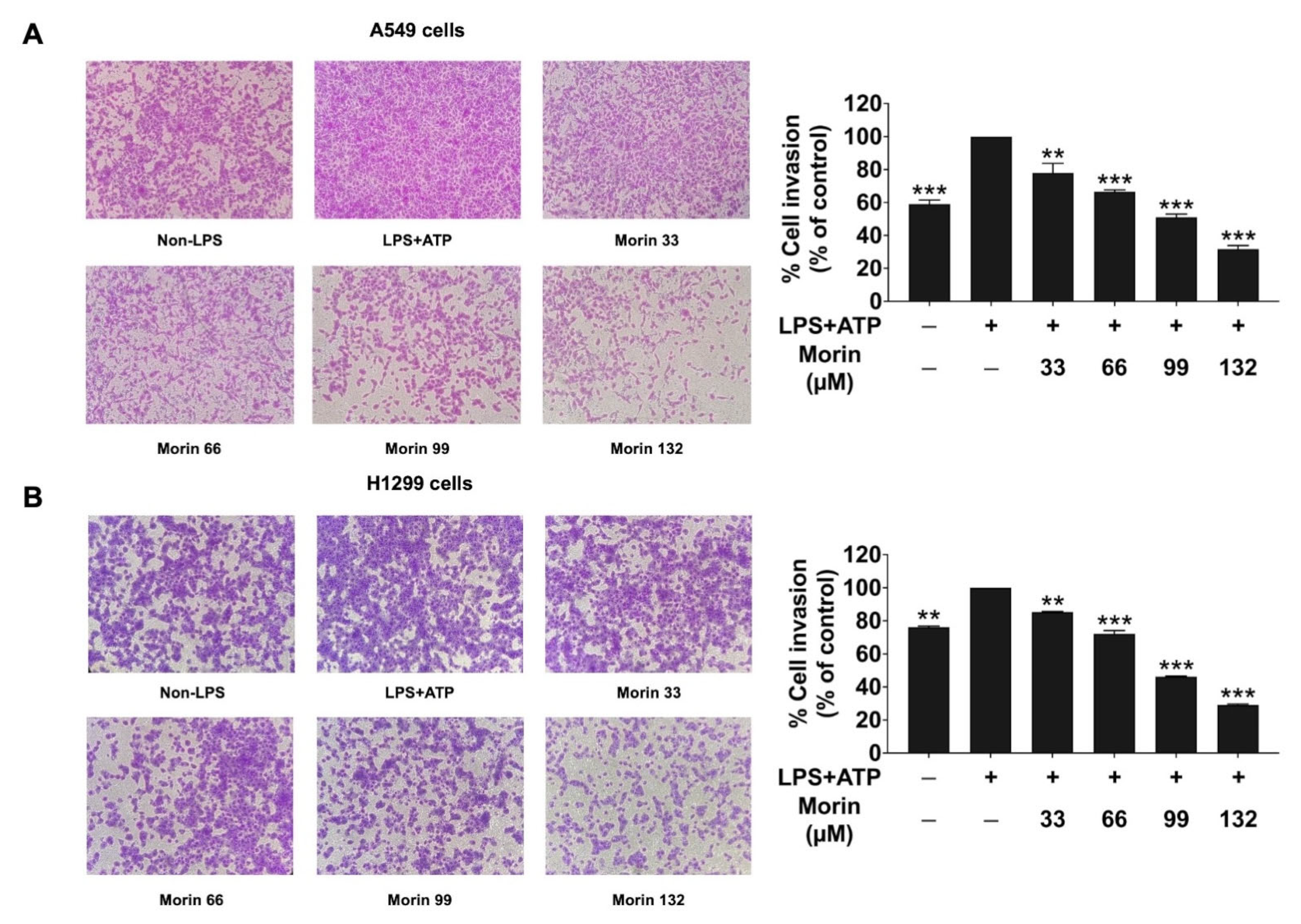
3.4. Effects of Morin on Anti-Invasion Properties in LPS+ATP-Stimulated NSCLC Cells
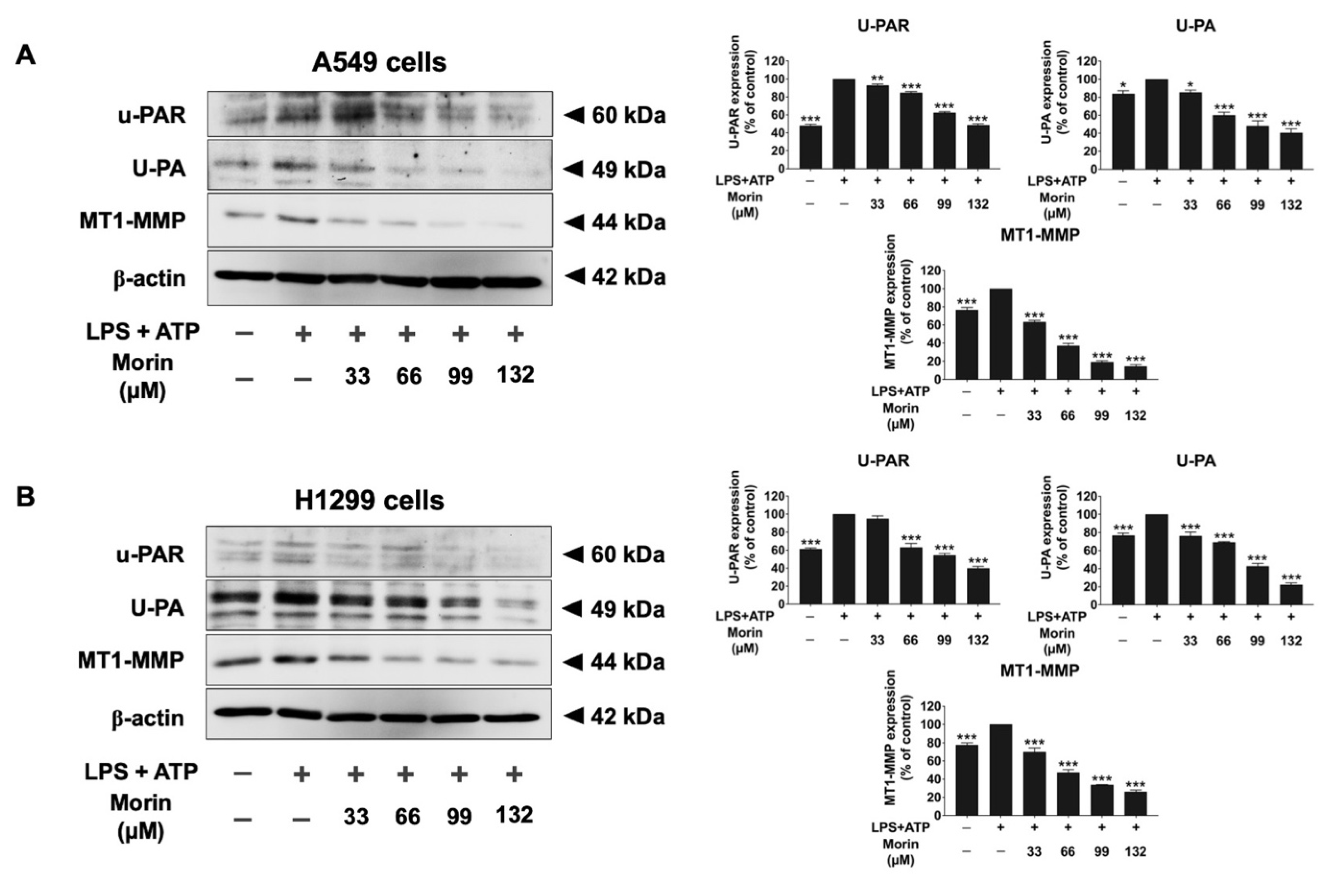
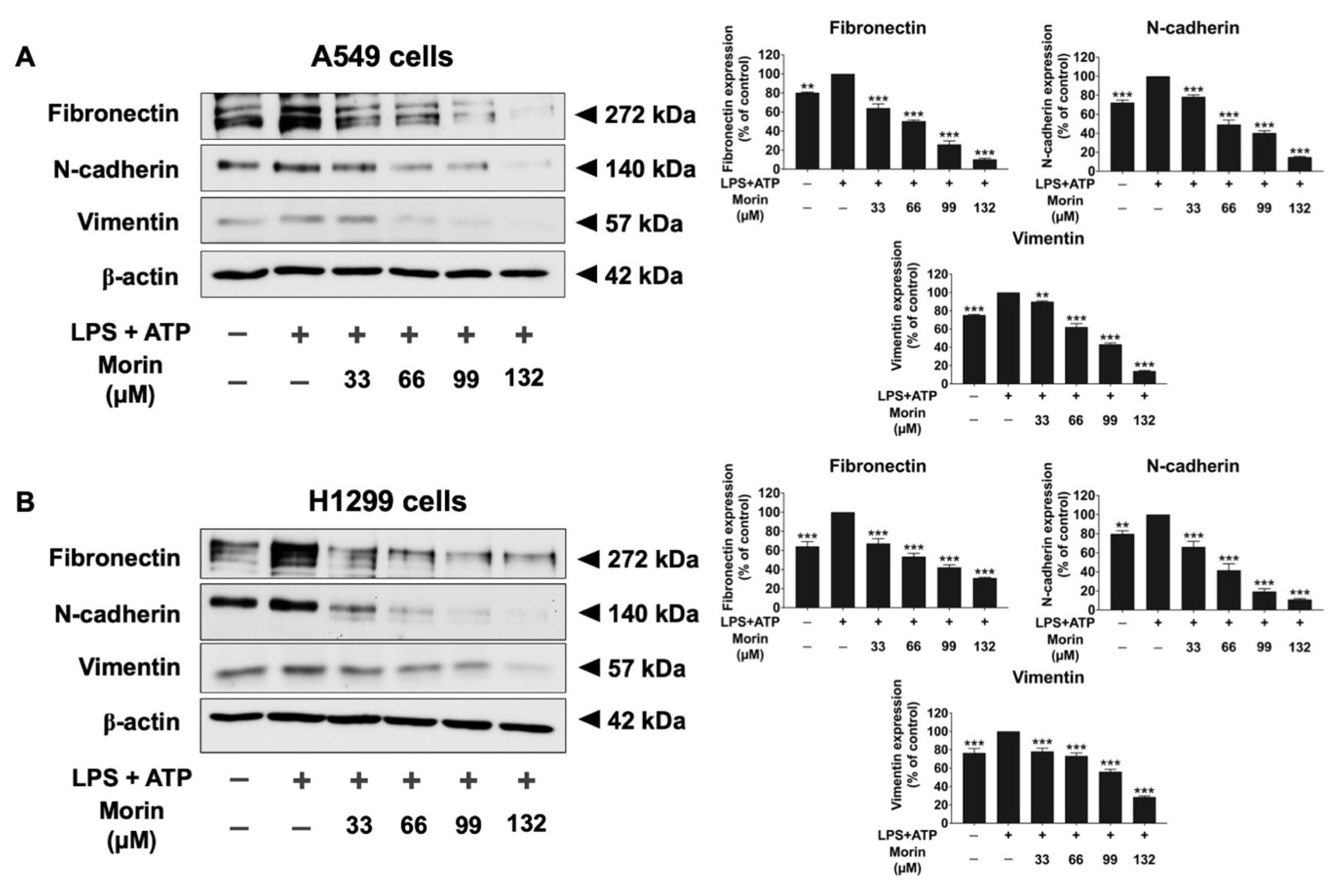
3.5. Effects of Morin on the Expression of Invasive Proteins and Mesenchymal Markers in LPS+ATP-Stimulated NSCLC Cells
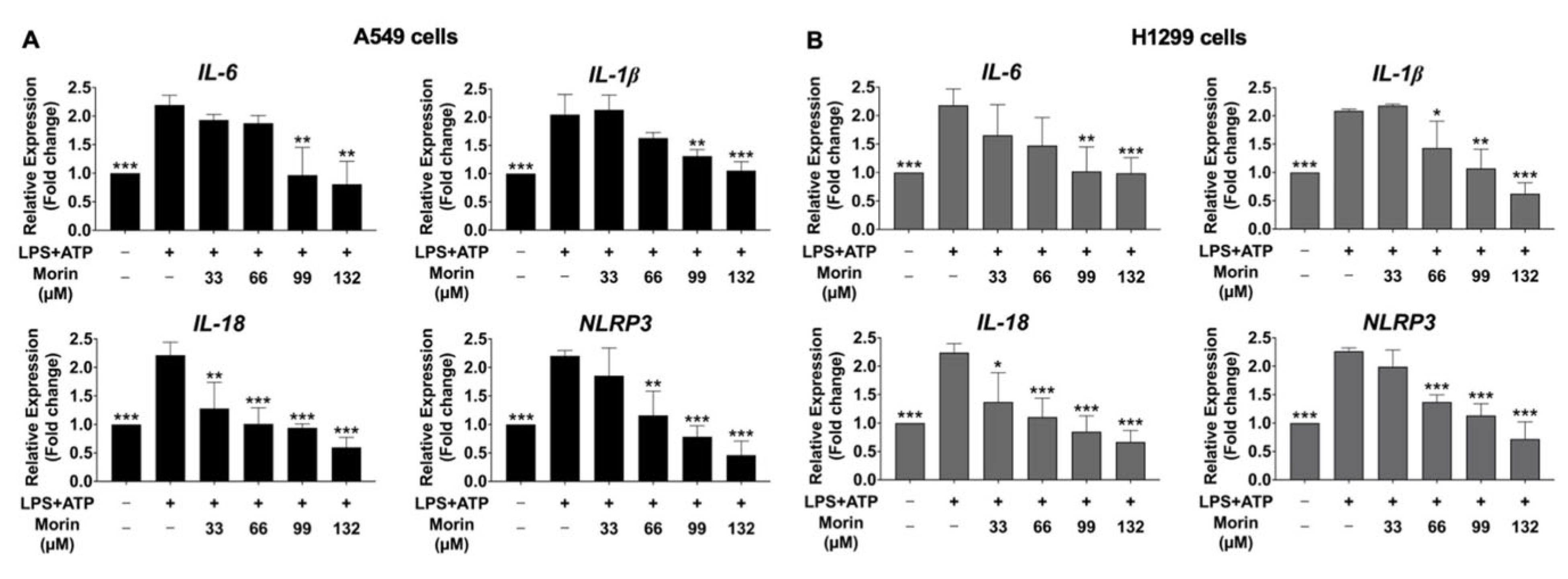
3.6. Inhibitory Effects of Morin on the Expression of Pro-Inflammatory Cytokines and NLRP3 Genes in LPS+ATP-Stimulated NSCLC Cells
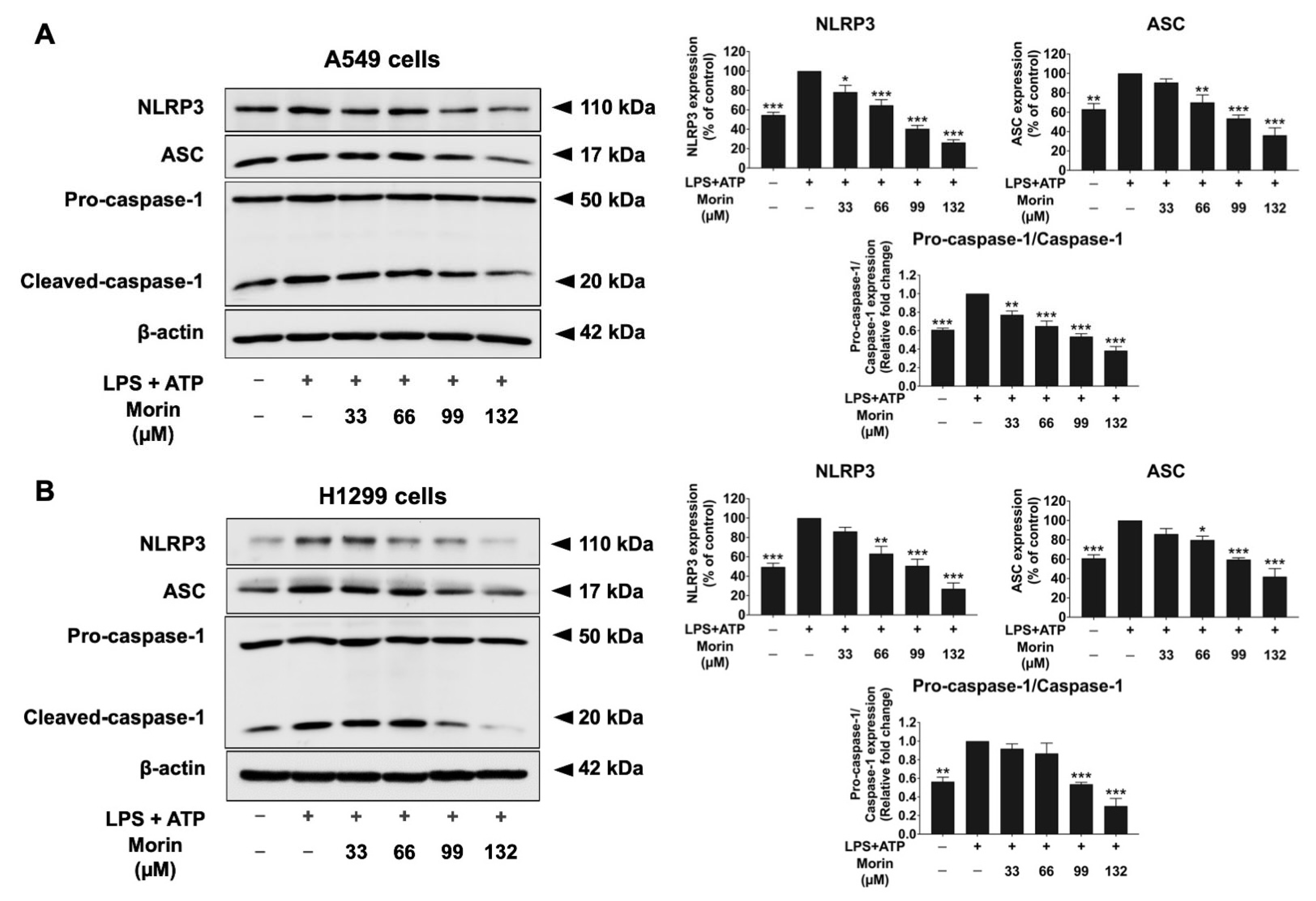
3.7. Effects of Morin on Expression of Protein-Related NLRP3 Inflammasome Pathway in LPS+ATP-Stimulated NSCLC Cells
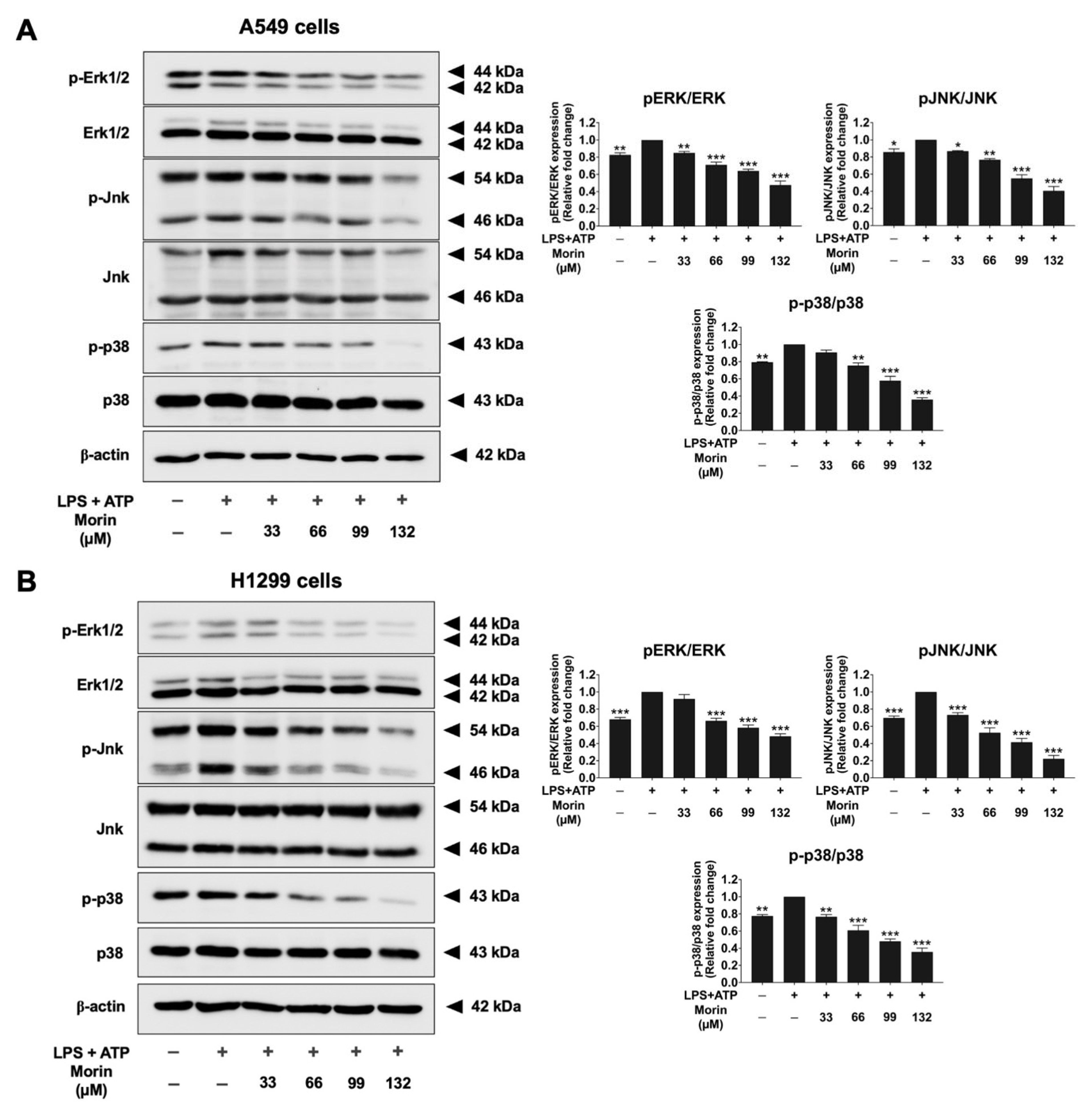
3.8. Effects of Morin on the MAPK Signaling Pathway in LPS+ATP-Stimulated NSCLC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Nelson, R.A.; Bogardus, A.; Grannis, F.W., Jr. Five-year lung cancer survival: Which advanced stage nonsmall cell lung cancer patients attain long-term survival? Cancer 2010, 116, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deng, J.; Liu, Y.; Wang, H.; Zhao, S.; He, Y.; Zhou, C. Analysis of metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Ann. Transl. Med. 2021, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.Y.; Zhou, Q.; Yang, J.J.; Zhong, W.Z.; Chen, Z.H.; Deng, W.; He, Y.Y.; Chen, H.J.; Zeng, Z.; Ke, E.E.; et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, G.H.; Toguri, D.; Dahele, M.; Warner, A.; de Haan, P.F.; Rodrigues, G.B.; Slotman, B.J.; Yaremko, B.P.; Senan, S.; Palma, D.A. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): Patient outcomes and prognostic factors. Lung Cancer 2013, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Lin, T.Y.; Tsai, M.C.; Tu, W.; Yeh, H.C.; Wang, S.C.; Huang, S.P.; Li, C.Y. Role of the NLRP3 Inflammasome: Insights Into Cancer Hallmarks. Front. Immunol. 2020, 11, 610492. