The Recent Progress of Tricyclic Aromadendrene-Type Sesquiterpenoids: Biological Activities and Biosynthesis
Abstract
1. Introduction
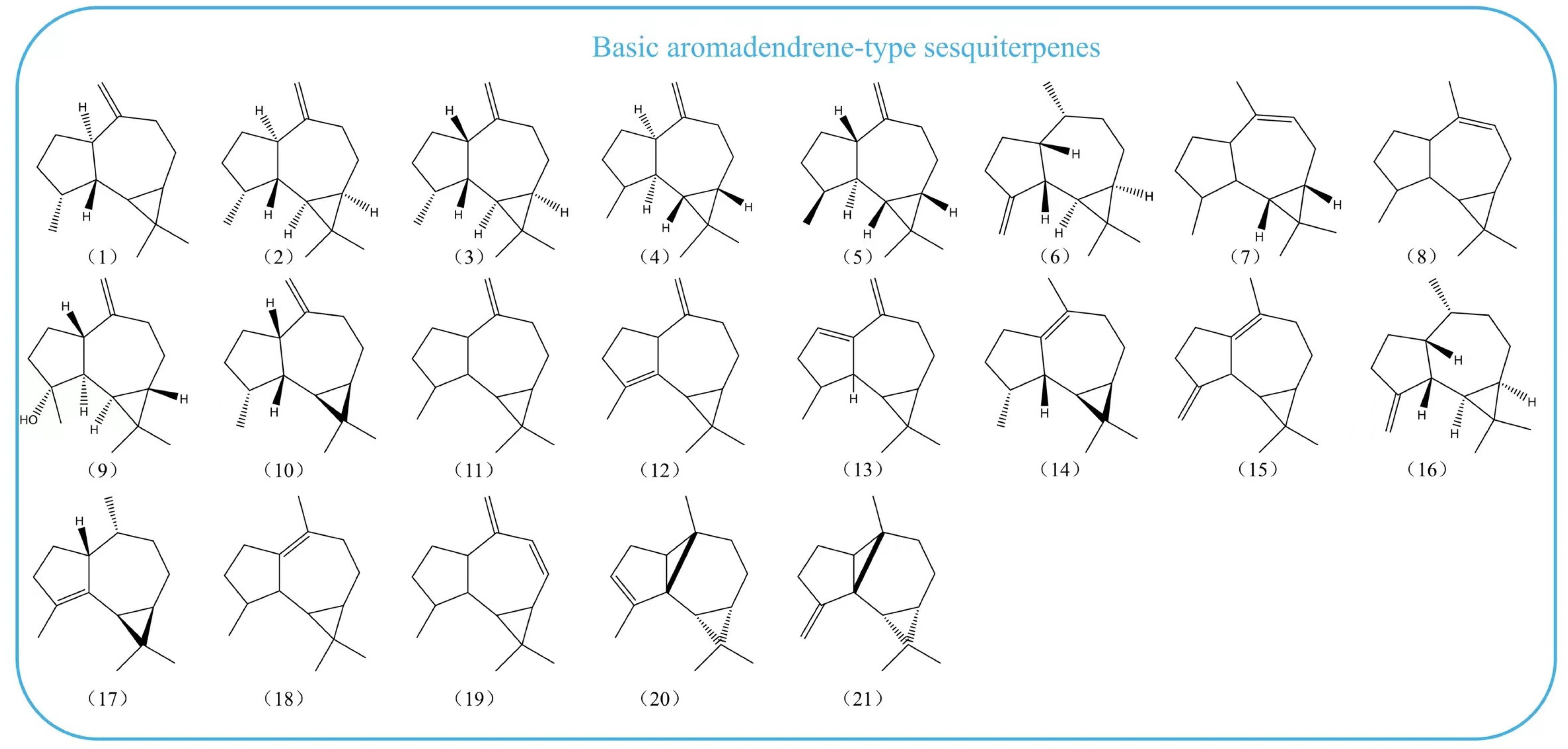
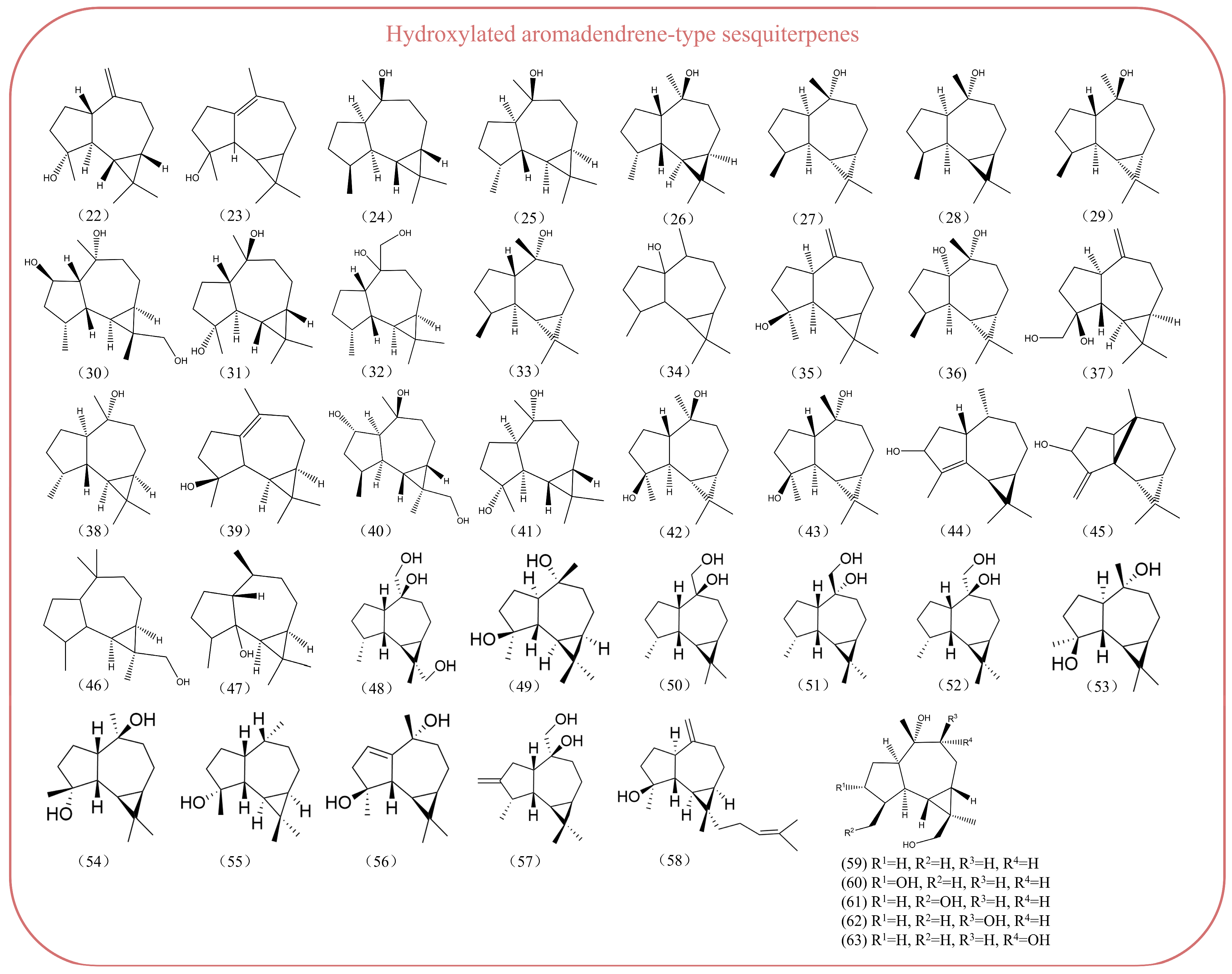
2. Distribution Characteristics of Aromadendrene-Type Sesquiterpenes
2.1. Aromadendrene-Type Sesquiterpenoids in Plant Essential Oils
2.2. Aromadendrene-Type Sesquiterpenoid in Microorganisms
3. Biological Activities of Aromadendrene-Type Sesquiterpenes and Their Related Plant Essential Oils
3.1. Anti-Inflammatory and Analgesic Activities
3.2. Antibacterial and Insecticidal Activity
3.3. Antioxidant Activity and Cytotoxicity
3.4. Other Activities
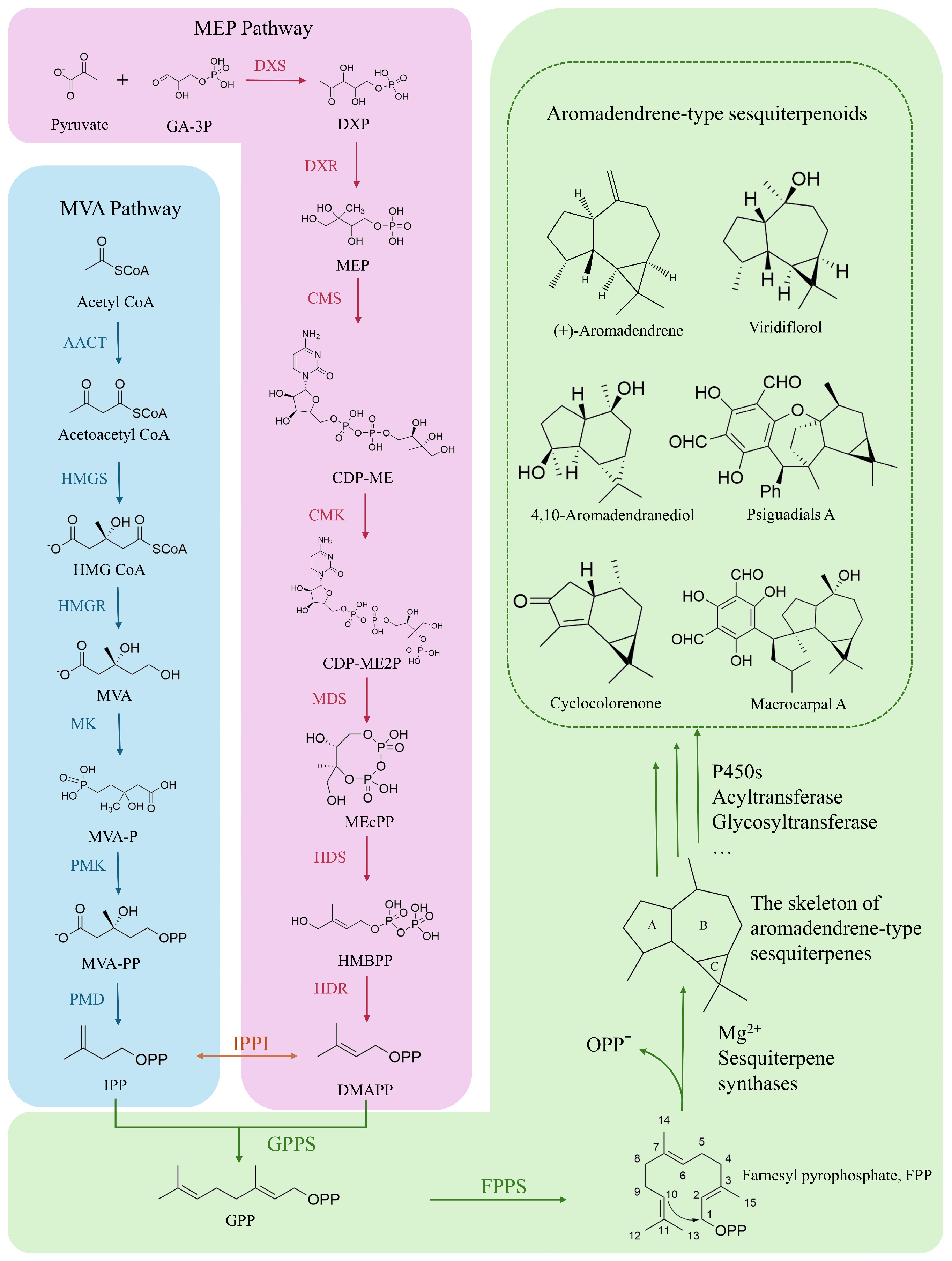
4. Synthetic Biology Research on Aromadendrene-Type Sesquiterpenes
4.1. Discovery and Modification of Key Enzymes for the Synthesis of Aromadendrene-Type Sesquiterpenes
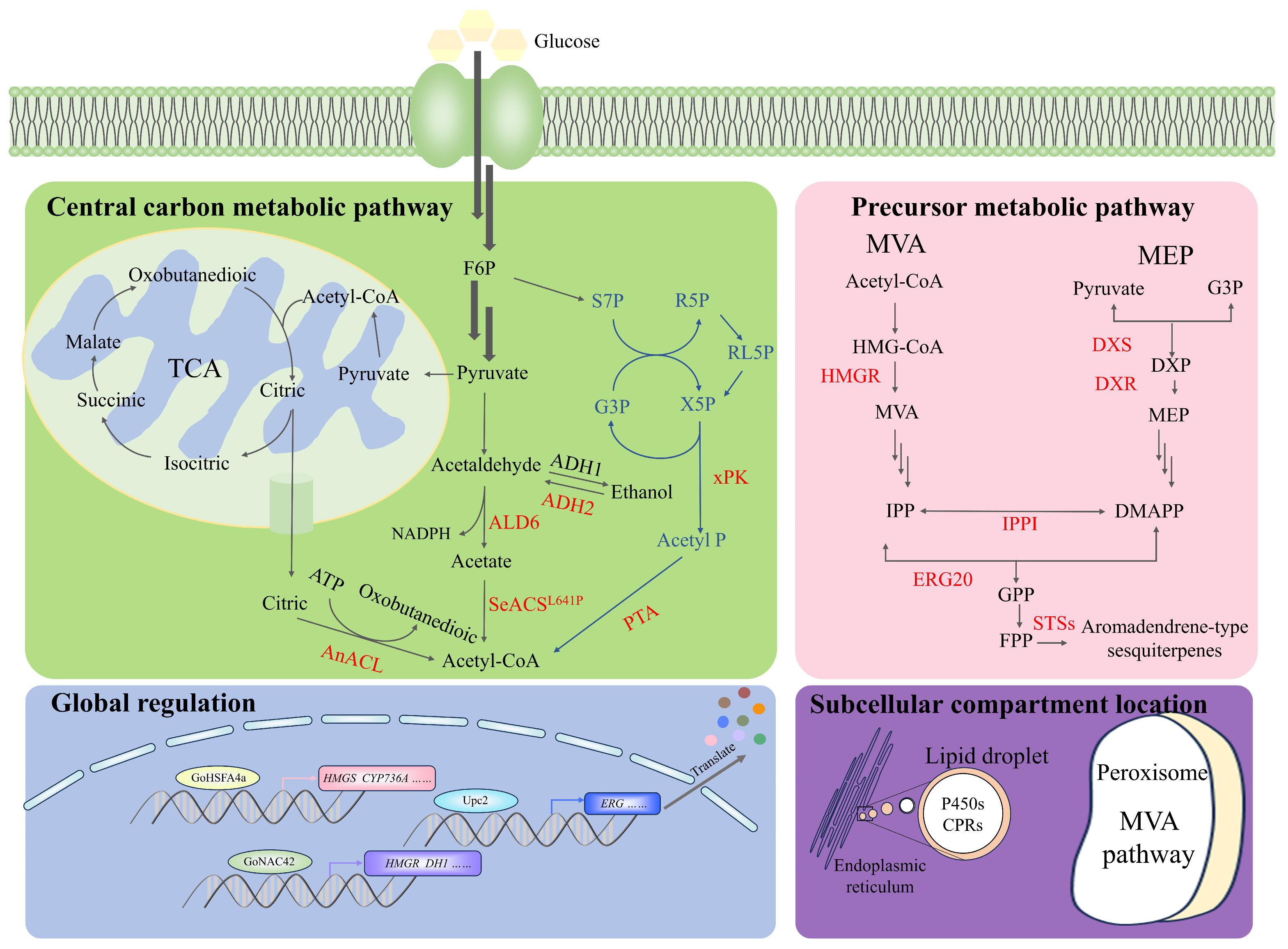
4.2. Selection of Different Hosts for the Synthesis of Aromadendrene-Type Sesquiterpenes
4.3. Metabolic Engineering to Improve Sesquiterpenoid Production
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Silva, G.N.; Rezende, L.C.; Emery, F.S.; Gosmann, G.; Gnoatto, S.C. Natural and semi synthetic antimalarial compounds: Emphasis on the terpene class. Mini Rev. Med. Chem. 2015, 15, 809–836. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, R.; Yan, X.; Liang, D.; Zhang, L.; Qin, X.; Caiyin, Q.; Zhao, G.; Xiao, W.; Hu, Z.; et al. De novo leaf and root transcriptome analysis to explore biosynthetic pathway of Celangulin V in Celastrus angulatus maxim. BMC Genom. 2019, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Buijs, N.A.; Siewers, V.; Nielsen, J. Advanced biofuel production by the yeast Saccharomyces cerevisiae. Curr. Opin. Chem. Biol. 2013, 17, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Williams, T.C.; Peng, B.; Cherry, J. Recent advances in synthetic biology for engineering isoprenoid production in yeast. Curr. Opin. Chem. Biol. 2017, 40, 47–56. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef]
- Lu, J.; Xie, L.; Liu, K.; Zhang, X.; Wang, X.; Dai, X.; Liang, Y.; Cao, Y.; Li, X. Bilobalide: A review of its pharmacology, pharmacokinetics, toxicity, and safety. Phytother. Res. 2021, 35, 6114–6130. [Google Scholar] [CrossRef]
- de Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential oils and isolated terpenes in nanosystems designed for topical administration: A review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Yadav, P.; Mohapatra, S.; Jaiswal, P.O.; Dokka, N.; Tyagi, S.; Sreevathsa, R.; Shasany, A.K. Characterization of a novel cytosolic sesquiterpene synthase MpTPS4 from Mentha × piperita as a bioresource for the enrichment of invaluable viridiflorol in mentha essential oil. Int. J. Biol. Macromol. 2024, 227, 134214. [Google Scholar] [CrossRef]
- Shukal, S.; Chen, X.; Zhang, C. Systematic engineering for high-yield production of viridiflorol and amorphadiene in auxotrophic Escherichia coli. Metab. Eng. 2019, 55, 170–178. [Google Scholar] [CrossRef]
- Gilardoni, G.; Ramirez, J.; Montalvan, M.; Quinche, W.; Leon, J.; Benitez, L.; Morocho, V.; Cumbicus, N.; Bicchi, C. Phytochemistry of three Ecuadorian lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling. Plants 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Medjahed, F.; Merouane, A.; Saadi, A.; Bader, A.; Cioni, P.L.; Flamini, G.J. Chemical profile and antifungal potential of essential oils from leaves and flowers of Salvia algeriensis (Desf.): A comparative study. Chil. J. Agric. Res. 2016, 76, 195–200. [Google Scholar] [CrossRef][Green Version]