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu Rev Immunol 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Zhu, X.; Zhou, B.; Jin, K.; Dai, J.; Jiang, G. Effect of NLRP3 Inflammasome on Lung Cancer Immune Microenvironment Activation and Its Mechanism. Altern. Ther. Health Med. 2024, 30, 86–91. [Google Scholar]
- Okamoto, M.; Liu, W.; Luo, Y.; Tanaka, A.; Cai, X.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J. Biol. Chem. 2010, 285, 6477–6488. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Lu, L.; Li, B.; Shi, X.; Jin, H.; Hu, W. The roles of inflammasomes in cancer. Front. Immunol. 2023, 14, 1195572. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Q.; Feng, X.; Zhang, R.; Li, J.; Chen, F. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer 2018, 18, 500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Xu, Y.; Peng, T.; Meng, X.; Zou, F. NLRP3 induces the autocrine secretion of IL-1β to promote epithelial-mesenchymal transition and metastasis in breast cancer. Biochem. Biophys. Res. Commun. 2021, 560, 72–79. [Google Scholar] [CrossRef]
- Lee, H.E.; Lee, J.Y.; Yang, G.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Inhibition of NLRP3 inflammasome in tumor microenvironment leads to suppression of metastatic potential of cancer cells. Sci. Rep. 2019, 9, 12277. [Google Scholar] [CrossRef]
- Zou, J.; Yang, Y.; Yang, Y.; Liu, X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomed. Pharmacother. 2018, 108, 130–136. [Google Scholar] [CrossRef]
- Rueankham, L.; Panyajai, P.; Saiai, A.; Rungrojsakul, M.; Tima, S.; Chiampanichayakul, S.; Yeerong, K.; Somwongin, S.; Chaiyana, W.; Dejkriengkraikul, P.; et al. Biological activities of extracts and compounds from Thai Kae-Lae (Maclura cochinchinensis (Lour.) Corner). BMC Complement. Med. Ther. 2023, 23, 191. [Google Scholar] [CrossRef]