- Danijela, V.; Milka, M.; Sanja, C.Z.; Marija, E.S. Comparison of essential oil prof iles of Satureja montana L. and Endemic satureja visianii šilic. J. Essent. Oil Bear. Plants 2009, 12, 273–281. [Google Scholar]
- Jäger, A.K.; Almqvist, J.P.; Vangsøe, S.A.K.; Stafford, G.I.; Adsersen, A.; Van Staden, J. Compounds from Mentha aquatica with affinity to the GABA-benzodiazepine receptor. S. Afr. J. Bot. 2007, 73, 518–521. [Google Scholar] [CrossRef]
- Catinella, G.; Badalamenti, N.; Ilardi, V.; Rosselli, S.; Martino, L.D.; Bruno, M.J.M. The essential oil compositions of three Teucrium taxa growing wild in sicily: HCA and PCA analyses. Molecules 2021, 26, 643. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Segura, C.; Padovan, A.; Kainer, D.; Foley, W.J.; Kulheim, C. Transcriptome analysis of terpene chemotypes of Melaleuca alternifolia across different tissues. Plant Cell Environ. 2017, 40, 2406–2425. [Google Scholar] [CrossRef]
- Maghsoodlou, M.T.; Kazemipoor, N.; Valizadeh, J.; Falak Nezhad Seifi, M.; Rahneshan, N. Essential oil composition of Eucalyptus microtheca and Eucalyptus viminalis. Avicenna J. Phytomed. 2015, 5, 540–552. [Google Scholar]
- Lam, N.S.; Long, X.; Su, X.Z.; Lu, F. Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed. Pharmacother. 2020, 130, 110624. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.D.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Liu, B.; Yan, J.; Li, W.; Yin, L.; Li, P.; Yu, H.; Xing, L.; Cai, M.; Wang, H.; Zhao, M.; et al. Mikania micrantha genome provides insights into the molecular mechanism of rapid growth. Nat. Commun. 2020, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Al-Gendy, A.; Al-Youssef, H.; El-Shazely, A. Chemical constituents and biological activities of Senecio aegyptius var. discoideus Boiss. Z. Naturforsch. C. 2012, 67, 144–150. [Google Scholar] [PubMed]
- Magura, J.; Moodley, R.; Maduray, K.; Mackraj, I. Phytochemical constituents and in vitro anticancer screening of isolated compounds from Eriocephalus africanus (double dagger). Nat. Prod. Res. 2021, 35, 4173–4176. [Google Scholar] [CrossRef]
- Brophy, J.J.; Forster, P.I.; Goldsack, R.J. Coconut Laurels: The leaf essential oils from four endemic Australian Cryptocarya species: C. bellendenkerana, C. cocosoides, C. cunninghamii and C. lividula (Lauraceae). Nat. Prod. Commun. 2016, 11, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Li, W.H.; Hsu, F.L.; Yen, P.L.; Chang, S.T.; Liao, V.H. Essential oil alloaromadendrene from mixed-type Cinnamomum osmophloeum leaves prolongs the lifespan in Caenorhabditis elegans. J. Agric. Food Chem. 2014, 62, 6159–6165. [Google Scholar] [CrossRef]
- Trevizan, L.N.; Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.; Cardoso, C.A.; Vieira, M.D.; Moreira, F.M.; Croda, J.; Formagio, A.S. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 192, 510–515. [Google Scholar]
- Xue, L.; He, Z.; Bi, X.; Xu, W.; Wei, T.; Wu, S.; Hu, S. Transcriptomic profiling reveals MEP pathway contributing to ginsenoside biosynthesis in Panax ginseng. BMC Genom. 2019, 20, 383. [Google Scholar] [CrossRef]
- Morocho, V.; Benitez, A.; Carrion, B.; Cartuche, L. Novel study on chemical characterization and antimicrobial, antioxidant, and anticholinesterase activity of essential oil from Ecuadorian bryophyte Syzygiella rubricaulis (Nees) Stephani. Plants 2024, 13, 935. [Google Scholar] [CrossRef]
- Kokilananthan, S.; Bulugahapitiya, V.P.; Manawadu, H.; Gangabadage, C.S. Investigations of chemical compositions and antioxidative potential of essential oils isolated from the leaves of two Garcinia species. J. Adv. Pharm. Technol. 2023, 14, 12–17. [Google Scholar] [CrossRef]
- Jesionek, A.; Poblocka-Olech, L.; Zabiegala, B.; Bucinski, A.; Krauze-Baranowska, M.; Luczkiewicz, M. Validated HPTLC method for determination of ledol and alloaromadendrene in the essential oil fractions of Rhododendron tomentosum plants and in vitro cultures and bioautography for their activity screening. J. Chromatogr. B 2018, 1086, 63–72. [Google Scholar] [CrossRef]
- Erkan, N.; Tao, Z.; Rupasinghe, H.P.; Uysal, B.; Oksal, B.S. Antibacterial activities of essential oils extracted from leaves of Murraya koenigii by solvent-free microwave extraction and hydro-distillation. Nat. Prod. Commun. 2012, 7, 121–124. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Cianfaglione, K.; Blomme, E.E.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Dall’Acqua, S.; Maggi, F. Cytotoxic essential oils from Eryngium campestre and Eryngium amethystinum (Apiaceae) growing in central Italy. Chem. Biodivers. 2017, 14, e1700096. [Google Scholar] [CrossRef] [PubMed]
- de Matos Balsalobre, N.; dos Santos, E.; Mariano dos Santos, S.; Arena, A.C.; Konkiewitz, E.C.; Ziff, E.B.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Potential anti-arthritic and analgesic properties of essential oil and viridiflorol obtained from Allophylus edulis leaves in mice. J. Ethnopharmacol. 2023, 301, 115785. [Google Scholar] [CrossRef] [PubMed]
- Hackl, T.; Konig, W.A.; Muhle, H. Isogermacrene A, a proposed intermediate in sesquiterpene biosynthesis. Phytochemistry 2004, 65, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, W.; Liang, D.; Caiyin, Q.; Zhao, G.; Zhang, Z.; Wenzhang, M.; Qiao, J. De novo assembly of the Mylia taylorii transcriptome and identification of sesquiterpene synthases. Arch. Biochem. Biophys. 2021, 698, 108742. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Li, W.; Liang, D.; Nie, S.; Chen, R.; Qiao, J.; Wen, M.; Caiyin, Q. Transcriptome analysis and identification of sesquiterpene synthases in liverwort Jungermannia exsertifolia. Bioengineering 2023, 10, 569. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Orban, A.; Shukal, S.; Birk, F.; Too, H.P.; Ruehl, M. Agrocybe aegerita serves as a gateway for identifying sesquiterpene biosynthetic enzymes in higher fungi. ACS Chem. Biol. 2020, 15, 1268–1277. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Oropeza, F.; Duarte, G.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Anaya, A.L. Allelochemical effects of volatile compounds and organic extracts from Muscodor yucatanensis, a tropical endophytic fungus from Bursera simaruba. J. Chem. Ecol. 2010, 36, 1122–1131. [Google Scholar] [CrossRef]
- Nakamori-Maehara, T.; Miyaura, R.; Morikawa, C.I.O.; Pérez de Molas, L.F.; Fujii, Y. Screening of 239 Paraguayan plant species for allelopathic activity using the sandwich method. Allelopathy J. 2018, 44, 245–260. [Google Scholar] [CrossRef]
- Cheng, M.J.; Chen, J.J.; Wu, M.D.; Yang, P.S.; Yuan, G.F. Isolation and structure determination of one new metabolite isolated from the red fermented rice of Monascus purpureus. Nat. Prod. Res. 2010, 24, 979–988. [Google Scholar] [CrossRef]