- Balaga, V.K.R.; Pradhan, A.; Thapa, R.; Patel, N.; Mishra, R.; Singla, N. Morin: A Comprehensive Review on Its Versatile Biological Activity and Associated Therapeutic Potential in Treating Cancers. Pharmacol. Res.—Mod. Chin. Med. 2023, 7, 100264. [Google Scholar] [CrossRef]
- Solairaja, S.; Andrabi, M.Q.; Dunna, N.R.; Venkatabalasubramanian, S. Overview of Morin and Its Complementary Role as an Adjuvant for Anticancer Agents. Nutr. Cancer 2021, 73, 927–942. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.Q.; Yan, H.C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef]
- Gálvez, J.; Coelho, G.; Crespo, M.E.; Cruz, T.; Rodríguez-Cabezas, M.E.; Concha, A.; Gonzalez, M.; Zarzuelo, A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001, 15, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ge, A.; Zhu, W.; Liu, Y.N.; Ji, N.F.; Zha, W.J.; Zhang, J.X.; Zeng, X.N.; Huang, M. Morin Attenuates Ovalbumin-Induced Airway Inflammation by Modulating Oxidative Stress-Responsive MAPK Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 5843672. [Google Scholar] [CrossRef]
- Jin, H.; Lee, W.S.; Eun, S.Y.; Jung, J.H.; Park, H.S.; Kim, G.; Choi, Y.H.; Ryu, C.H.; Jung, J.M.; Hong, S.C.; et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014, 45, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio-Protoc 2016, 6, e1984. [Google Scholar] [CrossRef]
- Arjsri, P.; Srisawad, K.; Mapoung, S.; Semmarath, W.; Thippraphan, P.; Umsumarng, S.; Yodkeeree, S.; Dejkriengkraikul, P. Hesperetin from Root Extract of Clerodendrum petasites S. Moore Inhibits SARS-CoV-2 Spike Protein S1 Subunit-Induced NLRP3 Inflammasome in A549 Lung Cells via Modulation of the Akt/MAPK/AP-1 Pathway. Int. J. Mol. Sci. 2022, 23, 10346. [Google Scholar] [CrossRef] [PubMed]