- Amirzakariya, B.Z.; Shakeri, A. Bioactive terpenoids derived from plant endophytic fungi: An updated review (2011–2020). Phytochemistry 2022, 197, 113130. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; Araújo, A.C.J.D.; Freitas, P.R.; Almeida, R.S.D.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as bacterial efflux pump inhibitors: A systematic review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Budiene, J.; Svediene, J.; Garjonyte, R. Toxic, radical scavenging, and antifungal activity of Rhododendron tomentosum H. essential oils. Molecules 2020, 25, 1676. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.R.R.; Fernandes, C.C.; Gonçalves, D.S.; Martins, C.H.G.; Miranda, M.L.D. Chemical composition and anti-Xanthomonas citri activities of essential oils from Schinus molle L. fresh and dry leaves and of its major constituent spathulenol. Nat. Prod. Res. 2023, 1–5. [Google Scholar] [CrossRef]
- Amoah, S.K.; Dalla Vecchia, M.T.; Pedrini, B.; Carnhelutti, G.L.; Goncalves, A.E.; Dos Santos, D.A.; Biavatti, M.W.; de Souza, M.M. Inhibitory effect of sesquiterpene lactones and the sesquiterpene alcohol aromadendrane-4beta,10alpha-diol on memory impairment in a mouse model of Alzheimer. Eur. J. Pharmacol. 2015, 769, 195–202. [Google Scholar] [CrossRef]
- Gilabert, M.; Marcinkevicius, K.; Andujar, S.; Schiavone, M.; Arena, M.E.; Bardon, A. Sesqui- and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine 2015, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Behiry, S.I.; El-Hefny, M.; Salem, M.Z.M. Toxicity effects of Eriocephalus africanus L. leaf essential oil against some molecularly identified phytopathogenic bacterial strains. Nat. Prod. Res. 2020, 34, 3394–3398. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhaes, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Akiel, M.A.; Alshehri, O.Y.; Aljihani, S.A.; Almuaysib, A.; Bader, A.; Al-Asmari, A.I.; Alamri, H.S.; Alrfaei, B.M.; Halwani, M.A. Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J. Biol. Sci. 2022, 29, 816–821. [Google Scholar] [CrossRef]
- Chang, S.; Ruan, W.C.; Xu, Y.Z.; Wang, Y.J.; Pang, J.; Zhang, L.Y.; Liao, H.; Pang, T. The natural product 4,10-aromadendranediol induces neuritogenesis in neuronal cells in vitro through activation of the ERK pathway. Acta Pharmacol. Sin. 2017, 38, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.L.; Li, W.; Zhou, B.H.; Chen, L.; Weng, H.Z.; Zou, Y.H.; Tang, G.H.; Bu, X.Z.; Yin, S. (+)-Isobicyclogermacrenal and spathulenol from Aristolochia yunnanensis alleviate cardiac fibrosis by inhibiting transforming growth factor β/small mother against decapentaplegic signaling pathway. Phytother. Res. 2019, 33, 214–223. [Google Scholar] [CrossRef]
- Schmidt, C.O.; Bouwmeester, H.J.; Bulow, N.; Konig, W.A. Isolation, characterization, and mechanistic studies of (-)-alpha-gurjunene synthase from Solidago canadensis. Arch. Biochem. Biophys. 1999, 364, 167–177. [Google Scholar] [CrossRef]
- Zha, W.; An, T.; Li, T.; Zhu, J.; Gao, K.; Sun, Z.; Xu, W.; Lin, P.; Zi, J. Reconstruction of the biosynthetic pathway of santalols under control of the GAL regulatory system in yeast. ACS Synth. Biol. 2020, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lin, Y.C.; Guo, J.J.; Du, M.M.; Tao, X.; Gao, B.; Zhao, M.; Ma, Y.; Wang, F.Q.; Wei, D.Z. High-level production of sesquiterpene patchoulol in Saccharomyces cerevisiae. ACS Synth. Biol. 2021, 10, 158–172. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.; Zhang, Y.; Liang, D.; Caiyin, Q.; Qiao, J. Characterization of trans-nerolidol synthase from Celastrus angulatus Maxim and production of trans-nerolidol in engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Kumar, S.; Kempinski, C.; Zhuang, X.; Norris, A.; Mafu, S.; Zi, J.; Bell, S.A.; Nybo, S.E.; Kinison, S.E.; Jiang, Z.; et al. Molecular diversity of terpene synthases in the liverwort Marchantia polymorpha. Plant Cell 2016, 28, 2632–2650. [Google Scholar] [CrossRef]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for the volatile terpene emissions in flowers of Freesia hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Kitaoka, T. Insight into metabolic diversity of the brown-rot basidiomycete Postia placenta responsible for sesquiterpene biosynthesis: Semi-comprehensive screening of cytochrome P450 monooxygenase involved in protoilludene metabolism. Microb. Biotechnol. 2018, 11, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, E.; Huang, X.; Kou, J.; Teng, D.; Lv, B.; Han, X.; Zhang, Y. Characterization of a novel insect-induced sesquiterpene synthase GbTPS1 based on the transcriptome of Gossypium barbadense feeding by Cotton Bollworm. Front. Plant Sci. 2022, 13, 898541. [Google Scholar] [CrossRef]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-Ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Ntana, F.; Bhat, W.W.; Johnson, S.R.; Jorgensen, H.J.L.; Collinge, D.B.; Jensen, B.; Hamberger, B. A sesquiterpene synthase from the endophytic fungus Serendipita indica catalyzes formation of viridiflorol. Biomolecules 2021, 11, 898. [Google Scholar] [CrossRef]
- Chou, W.K.; Fanizza, I.; Uchiyama, T.; Komatsu, M.; Ikeda, H.; Cane, D.E. Genome mining in Streptomyces avermitilis: Cloning and characterization of SAV_76, the synthase for a new sesquiterpene, avermitilol. J. Am. Chem. Soc. 2010, 132, 8850–8851. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, J.; Chen, S.; Yang, Z.; Wang, Z.; Xu, H.M.; Dong, L.B. Discovery of sesquiterpenoids from an actinomycete Crossiella cryophila through genome mining and heterologous expression. Bioorg. Chem. 2024, 146, 107308. [Google Scholar] [CrossRef]
- Padovan, A.; Keszei, A.; Kollner, T.G.; Degenhardt, J.; Foley, W.J. The molecular basis of host plant selection in Melaleuca quinquenervia by a successful biological control agent. Phytochemistry 2010, 71, 1237–1244. [Google Scholar] [CrossRef]
- Shasany, A.K.; Yadav, P.; Rastogi, S.; Jalil, S.U.; Bhakuni, R.S. Method for Increasing Viridiflorol Content in Tissues. Patent US2021403827A1, 30 December 2021. [Google Scholar]
- Baer, P.; Rabe, P.; Fischer, K.; Citron, C.A.; Klapschinski, T.A.; Groll, M.; Dickschat, J.S. Induced-fit mechanism in class I terpene cyclases. Angew. Chem. Int. Ed. 2014, 53, 7652–7656. [Google Scholar] [CrossRef]
- Baer, P.; Rabe, P.; Citron, C.A.; de Oliveira Mann, C.C.; Kaufmann, N.; Groll, M.; Dickschat, J.S. Hedycaryol synthase in complex with nerolidol reveals terpene cyclase mechanism. Chembiochem 2014, 15, 213–216. [Google Scholar] [CrossRef]
- Li, J.X.; Fang, X.; Zhao, Q.; Ruan, J.X.; Yang, C.Q.; Wang, L.J.; Miller, D.J.; Faraldos, J.A.; Allemann, R.K.; Chen, X.Y.; et al. Rational engineering of plasticity residues of sesquiterpene synthases from Artemisia annua: Product specificity and catalytic efficiency. Biochem. J. 2013, 451, 417–426. [Google Scholar] [CrossRef]
- Leferink, N.G.H.; Scrutton, N.S. Predictive engineering of class I terpene synthases using experimental and computational approaches. Chembiochem 2021, 23, e202100484. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.L.; Johns, S.T.; Walters, R.; Miller, D.J.; Van der Kamp, M.W.; Allemann, R.K. Active site loop engineering abolishes water capture in hydroxylating sesquiterpene synthases. ACS Catal. 2023, 13, 14199–14204. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Cramer, N. Biomimetic synthesis of (+)-ledene, (+)-viridiflorol, (-)-palustrol, (+)-spathulenol, and psiguadial A, C, and D via the platform terpene (+)-bicyclogermacrene. Chemistry 2014, 20, 10654–10660. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, J.; Ge, J.; Li, W.; Liang, D.; Singh, W.; Black, G.; Nie, S.; Liu, J.; Sun, M.; et al. Computer-informed engineering: A new class I sesquiterpene synthase JeSTS4 for the synthesis of an unusual C10-(S)-bicyclogermacrene. ACS Catal. 2022, 12, 4037–4045. [Google Scholar] [CrossRef]
- Starks, C.M.; Noel, J.P. Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 1997, 277, 1815–1820. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, N.; Zhou, J.; Wu, R. Protonation-dependent diphosphate cleavage in FPP cyclases and synthases. ACS Catal. 2016, 6, 6918–6929. [Google Scholar] [CrossRef]
- Srivastava, P.L.; Escorcia, A.M.; Huynh, F.; Miller, D.J.; Allemann, R.K.; van der Kamp, M.W. Redesigning the molecular choreography to prevent hydroxylation in germacradien-11-ol synthase catalysis. ACS Catal. 2021, 11, 1033–1041. [Google Scholar] [CrossRef]
- Grundy, D.J.; Chen, M.; Gonzalez, V.; Leoni, S.; Miller, D.J.; Christianson, D.W.; Allemann, R.K. Mechanism of germacradien-4-ol synthase-controlled water capture. Biochemistry 2016, 55, 2112–2121. [Google Scholar] [CrossRef]
- Allemann, R.K.; Loizzi, M.; Gonzalez, V.; Miller, D. Nucleophilic water capture or proton loss: Single amino acid switch converts δ-cadinene synthase to germacradienl synthase. Chembiochem 2018, 19, 100–105. [Google Scholar]
- Gonzalez Requena, V.; Srivastava, P.; Miller, D.; Allemann, R.K. Single point mutation abolishes water capture in germacradien-4-ol synthase. Chembiochem 2024, e202400290. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.L.; Johns, S.T.; Voice, A.; Morley, K.; Escorcia, A.M.; Miller, D.J.; Allemann, R.K.; van der Kamp, M.W. Simulation-guided engineering enables a functional switch in selinadiene synthase toward hydroxylation. ACS Catal. 2024, 14, 11034–11043. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yu, W.; Chen, Y.; Yang, S.; Zhao, Z.K.; Nielsen, J.; Luan, H.; Zhou, Y.J. Engineering yeast for high-level production of diterpenoid sclareol. Metab. Eng. 2023, 75, 19–28. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, X.; Jiang, G.; Wu, J.; Zhang, J.L.; Lei, D.; Yuan, Y.J.; Qiao, J.; Zhao, G.R. Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae. ACS Synth. Biol. 2019, 8, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.L.; Xu, J.Z.; Zhang, W.G. Dual regulation of cytoplasm and peroxisomes for improved alpha-farnesene production in recombinant Pichia pastoris. ACS Synth. Biol. 2021, 10, 1563–1573. [Google Scholar] [CrossRef]
- Zuo, Y.; Xiao, F.; Gao, J.; Ye, C.; Jiang, L.; Dong, C.; Lian, J. Establishing Komagataella phaffii as a cell factory for efficient production of sesquiterpenoid alpha-santalene. J. Agric. Food Chem. 2022, 70, 8024–8031. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Cui, Z.; Wang, Z.; Qi, Q.; Hou, J. Engineering the oleaginous yeast Yarrowia lipolytica for production of alpha-farnesene. Biotechnol. Biofuels 2019, 12, 296. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, T.Q.; Peng, Q.Q.; Sun, X.M.; Ji, X.J.; Huang, H. Harnessing Yarrowia lipolytica peroxisomes as a subcellular factory for α-humulene overproduction. J. Agric. Food Chem. 2021, 69, 13831–13837. [Google Scholar] [CrossRef]
- Jia, D.; Xu, S.; Sun, J.; Zhang, C.; Li, D.; Lu, W. Yarrowia lipolytica construction for heterologous synthesis of alpha-santalene and fermentation optimization. Appl. Microbiol. Biotechnol. 2019, 103, 3511–3520. [Google Scholar] [CrossRef]