- Semmarath, W.; Srisawad, K.; Arjsri, P.; Umsumarng, S.; Yodkeeree, S.; Jamjod, S.; Prom, U.T.C.; Dejkriengkraikul, P. Protective Effects of Proanthocyanidin-Rich Fraction from Red Rice Germ and Bran on Lung Cell Inflammation via Inhibition of NF-κB/NLRP3 Inflammasome Pathway. Nutrients 2023, 15, 3793. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.S.; Zhao, Y.H.; Zhong, Y.; Li, X.Z.; Pu, J.X.; Luo, Y.C.; Zhou, Q.L. Neferine inhibits LPS-ATP-induced endothelial cell pyroptosis via regulation of ROS/NLRP3/Caspase-1 signaling pathway. Inflamm. Res. 2019, 68, 727–738. [Google Scholar] [CrossRef]
- Bobadilla, A.V.P.; Arévalo, J.; Sarró, E.; Byrne, H.M.; Maini, P.K.; Carraro, T.; Balocco, S.; Meseguer, A.; Alarcón, T. In vitro cell migration quantification method for scratch assays. J. R. Soc. Interface 2019, 16, 20180709. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Arjsri, P.; Srisawad, K.; Semmarath, W.; Umsumarng, S.; Rueankham, L.; Saiai, A.; Rungrojsakul, M.; Katekunlaphan, T.; Anuchapreeda, S.; Dejkriengkraikul, P. Suppression of inflammation-induced lung cancer cells proliferation and metastasis by exiguaflavanone A and exiguaflavanone B from Sophora exigua root extract through NLRP3 inflammasome pathway inhibition. Front. Pharmacol. 2023, 14, 1243727. [Google Scholar] [CrossRef]
- Chittasupho, C.; Umsumarng, S.; Srisawad, K.; Arjsri, P.; Phongpradist, R.; Samee, W.; Tingya, W.; Ampasavate, C.; Dejkriengkraikul, P. Inhibition of SARS-CoV-2-Induced NLRP3 Inflammasome-Mediated Lung Cell Inflammation by Triphala-Loaded Nanoparticle Targeting Spike Glycoprotein S1. Pharmaceutics 2024, 16, 751. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Choi, S.I.; Kim, E.K. Uptake of cell debris and enhanced expression of inflammatory factors in response to dead cells in corneal fibroblast cells. Exp. Eye Res. 2020, 194, 108017. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Parbin, S.; Kausar, C.; Kar, S.; Mawatwal, S.; Das, L.; Deb, M.; Sengupta, D.; Dhiman, R.; Patra, S.K. Paederia foetida induces anticancer activity by modulating chromatin modification enzymes and altering pro-inflammatory cytokine gene expression in human prostate cancer cells. Food Chem. Toxicol. 2019, 130, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Plotnikova, M.A.; Klotchenko, S.A.; Vasin, A.V. Development of a multiplex quantitative PCR assay for the analysis of human cytokine gene expression in influenza A virus-infected cells. J. Immunol. Methods 2016, 430, 51–55. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zheng, J.H.; Li, X.; Yao, Y.L.; Wu, Y.L.; Song, S.Z.; Sun, P.; Nan, J.X.; Lian, L.H. Potentiation of hepatic stellate cell activation by extracellular ATP is dependent on P2X7R-mediated NLRP3 inflammasome activation. Pharmacol. Res. 2017, 117, 82–93. [Google Scholar] [CrossRef]
- Balahura Stămat, L.R.; Dinescu, S. Inhibition of NLRP3 inflammasome contributes to paclitaxel efficacy in triple negative breast cancer treatment. Sci. Rep. 2024, 14, 24753. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Nguyen, T.Q.C.; Duy Binh, T.; Pham, T.L.A.; Nguyen, Y.D.H.; Thi Xuan Trang, D.; Nguyen, T.T.; Kanaori, K.; Kamei, K. Anti-Inflammatory Effects of Lasia spinosa Leaf Extract in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2020, 21, 3439. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Yang, X.; Wang, W.; Zhang, J.; He, Y.; Zhang, W.; Jing, T.; Wang, B.; Lin, R. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol. Immunol. 2016, 77, 148–156. [Google Scholar] [CrossRef]
- Zhao, L.R.; Xing, R.L.; Wang, P.M.; Zhang, N.S.; Yin, S.J.; Li, X.C.; Zhang, L. NLRP1 and NLRP3 inflammasomes mediate LPS/ATP-induced pyroptosis in knee osteoarthritis. Mol. Med. Rep. 2018, 17, 5463–5469. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Shen, W.W.; Zhong, J.; Huang, C.; Zhang, L.; Li, J. Lipopolysaccharide/adenosine triphosphate induces IL-1β and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int. J. Mol. Med. 2014, 34, 341–349. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, H.; Zeng, X.; Liu, W.; Wang, Z.; Yan, X.; Wang, H.; Xie, W. Activation of NLRP3 inflammasome enhances the proliferation and migration of A549 lung cancer cells. Oncol. Rep. 2016, 35, 2053–2064. [Google Scholar] [CrossRef]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Bonina, F.; Cardile, V. Hydroxytyrosol from olive fruits prevents blue-light-induced damage in human keratinocytes and fibroblasts. J. Cell. Physiol. 2019, 234, 9065–9076. [Google Scholar] [CrossRef]
- Gouravani, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. The NLRP3 inflammasome: A therapeutic target for inflammation-associated cancers. Expert Rev. Clin. Immunol. 2020, 16, 175–187. [Google Scholar] [CrossRef]
- Shin, S.S.; Won, S.Y.; Noh, D.H.; Hwang, B.; Kim, W.J.; Moon, S.K. Morin Inhibits Proliferation, Migration, and Invasion of Bladder Cancer EJ Cells via Modulation of Signaling Pathways, Cell Cycle Regulators, and Transcription Factor-Mediated MMP-9 Expression. Drug Dev. Res. 2017, 78, 81–90. [Google Scholar] [CrossRef]
- Nie, Z.Y.; Yang, L.; Liu, X.J.; Yang, Z.; Yang, G.S.; Zhou, J.; Qin, Y.; Yu, J.; Jiang, L.L.; Wen, J.K.; et al. Morin Inhibits Proliferation and Induces Apoptosis by Modulating the miR-188-5p/PTEN/AKT Regulatory Pathway in CML Cells. Mol. Cancer Ther. 2019, 18, 2296–2307. [Google Scholar] [CrossRef]
- Jung, J.S.; Choi, M.J.; Lee, Y.Y.; Moon, B.I.; Park, J.S.; Kim, H.S. Suppression of Lipopolysaccharide-Induced Neuroinflammation by Morin via MAPK, PI3K/Akt, and PKA/HO-1 Signaling Pathway Modulation. J. Agric. Food Chem. 2017, 65, 373–382. [Google Scholar] [CrossRef]