- Vavitsas, K.; Fabris, M.; Vickers, C.E. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes 2018, 9, 520. [Google Scholar] [CrossRef]
- Ma, H.; Steede, T.; Dewey, R.E.; Lewis, R.S. Engineering sclareol production on the leaf surface of Nicotiana tabacum. J. Agric. Food Chem. 2024, 72, 13812–13823. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Nishimoto, M.; Chow, R.W.; Baidoo, E.E.; Wang, G.; Martin, J.; Schackwitz, W.; Chan, R.; Fortman, J.L.; Keasling, J.D. Enhancing terpene yield from sugars via novel routes to 1-deoxy-d-xylulose 5-phosphate. Appl. Environ. Microbiol. 2015, 81, 130–138. [Google Scholar] [CrossRef]
- Nielsen, J. Cell factory engineering for improved production of natural products. Nat. Prod. Rep. 2019, 36, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.C.; Williams, T.C.; Schulz, B.L.; Palfreyman, R.W.; Krömer, J.O.; Nielsen, L.K. Evolutionary engineering improves tolerance for replacement jet fuels in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015, 81, 3316–3325. [Google Scholar] [CrossRef]
- Jorda, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Weston, N.; Sharma, P.; Ricci, V.; Piddock, L.J.V. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res. Microbiol. 2018, 169, 425–431. [Google Scholar] [CrossRef]
- Zhang, C.; Seow, V.Y.; Chen, X.; Too, H.P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018, 9, 1858. [Google Scholar] [CrossRef]

| Compound Name | Species | Family and Genus | Content (%) | Ref. | |
|---|---|---|---|---|---|
| Plants | |||||
| Viridiflorene (18) | Lepechinia heteromorpha | Lamiaceae | Lepechinia | 27.30% | [12] |
| Viridiflorol (26) | Salvia algeriensis | Salvia | 71.10% | [13] | |
| Viridiflorol (26) | Satureja visianii | Satureja | 17.90% | [14] | |
| Viridiflorol (26) | Mentha aquatica | Mentha | 11.30% | [15] | |
| Ledol (28) | Lepechinia heteromorpha | Lepechinia | 21.20% | [12] | |
| epi-Globulol (25) | Teucrium montanum | Teucrium | [16] | ||
| α-Gurjunene (17) | Melaleuca alternifolia | Myrtaceae | Melaleuca | Stem (1.1%) | [17] |
| Aromadendrene (11) | Eucalyptus microtheca | Eucalyptus | Leaves (12.773%), flowers (7.444%) | [18] | |
| Aromadendrene (11) | Melaleuca alternifolia | Melaleuca | Stem (1.6%) | [19] | |
| Viridiflorene (18) | Stem (1.6%) | [18] | |||
| Viridiflorol (26) | Stem (1.3%) | ||||
| Globulol (38) | Stem (1.4%) | ||||
| Globulol (38) | Eucalyptus microtheca | Eucalyptus | Leaves (5.997%), flowers (5.419%) | [18] | |
| Globulol (38) | Eucalyptus viminalis | Leaves (3.054%) | [18] | ||
| Spathulenol (35) | Psidium guineense | Psidium | Leaves (80.7%) | [20] | |
| α-Gurjunene (17) | Mikania micrantha | Asteraceae | Mikania | 9% | [21] |
| Viridiflorol (26) | Senecio rowleyanus | Senecio | 11.00% | [22] | |
| Ledol (28) | Eriocephalus africanus | Eriocephalus | Leaves (19.92%) | [23] | |
| Isospathulenol (39) | Anthemis pignattiorum | Anthemis | 10.60% | [23] | |
| Viridiflorene (18) | Cryptocarya bellendenkerana | Lauraceae | Cryptocarya | [24] | |
| Alloaromadendrene (10) | Cryptocarya osmophloeum | Leaves (5.0%) | [25] | ||
| Viridiflorol (26) | Cryptocarya bellendenkerana | [24] | |||
| α-Gurjunene (17) | Dimocarpus longan | Sapindaceae | Dimocarpus | Peel (11–24%) | |
| Viridiflorol (26) | Allophylus edulis | Allophylus | Leaves (30.88%) | [26] | |
| α-Gurjunene (17) | Panax ginsengr | Araliaceae | Panax | Root (8%) | [27] |
| Viridiflorene (18) | Syzygiella rubricaulis | Cryptocapsaceae | Syzygiella | [28] | |