- Mo, J.S.; Choi, D.; Han, Y.R.; Kim, N.; Jeong, H.S. Morin has protective potential against ER stress induced apoptosis in renal proximal tubular HK-2 cells. Biomed. Pharmacother. 2019, 112, 108659. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, Z.Q.; Wang, H.Y.; Cheng, Y.N.; Yu, L.G.; Zhang, X.K.; Sun, Y.; Guo, X.L. The anti-inflammatory effects of Morin hydrate in atherosclerosis is associated with autophagy induction through cAMP signaling. Mol. Nutr. Food Res. 2017, 61, 1600966. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Yu, D.; Changyong, E.; Yang, J. Morin Protects LPS-Induced Mastitis via Inhibiting NLRP3 Inflammasome and NF-κB Signaling Pathways. Inflammation 2020, 43, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, T.; Wang, G.; Wang, H.; Che, X.; Gao, X.; Liu, H. Rac3 Regulates Cell Invasion, Migration and EMT in Lung Adenocarcinoma through p38 MAPK Pathway. J. Cancer 2017, 8, 2511–2522. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef]
- Pradhan, R.; Singhvi, G.; Dubey, S.K.; Gupta, G.; Dua, K. MAPK pathway: A potential target for the treatment of non-small-cell lung carcinoma. Future Med. Chem. 2019, 11, 793–795. [Google Scholar] [CrossRef]
- Zheng, G.; Shen, Z.; Chen, H.; Liu, J.; Jiang, K.; Fan, L.; Jia, L.; Shao, J. Metapristone suppresses non-small cell lung cancer proliferation and metastasis via modulating RAS/RAF/MEK/MAPK signaling pathway. Biomed. Pharmacother. 2017, 90, 437–445. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Yang, Y.; Liu, X.; Li, R.; Zhang, M.; Yin, Y.; Qu, Y. Synaptotagmin 7 in twist-related protein 1-mediated epithelial—Mesenchymal transition of non-small cell lung cancer. EBioMedicine 2019, 46, 42–53. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell. Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Inthanon, S.; Dejkriengkraikul, P.; Yodkeeree, S. Notopterol Suppresses IL-17-Induced Proliferation and Invasion of A549 Lung Adenocarcinoma Cells via Modulation of STAT3, NF-κB, and AP-1 Activation. Int. J. Mol. Sci. 2023, 24, 15057. [Google Scholar] [CrossRef]