| Alloaromadendrene (10) | Garcinia quaesita | Lutaceae | Garcinia | Leaves (11.12%) | [29] |
| Ledol (28) | Rhododendron tomentosum | Ericaceae | Rhododendron | [30] | |
| Palustrol (34) | |||||
| β-Gurjunene (6) | Murraya koenigii | Rutaceae | Murraya | 25% | [31] |
| Isospathulenol (39) | Murraya paniculata | ||||
| Ledol (28) | Hagenia abyssinica | Rosaceae | Hagenia | Flowers (58.57%) | [32] |
| Ledol (28) | Eryngium campestre, Eryngium amethystinum | Apiaceae | Eryngium | [33] | |
| Spathulenol (35) | Eryngium campestre, Eryngium amethystinum | ||||
| Spathulenol (35) | Schinus molle | Burseraceae | Schinus | [34] | |
| Myli-4(15)-ene (21) | Mylia taylorii, Mylia nuda | Jungermanniaceae | Mylia | [35,36] | |
| Aromadendrene (11) | |||||
| Aromadendra-4,10(14)-diene (12) | |||||
| α-Gurjunene (17) | |||||
| Anastreptene (20) | |||||
| Viridiflorene (18) | |||||
| Viridiflorol (26) | |||||
| Globulol (38) | |||||
| Myliol (45) | |||||
| Spathulenol (35) | |||||
| Aromadendrene (11) | Jungermannia exsertifolia | Jungermanniaceae | Jungermannia | [37] | |
| Viridiflorol (26) | |||||
| Microorganisms | |||||
| Viridiflorene (18) | Agrocybe aegerita | Strophariaceae | Agrocybe | [38] | |
| β-Gurjunene (6) | |||||
| Viridiflorol (26) | |||||
| Aromadendrene (11) | Muscodor yucatanensis | Muscodor | [39] | ||
| Spathulenol (35) | Monascus purpureus | Monascus | [40] | ||
| Name | ID | Products | Species | Ref. |
|---|---|---|---|---|
| MpMTPSL4 | KU664191 | α-Gurjunene (17) | Marchantia polymorpha | [60] |
| FhTPS8 | Unigene_80141 | α-Gurjunene (17) | Freesia x hybrida | [61] |
| PpSTP06 | - | α-Gurjunene (17) | Postpartum | [62] |
| GbTPS1 | GB_D01G0996 | α-Gurjunene (17) | Gossypium barbadense | [63] |
| Agr2 | A0A5Q0QNJ2 | Viridiflorene (18) | Agrocybe aegerita | [38] |
| SLT18 | BAP82213.1 | Viridiflorene (18) | Streptomyces lactacystinaeus | [64] |
| SiTPS | JGI Protein Id: 77541 | Viridiflorene (18); Viridiflorol (26) | Serendipita indica | [65] |
| Sav_76 | BA000030.4 | Avermitilol; Viridiflorol (26) | Streptomyces avermitilis | [66] |
| Agr5 | A0A5Q0QSI8.1 | Viridiflorol (26) | Agrocybe aegerita | [38] |
| CryA | A0A7W7FVV8 | ent-Viridiflorol (27) | Crossiella cryophila | [67] |
| MqTPS1 | - | Viridiflorol (26) | Melaleuca quinquenervia | [68] |
| MpTPS4 | MH790402.1 | Viridiflorol (26) | Mentha piperita | [10,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Lin, J.; Liu, Z.; David, S.D.; Liang, D.; Nie, S.; Ge, M.; Xue, Z.; Li, W.; Qiao, J. The Recent Progress of Tricyclic Aromadendrene-Type Sesquiterpenoids: Biological Activities and Biosynthesis. Biomolecules 2024, 14, 1133. https://doi.org/10.3390/biom14091133
Yan X, Lin J, Liu Z, David SD, Liang D, Nie S, Ge M, Xue Z, Li W, Qiao J. The Recent Progress of Tricyclic Aromadendrene-Type Sesquiterpenoids: Biological Activities and Biosynthesis. Biomolecules. 2024; 14(9):1133. https://doi.org/10.3390/biom14091133
Chicago/Turabian StyleYan, Xiaoguang, Jiaqi Lin, Ziming Liu, Sichone Daniel David, Dongmei Liang, Shengxin Nie, Mingyue Ge, Zhaohui Xue, Weiguo Li, and Jianjun Qiao. 2024. "The Recent Progress of Tricyclic Aromadendrene-Type Sesquiterpenoids: Biological Activities and Biosynthesis" Biomolecules 14, no. 9: 1133. https://doi.org/10.3390/biom14091133
APA StyleYan, X., Lin, J., Liu, Z., David, S. D., Liang, D., Nie, S., Ge, M., Xue, Z., Li, W., & Qiao, J. (2024). The Recent Progress of Tricyclic Aromadendrene-Type Sesquiterpenoids: Biological Activities and Biosynthesis. Biomolecules, 14(9), 1133. https://doi.org/10.3390/biom14091133