- Alisson-Silva, F.; Freire-de-Lima, L.; Donadio, J.L.; Lucena, M.C.; Penha, L.; Sá-Diniz, J.N.; Dias, W.B.; Todeschini, A.R. Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells. PLoS ONE 2013, 8, e60471. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol. Sci. 2014, 35, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, Y.; Saya, H.; Arima, Y. Prospects for new lung cancer treatments that target EMT signaling. Dev. Dyn. 2018, 247, 462–472. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arjsri, P.; Srisawad, K.; Umsumarng, S.; Thippraphan, P.; Anuchapreeda, S.; Dejkriengkraikul, P. Anti-Inflammatory and Anti-Migratory Effects of Morin on Non-Small-Cell Lung Cancer Metastasis via Inhibition of NLRP3/MAPK Signaling Pathway. Biomolecules 2025, 15, 103. https://doi.org/10.3390/biom15010103
Arjsri P, Srisawad K, Umsumarng S, Thippraphan P, Anuchapreeda S, Dejkriengkraikul P. Anti-Inflammatory and Anti-Migratory Effects of Morin on Non-Small-Cell Lung Cancer Metastasis via Inhibition of NLRP3/MAPK Signaling Pathway. Biomolecules. 2025; 15(1):103. https://doi.org/10.3390/biom15010103
Chicago/Turabian StyleArjsri, Punnida, Kamonwan Srisawad, Sonthaya Umsumarng, Pilaiporn Thippraphan, Songyot Anuchapreeda, and Pornngarm Dejkriengkraikul. 2025. "Anti-Inflammatory and Anti-Migratory Effects of Morin on Non-Small-Cell Lung Cancer Metastasis via Inhibition of NLRP3/MAPK Signaling Pathway" Biomolecules 15, no. 1: 103. https://doi.org/10.3390/biom15010103
APA StyleArjsri, P., Srisawad, K., Umsumarng, S., Thippraphan, P., Anuchapreeda, S., & Dejkriengkraikul, P. (2025). Anti-Inflammatory and Anti-Migratory Effects of Morin on Non-Small-Cell Lung Cancer Metastasis via Inhibition of NLRP3/MAPK Signaling Pathway. Biomolecules, 15(1), 103. https://doi.org/10.3390/biom15010103










